Abstract
The downstream processing of efficient biomass-based microbial biopesticides is heavily reliant on obtaining the largest concentration of viable cells in the most cost-effective manner. The goal of this research was to assess the ability of chitosan flocculation to recover bacterial Bacillus sp. BioSol021 biomass from the broth after biological treatment of wastewaters from the dairy and wine industries. Second-order factorial design models were used to estimate the effect of chitosan concentration and mixing speed on flocculation efficiency, settling velocity, and antimicrobial activity against Aspergillus flavus, i.e., inhibition zone diameter. Response surface methodology was followed by multi-objective optimization by applying the desirability function (DF) and genetic algorithm (GA). The optimum values for flocculation efficiency, settling velocity, and inhibition zone diameter for cheese whey effluent were 88%, 0.10 mm/s, and 51.00 mm, respectively. In the case of winery flotation effluent, the optimum values were flocculation efficiency 95% and settling velocity 0.05 mm/s, while the inhibition zone diameter was 48.00 mm. These results indicate that utilizing chitosan as a flocculation agent not only fits the criteria for effective downstream processing, but also has a synergistic effect on Bacillus sp. antibacterial activity.
1. Introduction
Food spoilage organisms are widely regarded as a severe public health threat and provide significant health concerns to consumers and the food industry [1,2]. The use of natural antimicrobial agents represents a highly effective approach for biopreservation of food products and a significant part of the green food revolution. Some plant pathogens, such as fungi Aspergillus flavus, beside the problem of food spoilage, release mycotoxin contamination that could influence human health. A well-known opportunistic pathogen of numerous crops with economic value, such as maize, peanuts, and cotton, Aspergillus flavus is also a noteworthy producer of aflatoxins [3,4]. Aflatoxin B1 (AFB1) is categorized by the International Agency for Research on Cancer (IARC) in the 1a group of substances that are carcinogenic to both humans and animals. AFB1 is one of the few mycotoxins that have been used in the development of biological weapons and is regarded as the most potent natural carcinogen [5,6].
Plant growth promotion, induction of systemic plant resistance, biofilm formation, competition for nutrients and space, lytic effect, and antibiotic production are just a few of the diverse mechanisms of action used by various bacterial biopesticides of the Bacillus genus whose cells and active metabolite components display intense antibacterial and antifungal activity against the different plant pathogens [7]. The substitution of chemical pesticides by biological ones is a foreseeable trend. However, a number of obstacles have prevented the commercial production and use of microbial products. Despite the substantial potential of currently known and utilized biopesticides, there are still only a few products on the market at large. The high cost of biotechnological production, with a focus on the costs of capital equipment, sterility requirements, and commercially available nutritive media, is recognized for the lack of full commercialization [4].
The utilization of alternative complex media based on industrial effluents is the solution to the current limitations. It may be advantageous from the perspective of the designed bioprocess’s economic efficiency, but at the same time, can be regarded as an environmentally friendly solution aimed at the sustainability of the production processes used to create microbial biocontrol agents [4,8]. Wastewaters from the food industry are of particular interest as potential raw materials for the production of microbial biopesticides. The high organic and inorganic content of these effluents, which makes their treatment before disposal mandatory, has been the subject of numerous studies, including their biotechnological valorization [9]. In accordance with the principles of the circular economy and industrial symbiosis, this may also offer a way to recycle water and reduce the consumption of fresh water by the food industry [10].
Moreover, the choice of the growth medium, due to the low concentration and microorganisms’ dimensions (the majority are small and have a negative surface charge), submerged fermentation microorganism harvesting is frequently challenging [11]. However, the effective recovery of the cultivation broth components, particularly microbial biomass, has not yet been thoroughly discussed in relation to the production of Bacillus-based biopesticides [12]. Various separation technologies have been developed for microorganism harvesting such as centrifugation, coagulation, filtration, and flocculation [13]. Flocculation has often been used as an aid to enhance the effectiveness of the downstream processes, sedimentation, centrifugal recovery, and filtration, through particle size enlargement [14].
The choice of coagulants/flocculant is essential to ensure that its use does not adversely impact downstream processing, i.e., flocculants should not hinder downstream processes taking into consideration the requirement to preserve cell viability. The usual choices are synthetic organic polymers (polyacrylamide derivatives and polyethylene amine) and inorganic metal salts (aluminum and ferric sulfates and chlorides), particularly in water treatment processes [15]. Although these substances have been shown to be an efficient for cell separations, if the biomass is to be used for food, aquaculture, animal fodder, or fertilizer, they also may have effects on human health [16].
Chitosan, starch, cellulose, tannins, and microbial basic materials make up the majority of natural flocculants that can substitute synthetic organic polymers [17]. Chitosan has been suggested for use as a flocculant because it is non-toxic, non-corrosive, and safe to handle. Additionally, it is biodegradable and biocompatible while also having characteristics of both coagulants and flocculants [17,18]. Chitosan has a high cationic charge density, which enables it to successfully destabilize other particles by strongly adsorbing negative regions on them. This process may operate via charge neutralization (electrostatic patch effects) or polymer bridging [16]. Numerous industries, including cosmetics, biomedical engineering, pharmaceuticals, ophthalmology, biotechnology, agriculture, textiles, oenology, food processing, and nutrition, use chitosan due to its wide range of physico-chemical and biological properties [18,19].
The primary goal of this study was to assess the potential of chitosan for flocculation of fermentation media in order to concentrate Bacillus sp. BioSol021 biomass for biopesticide production. Another goal was to evaluate a possible synergetic effect of chitosan combined with Bacillus sp. BioSol021 biomass against plant pathogens as it is a fungus known to be a producer of aflatoxins Aspergillus flavus. The present study investigated the influence of chitosan dosage and mixing speed on the flocculation process of Bacillus sp. BioSol021 broth coming from a biological treatment of wastewaters from the dairy and wine industries (cheese whey and winery flotation wastewaters). Besides that, the factor influence on the settling velocity and antimicrobial activity was evaluated. Multi-criteria optimization was performed in order to optimize the process of cell separation in accordance with other goals of flocculation using chitosan.
2. Materials and Methods
2.1. Microorganisms and Cultivation Media
Bacillus sp. strain BioSol021, which was isolated from the rhizosphere of common beans or kidney beans (Phaseolus vulgaris), was the producing microorganism used in this study. It belongs to the operational group Bacillus amyloliquefaciens [4]. The investigated phytopathogen was Aspergillus flavus strain PA2D SS isolated from maize with proven aflatoxigenic potential [20]. The details about storage can be found in previously published paper [4].
Cultivations of the producing microorganism were performed using media based on cheese whey (CW) as the dairy industry effluents and winery flotation wastewater (WFW), and the details about cultivation are described in reference [4].
2.2. Flocculation and Settling Tests
The jar tests were applied to determine the optimal experimental conditions in terms of the selected flocculation process variables.
Chitosan was provided by Acros Organics (Geel, Belgium) (90% deacetylated, molecular mass 600,000–800,000 Da). Chitosan solution was prepared by mixing 100 mg (dry weight) of chitosan with 100 mL of 0.1 M HCl solution with continuous stirring by the magnetic stirrer (25 °C, 100 rpm). Stirring was carried out until chitosan was completely dissolved in the solution. Only freshly prepared solutions (stored at 4 °C for less than 24 h) were used in all flocculation experiments.
Flocculation assays were conducted in 400 mL glass beakers with 250 mL of the Bacillus sp. BioSol021 cultivation broth, obtained after the 96 h bioreactor cultivation to determine the optimal experimental conditions in terms of the selected flocculation process variables. Following the addition of the chitosan to the selected dosage (varied in experiments), pH was adjusted by dropwise addition of 0.1 M NaOH/0.1 M HCl to a final pH of 5.0. After that, the sample beaker was placed on a magnetic stirrer, first for 5 min of rapid mixing (varied in experiments) to disperse the flocculant, and then, the speed was reduced to a slow mixing speed (50 rpm) for 30 min to enhance particle collisions. All flocculation experiments were conducted at the same temperature (25 °C).
The flocculation efficiency, after 60 min of settling, was calculated by the spectrophotometric measurements of the microbial suspension optical density (absorbance at 600 nm, UV 1800, Shimadzu, Kyoto, Japan) using Equation (1):
where OD0 and OD are the optical densities of the samples taken before and after the flocculation, respectively.
Settling test procedures in the field of wastewater treatment were used [21,22]. Static column settling experiments were conducted to measure the settling velocity of the formed flocs (height, 20 cm; internal diameter, 2.6 cm). The flocculated broth samples were gently poured into a column to prevent any breakage; then, the settling test began, and no further disturbances were allowed. The mud line, the decline of the solids/liquid interface, was carefully observed and recorded as a function of settling time at one minute intervals over 60 minutes. Settling velocity was determined using the slope of the linear section of the sedimentation curve [22]. Tests run without flocculant addition and without agitation were completed to determine the settling velocity of the culture broth.
2.3. Antimicrobial Activity
After the flocculation tests, antimicrobial activity of the precipitate against the phytopathogen Aspergillus flavus strain PA2D SS was examined in vitro using the well diffusion method. In 90 mm Petri plates, a mixture of the SMA medium (15 mL) and fungal pathogen suspension in sterile saline (1 mL–105 CFU/mL), homogenized using the vortex mixer, was spread. After the medium solidification, three wells per plate with a diameter of 10 mm were made. In each well, 100 μL of the flocculated sample was added. The incubation was performed at 26 °C for 96 h, followed by measurements of the inhibition zone diameters.
2.4. Modeling and Optimization
The response surface methodology (RSM) models were used to build the relationship between the factors and responses. The experimental design (Table 1 and Table 2) was based on a central composite design for two factors (alpha = 1.41421), which was a second-order factorial design. In a central composite design, alpha is the distance between each axial point (also known as a star point) and the center. Each variable in the design has new extreme values (low and high) represented by the star points. The independent variables were chitosan dosage (mg/L) and mixing speed (rpm). The selected range for mixing speed was 150–600 rpm, while in the case chitosan dosage, two ranges were selected: 30–150 mg/L for cheese whey effluent and 120–400 mg/L for winery flotation effluent.

Table 1.
Experimental design and responses for cheese whey effluent.

Table 2.
Experimental design and responses for winery flotation effluent.
From the experimental plan, the following second-order polynomial function Equation (2) was obtained:
where Xi represents design variables (X1–chitosan dosage (mg/L) and X2-he mixing speed (rpm)); b0 represents the intercept (constant), bi the linear, bii the quadratic, and bij the interaction effect of the factors; Y represents the response (flocculation efficiency (%), settling velocity (mm/s), and inhibition zone diameter (mm)).
Optimization of experimental results was performed through numerical optimization, i.e., the desirability function (DF) and the genetic algorithm (GA) combined with the response surface models. The RSM numerical optimization provides a local solution to the non-linear model, whereas GA provides a set of non-dominated Pareto optimal solutions [23]. The optimization problem studied is represented mathematically as
For creating polynomial RSM models and optimization using the desirability function technique, Stat-Ease, Inc.’s Design-Expert software, version 8.1, was used, whilst MATLAB software (R2015b, MathWorks, USA) was used for GA optimization.
2.5. Statistical Analysis
Using multiple regression analysis on the experimental data, the quadratic equation was defined to relate responses and design variables. Statistical analysis of the quadratic polynomial model was performed by the analysis of variance (ANOVA) to check each model’s adequacy.
ANOVA consists of calculations that provide information about levels of variability within a regression model and form a basis for tests of significance. ANOVA has a number of parameters, which are summarized in a table, the degrees of freedom (df), sum of squares (SS), and mean square (MS). These parameters are calculated for each model and error. Tests of significance are conducted by analyzing p-values.
The p-values obtained are used to assess the statistical significance of the models and their coefficients, while the quality of the experimental data fitting is estimated using the lack-of-fit, pure error, and R2 (coefficient of determination) values. The coefficient of determination value can be defined as the proportion of variability around the mean for the dependent variable that the model can account for, in range between 0 and 1 (the ideal fit). Due to measurement errors or relationships between responses and factors that the selected model cannot describe, a selected second-order polynomial model cannot fit the measured values perfectly. Actually, this fact causes deviations between predicted and measured values, which are referred to as residuals. At the design points, therefore, so-called residual values exist [12].
The significance of the lack of fit can also be determined where some runs are replicated, such as central composite design. A statistical test based on splitting the residual error sum of squares into two components, lack-of-fit sum of squares (associated with variation due to factors other than measurement error) and pure error sum of squares (associated with random variation caused by measurement error), is used to evaluate the model’s acceptability. A low p-value, below 0.05, for lack-of-fit in the ANOVA table shows that the analyzed model does not adequately fit the experimental data [12,23]. All statistical analyses were performed at the significance level of 95%.
3. Results
The experimental design data and responses are presented in Table 1 and Table 2 for the cheese whey and winery flotation effluent, respectively. The design has nine different experimental conditions, with two replications in center point.
3.1. Flocculation Efficiency
A summary of the analysis of variance of the second-degree polynomial models is presented in Table 3. The models developed for flocculation efficiency proved significant for both effluents, with p-values 0.0011 and 0.0003 for cheese whey and winery flotation effluent, respectively. Moreover, in the ANOVA results (Table 3), high values of the adjusted coefficient of the determination (Adj. R2) are showed: 98.43% and 99.18% for CW and WFW, respectively.

Table 3.
Analysis of variance (ANOVA) for flocculation efficiency.
In addition to the high coefficient of the determination values for both effluents, the proposed second-degree polynomial models had a non-significant lack-of-fit (p-values CW: 0.5355 and WFW: 04743). Adequate precision is an indicator of signal to noise ratio and a ratio greater than 4 is desirable. The ratios of 20.9669 and 27.0894 indicated an adequate signal for the responses of cheese whey and winery flotation effluent, respectively.
Coefficients of regression equations for flocculation efficiency in terms of coded and actual variables’ values and the corresponding p-values are given in Table 4. The linear (b1, b2), quadratic (b11, b22), and interaction (b12) coefficients of each model are statistically significant if the p-values are less than 0.05. The obtained results indicated statistical significance of linear and quadratic effects of chitosan dosage for the model of broth grown on cheese whey effluent. In the regression equation for winery flotation wastewater, the significant terms are the linear effect of chitosan dosage, whilst the quadratic effects are significant for both factors.

Table 4.
Coefficients of regression equations for flocculation efficiency.
The 3D-fitted response surfaces for flocculation efficiency are presented in Figure 1. The flocculation efficiency increases with rising chitosan dosage in the measured range for both effluents used for cultivation. This trend is evident for all mixing speeds. These results are in agreement with the chitosan flocculation for microalgal–bacterial biomass harvesting [14]. To achieve the necessary flocculation efficiency, a high concentration of nutrient medium requires relatively high flocculant dosage [24]. As shown in Figure 1a, in the case of cheese whey effluent, efficiency higher than 80% was achieved for values of chitosan dosage greater than 100 mg/L at all mixing speeds. The maximum value of flocculation efficiency in the selected experimental plan of 97.1% was obtained with 175 mg/L chitosan addition (Table 1).
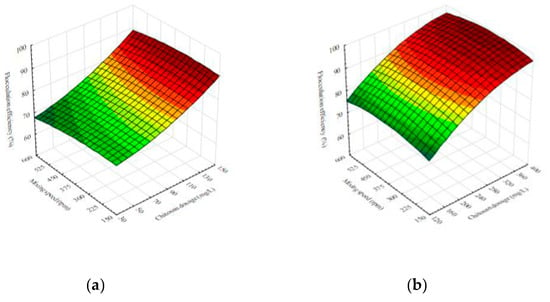
Figure 1.
Response surface illustrating the interaction effects of factors on the flocculation efficiency of Bacillus sp. BioSol021 flocculation using chitosan in (a) cheese whey effluent and (b) winery flotation effluent.
As for the effects of the mixing speed, it seems to have less impact on the flocculation performances in comparison with chitosan dosage for broths cultivated with both effluent types (Figure 1). The findings of mixing speed influence on the flocculation efficiency for broth cultivated on cheese whey effluent, Figure 1a, indicate that increase in mixing speed results in slightly lower efficiency values. Higher mixing speed tends to disperse the flocculated cells and reintroduce them into the media, breaking up the flocs in the process [25].
In this investigation, a higher concentration of chitosan was needed for flocculation of broth based on the winery flotation effluent, Figure 1b. The chitosan dosage needed to achieve efficiency greater than 80% was 300 mg/L, three times higher compared to broth based on cheese whey effluent. The reason for this can be found in the fact that the dry matter content of winery flotation effluent is higher compared to cheese whey effluent [4,26].
In the case of fermentation medium based on the winery flotation effluent, Figure 1b, an increase in mixing speed results in a minor flocculation efficiency rise. The increase is apparent for a mixing speed interval up to around 350 rpm. Further increase in the mixing speed results in an insignificant decrease in flocculation efficiencies. This behavior is registered at all chitosan dosage values. At low levels of suspension agitation, the polymer and particle surfaces cannot sufficiently make contact, so the increase in efficiency is achieved by raising the mixing speed [27]. On the other hand, the flocs may be broken up by vigorous agitation, and the extended polymer molecules may be adsorbed on the same particle surface again, which further prevents the bridging [27]. As an outcome, the particles are re-dispersed again, and, so, efficiency decreases.
3.2. Settling Velocity
Table 5 presents an ANOVA for the quadratic model of the settling velocity for both cheese whey and winery flotation effluent. Regression models are significant with low p-values (<0.05). For the second-degree polynomial models, p-values are 0.0047 and 0.0045 for cheese whey and winery flotation effluent, respectively.

Table 5.
Analysis of variance (ANOVA) for settling velocity.
The quantity of model p-value for lack-of-fit should be over 0.05. In this study, as shown in Table 5, the p-values for the models’ lack-of-fit for settling velocity was 0.1607 for CW and 0.1805 for WFW, showing that the models adequately fit the experimental data.
The coefficient of determination for the settling velocity of flocculated broth based on cheese whey effluent showed that around 4% of the changes cannot be described using the quadratic model. For the settling velocity of flocculated broth based on winery flotation effluent, around 3% of the changes cannot be described using the quadratic model. The ratios of 14.1569 and 12.8134 indicated an adequate signal for the responses of cheese whey and winery flotation effluent, respectively.
Coefficients of regression equations for the settling velocity in terms of coded and actual variables’ values and the corresponding p-values are presented in Table 6. The results indicated statistical significance of linear and quadratic effects of the mixing speed for the model of broth grown on cheese whey effluent. In the regression equation for winery flotation wastewater, the significant terms are the linear effect of chitosan dosage and the quadratic effect for mixing speed.

Table 6.
Coefficients of regression equations for settling velocity.
For unflocculated fermentation suspensions, the settling velocities were 0.061 mm/s and 0.004 mm/s for cheese whey and winery flotation effluents, respectively. Winery flotation wastewater is higher in suspended solids and has a lower percentage of settleable solids, so settling problems of biomass produced during biological treatment limit process reliability [28]. Similar problems, with reduced settling of sludge, are reported for most dairy wastewater treatment plants with aerobic processes [29].
The effects of chitosan dosage and mixing speed on the settling velocity of flocculated broths for both effluents used for cultivation are shown in Figure 2. The increase in floc size after adding chitosan enhances the floc settling velocity and consequently decreases the settling time, but the velocities are still relatively slow. The reason for this behavior can be found in the fact that chitosan develops a longer chain in acidic liquids, resulting in smaller, looser flocs [14].
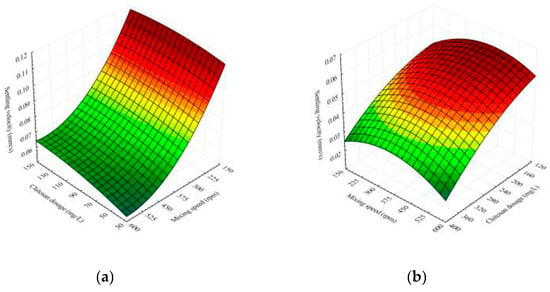
Figure 2.
Response surface illustrating the interaction effects of factors on the settling velocity of Bacillus sp. BioSol021 after flocculation using chitosan in (a) cheese whey effluent and (b) winery flotation effluent.
It is evident from Figure 2a that mixing speed has significant influence on the settling velocity of broth grown on the cheese whey compared to the influence of chitosan dosage in the experimental range. Particles (flocs) settled more quickly for experiments with lower agitation speeds. High mixing velocities may shatter the flocs, making them more difficult to settle [14]. On the other side, the increase in chitosan dosage results in a moderate settling velocity increase. The increase in the settling velocity is more noticeable at higher agitation speeds.
The results of the settling experiments for winery flotation effluent are presented in Figure 2b. The increase in chitosan dosage values show a rather altered trend compared to the results for cheese whey illustrated in Figure 2a. A chitosan dosage increases results in the settling velocity’s austere decrease. The cause of this behavior can be traced to the experimental range of chitosan dosage, which is significantly higher for the broth grown on the winery flotation effluent, Table 2.
The results show that when the chitosan dosage is increased from 120 mg/L to 400 mg/L while mixing at the greatest speed (600 rpm), the settling velocity is halved. The decreases in values of the settling velocity at other mixing speeds also follow the same trend. This is because a higher dosage of chitosan adsorption increases the positive charge of the particles, resulting in a strong electrostatic repulsive force between particles that hinders settling [16,30,31].
Regarding the influence of the mixing speed, it can be observed that increasing mixing intensity (up to 350 rpm) causes a minor increase in settling velocity, whereas further increase causes a reduction in velocity, Figure 2b. After adding chitosan, the suspension is better mixed, which leads to the formation of larger flocs that settle more quickly. However, an increase in mixing speed above 350 rpm causes flocs to break and makes flocs more difficult to settle [14].
3.3. Inhibition Zone Diameter
The results of the ANOVA for analyzing a quadratic model of inhibition zone diameter for cheese whey and winery flotation effluent are shown in Table 7.

Table 7.
Analysis of variance (ANOVA) for inhibition zone diameter.
By examining the p-values, which were determined to be 0.0229 and 0.0491 for CW and WFW, respectively, the significance of the model regression equations is confirmed. In this study, as shown in Table 7, the p-values for the models’ lack-of-fit for inhibition zone diameter were 0.9379 for CW and 0.5325 for WFW, showing that the models adequately fit the experimental data. Relatively high values of R2 (around and higher than 0.75) show the suitability of the model for satisfactory empirical data representation. The adequate precision ratios of 7.5661 and 8.3791 for CW and WFW, respectively, indicate an adequate signal for the responses of inhibition zone diameter for both effluents.
Coefficients of regression equations for inhibition zone diameter in terms of coded and actual variables’ values and the corresponding p-values are provided in Table 8. The obtained results indicated statistical significance of linear and quadratic effects of the mixing speed for the model of broth grown on cheese whey effluent. The quadratic effect of the chitosan dosage is also significant. In the regression equation for winery flotation wastewater, the significant terms are the linear effect of the chitosan dosage and the interaction effect of the chitosan dosage and mixing speed.

Table 8.
Coefficients of regression equations for inhibition zone diameter.
The effects of the chitosan dosage and mixing speed on the inhibition zone diameter against Aspergillus flavus of flocculated broths for both effluents used for cultivation are shown in Figure 3. In the current study, both broth and chitosan were found to have a positive influence on the bactericidal activity against Aspergillus flavus. The antimicrobial activity of unflocculated broth samples is 42 mm and 35 mm for broth cultivated at cheese whey effluent and winery flotation effluent, respectively. As chitosan is known for its antimicrobial properties, the inhibition zone diameters of chitosan solution were investigated; their range was between 13 and 15 mm. The results of the flocculated broth samples (bigger inhibition diameter) indicate that there is some synergistic effect between broth and chitosan solution. Similar results are reported in the literature [16,32,33].
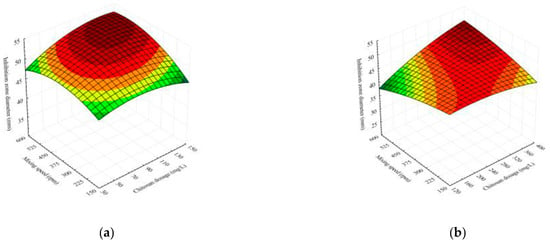
Figure 3.
Response surface illustrating the interaction effects of factors on the inhibition zone diameter of Bacillus sp. BioSol021 after flocculation using chitosan in (a) cheese whey effluent and (b) winery flotation effluent.
The inhibition zone appears to have maximum value in the range of maximum chitosan dosage as well as mixing speeds values for both effluent types. Although the increase in agitation speed resulted in slightly less effective cell flocculation (Figure 1), the increase in the inhibition zone diameter was documented for the majority of the experimental data (Figure 3).
The reason for this behavior can be found in the fact that fermentation suspensions cultivated on wastewater effluents are of complex nature. Chitosan is a well-known adsorbent of various substances such as pharmaceuticals, enzymes, dyes, and other organic compounds [34,35,36]. In addition to microbial cells, cultivation broth also includes a variety of extracellular components and leftover medium nutrients. All of these components interact with chitosan, altering the flocculation efficiency and, in consequence, the antimicrobial activity of the flocculated samples. Some of these extracellular compounds have antimicrobial properties that contribute to the increase in the flocculated samples’ inhibition zone diameter.
Hughes et al. [37] reported that flocculation of bacterial cells can be altered by the presence of various adsorbed materials, but with washed cells, these complications are removed. It is possible that the flocculants’ interactions with the proteins, polysaccharides, and nucleic acids generated during cell growth are what cause this effect [37]. One of such substances is surfactin, which is present in the broth; the CW medium concentration is 1.54 g/L, while in the WFW-based medium, it was 2.65 g/L [4]. It is a lipopeptide that is mostly known for its potent biosurfactant capabilities, but numerous studies have demonstrated its important role in the inhibition of fungal pathogens [38].
The initial decline of inhibition zone diameter obtained for the lower chitosan dosages with the increase in the mixing speed, Figure 3b, can be attributed to the smaller chitosan concentrations in suspension. This concentration may be insufficient to flocculate other substances in broth cultivated on winery flotation effluent with already high dry matter content compared to cheese whey effluent [4,25].
3.4. Optimization
Optimization of the operational conditions during flocculation harvesting of Bacillus sp. BioSol021 biomass from cultivation broth was performed using the desirability function (DF) and genetic algorithm approach (GA). The results of the GA optimization are illustrated in Figure 4. Both optimization methods were aimed at obtaining maximum values of the three responses modeled by applying RSM.
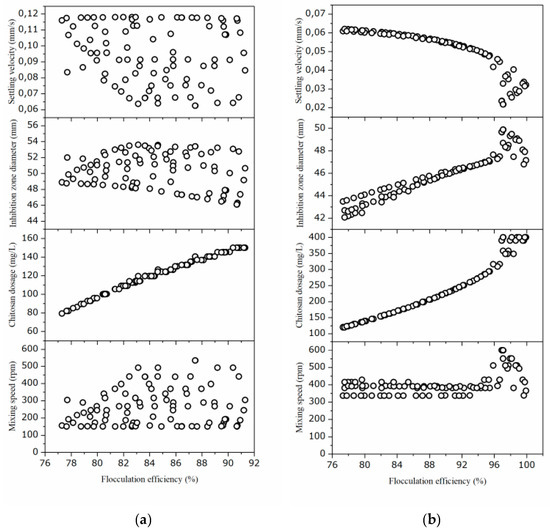
Figure 4.
The Pareto front plot and decision space of the optimal solution set obtained from the multi-objective genetic algorithm with (a) cheese whey effluent and (b) winery flotation effluent.
DF optimization is the approach used to aggregate several responses into a single response. The chosen responses are converted into particular desirability scores, which range from 0 to 1. The geometric mean of individual desirability functions is used to obtain the process’ overall desirability [12].
The optimal solution for broth grown on the cheese whey effluent had the overall desirability of 0.553. This solution was achieved with a chitosan dosage of 141.23 mg/L and a mixing speed of 263.6 rpm. At these experimental conditions, the flocculation efficiency was 89% and the settling velocity was 0.09 mm/s, while the inhibition zone diameter was 50.18 mm.
The reason for the rather low overall desirability value can be found in the contradictory influence of speed mixing on the flocculation efficiency and settling velocity. While flocculation efficiency is reduced to a smaller degree by increasing the mixing speed, the decrease in settling velocity is more pronounced. As said earlier, high mixing velocities may shatter the flocs, making them more difficult to settle [14]. As for chitosan dosage, the optimal value is closer to the higher dosages of the experimental range.
The optimal solution for broth grown on the winery flotation effluent had an overall desirability of 0.727. This solution was achieved with a chitosan dosage of 280.60 mg/L and a mixing speed of 384.95 rpm. At these experimental conditions, the flocculation efficiency was 94% and the settling velocity was 0.05 mm/s, while the inhibition zone diameter was 46.89 mm.
The optimal mixing speed is in agreement with the results of the flocculation efficiency and settling velocity as the optimum values of these responses are achieved around speed of 350 rpm. As can be seen from Figure 1b and Figure 2b, there is a ridge of maximum response values around this mixing speed for all selected chitosan dosages. As for chitosan dosage, the optimum value (280.60 mg/L) is a compromise between increased flocculation efficiency with dosage rise and settling velocity decrease with the aforementioned rise in the chitosan dosage.
Confirmation experiments for both effluents at the optimum solution experimental values gave satisfactory agreement between predicted and experimental response values. This fact confirms the validity of the proposed second-degree polynomial models for representation of experimental results. The experimental optimum values for flocculation efficiency, settling velocity, and inhibition zone diameter for cheese whey effluent were 88%, 0.10 mm/s, and 51.00 mm, respectively. In the case of winery flotation effluent, the optimum values of conformation experiment were flocculation efficiency 95% and settling velocity 0.05 mm/s, while the inhibition zone diameter was 48.00 mm.
The results of three-objective GA optimization are illustrated in Figure 4. The optimization problem is solved using coded NSGA-II in MATLAB to achieve a set of Pareto-optimal solutions. Scatter diagrams and parallel coordinates are two common types of visualization methods for multi-dimensional visualization of the Pareto front [39,40,41].
The Pareto front and the best prediction made using the desirability function approach coincide. The challenge of selecting a compromise solution remains despite the Pareto front’s many optimal solutions. The desired optimization’s goal(s) can be considered while choosing a suitable solution, although trade-offs between various objectives are required. [12]. The Pareto front reveals the conflicting relationship between flocculation efficiency and settling velocity, especially in the case of WFW, Figure 4b; with the increase in chitosan dosage, flocculation efficiency rises while settling velocity decreases.
4. Conclusions
Chitosan was found to be an effective natural flocculant for harvesting Bacillus sp. BioSol021 biomass from broth after biological treatment of wastewaters from the dairy and wine industries (cheese whey and winery flotation wastewaters). It was found that flocculation efficiencies up to 97% could be achieved with a chitosan dosage of 175 mg/L for broth grown on cheese whey effluent. The higher concentration of chitosan was needed for flocculation of broth based on the winery flotation effluent; to achieve flocculation efficiencies higher than 92%, the dosage needed to be 260 mg/L and above.
Second-order factorial design models were used to estimate the effect of chitosan concentration and mixing speed on flocculation efficiency, settling velocity, and antimicrobial activity against Aspergillus flavus, i.e., inhibition zone diameter. Response surface methodology was followed by multi-objective optimization by applying desirability function (DF) and the genetic algorithm (GA).
When pH was adjusted (pH = 5), it was discovered that chitosan concentration primarily determined flocculation efficiency, whereas agitation speed had little effect on it. On the other hand, settling velocity was mainly determined by flocs breakage at higher mixing speeds. Inhibition zone diameter against Aspergillus flavus was a result not only of BioSol021 biomass but also of the antimicrobial activity of metabolites present in the broth, such as surfactin.
The optimal results of inhibition zone diameters suggest that there is a synergistic effect of chitosan and Bacillus sp. BioSol021 biomass against Aspergillus flavus.
The study is indicative that chitosan use as a flocculation agent can lead to cost savings and environmental benefits for the dairy and wine industry through production of added value to the wastewater treatment. More research is needed to assess the impact of other factors (such as initial pH and biomass concentration) on chitosan flocculation and, as a result, optimize the bacterial harvesting method, as well as the economic impacts on downstream processing.
Author Contributions
Conceptualization, A.J. and S.D.; methodology, A.J. and J.G.; software, A.J. and N.L.; validation, S.D.; formal analysis, S.D., I.P. and V.V.; investigation, S.D., I.P. and V.V.; resources, A.J. and J.G.; data curation, A.J. and N.L.; writing—original draft preparation, S.D. and A.J.; writing—review and editing, I.P. and J.G.; visualization, A.J. and N.L.; supervision, A.J.; project administration, A.J. and J.G.; funding acquisition, A.J. and J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the program 451-03-47/2023-01/200134 of the Ministry of Science, Technological Development and Innovations of the Republic of Serbia, the project 142-451-3187/2022-01/01 “Development of industrial symbiosis in the AP Vojvodina through valorization of fruit processing by-products using green technologies” financed by the Provincial Secretariat for Higher Education and Scientific Research, the Autonomous Province of Vojvodina, Republic of Serbia, and by the Science Fund of the Republic of Serbia, PROMIS, #6064541, BioSolAfla.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68. [Google Scholar] [CrossRef]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Dmitrović, S.; Pajčin, I.; Vlajkov, V.; Grahovac, M.; Jokić, A.; Grahovac, J. Dairy and wine industry effluents as alternative media for the production of Bacillus-based biocontrol agents. Bioengineering 2022, 9, 663. [Google Scholar] [CrossRef]
- Khan, R.; Ghazali, F.M.; Mahyudin, N.A.; Samsudin, N.I.P. Biocontrol of aflatoxins using non-aflatoxigenic Aspergillus flavus: A literature review. J. Fungi 2021, 7, 381. [Google Scholar] [CrossRef]
- Amare, M.G.; Keller, N.P. Molecular mechanisms of Aspergillus flavus secondary metabolism and development. Fungal Genet. Biol. 2014, 66, 11–18. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Diseases. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 274–302. [Google Scholar]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Yi, H.; Li, M.; Huo, X.; Zeng, G.; Lai, C.; Huang, D.; An, Z.; Qin, L.; Liu, X.; Li, B.; et al. Recent development of advanced biotechnology for wastewater treatment. Crit. Rev. Biotechnol. 2019, 40, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.; Godina, R.; Azevedo, S.G.; Matias, J.C.O. A comprehensive review of industrial symbiosis. J. Clean. Prod. 2020, 247, 119113. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R. Recent advances in downstream processing and formulations of Bacillus thuringiensis based biopesticides. Process. Biochem. 2006, 41, 323–342. [Google Scholar] [CrossRef]
- Jokić, A.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Kiralj, A.; Dmitrović, S.; Grahovac, J. Modeling and optimization of gas sparging-assisted bacterial cultivation broth microfiltration by response surface methodology and genetic algorithm. Membranes 2021, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Mu, D.; Mu, X.; Xu, Z.; Du, Z.; Chen, G. Removing Bacillus subtilis from fermentation broth using alumina nanoparticles. Bioresour. Technol. 2015, 197, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Riaño, B.; Molinuevo, B.; García-González, M.C. Optimization of chitosan flocculation for microalgal-bacterial biomass harvesting via response surface methodology. Ecol. Eng. 2012, 38, 110–113. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Mat Yasin, N.H.; Derek, C.J.C.; Lim, J.K. Optimization of microalgae coagulation process using chitosan. Chem. Eng. J. 2011, 173, 879–882. [Google Scholar] [CrossRef]
- Dmitrović, S.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Jokić, A. Taguchi grey relational analysis for multi-response optimization of Bacillus bacteria flocculation recovery from fermented broth by chitosan to enhance biocontrol efficiency. Polymers 2022, 14, 3282. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.-M.; Crini, G. Chitosan for coagulation/flocculation processes–An eco-friendly approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Picos-Corrales, L.A.; Morales-Burgos, A.M.; Ruelas-Leyva, J.P.; Crini, G.; García-Armenta, E.; Jimenez-Lam, S.A.; Ayón-Reyna, L.E.; Rocha-Alonzo, F.; Calderón-Zamora, L.; Osuna-Martínez, U.; et al. Chitosan as an outstanding polysaccharide improving health-commodities of humans and environmental protection. Polymers 2023, 15, 526. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens-the IARC monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef]
- Luna-Finkler, C.L.; Finkler, L. Production of concentrates of bacterial bio-insecticide Bacillus thuringiensis var. israelensis by flocculation/sedimentation. Acta Trop. 2008, 107, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.V.; Moorthy, I.G.; Pugazhenthi, G. Modelling and optimization of critical parameters by hybrid RSM-GA for the separation of BSA using a tubular configured MFI-type zeolite microfiltration membrane. RSC Adv. 2015, 5, 87645–87659. [Google Scholar] [CrossRef]
- Yang, Z.; Degorce-Dumas, J.R.; Yang, H.; Guibal, E.; Li, A.; Cheng, R. Flocculation of Escherichia coli using a quaternary ammonium salt grafted carboxymethyl chitosan flocculant. Environ. Sci. Technol. 2014, 48, 6867–6873. [Google Scholar] [CrossRef] [PubMed]
- Hadiyanto, H.; Widayat, W.; Christwardana, M.; Pratiwi, M.E. The flocculation process of Chlorella sp. using chitosan as a bio-flocculant: Optimization of operating conditions by response surface methodology. Curr. Res. Green Sustain. Chem. 2022, 5, 100291. [Google Scholar] [CrossRef]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Duzyol, S. Turbidity removal of fine coal–water suspension by flocculation using Taguchi (L16) experimental design. Part. Sci. Technol. 2016, 36, 351–356. [Google Scholar] [CrossRef]
- Bolzonella, D.; Papa, M.; Da Ros, C.; Anga Muthukumar, L.; Rosso, D. Winery wastewater treatment: A critical overview of advanced biological processes. Crit. Rev. Biotechnol. 2019, 39, 489–507. [Google Scholar] [CrossRef]
- Slavov, A.K. General characteristics and treatment possibilities of dairy wastewater-A review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.; Zhu, X.; Huang, W. The settling behavior of quartz using chitosan as flocculant. J. Mater. Res. Technol. 2017, 6, 71–76. [Google Scholar] [CrossRef]
- Lichtfouse, E.; Morin-Crini, N.; Fourmentin, M.; Zemmouri, H.; do Carmo Nascimento, I.O.; Queiroz, L.M.; Tadza, M.Y.M.; Picos-Corrales, L.A.; Pei, H.; Wilson, L.D.; et al. Chitosan for direct bioflocculation of wastewater. Environ. Chem. Lett. 2019, 17, 1603–1621. [Google Scholar] [CrossRef]
- Rkhaila, A.; Chtouki, T.; Erguig, H.; El Haloui, N.; Ounine, K. Chemical proprieties of biopolymers (chitin/chitosan) and their synergic effects with endophytic Bacillus species: Unlimited applications in agriculture. Molecules 2021, 26, 1117. [Google Scholar] [CrossRef]
- Mingzheng, Z.; Bo, L.; Naiquan, L.; Chuanqing, R.; Jianglin, L. Factors affecting flocculation of fermentation broth of Bacillus thuringiensis by chitosan. Chin. J. Biol. Control 2009, 25, 133–137. [Google Scholar]
- Rizzi, V.; Romanazzi, F.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Petrella, A.; Cosma, P. Chitosan film as eco-friendly and recyclable bio-adsorbent to remove/recover Diclofenac, Ketoprofen, and their mixture from wastewater. Biomolecules 2019, 9, 571. [Google Scholar] [CrossRef] [PubMed]
- Bodakowska-Boczniewicz, J.; Garncarek, Z. Immobilization of naringinase from Penicillium decumbens on chitosan microspheres for debittering grapefruit juice. Molecules 2019, 24, 4234. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Ainali, N.M.; Tolkou, A.K.; Mitropoulos, A.C.; Lambropoulou, D.A.; Bikiaris, D.N.; Kyzas, G.Z. Removal of heavy metal ions from wastewaters by using chitosan/poly (vinyl alcohol) adsorbents: A review. Macromol 2022, 2, 403–425. [Google Scholar] [CrossRef]
- Hughes, J.; Ramsden, D.K.; Symes, K.C. The flocculation of bacteria using cationic synthetic flocculants and chitosan. Biotechnol. Tech. 1990, 4, 55–60. [Google Scholar] [CrossRef]
- Kirishnan, N.; Velramar, B.; Velu, R.K. Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol. 2019, 155, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Guria, C.; Maiti, S.K. Fertilizer assisted optimal cultivation of microalgae using response surface method and genetic algorithm for biofuel feedstock. Energy 2016, 115, 1272–1290. [Google Scholar] [CrossRef]
- Yang, K.; He, Y.; Ma, Z. Multi-objective steady-state optimization of two-chamber microbial fuel cells. Chin. J. Chem. Eng. 2017, 25, 1000–1012. [Google Scholar] [CrossRef]
- Yan, N.; Ren, B.; Wu, B.; Bao, D.; Zhang, X.; Wang, J. Multi-objective optimization of biomass to biomethane system. Green Energy Environ. 2016, 1, 156–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).