Effect of Potassium Ferrate as a Dewatering Conditioner on Sludge Pyrolysis Characteristics and the Releasing Characteristics of Nitrogen, Sulfur, and Chlorine during Sewage Sludge Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Preparation of Sludge Biochar
2.3. Physical and Chemical Properties
2.4. TG-MS and Py-GC/MS Analysis
2.5. Dynamic Analysis
3. Results and Discussion
3.1. Effect of Potassium Ferrate on the Physicochemical Properties of Sludge
3.2. Effect of Potassium Ferrate on the Sludge Thermal Decomposition Characteristics
3.3. Kinetics Analysis
3.4. Pyrolytic Gas Evolution
3.4.1. Release of C/H/O-Containing Substances
3.4.2. N-Containing Pollutants
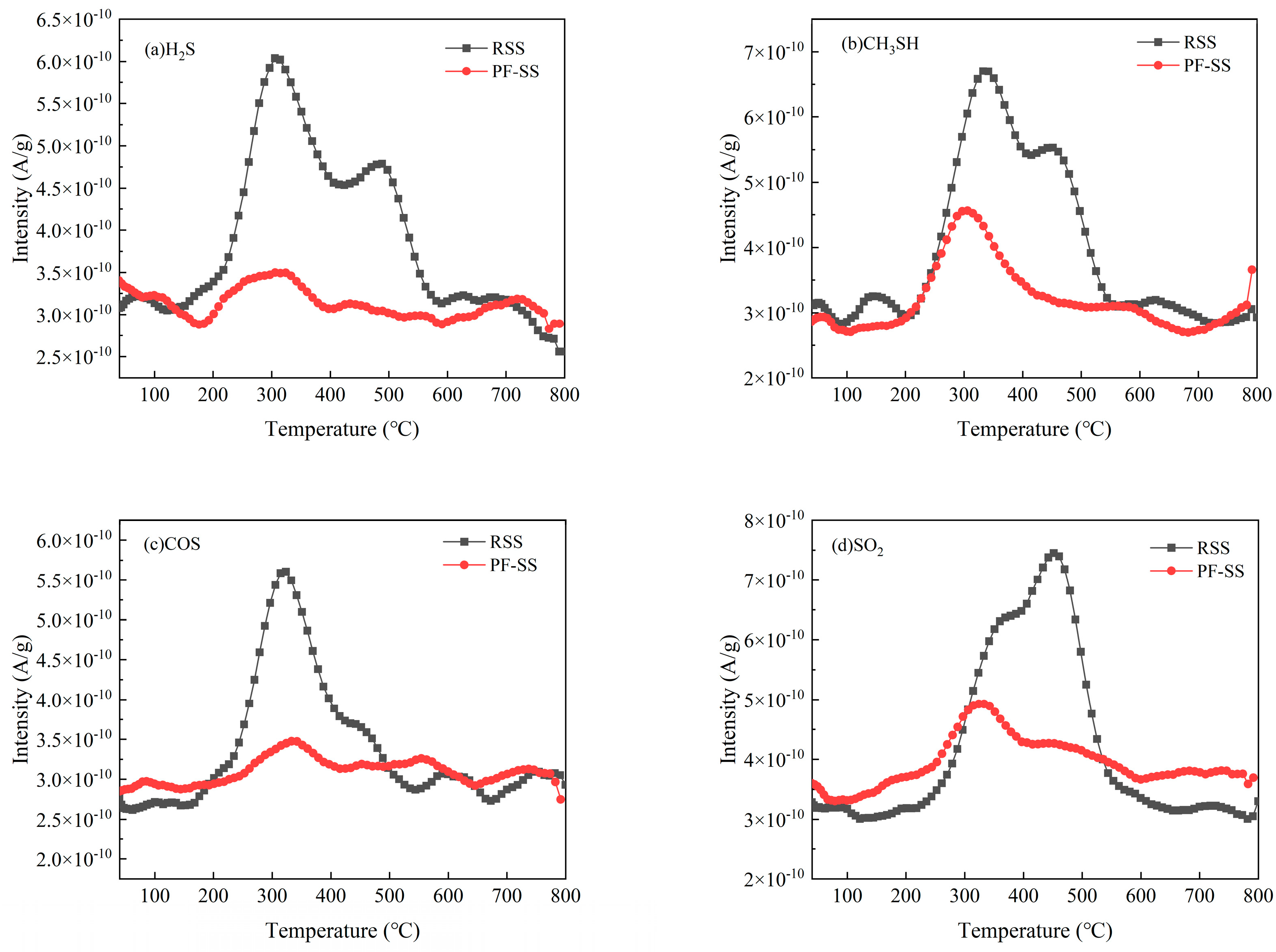
3.4.3. S-Containing Pollutants
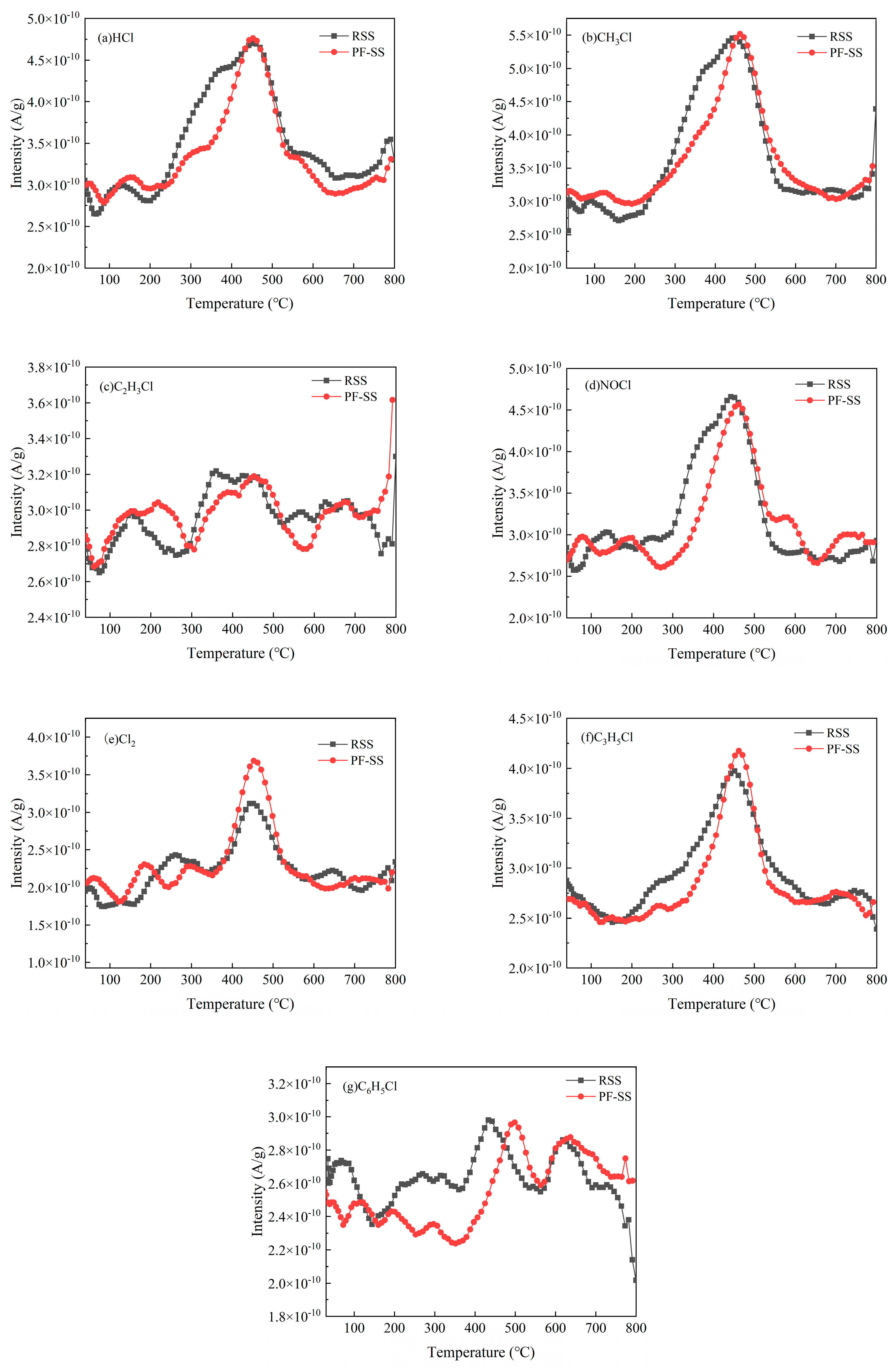
3.4.4. Cl-Containing Pollutants
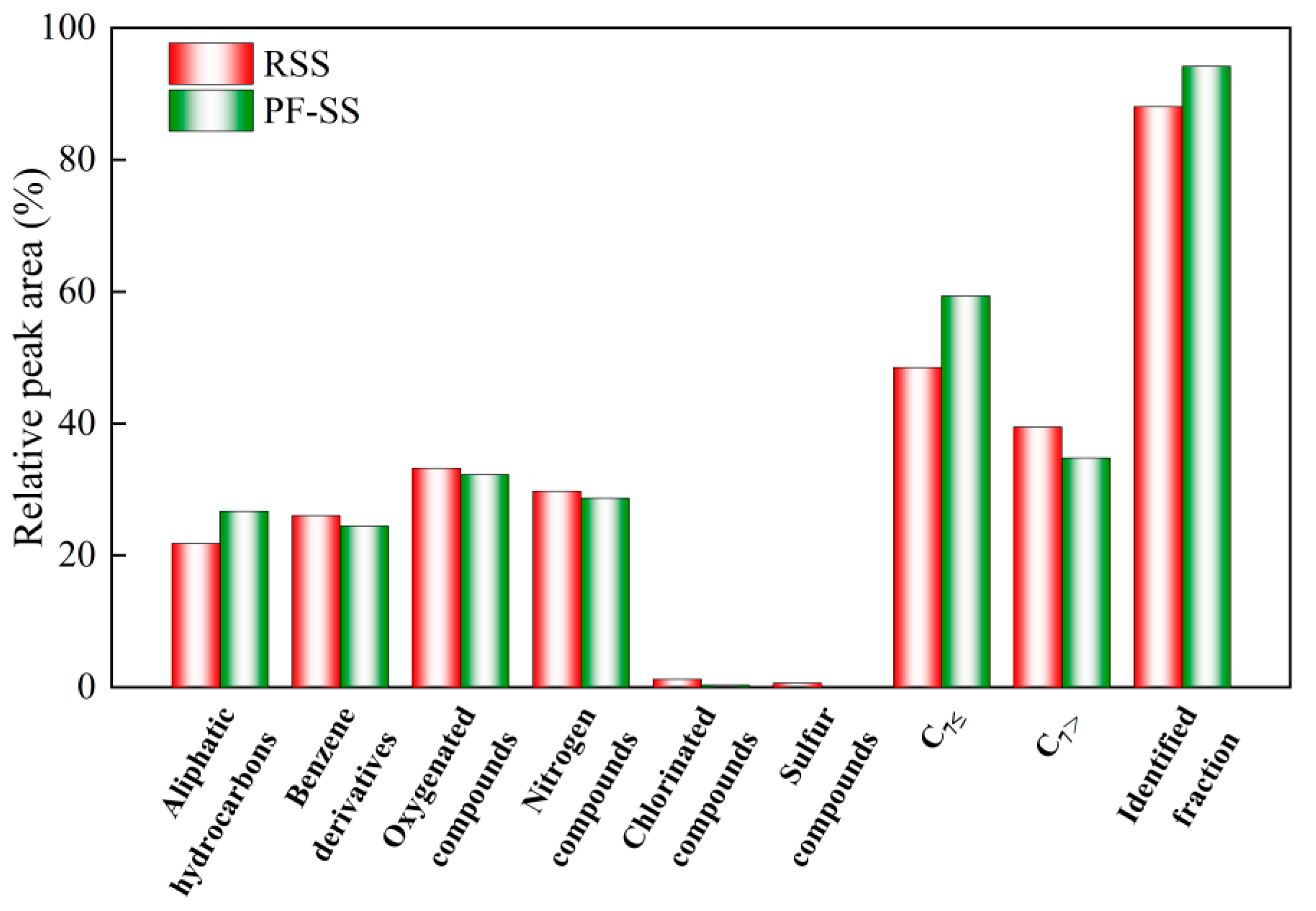
3.5. Pyrolytic Volatiles Products
4. Conclusions
- (1)
- The pyrolysis of sludge samples was divided into three stages (room temperature–180 °C, 180–550 °C, and above 550 °C). The largest weight loss occurred in the 180–550 °C interval, which primarily involved the pyrolysis of organic matter and fixed carbon, with less residual mass in the PF-SS than the RSS conditions. The dewatering pretreatment with K2FeO4 had a facilitatory effect on sludge pyrolysis.
- (2)
- Sludge conditioned with K2FeO4 requires less energy for the pyrolysis process. This step also reduces the activation energy, and the type and complexity of volatiles in sludge pyrolysis, making the sludge more susceptible to the reaction.
- (3)
- Compared with RSS, PF-SS promoted the release of C/H/O gases but inhibited that of N/S/Cl-contaminated gases.
- (4)
- PF-SS increased the calorific value of tar and improved the chemical stability of bio-oil. The addition of iron promotes the cracking of macromolecular compounds into light tar and syngas as well as the formation of aliphatic hydrocarbons and the catalytic reformation and decomposition of benzene. Moreover, the PF-SS treatment moderately reduces the formation of compounds containing nitrogen, sulfur, and chlorine. Compared to the results of the previous study, the amount of CH4 and CO as well as that of the N-containing and S-containing pollutant gas products produced by PF-SS was lower than that produced by FC-SS, which may be due to the low content of C, H, O, N, and S in PF-SS. The amount of all the Cl-containing pollutant gas products produced by PF-SS was lower than that produced by FC-SS, but higher than that of FS-SS.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paz-Ferreiro, J.; Nieto, A.; Mendez, A.; Askeland, M.P.J.; Gasco, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Wang, X.; Chang, V.W.-C.; Li, Z.; Chen, Z.; Wang, Y. Co-pyrolysis of sewage sludge and organic fractions of municipal solid waste: Synergistic effects on biochar properties and the environmental risk of heavy metals. J. Hazard. Mater. 2021, 412, 125200. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.Y.; Liu, C.; Wang, M.Q.; Lan, G.X.; Zhong, Y.H.; Wu, Y.; Fu, C.A.; Shi, H.Y.; Zhu, R.; Zhou, L.L. Effect of dewatering conditioners on phosphorus removal efficiency of sludge biochar. Environ. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Pan, X.; Wang, M.; Wang, X.; Xia, Y.; Liu, C.; Wu, Y.; Fu, C.; Hu, K.; Su, K.; Zhang, Z. Comparative study on the effect of different dewatering skeleton conditioners on sludge pyrolysis products. J. Environ. Chem. Eng. 2021, 9, 106527. [Google Scholar] [CrossRef]
- Bao, D.; Li, Z.; Tang, R.; Wan, C.; Zhang, C.; Tan, X.; Liu, X. Metal-modified sludge-based biochar enhance catalytic capacity: Characteristics and mechanism. J. Environ. Manag. 2021, 284, 112113. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Y.; Lan, G.; Ji, X.; Xia, Y.; Fu, C.; Shen, J.; Gui, J.; Liu, Y.; Qu, Y.; et al. CO2 capture performance of biochar prepared from sewage sludge after conditioning with different dewatering agents. J. Environ. Chem. Eng. 2022, 10, 108318. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, W.; Chen, J.; Wang, X.; Xing, D.; Dong, W.; Wang, H.; Wang, J. The combination of aerobic digestion and bioleaching for heavy metal removal from excess sludge. Chemosphere 2022, 290, 133231. [Google Scholar] [CrossRef]
- Jiang, L.-B.; Yuan, X.-Z.; Li, H.; Chen, X.-H.; Xiao, Z.-H.; Liang, J.; Leng, L.-J.; Guo, Z.; Zeng, G.-M. Co-pelletization of sewage sludge and biomass: Thermogravimetric analysis and ash deposits. Fuel Process. Technol. 2016, 145, 109–115. [Google Scholar] [CrossRef]
- Sun, K.; Themelis, N.J.; Bourtsalas, A.C.; Huang, Q. Selective production of aromatics from waste plastic pyrolysis by using sewage sludge derived char catalyst. J. Clean. Prod. 2020, 268, 122038. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, F.; Yu, B.; Hu, L.; Zhang, J.; Qu, X.; Yang, H.; Luo, Z. Full-scale municipal sludge pyrolysis in China: Design fundamentals, environmental and economic assessments, and future perspectives. Sci. Total Environ. 2021, 795, 148832. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Tang, L.; Wang, Y.; Liu, R. Research and application of polyethylene glycol solution dialysis combined with vacuum pressure in sludge dewatering. Environ. Technol. Innov. 2021, 23, 101611. [Google Scholar] [CrossRef]
- Wu, C.; Jin, L.; Zhang, P.; Zhang, G. Effects of potassium ferrate oxidation on sludge disintegration, dewaterability and anaerobic biodegradation. Int. Biodeterior. Biodegrad. 2015, 102, 137–142. [Google Scholar] [CrossRef]
- Hu, J.; Li, Z.; Zhang, A.; Mao, S.; Jenkinson, I.R.; Tao, W. Using a strong chemical oxidant, potassium ferrate (K2FeO4), in waste activated sludge treatment: A review. Environ. Res. 2020, 188, 109764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cao, B.; Wang, D.; Ma, T.; Yu, D. Variations in distribution and composition of extracellular polymeric substances (EPS) of biological sludge under potassium ferrate conditioning: Effects of pH and ferrate dosage. Biochem. Eng. J. 2016, 106, 37–47. [Google Scholar] [CrossRef]
- He, Z.-W.; Liu, W.-Z.; Gao, Q.; Tang, C.-C.; Wang, L.; Guo, Z.-C.; Zhou, A.-J.; Wang, A.-J. Potassium ferrate addition as an alternative pre-treatment to enhance short-chain fatty acids production from waste activated sludge. Bioresour. Technol. 2018, 247, 174–181. [Google Scholar] [CrossRef]
- Zhu, A.; Wu, Y.; Wang, M.; Lan, G.; Xia, Y.; Liu, C.; Ji, X.; Shen, J.; Li, T.; Fu, C.; et al. Effect of FeCl3 combined with biochar as dewatering conditioners on sludge pyrolysis products. Chem. Eng. Res. Des. 2022, 186, 628–637. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, S.; Xiao, X.; Lai, F. Impact of persulphate mixed with paper sludge on activated sludge dewaterability. Water Environ. 2020, 34, 884–892. [Google Scholar] [CrossRef]
- Wang, M.; Pan, X.; Xia, Y.; Zhu, A.; Wu, Y.; Fu, C.; Zhang, P.; Zhao, J.; Li, J.; Fu, J. Effect of dewatering conditioners on pollutants with nitrogen, sulfur, and chlorine releasing characteristics during sewage sludge pyrolysis. Fuel 2022, 307, 121834. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, Y.; Yu, Z.; Fang, S.; Ma, X. A study on co-pyrolysis of bagasse and sewage sludge using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2017, 151, 190–198. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Naqvi, M.; Chen, W.-H.; Ceylan, S.; Rashid, H.; Ahmad, J.; Taqvi, S.A.; et al. Pyrolysis of high ash sewage sludge: Kinetics and thermodynamic analysis using Coats-Redfern method. Renew. Energy 2019, 131, 854–860. [Google Scholar] [CrossRef]
- Gao, N.; Li, J.; Qi, B.; Li, A.; Duan, Y.; Wang, Z. Thermal analysis and products distribution of dried sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2014, 105, 43–48. [Google Scholar] [CrossRef]
- Yang, J.; Xu, X.; Liang, S.; Guan, R.; Li, H.; Chen, Y.; Liu, B.; Song, J.; Yu, W.; Xiao, K.; et al. Enhanced hydrogen production in catalytic pyrolysis of sewage sludge by red mud: Thermogravimetric kinetic analysis and pyrolysis characteristics. Int. J. Hydrogen Energy 2018, 43, 7795–7807. [Google Scholar] [CrossRef]
- Karayigit, A.I.; Oskay, R.G.; Çelik, Y. Mineralogy, petrography, and Rock-Eval pyrolysis of late Oligocene coal seams in the Malkara coal field from the Thrace Basin (NW Turkey). Int. J. Coal Geol. 2021, 244, 103814. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-pyrolysis behavior of sewage sludge and rice husk by TG-MS and residue analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Wang, C.; Bi, H.; Lin, Q.; Jiang, X.; Jiang, C. Co-pyrolysis of sewage sludge and rice husk by TG–FTIR–MS: Pyrolysis behavior, kinetics, and condensable/non-condensable gases characteristics. Renew. Energy 2020, 160, 1048–1066. [Google Scholar] [CrossRef]
- Zou, C.; Ma, C.; Zhao, J.; Shi, R.; Li, X. Characterization and non-isothermal kinetics of Shenmu bituminous coal devolatilization by TG-MS. J. Anal. Appl. Pyrolysis 2017, 127, 309–320. [Google Scholar] [CrossRef]
- Zi-Zhao, D.; Zhang, S.; Qiang, L.; Ming-Hui, D.; Rui, G.; Jie-Ping, W.; Guang-Yue, L.; Ying-Hua, L. Boudouard reaction accompanied by graphitization of wrinkled carbon layers in coke gasification: A theoretical insight into the classical understanding. Fuel 2021, 297, 120747. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Liu, Y.; Deng, Z.; Li, X.; Wang, L.; Dai, J.; Song, Y.; Jiang, Z.; Qu, J.; et al. Co-pyrolysis of textile dyeing sludge and red wood waste in a continuously operated auger reactor under microwave irradiation. Energy 2021, 218, 119398. [Google Scholar] [CrossRef]
- Xiao, K.; Guan, R.; Yang, J.; Li, H.; Yu, Z.; Liang, S.; Yu, W.; Hu, J.; Hou, H.; Liu, B. Effects of red mud on emission control of NOx precursors during sludge pyrolysis: A protein model compound study. Waste Manag. 2019, 85, 452–463. [Google Scholar] [CrossRef]
- Meng, J.; Wang, J.; Yang, F.; Cheng, F. Study on the multiple roles of CaO on nitrogen evolution mechanism of protein inside sewage sludge pyrolysis. Chem. Eng. J. 2023, 458, 141039. [Google Scholar] [CrossRef]
- Han, H.; Li, A.; Zhu, M.; Hu, S.; Xu, J.; Xiong, Z.; Ren, Q.; Wang, Y.; Jiang, L.; Su, S.; et al. Heavy tar evolution characteristics during advanced sludge pyrolysis and biomass gasification integrated process. Sci. Total Environ. 2022, 853, 158107. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Yu, Z.; Wang, H.; Yang, J.; Liang, S.; Hu, J.; Hou, H.; Liu, B. Investigation on emission control of NOx precursors and phosphorus reclamation during pyrolysis of ferric sludge. Sci. Total Environ. 2019, 670, 932–940. [Google Scholar] [CrossRef] [PubMed]
- He, Z.-W.; Tang, C.-C.; Liu, W.-Z.; Ren, Y.-X.; Guo, Z.-C.; Zhou, A.-J.; Wang, L.; Yang, C.-X.; Wang, A.-J. Enhanced short-chain fatty acids production from waste activated sludge with alkaline followed by potassium ferrate treatment. Bioresour. Technol. 2019, 289, 121642. [Google Scholar] [CrossRef]
- Chen, G.; Li, J.; Li, K.; Lin, F.; Tian, W.; Che, L.; Yan, B.; Ma, W.; Song, Y. Nitrogen, sulfur, chlorine containing pollutants releasing characteristics during pyrolysis and combustion of oily sludge. Fuel 2020, 273, 117772. [Google Scholar] [CrossRef]
- Liu, W.-J.; Shao, Z.-G.; Xu, Y. Emission characteristics of nitrogen and sulfur containing pollutants during the pyrolysis of oily sludge with and without catalysis. J. Hazard. Mater. 2021, 401, 123820. [Google Scholar] [CrossRef]
- Nsaful, F.; Collard, F.-X.; Görgens, J.F. Lignocellulose thermal pretreatment and its effect on fuel properties and composition of the condensable products (tar precursors) from char devolatilization for coal substitution in gasification application. Fuel Process. Technol. 2018, 179, 334–343. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, C.; Zhang, Z. Effect of inherent minerals on sewage sludge pyrolysis: Product characteristics, kinetics and thermodynamics. Waste Manag. 2018, 80, 175–185. [Google Scholar] [CrossRef]
- Ibrahim, S.; Rahman, R.K.; Raj, A. Effects of H2O in the Feed of Sulfur Recovery Unit on Sulfur Production and Aromatics Emission from Claus Furnace. Ind. Eng. Chem. Res. 2017, 56, 11713–11725. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, H.; Han, D. Ferrocene addition for suppression of hydrogen sulfide formation during thermal recovery of oil sand bitumen. Energy 2021, 230, 120744. [Google Scholar] [CrossRef]
- Zhou, B.; Dichiara, A.; Zhang, Y.; Zhang, Q.; Zhou, J. Tar formation and evolution during biomass gasification: An experimental and theoretical study. Fuel 2018, 234, 944–953. [Google Scholar] [CrossRef]
- Paul, K.; Jalal, S.; Kundal, S.; Jana, U. Synthesis of Fused Dibenzofuran Derivatives via Palladium-Catalyzed Domino C–C Bond Formation and Iron-Catalyzed Cycloisomerization/Aromatization. J. Org. Chem. 2016, 81, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Chen, D.; Arena, U.; Huang, Z.; Dai, X. Reforming sewage sludge pyrolysis volatile with Fe-embedded char: Minimization of liquid product yield. Waste Manag. 2018, 73, 464–475. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Y.; Song, Q.; Liu, S.; Ma, L.; Shu, X. Catalytic reforming of volatiles from co-pyrolysis of lignite blended with corn straw over three iron ores: Effect of iron ore types on the product distribution, carbon-deposited iron ore reactivity and its mechanism. Fuel 2021, 286, 119398. [Google Scholar] [CrossRef]

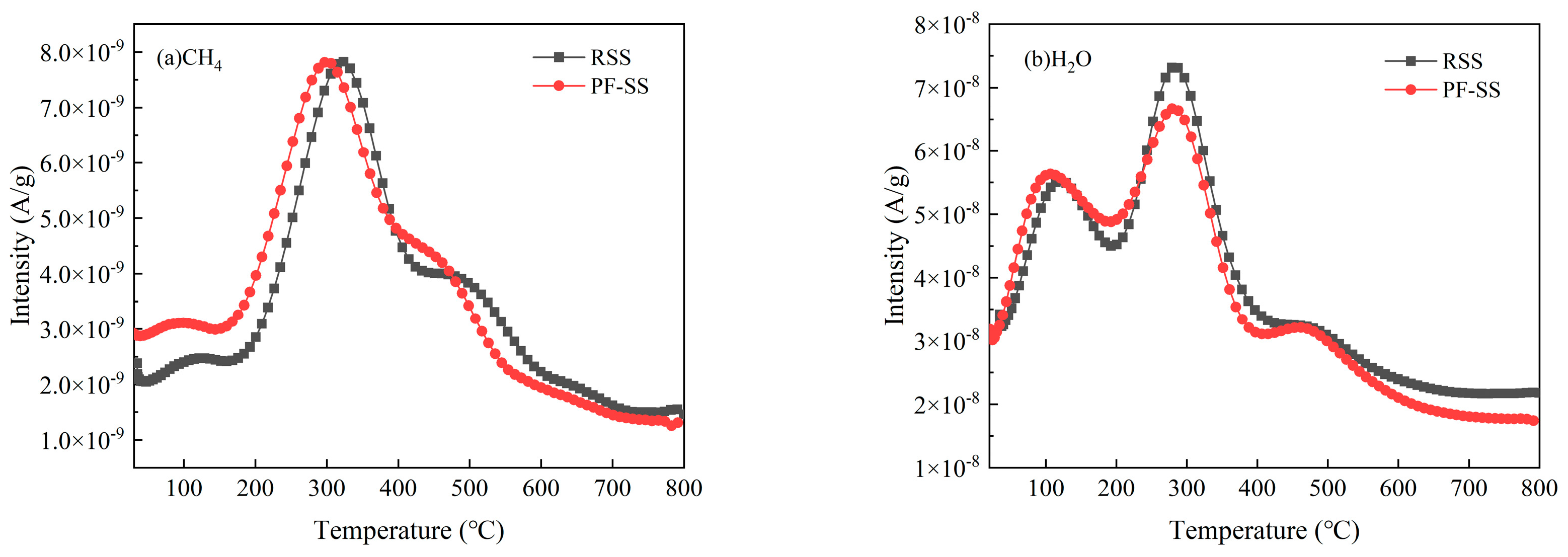
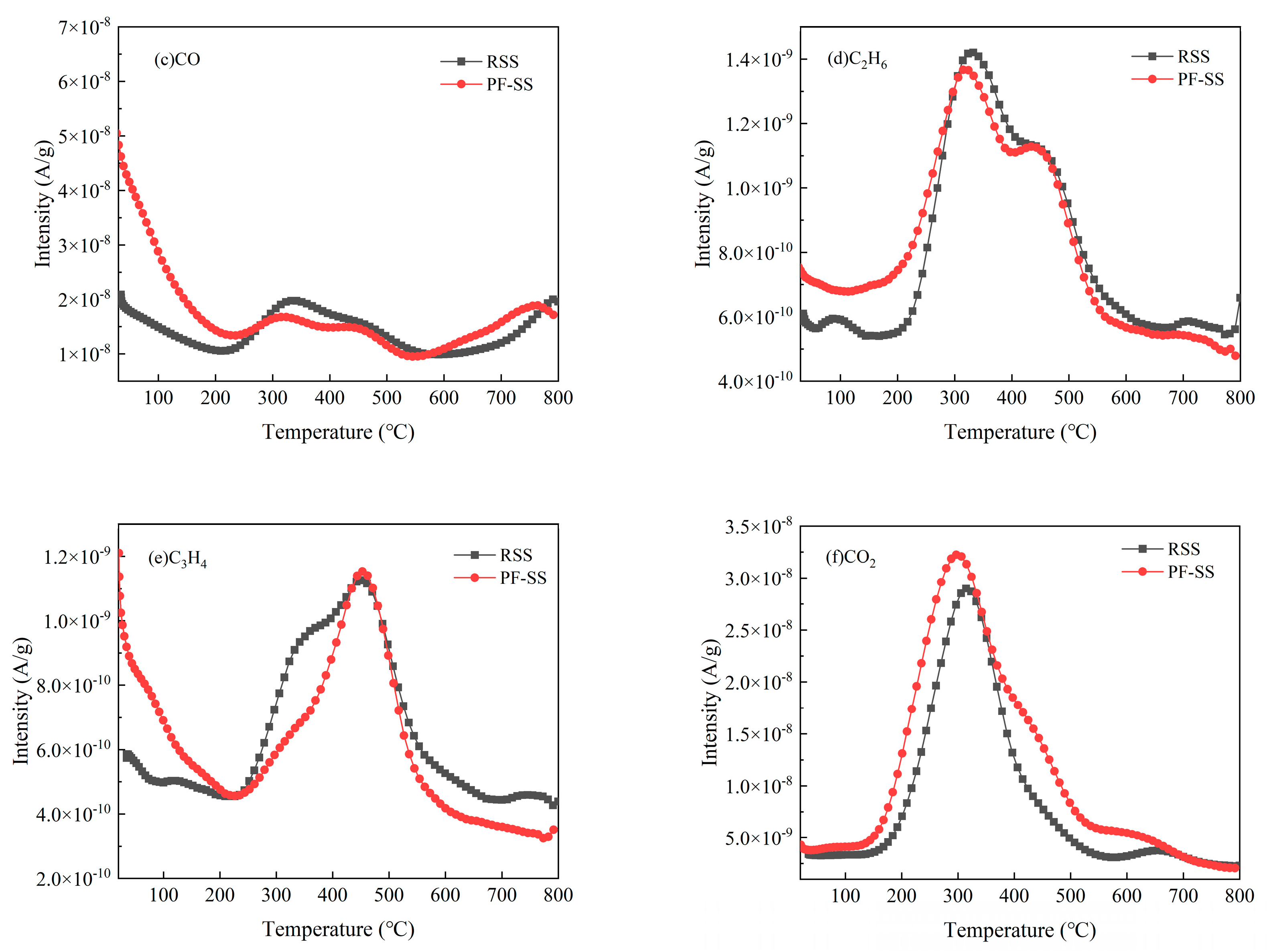
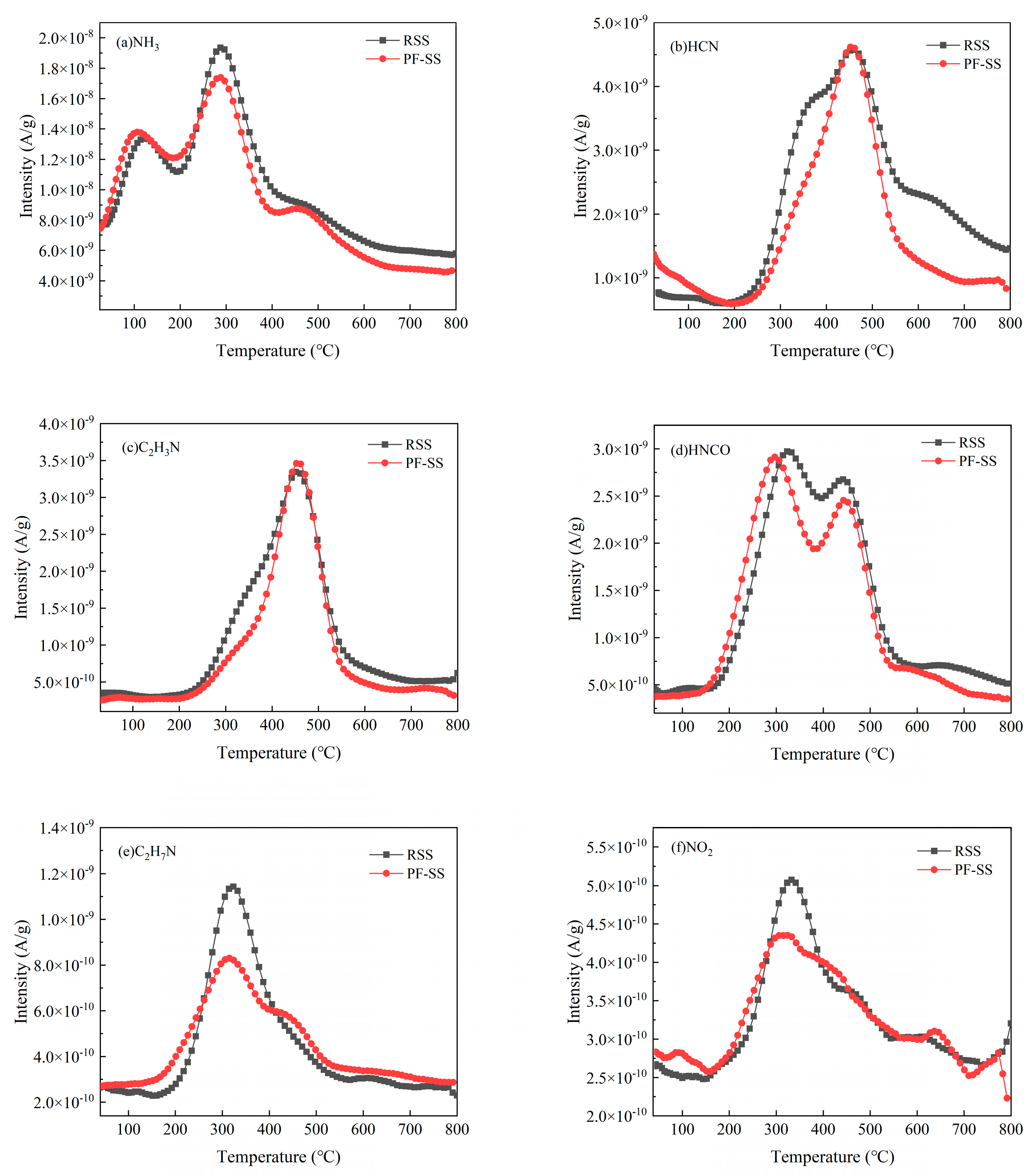
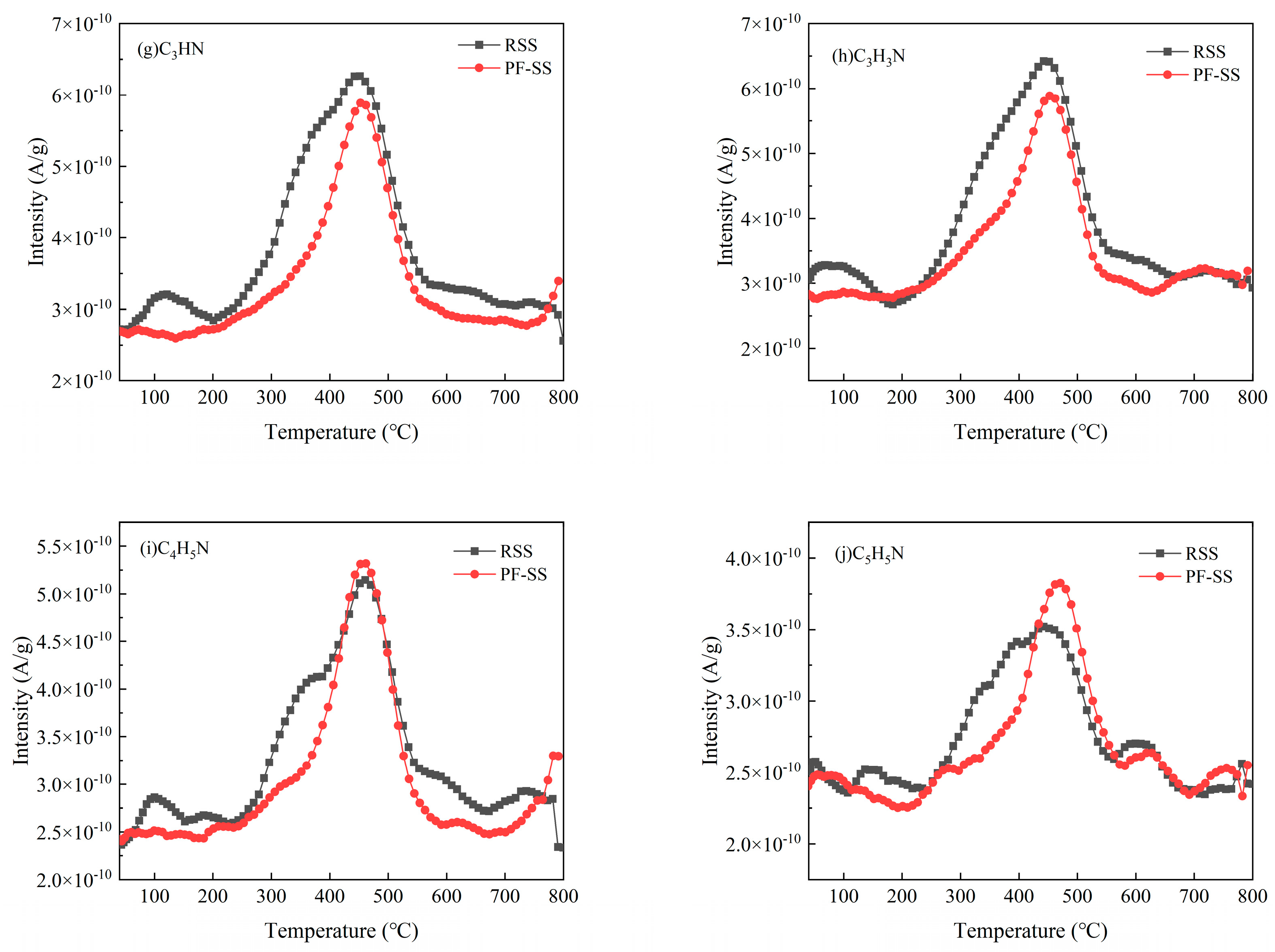

| Sample | Proximate Analysis (wt %) | Ultimate Analysis (wt %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vad | FCad | C | H | O 1 | N | S | Cl | Fe | |
| RSS 2 | 1.88 | 31.00 | 60.63 | 6.49 | 34.47 | 5.25 | 21.48 | 6.68 | 0.913 | 0.21 | 2.23 |
| PF–SS | 1.04 | 39.45 | 55.24 | 4.27 | 30.46 | 4.70 | 18.71 | 5.46 | 0.94 | 0.28 | 9.59 |
| Compound | Molecule | m/z | RSS 1 | PF-SS |
|---|---|---|---|---|
| Methane | CH4 | 16 | 9.09 × 10−7 | 1.14 × 10−6 |
| Water | H2O | 18 | 1.02 × 10−5 | 1.08 × 10−5 |
| Carbon monoxide | CO | 28 | 2.30 × 10−6 | 2.71 × 10−6 |
| Ethane | C2H6 | 30 | 1.83 × 10−7 | 1.70 × 10−7 |
| Propyne | C3H4 | 40 | 1.36 × 10−7 | 1.05 × 10−7 |
| Carbon dioxide | CO2 | 44 | 4.00 × 10−6 | 4.84 × 10−6 |
| Compound | Molecule | m/z | RSS 1 | PF-SS |
|---|---|---|---|---|
| Ammonia | NH3 | 17 | 2.72 × 10−6 | 2.49 × 10−6 |
| Hydrogen cyanide | HCN | 27 | 9.04 × 10−7 | 6.98 × 10−7 |
| Acetonitrile | C2H3N | 41 | 5.23 × 10−7 | 4.52 × 10−7 |
| Isocyanic acid | HNCO | 43 | 5.49 × 10−7 | 4.75 × 10−7 |
| Ethylamine | C2H7N | 45 | 1.38 × 10−7 | 8.78 × 10−8 |
| Nitrogen dioxide | NO2 | 46 | 4.93 × 10−8 | 4.27 × 10−8 |
| Cyanoacetylene | C3HN | 51 | 6.55 × 10−8 | 4.58 × 10−8 |
| Acrylonitrile | C3H3N | 53 | 7.37 × 10−8 | 4.59 × 10−8 |
| Pyrrole | C4H5N | 67 | 4.47 × 10−8 | 3.99 × 10−8 |
| Pyridine | C5H5N | 79 | 2.29 × 10−8 | 2.44 × 10−8 |
| Compound | Molecule | m/z | RSS 1 | PF-SS |
|---|---|---|---|---|
| Hydrogen sulfide | H2S | 34 | 5.74 × 10−8 | 1.61 × 10−8 |
| Methyl mercaptan | CH3SH | 48 | 7.14 × 10−8 | 2.57 × 10−8 |
| Carbonyl sulfide | COS | 60 | 3.91 × 10−8 | 1.14 × 10−8 |
| Sulfur dioxide | SO2 | 64 | 7.70 × 10−8 | 2.16 × 10−8 |
| Carbon disulfide | CS2 | 76 | 7.89 × 10−9 | 9.35 × 10−9 |
| Dimethyl sulfoxide | C2H6OS | 78 | 1.56 × 10−8 | 2.59 × 10−8 |
| Dimethyl sulfone | C2H6O2S | 94 | 1.98 × 10−8 | 1.53 × 10−8 |
| Compound | Molecule | m/z | RSS 1 | PF-SS |
|---|---|---|---|---|
| Hydrogen chloride | HCl | 37 | 4.32 × 10−8 | 2.79 × 10−8 |
| Methyl chloride | CH3Cl | 50 | 5.49 × 10−8 | 4.26 × 10−8 |
| Vinyl chloride | C2H3Cl | 62 | 1.02 × 10−8 | 8.49 × 10−9 |
| Nitrosyl chloride | NOCl | 65 | 3.10 × 10−8 | 3.02 × 10−8 |
| Chlorine | Cl2 | 71 | 1.63 × 10−8 | 2.05 × 10−8 |
| Allyl chloride | C3H5Cl | 77 | 2.74 × 10−8 | 2.23 × 10−8 |
| Chlorobenzene | C6H5Cl | 113 | 6.18 × 10−9 | 1.48 × 10−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Tao, W.; Hou, M.; Ran, M.; Chen, C.; Liu, J.; Tu, L.; Huang, L.; Deng, P.; Chen, D.; et al. Effect of Potassium Ferrate as a Dewatering Conditioner on Sludge Pyrolysis Characteristics and the Releasing Characteristics of Nitrogen, Sulfur, and Chlorine during Sewage Sludge Pyrolysis. Processes 2023, 11, 920. https://doi.org/10.3390/pr11030920
Zhang H, Tao W, Hou M, Ran M, Chen C, Liu J, Tu L, Huang L, Deng P, Chen D, et al. Effect of Potassium Ferrate as a Dewatering Conditioner on Sludge Pyrolysis Characteristics and the Releasing Characteristics of Nitrogen, Sulfur, and Chlorine during Sewage Sludge Pyrolysis. Processes. 2023; 11(3):920. https://doi.org/10.3390/pr11030920
Chicago/Turabian StyleZhang, Hua, Wenpan Tao, Mingming Hou, Maoqian Ran, Chi Chen, Jingcui Liu, Liang Tu, Lan Huang, Peiyao Deng, Dan Chen, and et al. 2023. "Effect of Potassium Ferrate as a Dewatering Conditioner on Sludge Pyrolysis Characteristics and the Releasing Characteristics of Nitrogen, Sulfur, and Chlorine during Sewage Sludge Pyrolysis" Processes 11, no. 3: 920. https://doi.org/10.3390/pr11030920
APA StyleZhang, H., Tao, W., Hou, M., Ran, M., Chen, C., Liu, J., Tu, L., Huang, L., Deng, P., Chen, D., & Wu, Y. (2023). Effect of Potassium Ferrate as a Dewatering Conditioner on Sludge Pyrolysis Characteristics and the Releasing Characteristics of Nitrogen, Sulfur, and Chlorine during Sewage Sludge Pyrolysis. Processes, 11(3), 920. https://doi.org/10.3390/pr11030920






