Inhibition of Survival Mechanisms and Cell Death Induction in Melanoma Following Photodynamic Therapy Mediated by Meso-5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of Meso-5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin (THPP)
2.2. Octanol Water Partition Coefficient of THPP
2.3. Partition Coefficient Measurements
2.4. Singlet Oxygen Quantum Yields
2.5. Bioassays
2.5.1. Cell Cultures
2.5.2. Light Source
2.5.3. Cytotoxicity assay
2.5.4. Experimental Design
2.5.5. Confocal Microscopy
2.5.6. Flowcytometry
2.5.7. Mitochondrial Membrane Potential
2.5.8. Cell Lysis
2.5.9. Spectrophotometry and Fluorometry
2.5.10. ELISA
2.5.11. Western Blot
2.5.12. Statistical Analysis
3. Results and Discussion
3.1. THPP Photosensitizer Properties
Octanol–Water Partition Coefficient and Singlet Oxygen Quantum Yields for THPP
3.2. Biological Assays
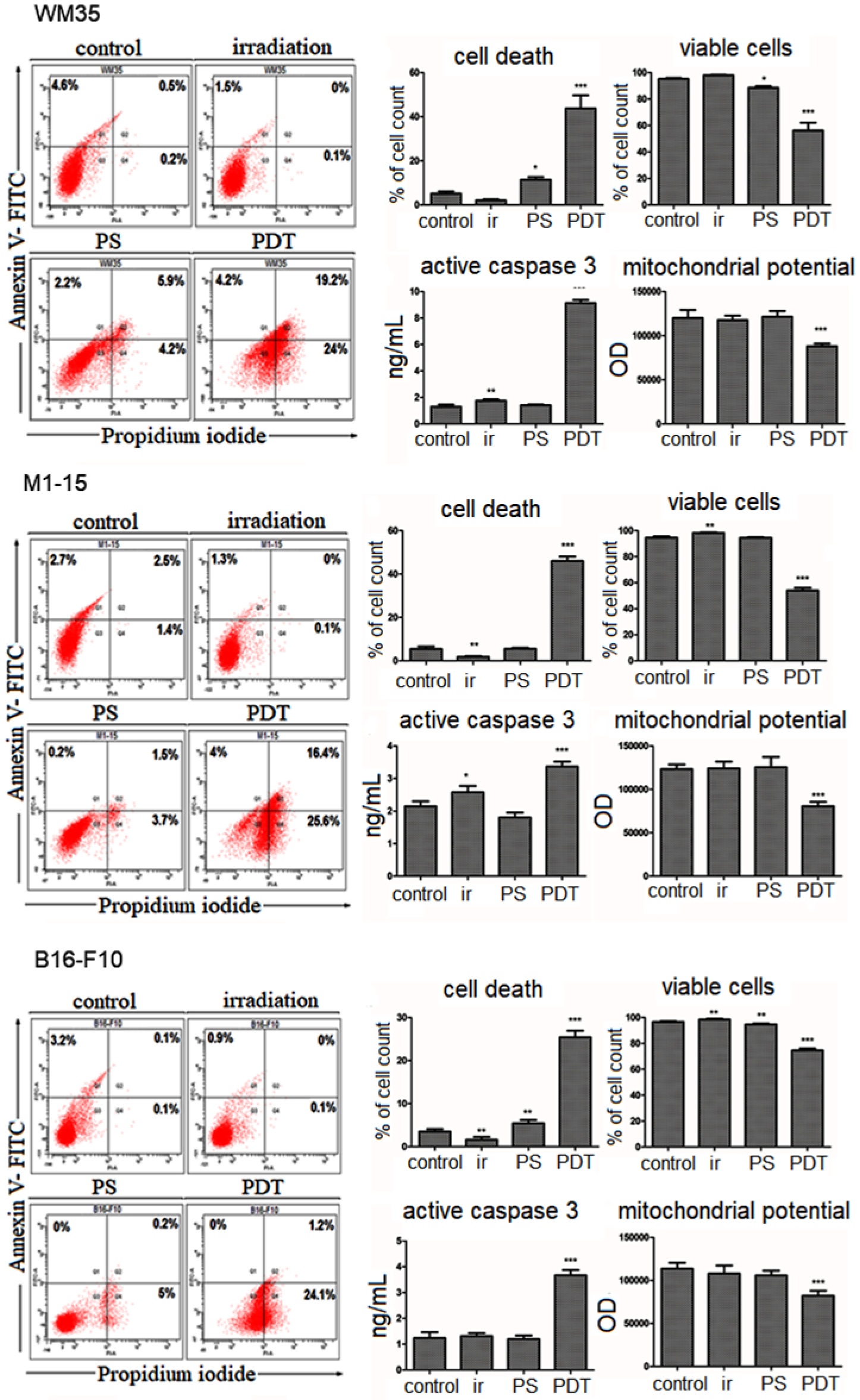
3.2.1. Cytotoxicity and Photo-Toxicity Assessment
3.2.2. Morphological Aspect and Cytoskeleton Alterations
3.2.3. Cell Death Mechanism
3.2.4. Oxidative Stress Induction
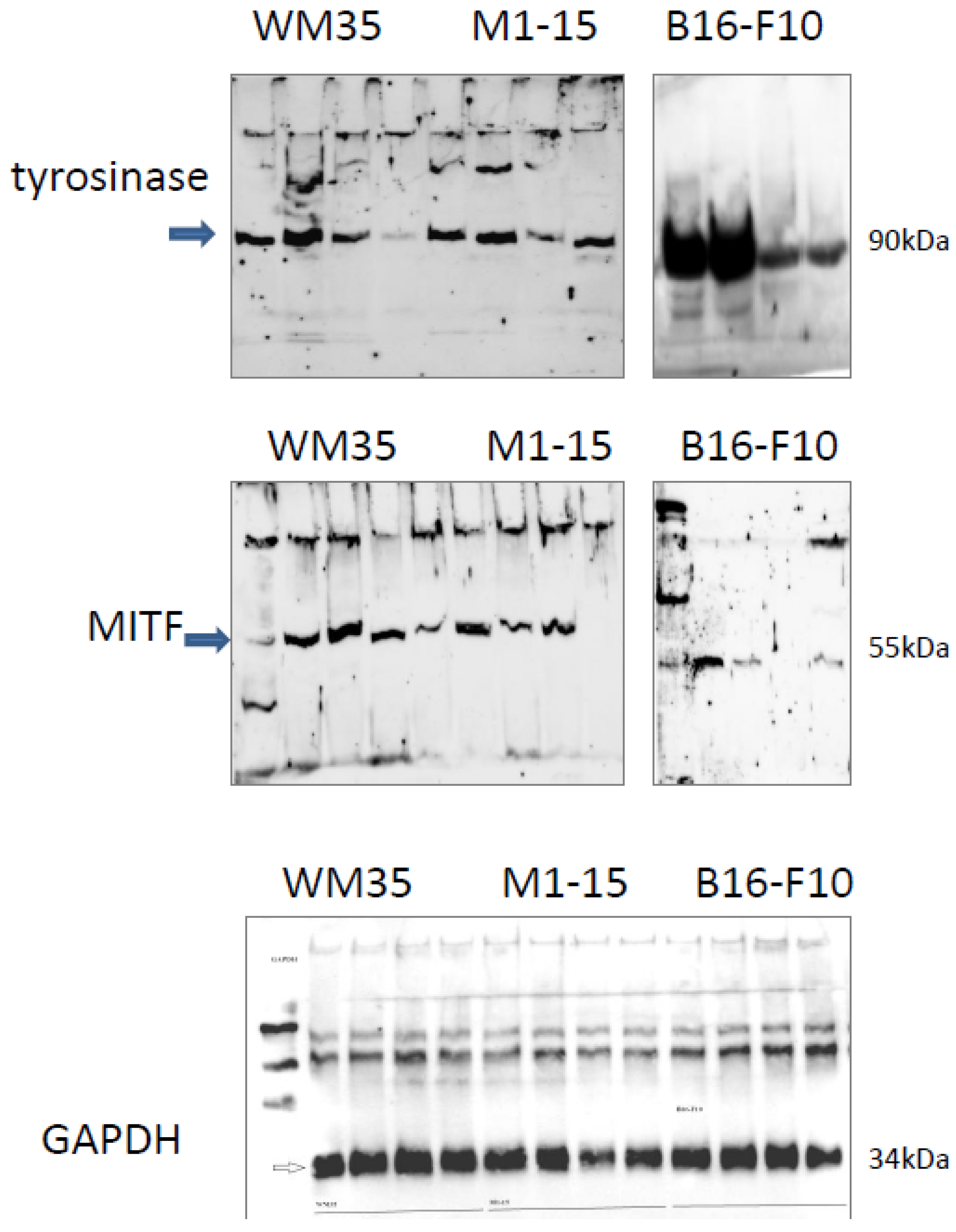
3.2.5. Melanogenesis
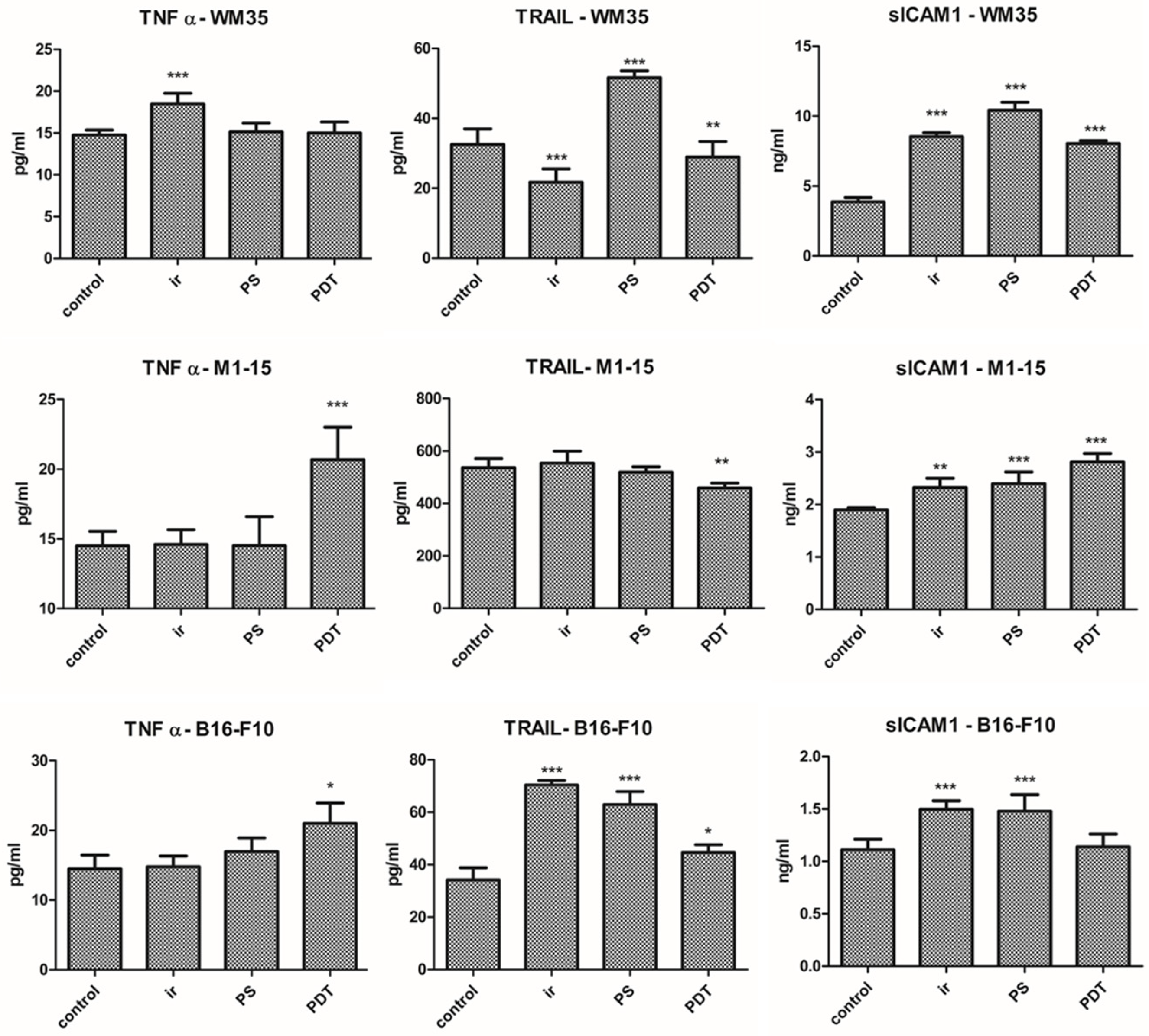
3.2.6. Inflammation
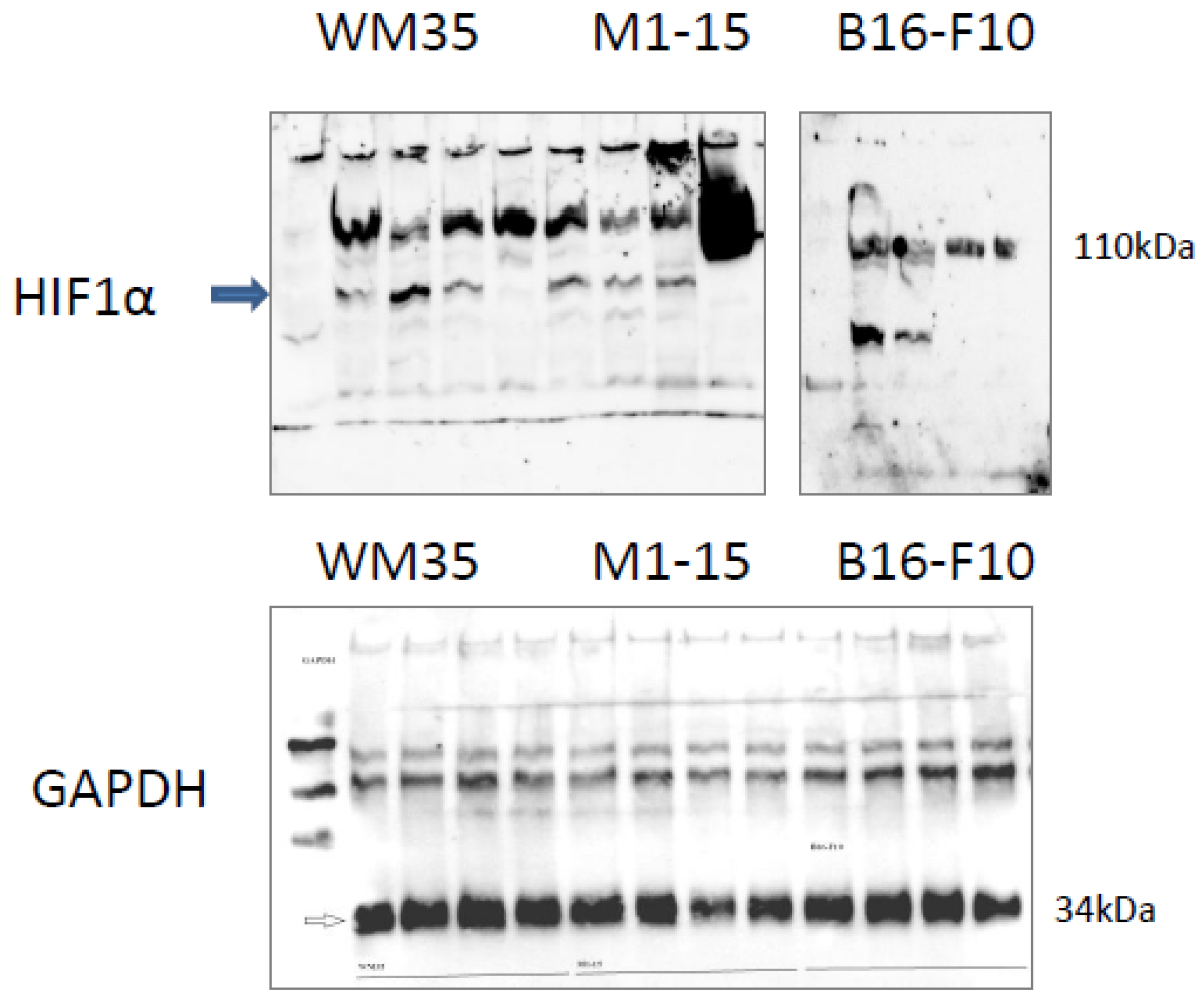
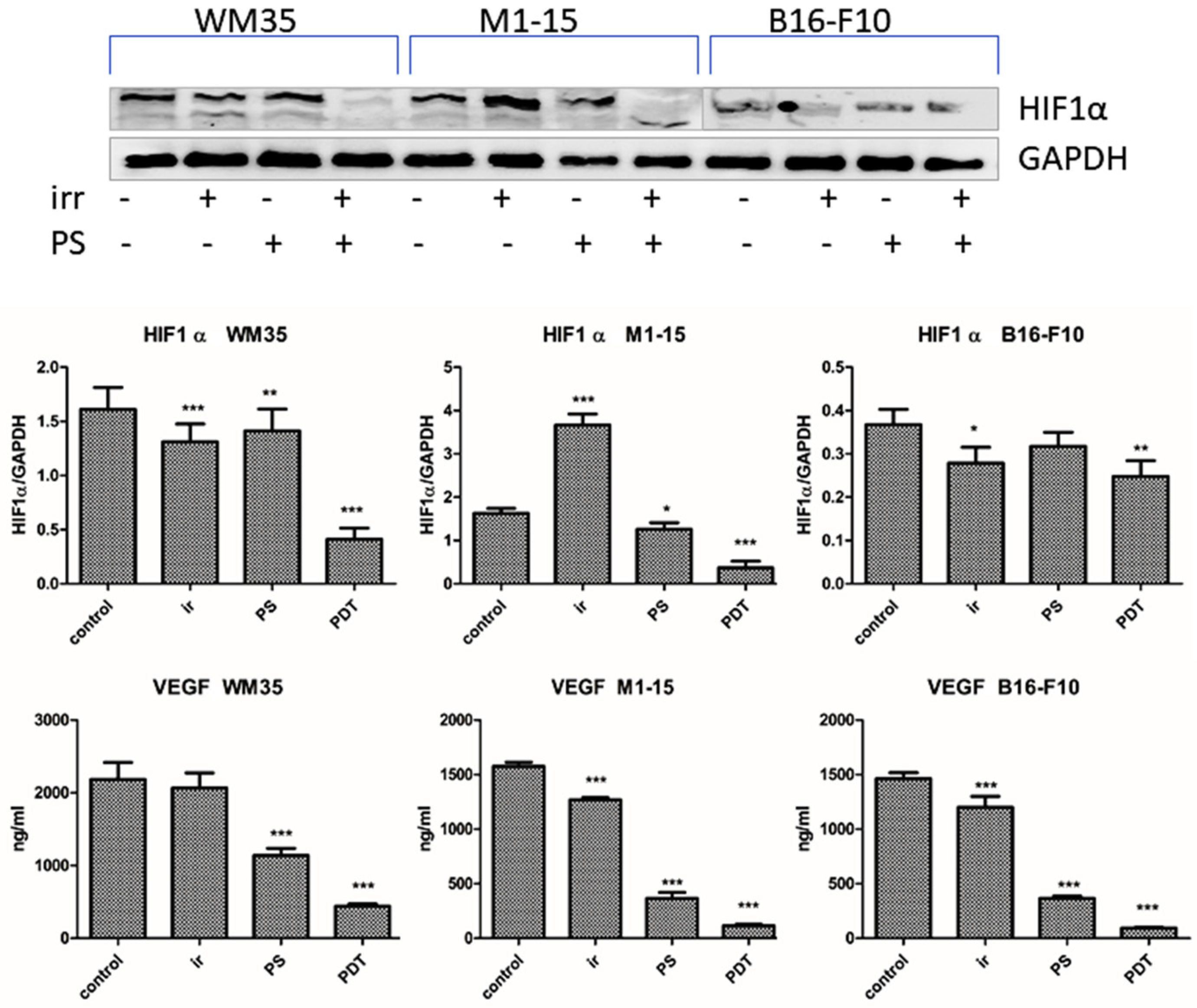
3.2.7. Angiogenesis Markers
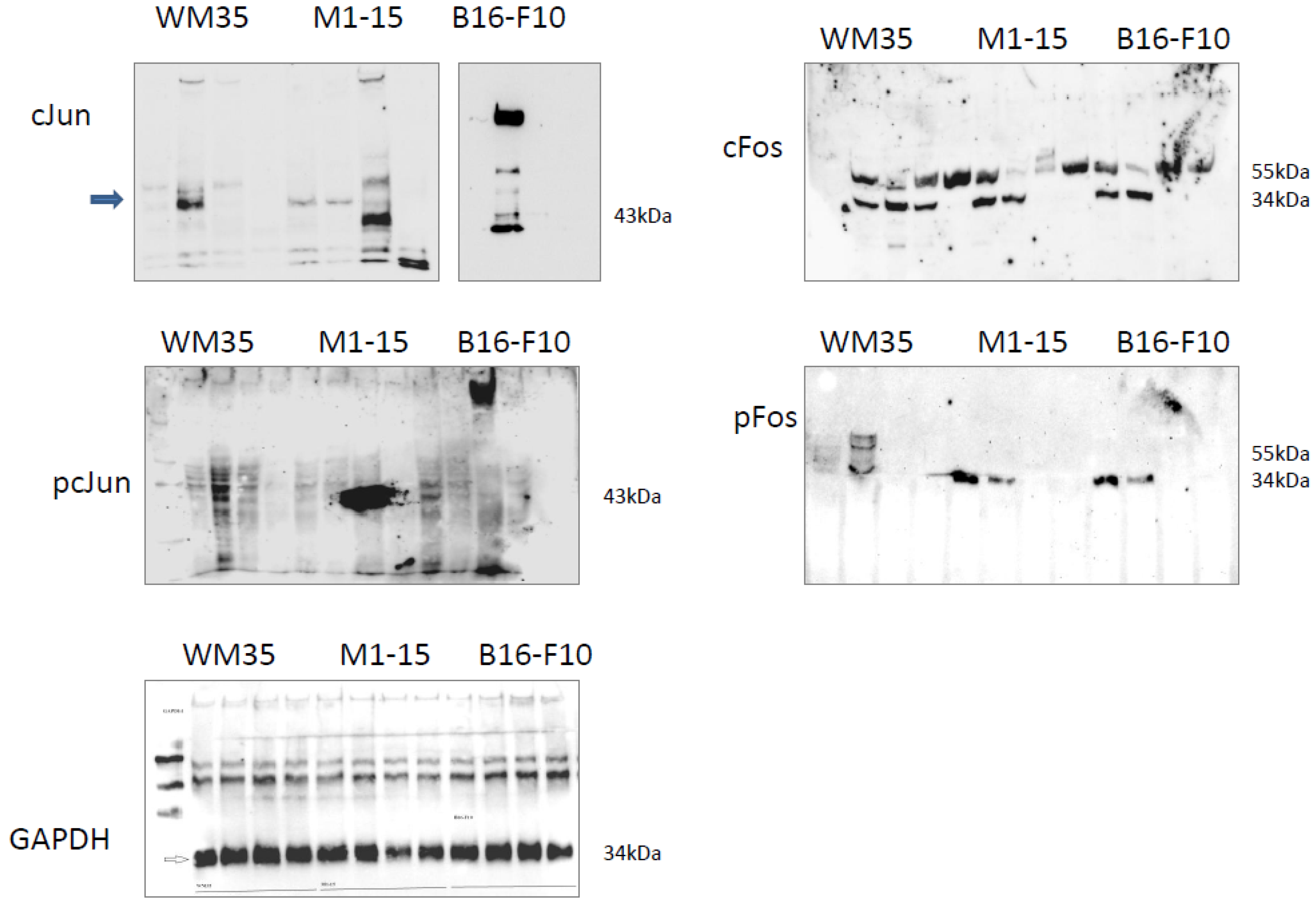
3.2.8. AP1 Transcription Factors
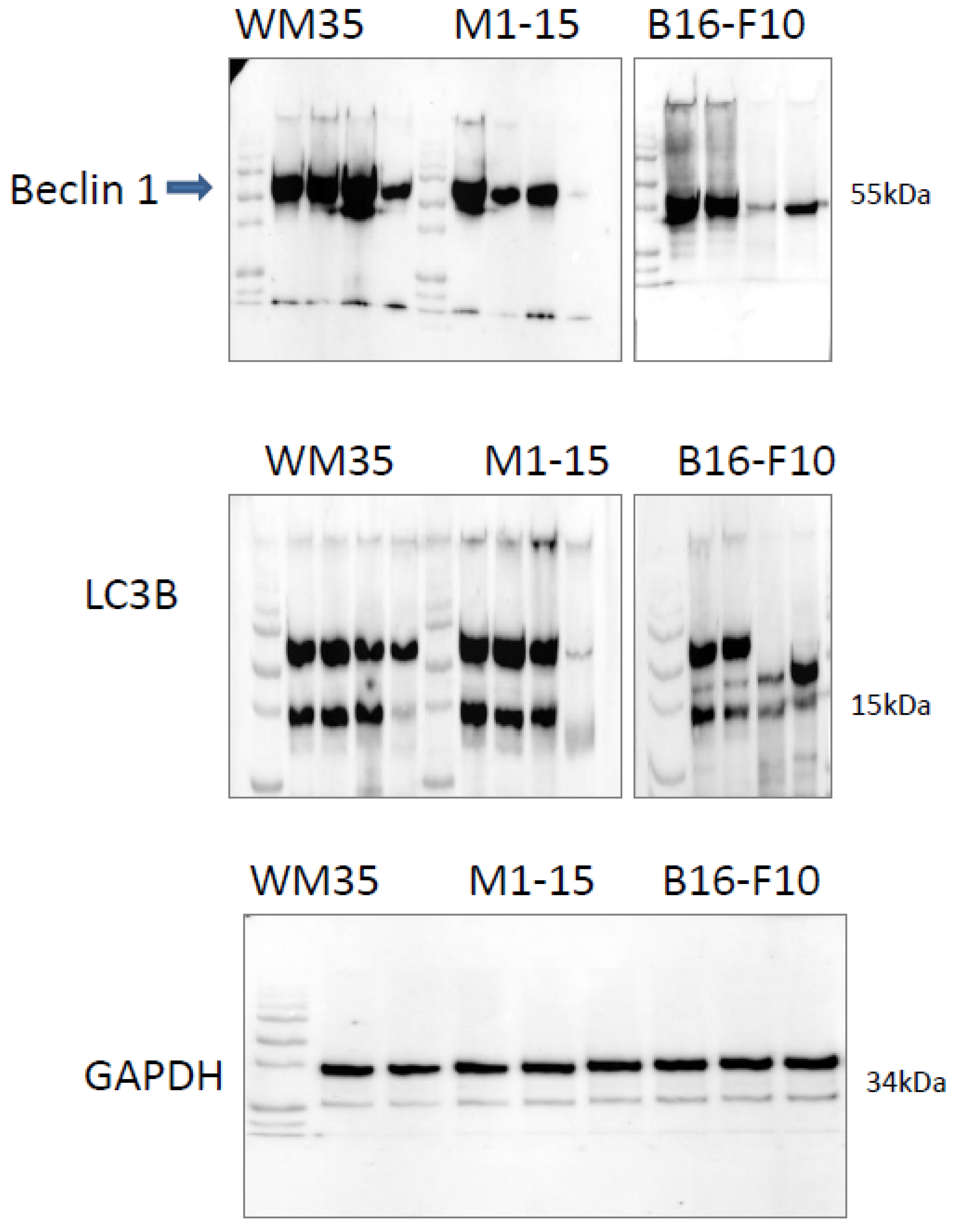
3.2.9. Autophagy
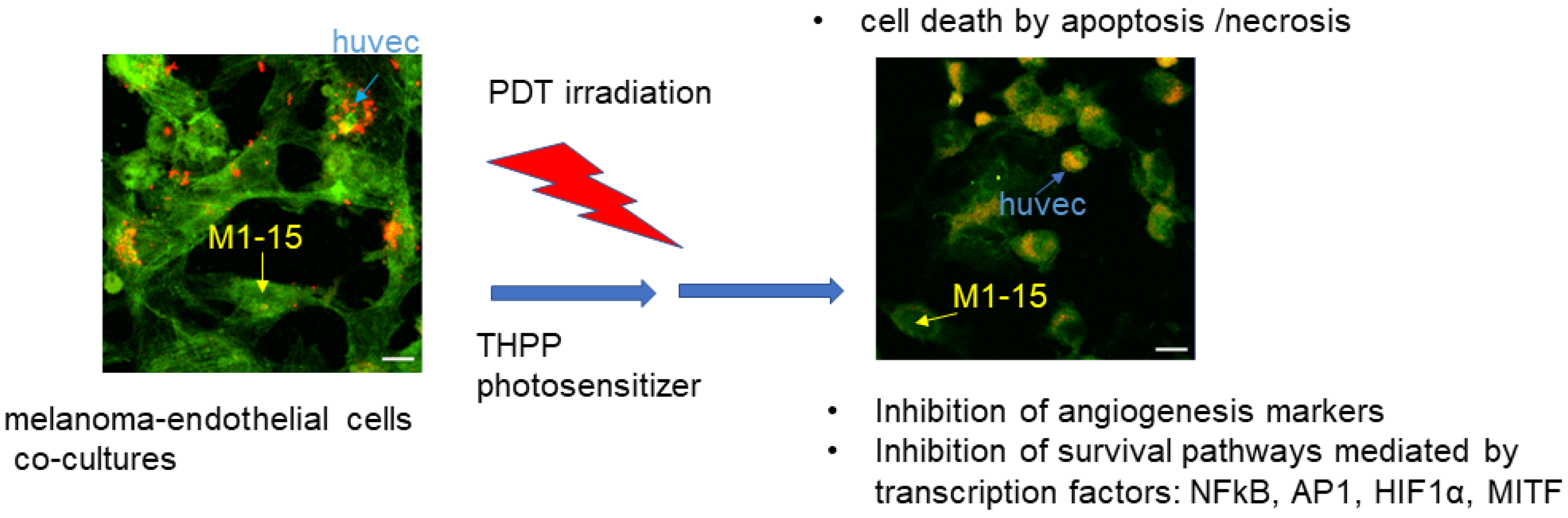
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; et al. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Baldea, I.; Filip, A.G. Photodynamic therapy in melanoma-an update. J. Physiol. Pharmacol. 2012, 63, 109–118. [Google Scholar]
- Dickson, P.V.; Gershenwald, J.E. Staging and Prognosis of Cutaneous Melanoma. Surg. Oncol. Clin. N. Am. 2011, 20, 1–17. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Vecchio, D.; Avci, P.; Yin, R.; Garcia-Diaz, M.; Hamblin, M.R. Melanoma resistance to photodynamic therapy: New insights. Biol. Chem. 2013, 394, 239–250. [Google Scholar] [CrossRef]
- Baldea, I.; Giurgiu, L.; Teacoe, I.D.; Olteanu, D.E.; Olteanu, F.C.; Clichici, S.; Filip, G.A. Photodynamic Therapy in Melanoma—Where do we Stand? Curr. Med. Chem. 2018, 25, 5540–5563. [Google Scholar] [CrossRef]
- De Klerk, D.J.; de Keijzer, M.J.; Dias, L.M.; Heemskerk, J.; de Haan, L.R.; Kleijn, T.G.; Franki, L.P.; Heger, M. Strategies for improving photodynamic therapy through pharmacological modulation of the immediate early stress response. In Photodynamic Therapy. Methods in Molecular Biology; Broekgaarden, M., Zhang, H., Korbelik, M., Hamblin, M.R., Heger, M., Eds.; Humana: New York, NY, USA, 2022; Volume 2451, pp. 405–480. [Google Scholar]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef]
- Baldea, I.; Olteanu, D.E.; Bolfa, P.; Tabaran, F.; Ion, R.M.; Filip, G.A. Melanogenesis and DNA damage following photodynamic therapy in melanoma with two meso-substituted porphyrins. J. Photochem. Photobiol. 2016, 161, 402–410. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Hsu, H.C.; Schreiman, I.C. Synthesis of tetraphenylporphyrins under very mild conditions. Tetrahedron. Lett. 1986, 27, 4969–4970. [Google Scholar] [CrossRef]
- Baldea, I.; Olteanu, D.E.; Bolfa, P.; Ion, R.M.; Decea, N.; Cenariu, M.; Banciu, M.; Sesarman, A.V.; Filip, A.G. Efficiency of photodynamic therapy on WM35 melanoma with new synthetic porphyrins: Role of chemical structure, intracellular targeting and antioxidant defense. J. Photochem. Photobiol. B Biol. 2015, 151, 142–152. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- Scalise, I.; Durantini, E.N. Photodynamic effect of metallo 5-(4-carboxyphenyl)-10,15,20-tris(4-methylphenyl) porphyrins in biomimetic AOT reverse micelles containing urease. J. Photochem. Photobiol. A Chem. 2004, 162, 105–113. [Google Scholar] [CrossRef]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: An in vitro study on Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B Biology 2006, 85, 28–38. [Google Scholar] [CrossRef]
- Spiller, W.; Kliesch, H.; Wohrle, D.; Hackbarth, S.; Röder, B.; Schnurpfeil, G. Singlet oxygen quantum yields of different photosensitizers in polar solvents and micellar solutions. J. Porphyr. Phthalocyanines 1998, 2, 145–158. [Google Scholar] [CrossRef]
- Pineiro, M.; Carvalho, A.L.; Pereira, M.M.; Gonsalves, A.D.A.R.; Arnaut, L.G.; Formosinho, S.J. Photoacoustic measurements of porphyrin triplet-state quantum yields and singlet-oxygen efficiencies. Chem. Eur. J. 1998, 4, 2299. [Google Scholar] [CrossRef]
- Foletto, P.; Correa, F.; Dornelles, L.; Iglesias, B.A.; da Silveira, C.H.; Nogara, P.A.; de Rocha, J.B.T.; Faustino, M.A.F.; Rodrigues, O.E.D. A New Protocol for the Synthesis of New Thioaryl-Porphyrins Derived from 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin: Photophysical Evaluation and DNA-Binding Interactive Studies. Molecules 2018, 23, 2588. [Google Scholar] [CrossRef]
- Viecelli, V.; Chaves, O.A.; Araki, K.; Martins, P.R.; Iglesias, B.A. Synthesis, Characterization, Spectroelectrochemical, Photophysical and HSA-Binding Properties of Novel and Versatile meso-Tetra(4-pyridylvinylphenyl)porphyrins Coordinated to Ruthenium(II)-Polypyridyl Derivatives. J. Braz. Chem. Soc. 2020, 31, 2282–2298. [Google Scholar] [CrossRef]
- Hegyesi, H.; Somlai, B.; Varga, V.L.; Toth, G.; Kovacs, P.; Molnar, E.L.; Laszlov, V.; Karpati, S.; Rivera, E.; Falus, A.; et al. Suppression of melanoma cell proliferation by histidine decarboxylase specific antisense oligonucleotides. J. Invest. Dermatol. 2001, 117, 151–153. [Google Scholar] [CrossRef]
- Tudor, D.; Nenu, I.; Filip, G.A.; Olteanu, D.; Cenariu, M.; Tabaran, F.; Ion, R.M.; Gligor, L.; Baldea, I. Combined regimen of photodynamic therapy mediated by Gallium phthalocyanine chloride and Metformin enhances anti-melanoma efficacy. PLoS One 2017, 12, e0173241. [Google Scholar] [CrossRef]
- Ohkura, T.; Yamashita, K.; Mishima, Y.; Kobata, A. Purification of hamster melanoma tyrosinases and structural studies of their asparagine-linked sugar chains. Arch. Biochem. Biophys. 1984, 235, 63–77. [Google Scholar] [CrossRef]
- Tsolekile, N.; Ncapayi, V.; Parani, S.; Sakho, E.; Matoetoe, M.; Songca, S.; Oluwafemi, O. Synthesis of fluorescent CuInS2/ZnS quantum dots—Porphyrin conjugates for photodynamic therapy. MRS Commun. 2018, 8, 398–403. [Google Scholar] [CrossRef]
- Kenneth, K.N.; Lovell, J.F.; Vedadi, A.; Hajian, T.; Zheng, G. Self-Assembled Porphyrin Nanodiscs with Structure-Dependent Activation for Phototherapy and Photodiagnostic Applications. ACS Nano 2013, 7, 3484–3490. [Google Scholar]
- Salas-García, I.; Fanjul-Vélez, F.; Arce-Diego, J.L. Photosensitizer absorption coefficient modeling and necrosis prediction during photodynamic therapy. J. Photochem. Photobiol. B Biology. 2012, 114, 79–86. [Google Scholar] [CrossRef]
- Lazewski, D.; Kucinska, M.; Potapskiy, E.; Kuzminska, J.; Tezyk, A.; Popenda, L.; Jurga, S.; Teuberst, A.; Gdaniec, Z.; Kujawsky, J.; et al. Novel Short PEG Chain-Substituted Porphyrins: Synthesis, Photochemistry, and In Vitro Photodynamic Activity against Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10029. [Google Scholar] [CrossRef]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 2019, 9, 9679. [Google Scholar] [CrossRef]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Summers, F.A.; Zhao, B.; Ganini, D.; Mason, R.P. Photooxidation of amplex red to resorufin: Implications of exposing the amplex red assay to light. Methods Enzymol. 2013, 526, 1–17. [Google Scholar]
- Karakuzu, O.; Cruz, M.R.; Liu, Y.; Garsin, D.A. Amplex Red Assay for Measuring Hydrogen Peroxide Production from Caenorhabditis elegans. Bio-Protocol 2019, 5, e3409. [Google Scholar] [CrossRef]
- Mishin, V.; Gray, J.P.; Heck, D.E.; Laskin, D.L.; Laskin, J.D. Application of the Amplex red/horseradish peroxidase assay to measure hydrogen peroxide generation by recombinant microsomal enzymes. Free. Radic. Biol. Med. 2010, 48, 1485–1491. [Google Scholar] [CrossRef]
- Barui, A.K.; Nethi, S.K.; Patra, C.R. Investigation of the role of nitric oxide driven angiogenesis by zinc oxide nanoflowers. J. Mater. Chem. B 2017, 5, 3391–3403. [Google Scholar] [CrossRef]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. 2021, 84, 1219–1231. [Google Scholar] [CrossRef]
- Maddodi, N.; Jayanthy, A.; Setaluri, V. Shining light on skin pigmentation: The darker and the brighter side of effects of UV radiation. Photochem. Photobiol. 2012, 88, 1075–1082. [Google Scholar] [CrossRef]
- Liu, J.J.; Fisher, D.E. Lighting a path to pigmentation: Mechanisms of MITF induction by UV. Pigment. Cell Meloma. Res. 2010, 23, 741–745. [Google Scholar] [CrossRef]
- Sim, D.Y.; Sohng, J.K.; Jung, H.J. Anticancer activity of 7,8-dihydroxyflavone in melanoma cells via downregulation of α-MSH/cAMP/MITF pathway. Oncol. Rep. 2016, 36, 528–534. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Lee, S.-M.; Lin, C.-C.; Liu, C.-Y. Hispolon Decreases Melanin Production and Induces Apoptosis in Melanoma Cells through the Downregulation of Tyrosinase and Microphthalmia-Associated Transcription Factor (MITF) Expressions and the Activation of Caspase-3, -8 and -9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Schlatter, R.; Schmich, K.; Lutz, A.; Trefzger, J.; Sawodny, O.; Ederer, M.; Merfort, I. Modeling the TNFα-induced apoptosis pathway in hepatocytes. PLoS ONE 2011, 6, e18646. [Google Scholar] [CrossRef]
- Marini, P.; Schmid, A.; Jendrossek, V.; Faltin, H.; Daniel, P.T.; Budach, W.; Belka, C. Irradiation specifically sensitises solid tumour cell lines to TRAIL mediated apoptosis. BMC Cancer 2005, 5, 5. [Google Scholar] [CrossRef]
- Thorburn, A. Pathway of the Month. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) Pathway Signaling. J. Thorac. Oncol. 2007, 2, 461–465. [Google Scholar] [CrossRef]
- Bhuvaneswari, R.; Gan, Y.Y.; Lucky, S.S.; Chin, W.W.; Ali, S.M.; Soo, K.C.; Olivo, M. Molecular profiling of angiogenesis in hypericin mediated photodynamic therapy. Mol. Cancer 2008, 7, 56. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Borawska, M.H. Soluble intercellular adhesion molecule-1 (sICAM-1): An overview. Eur. Cytokine Netw. 2004, 15, 91–98. [Google Scholar]
- Zheng, Y.; Yin, G.; Le, V.; Zhang, A.; Chen, S.; Liang, X.; Liu, J. Photodynamic-therapy Activates Immune Response by disrupting Immunity Homeostasis of Tumor Cells, which Generates Vaccine for Cancer Therapy. Int. J. Biol. Sci. 2016, 12, 120–132. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A. Targeting Angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Otrock, Z.; Hatoum, H.; Awada, A.; Ishak, R.; Shamseddine, A. Hypoxia-inducible factor in cancer angiogenesis: Structure, regulation and clinical perspectives. Crit. Rev. Oncol. Hematol. 2009, 70, 93–102. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, X.; Frazier, D.L.; Panjehpour, M.; Breider, M.A. Tumor cell-enhanced sensitivity of vascular endothelial cells to photodynamic therapy. Lasers Surg. Med. 1994, 15, 342–350. [Google Scholar] [CrossRef]
- Chen, B.; Pogue, B.W.; Hoopes, P.J.; Hasan, T. Vascular and cellular targeting for photodynamic therapy. Crit. Rev. Eukaryot. Gene Expr. 2006, 16, 279–305. [Google Scholar] [CrossRef]
- Kappelmann, M.; Bosserhoff, A.; Kuphal, S. AP-1/c-Jun transcription factors: Regulation and function in malignant melanoma. Eur. J. Cell. Biol. 2014, 93, 76–81. [Google Scholar] [CrossRef]
- Kick, G.; Messer, G.; Plewig, G.; Kind, P.; Goetz, A.E. Strong and prolonged induction of c-jun and c-fos proto-oncogenes by photodynamic therapy. Br. J. Cancer 1996, 74, 30–36. [Google Scholar] [CrossRef]
- Gerosa, L.; Chidley, C.; Fröhlich, F.; Sanchez, G.; Lim, S.K.; Muhlich, J.; Chen, J.-Y.; Vallabhaneni, S.; Baker, G.J.; Schapiro, D.; et al. Receptor-Driven ERK Pulses Reconfigure MAPK Signaling and Enable Persistence of Drug-Adapted BRAF-Mutant Melanoma Cells. Cell Syst. 2020, 11, 478–494. [Google Scholar] [CrossRef]
- Baldea, I.; Olteanu, D.; Filip, G.A.; Pogacean, F.; Coros, M.; Suciu, M.; Tripon, S.C.; Cenariu, M.; Magerusan, L.; Stefan van-Staden, R.L.; et al. Cytotoxicity mechanisms of nitrogen-doped graphene obtained by electrochemical exfoliation of graphite rods, on human endothelial and colon cancer cells. Carbon 2020, 158, 267–281. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, H.; Liu, X.; Li, B.; Chen, Y.; Ren, X.; Liu, C.-G.; Yang, J.-M. Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy 2009, 5, 816–823. [Google Scholar] [CrossRef]
- Meijer, A.J. Autophagy research: Lessons from metabolism. Autophagy 2009, 5, 3–5. [Google Scholar] [CrossRef]
- Schmitt, D.; Bozkurt, S.; Henning-Domres, P.; Huesmann, H.; Eimer, S.; Bindila, L.; Behrends, C.; Boyle, E.; Wilfling, F.; Tascher, G.; et al. Lipid and protein content profiling of isolated native autophagic vesicles. EMBO Rep. 2022, 23, e53065. [Google Scholar] [CrossRef]
- Martins, W.K.; Belotto, R.; Maryana, N.S.; Grasso, D.; Suriani, M.D.; Lavor, T.S.; Itri, R.; Baptista, M.S.; Tsubone, T.M. Autophagy Regulation and Photodynamic Therapy: Insights to Improve Outcomes of Cancer Treatment. Front Oncol. 2021, 10, 610472. [Google Scholar] [CrossRef]
- Lim, C.K.; Li, F.M.; Peters, T.J. High-Performance Liquid Chromatography of Porphyrins. J. Chromatogr. 1988, 429, 123–153. [Google Scholar] [CrossRef]
- Lim, C.K.; Rideout, J.M.; Wright, D.J. Separation of Porphyrin Isomers by High-Performance Liquid Chromatography. Biochem. J. 1983, 211, 435–438. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldea, I.; Danescu, S.; Tabaran, F.; Filip, A.G.; Ion, R.M.; Olteanu, D.E.; Sevastre-Berghian, A.C.; Decea, R.M.; Iacovita, C.; Hanganu, D.; et al. Inhibition of Survival Mechanisms and Cell Death Induction in Melanoma Following Photodynamic Therapy Mediated by Meso-5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin. Processes 2023, 11, 917. https://doi.org/10.3390/pr11030917
Baldea I, Danescu S, Tabaran F, Filip AG, Ion RM, Olteanu DE, Sevastre-Berghian AC, Decea RM, Iacovita C, Hanganu D, et al. Inhibition of Survival Mechanisms and Cell Death Induction in Melanoma Following Photodynamic Therapy Mediated by Meso-5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin. Processes. 2023; 11(3):917. https://doi.org/10.3390/pr11030917
Chicago/Turabian StyleBaldea, Ioana, Sorina Danescu, Flaviu Tabaran, Adriana Gabriela Filip, Rodica Mariana Ion, Diana Elena Olteanu, Alexandra Cristina Sevastre-Berghian, Roxana Maria Decea, Cristian Iacovita, Daniela Hanganu, and et al. 2023. "Inhibition of Survival Mechanisms and Cell Death Induction in Melanoma Following Photodynamic Therapy Mediated by Meso-5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin" Processes 11, no. 3: 917. https://doi.org/10.3390/pr11030917
APA StyleBaldea, I., Danescu, S., Tabaran, F., Filip, A. G., Ion, R. M., Olteanu, D. E., Sevastre-Berghian, A. C., Decea, R. M., Iacovita, C., Hanganu, D., & Cenariu, M. (2023). Inhibition of Survival Mechanisms and Cell Death Induction in Melanoma Following Photodynamic Therapy Mediated by Meso-5,10,15,20-tetrakis-(4-hydroxyphenyl)-porphyrin. Processes, 11(3), 917. https://doi.org/10.3390/pr11030917










