Effects of Spray Drying, Freeze Drying and Gamma Irradiation on the Antioxidant Activities of Camel and Cow Milk Fractions
Abstract
1. Introduction
2. Materials and Methods
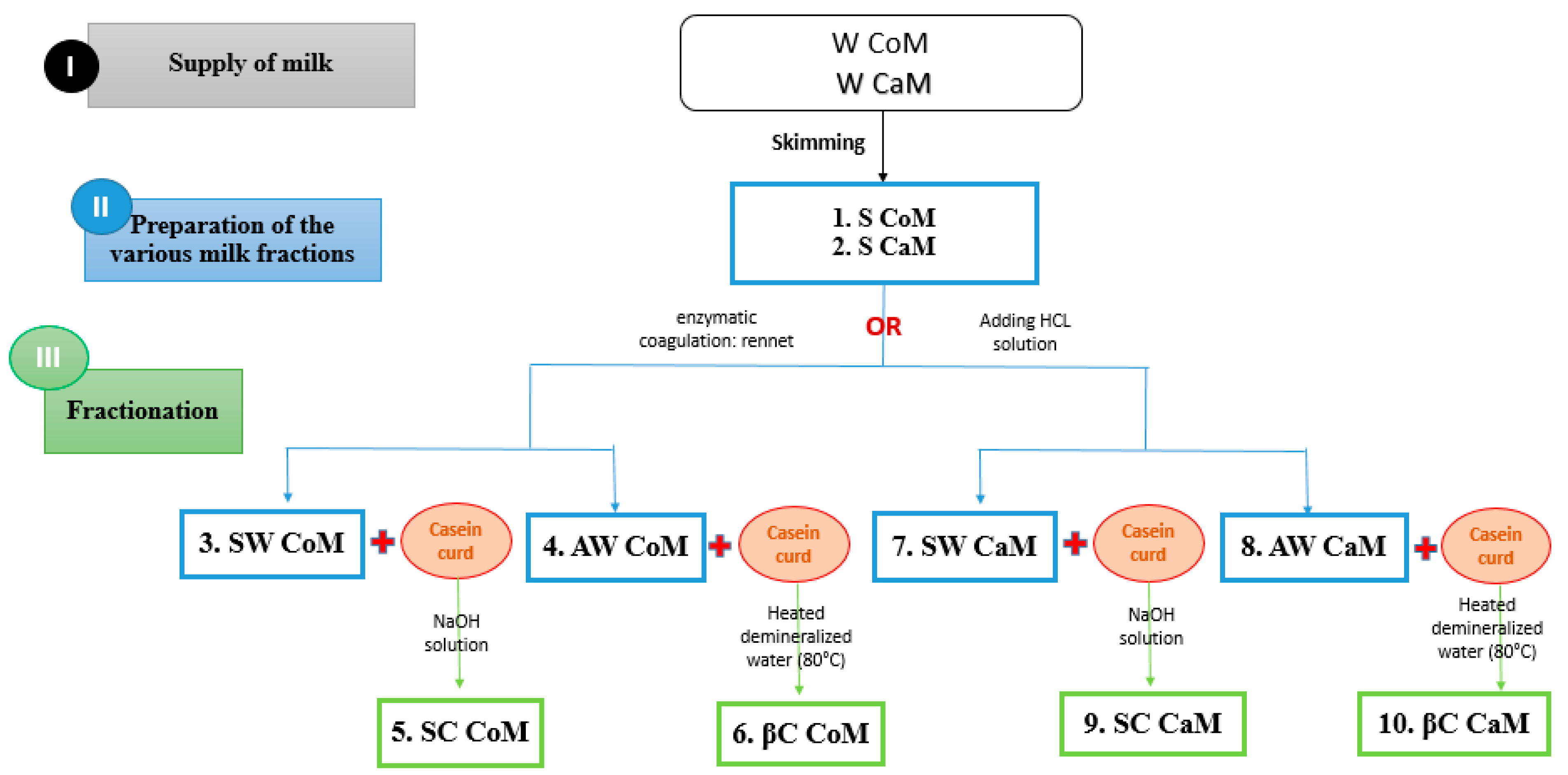
2.1. Raw Milk and Fractions Preparation
2.1.1. Milk Samples
2.1.2. Protein Fractions Preparation
- Acid whey
- Sodium caseinate
- Sweet whey
- β-Casein
2.2. Freeze Drying, Spray Drying and Irradiation
2.2.1. Spray Drying
2.2.2. Freeze Drying
2.2.3. Irradiation Experiments
2.3. Samples Preparation for Antioxidant Activities
2.4. Total Phenolic Content and Antioxidant Activities
2.5. Data Analysis
3. Results and Discussion
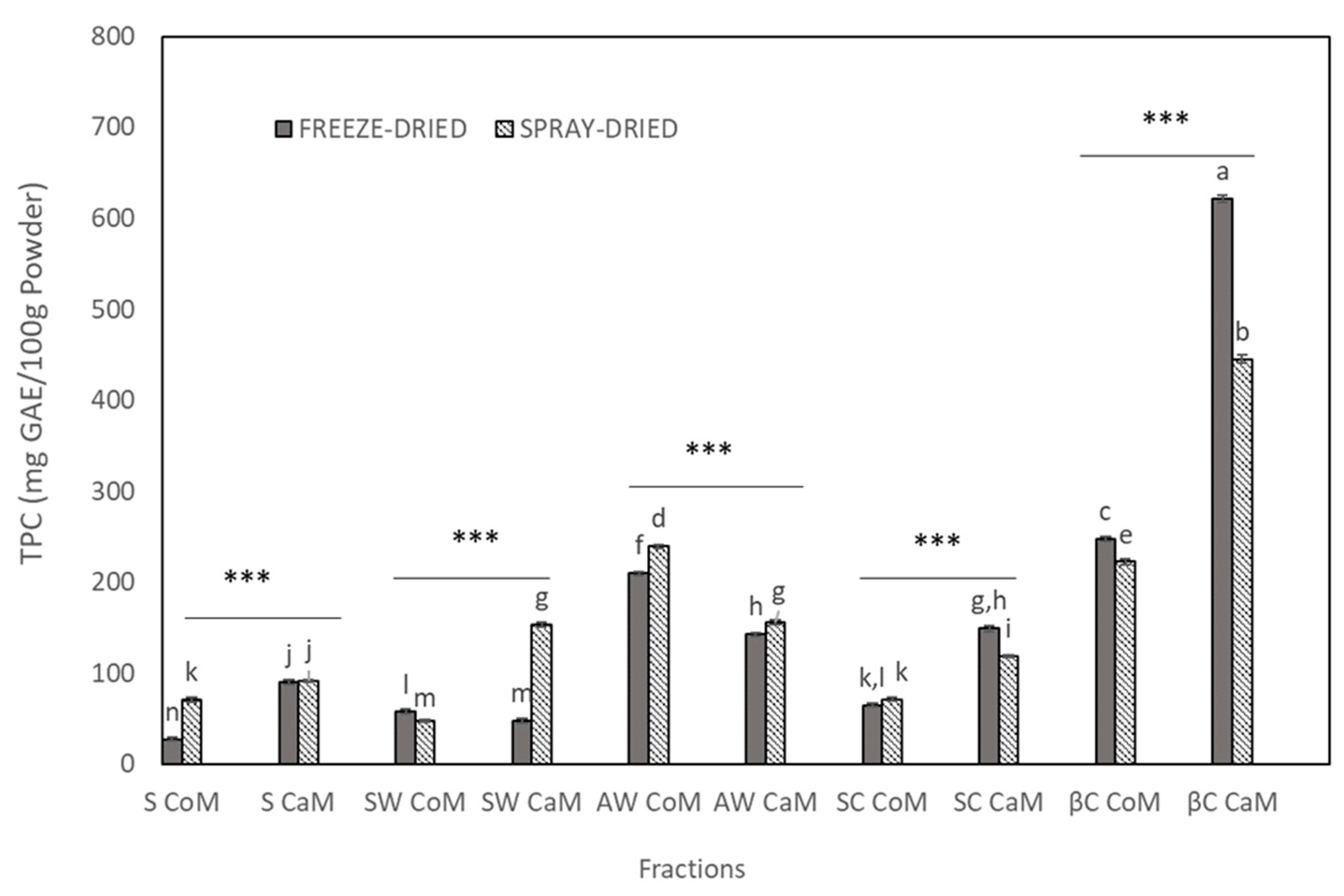
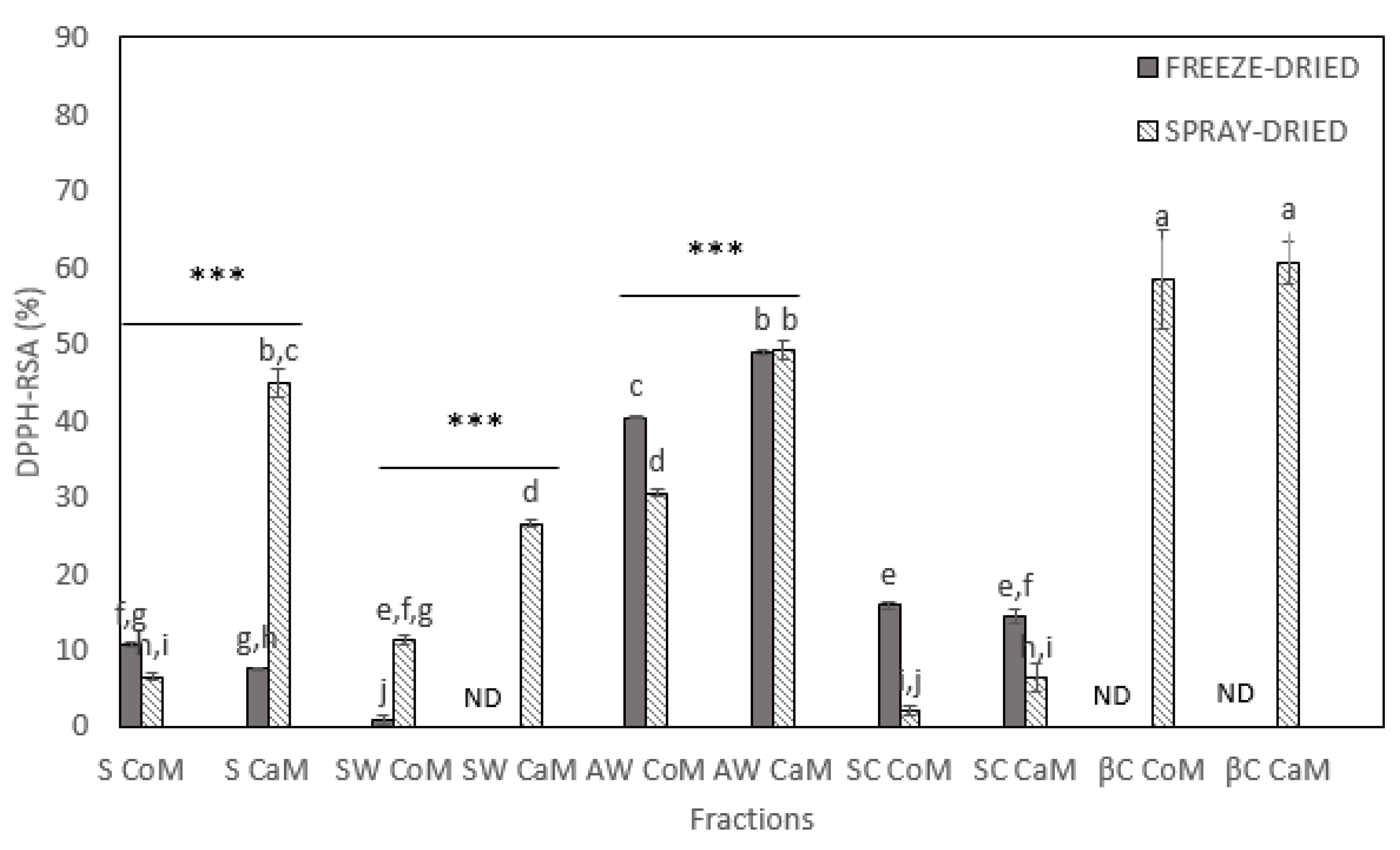
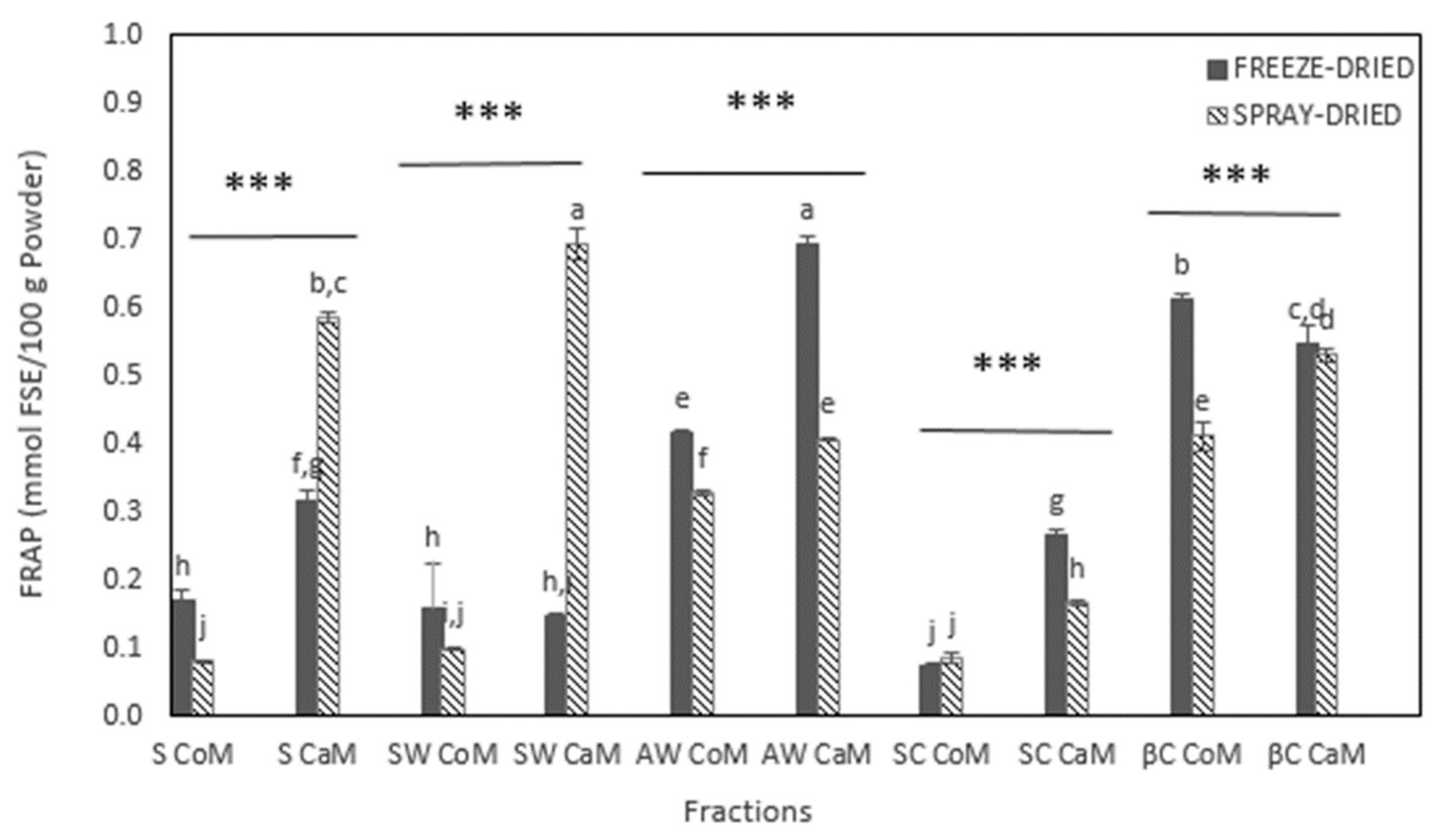
3.1. Total Phenolic Content and Antioxidant Activities of Dried Milk Fractions
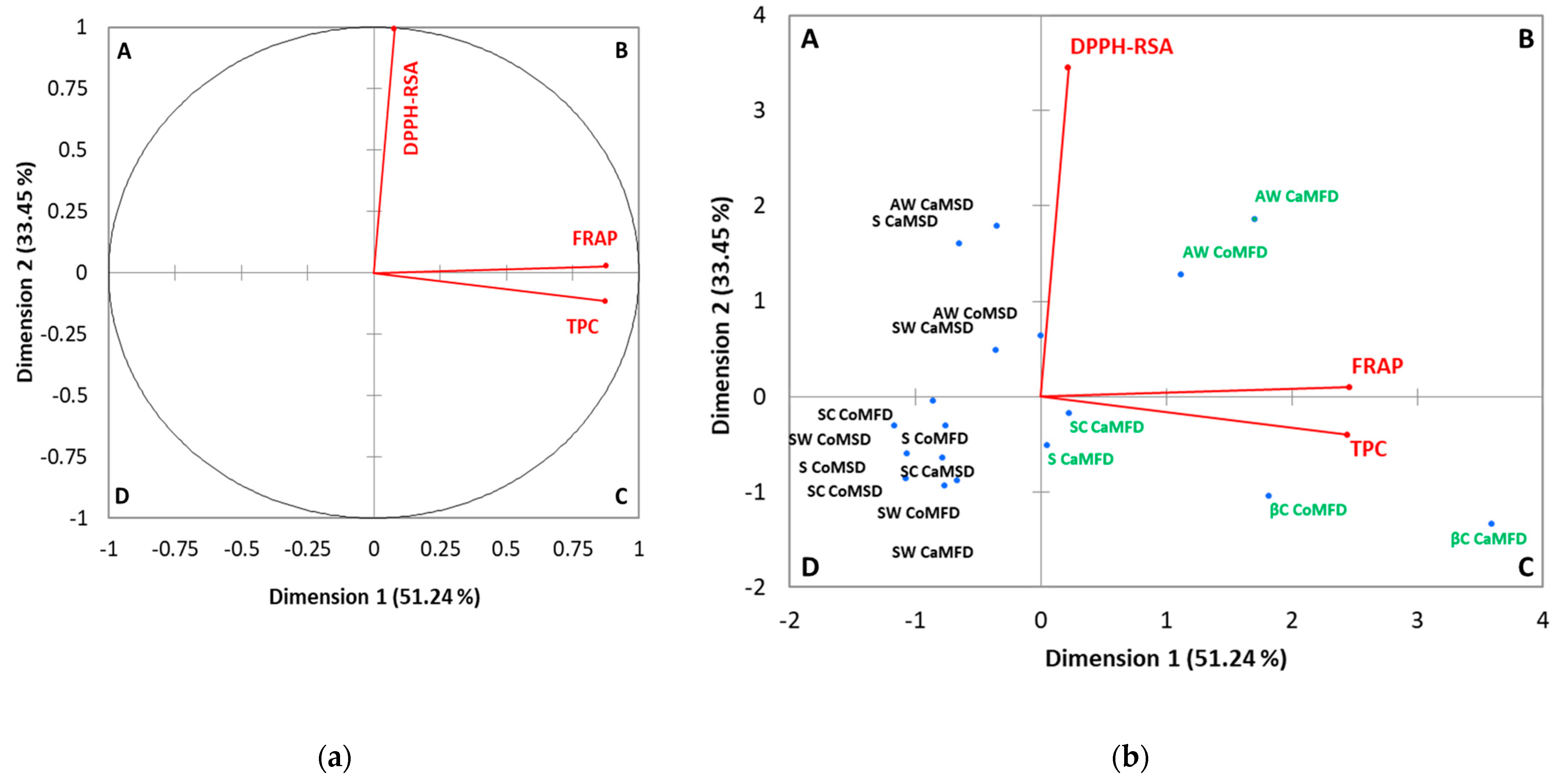
3.2. Principal Components Analysis
3.3. Effect of Gamma Radiation on the Antioxidant Activity of Milk Powder Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Hatmi, H.; Jrad, Z.; Salhi, I.; Aguibi, A.; Nadri, A.; Khorchani, T. Comparison of composition and whey protein fractions of human, camel, donkey, goat and cow’s milk. Mljekarstvo/Dairy 2015, 65, 159–167. [Google Scholar] [CrossRef]
- Nagy, P.P.; Skidmore, J.A.; Juhasz, J. Intensification of camel farming and milk production with special emphasis on animal health, welfare, and the biotechnology of reproduction. Anim. Front. 2022, 12, 35–45. [Google Scholar] [CrossRef] [PubMed]
- FAO. FAOSTAT. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 January 2022).
- Swelum, A.A.; El-Saadony, M.T.; Abdo, M.; Ombarak, R.; Elsayed, O.S.H.; Suliman, G.; Alhimaidi, A.R.; Ammari, A.A.; Ba-Awadh, H.; Taha, A.E.; et al. Nutritional, antimicrobial and medicinal properties of Camel’s milk: A review. Saudi J. Biol. Sci. 2021, 28, 3126–3136. [Google Scholar] [CrossRef]
- Mati, A.; Senoussi-Ghezali, C.; Zennia, S.S.A.; Almi-Sebbane, D.; El-Hatmi, H.; Girardet, J.M. Dromedary camel milk proteins, a source of peptides having biological activities—A review. Int. Dairy J. 2017, 73, 25–37. [Google Scholar] [CrossRef]
- Asunis, F.; De Gioannis, G.; Dessì, P.; Isipato, M.; Lens, P.N.L.; Muntoni, A.; Polettini, A.; Pomi, R.; Rossi, A.; Spiga, D. The dairy biorefinery: Integrating treatment processes for cheese whey valorisation. J. Environ. Manag. 2020, 276, 111240. [Google Scholar] [CrossRef]
- Figueroa Pires, A.; Marnote, N.G.; Díaz Rubio, O.; Cobos Garcia, A.; Dias Pereira, C. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.C.; Russo, M.; Novellino, E. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef]
- Borad, S.G.; Kumar, A.; Singh, A.K. Effect of processing on nutritive values of milk protein. Food Sci. Nutr. 2017, 57, 3690–3702. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Akabanda, F.; Agyei, D.; Jespersen, L. Microbial Safety of Milk Production and Fermented Dairy Products in Africa: Review. Microorganisms 2020, 8, 752. [Google Scholar] [CrossRef]
- Belkheir, B.; Zadi-Karam, H.; Karam, N.E.; Carballo, J.; Centeno, J.A. Effects of selected mesophilic Lactobacillus strains obtained from camel milk on the volatile and sensory profiles of a model short-ripened pressed cows’ milk cheese. Int. Dairy J. 2020, 109, 104738. [Google Scholar] [CrossRef]
- Konuspayeva, G.; Faye, B. Recent Advances in Camel Milk Processing: Review. Animals 2021, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Yirda, A.; Eshetu, M.; Babege, K. Current Status of Camel Dairy Processing and Technologies: A Review. Anim. Sci. 2020, 10, 362–377. [Google Scholar] [CrossRef]
- Sharma, A.; Jana, A.H.; Chavan, R.S. Functionality of milk powders and milk-based powders for end use applications—A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 518–528. [Google Scholar] [CrossRef]
- Indiarto, R.; Asyifaa, A.H.; Adiningsih, F.C.A.; Aulia, G.A.; Achmad, S.R. Conventional And Advanced Food-Drying Technology: A Current Review. Int. J. Sci. Technol. Res. 2021, 10, 99–107. [Google Scholar]
- Fang, Y.; Rogers, S.; Selomulya, C.; Chen, X.D. Functionality of milk protein concentrate: Effect of spray drying temperature. Biochem. Eng. J. 2012, 62, 101–105. [Google Scholar] [CrossRef]
- Salar, S.; Jafarian, S.; Mortazavi, A.; Nasiraie, L.R. Effect of hurdle technology of gentle pasteurisation and drying process on bioactive proteins, antioxidant activity and microbial quality of cow and buffalo colostrum. Int. Dairy J. 2021, 121, 105138. [Google Scholar] [CrossRef]
- Gaiani, C.; Moranda, M.; Sanchez, C.; Arab Tehranya, E.; Jacquot, M.; Schuck, P.; Jeantet, R.; Scher, J. How surface composition of high milk proteins powders is influenced by spray-drying temperature. Colloids Surf. B 2010, 75, 377–384. [Google Scholar] [CrossRef]
- Lajnaf, R.; Trigui, I.; Samet-Bali, O.; Attia, H.; Ayadi, M.A. Comparative study on emulsifying and physico-chemical properties of bovine and camel acid and sweet wheys. J. Food Eng. 2020, 268, 109741. [Google Scholar] [CrossRef]
- Fournaise, T.; Burgain, J.; Perroud, C.; Scher, J.; Gaiani, C.; Petit, J. Impact of formulation on reconstitution and flowability of spray-dried milk powders. Powder Technol. 2020, 372, 107–116. [Google Scholar] [CrossRef]
- Zouari, A.; Perrone, I.T.; Schuck, P.; Gaucheron, F.; Dolivet, A.; Attia, H.; Ayadi, M.A. Effect of outlet drying temperature and milk fat content on the physicochemical characteristics of spray-dried camel milk powder. Dry. Technol. 2018, 37, 1615–1624. [Google Scholar] [CrossRef]
- Stevanović, M. Chapter 11—Polymeric micro- and nanoparticles for controlled and targeted drug delivery. Nanostructures Drug Deliv. Micro Nano Technol. 2017, 355–378. Available online: https://dais.sanu.ac.rs/handle/123456789/2334 (accessed on 20 January 2023).
- Perusko, M.; Ghnimi, S.; Simovic, A.; Stevanovic, N.; Radomirovic, M.; Gharsallaoui, A.; Smiljanic, K.; Van Haute, S.; Stanic-Vucinic, D.; Velickovic, T.C. Maillard reaction products formation and antioxidative power of spray dried camel milk powders increases with the inlet temperature of drying. LWT 2021, 143, 111091. [Google Scholar] [CrossRef]
- Kostic, A.Z.; Milincic, D.D.; Stanisavljevic, N.S.; Gasic, U.M.; Levic, S.; Kojic, M.O.; Tesic, Z.L.; Nedovic, V.; Barac, M.B.; Pesic, M.B. Polyphenol bioaccessibility and antioxidant properties of in vitro digested spray-dried thermally-treated skimmed goat milk enriched with pollen. Food Chem. 2021, 351, 129310. [Google Scholar] [CrossRef] [PubMed]
- Jangam, S.V.; Mujumdar, A.S.; Adhikari, B. Drying: Physical and Structural Changes. Encycl. Food Health 2016, 2, 446–455. [Google Scholar]
- Oussaief, O.; Jrad, Z.; Dbara, M.; Khorchani, T.; El Hatmi, H. Physicochemical and antioxidant properties of freeze-dried dromedary skim colostrum and milk powder. Mljekarstvo 2021, 71, 69–78. [Google Scholar] [CrossRef]
- Tastemirova, U.; Mukhtarkhanova, R.; Alimardanova, M.; Alibekov, R.; Shingisov, A. Impact of vacuum freeze-drying on the reconstituted camel milk composition. Food Sci. Technol. 2022, 42, e61722. [Google Scholar] [CrossRef]
- Martysiak-Żurowska, D.; Rożek, P.; Puta, M. The effect of freeze-drying and storage on lysozyme activity, lactoferrin content, superoxide dismutase activity, total antioxidant capacity and fatty acid profile of freeze-dried human milk. Dry. Technol. 2020, 40, 615–625. [Google Scholar] [CrossRef]
- Syed, Q.A.; Hassan, A.; Sharif, S.; Ishaq, A.; Saeed, F.; Afzaal, M.; Hussain, M.; Anjum, F.M. Structural and functional properties of milk proteins as affected by heating, high pressure, Gamma and ultraviolet irradiation: A review. Int. J. Food Prop. 2021, 24, 871–884. [Google Scholar] [CrossRef]
- Madureira, J.; Barros, L.; Margaça, F.M.A.; Buelga, C.S.; Ferreira, I.C.F.R.; Verde, S.C. Chapter 14: Effects of Irradiation on Food Bioactives. In Retention of Bioactives in Food Processing; Springer International Publishing: Cham, Switzerland, 2022; pp. 429–465. [Google Scholar]
- Chatterton, D.E.; Aagaard, S.; Hansen, T.H.; Nguyen, D.N.; De Gobba, C.; Lametsch, R.; Sangild, P.T. Bioactive proteins in bovine colostrum and effects of heating, drying and irradiation. Food Funct. 2020, 11, 2309–2327. [Google Scholar] [CrossRef]
- Osaili, T.M.; Al-Nabulsi, A.A.; Shaker, R.R.; Ayyash, M.M.; Olaimat, A.N.; Abu Al-Hasan, A.S. Effects of extended dry storage of powdered infant milk formula on susceptibility of Enterobacter sakazakii to hot water and ionizing radiation. J. Food Prot. 2008, 71, 934–939. [Google Scholar] [CrossRef]
- Robichaud, V.; Bagheri, L.; Aguilar-Uscanga, B.R.; Millette, M.; Lacroix, M. Effect of ɣ-irradiation on the microbial inactivation, nutritional value, and antioxidant activities of infant formula. LWT Food Sci. Technol. 2020, 125, 109211. [Google Scholar] [CrossRef]
- Zouari, A.; Marchesseau, S.; Chevalier-Lucia, D.; Raffard, G.; Ayadi, M.A.; Picart-Palmade, L. Acid gelation of raw and reconstituted spray-dried dromedary milk: A dynamic approach of gel structuring. Int. Dairy J. 2018, 81, 95–103. [Google Scholar] [CrossRef]
- Lajnaf, R.; Gharsallah, H.; Attia, H.; Ayadi, M.A. Comparative study on antioxidant, antimicrobial, emulsifying and physico-chemical properties of purified bovine and camel β-casein. LWT Food Sci. Technol. 2021, 140, 110842. [Google Scholar] [CrossRef]
- Barkaoui, S.; Madureira, J.; Santos, P.M.P.; Margaça FMABMiloud, N.; Mankai, M.; Boudhrioua, N.; Cabo Verde, S. Effect of Ionizing Radiation and Refrigeration on the Antioxidants of Strawberries. Food Bioprocess Technol. 2020, 13, 1516–1527. [Google Scholar] [CrossRef]
- Whittaker, B.; Watts, M.F. The influence of dose rate, ambient temperature and time on the radiation response of Harwell PMMA dosimeters. Radiat. Phys. Chem. 2001, 60, 101–110. [Google Scholar] [CrossRef]
- Madureira, J.; Severino, A.; Cojocaru, M.; Garofalide, S.; Santos, P.M.P.; Carolino, M.M.; Margaça, F.M.A.; Cabo Verde, S. E-beam treatment to guarantee the safety and quality of cherry tomatoes. Innov. Food Sci. Emerg. Technol. 2019, 55, 57–65. [Google Scholar] [CrossRef]
- Harguindeguy, M.; Fissore, D. On the effects of freezedrying processes on the nutritional properties of foodstuff: A review. Dry. Technol. 2019, 38, 846–868. [Google Scholar] [CrossRef]
- Ertan, K.; Bayana, D.; Gokce, O.; Alatossava, T.; Yilmaz, Y.; Gursoy, O. Total Antioxidant Capacity and Phenolic Content of Pasteurized and UHT-Treated Cow Milk Samples Marketed in Turkey. Akad. Gıda 2017, 15, 103–108. [Google Scholar] [CrossRef]
- Zhang, C.; Veliky, C.V.; Birru, R.L.; Barinas-Mitchell, E.; Magnani, J.W.; Sekikawa, A. Potential Protective Effects of Equol (Soy Isoflavone Metabolite) on Coronary Heart Diseases—From Molecular Mechanisms to Studies in Humans. Nutrients 2021, 13, 3739. [Google Scholar] [CrossRef]
- Felfoul, I.; Lopez, C.; Gaucheronb, F.; Attiaa, H.; Ayadi, M.A. A laboratory investigation of cow and camel whey proteins deposition under different heat treatments. Food Bioprod Process 2015, 96, 256–263. [Google Scholar] [CrossRef]
- Felfoul, I.; Jardin, J.; Gaucheronb, F.; Attiaa, H.; Ayadi, M.A. Proteomic profiling of camel and cow milk proteins under heat treatment. Food Chem. 2016, 216, 161–169. [Google Scholar] [CrossRef]
- Amamcharla, J.K.; Metzger, L.E. Modification of the ferric reducing antioxidant power (FRAP) assay to determine the susceptibility of raw milk to oxidation. Int. Dairy J. 2014, 34, 177–179. [Google Scholar] [CrossRef]
- Najgebauer-Lejko, D.; Sady, M. Estimation of the antioxidant activity of the commercially available fermented milks. Acta Sci. Pol. Technol. Aliment. 2015, 14, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cekic, S.D.; Demir, A.; Baskan, K.S. Determination of total antioxidant capacity of milk by CUPRAC and ABTS methods with separate characterisation of milk protein fractions. J. Dairy Res. 2015, 82, 177–184. [Google Scholar] [CrossRef]
- Le Tien, C.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Milk protein coatings prevent oxidative browning of apples and potatoes. J Food Sci. 2001, 4, 512–516. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, C.; Manzocco, L.; Anese, M.; Nicoli, M.C. Effect of heat-treatment on the antioxidant and pro-oxidant activity of milk. Int. Dairy J. 2004, 14, 421–427. [Google Scholar] [CrossRef]
- Alothman, M.; Rajeev, B.; Karim, A.A. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Food Sci. Technol. 2009, 20, 201–212. [Google Scholar] [CrossRef]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Review. Animals 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, M.; Cichosz, G.; Czeczot, H. Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates—A review. Int. Dairy J. 2022, 127, 105208. [Google Scholar] [CrossRef]
- Ehlerman, D.A.E. Safety of food and beverages: Safety of irradiated foods. In Encyclopedia of Food Safety; Yasmine, M., Ed.; Academic: Waltham, MA, USA, 2014; Volume 3, pp. 447–452. [Google Scholar]
- Wang, X.B.; Wang, C.N.; Zhang, Y.C.; Liu, T.T.; Shen, L.J.P.; Guo, M.R. Effects of gamma radiation on microbial, physicochemical, and structural properties of whey protein model system. J. Dairy Sci. 2018, 101, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Kuana, Y.H.; Bhata, R.; Ankit, P.; Alias, A.K. Radiation processing of food proteins. A review on the recent developments. Food Sci. Technol. 2013, 30, 105–120. [Google Scholar] [CrossRef]
- Stefanova, R.; Vasilev, N.V.; Spassov, S.L. Irradiation of Food, Current Legislation Framework, and Detection of Irradiated Foods. Food Anal. Methods 2010, 3, 225–252. [Google Scholar] [CrossRef]
- Chawla, S.P.; Chander, R.; Sharma, A. Antioxidant properties of Maillard reaction products obtained by gamma-irradiation of whey proteins. Food Chem. 2009, 116, 122–128. [Google Scholar] [CrossRef]
| Skim Cow Milk (SCoM) | Acid Whey of Cow Milk (AWCoM) | β-Casein of Cow Milk (β CoM) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 kGy | 5 kGy | 11 kGy | 22 kGy | 0 kGy | 5 kGy | 11 kGy | 22 kGy | 0 kGy | 5 kGy | 11 kGy | 22 kGy | |
| TPC (mg GAE/100 g powder) | 27.2 ± 2.1 c | 33.9 ± 2.6 b | 32.9 ± 2.6 b,c | 44.9 ± 2 a | 209.6 ± 2.1 a | 184.9 ± 2 b | 182.9 ± 2 b | 153.2 ± 2.1 c | 247.2 ± 2.1 b | 244.6 ± 2.3 b | 241.9 ± 2.7 c | 259.9 ± 2.7 a |
| FRAP (mmol FSE/100 g powder) | 0.17 ± 0.02 a | 0.18 ± 0.04 a | 0.19 ± 0.02 a | 0.21 ± 0.02 a | 0.42 ± 0.01 a | 0.44 ± 0.03 a | 0.42 ± 0.02 a | 0.34 ± 0.01 b | 0.61 ± 0.01 b | 0.65 ± 0.01 b | 0.69 ± 0.01 a | 0.21 ± 0.01 c |
| DPPH RSA (%) | 10.5 ± 0.3 d | 14.2 ± 0.4 c | 16.2 ± 0.1 b | 20.6 ± 0.1 a | 40.3 ± 0.2 a | 39.6 ± 0.11 b | 36.5 ± 0.3 c | 33.6 ± 0.3 d | ND | ND | ND | ND |
| Skim Camel Milk (SCaM) | Acid Whey of Camel Milk (AW CaM) | Sodium Caseinate of Camel Milk (SC CaM) | β-Casein of Camel Milk (βC CaM) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 kGy | 5 kGy | 11 kGy | 22 kGy | 0 kGy | 5 kGy | 11 kGy | 22 kGy | 0 kGy | 5 kGy | 11 kGy | 22 kGy | 0 kGy | 5 kGy | 11 kGy | 22 kGy | |
| TPC (mg GAE/100 g powder) | 90.2 ± 2.5 b | 94.2 ± 2.1 b | 93.9 ± 2 b | 126.2 ± 2.5 a | 143 ± 2 a,b | 147 ± 2 a | 141 ± 2 b | 143 ± 2 a,b | 149 ± 3 a | 74 ± 3 c | 74 ± 0.6 c | 104 ± 2 b | 621 ± 4 a | 596 ± 4 b | 592 ± 4 b | 560 ± 1 c |
| FRAP (mmol FSE/100 g powder) | 0.32 ± 0.02 a,b | 0.3 ± 0.01 b | 0.26 ± 0.01 c | 0.33 ± 0.02 a | 0.69 ± 0.01 a | 0.64 ± 0.02 b | 0.58 ± 0.02 c | 0.57 ± 0.02c | 0.16 ± 0.01 c | 0.21 ± 0.01 b | 0.09 ± 0.001 d | 0.26 ± 0.003 a | 0.55 ± 0.03 a | 0.43 ± 0.02 b | 0.42 ± 0.02 b | 0.45 ± 0.01 b |
| DPPH RSA (%) | 7.5 ± 0.1 a | 7.2 ± 0.5 a | 4.4 ± 0.3 b | ND | 48.9 ± 0.4 a | 45.2 ± 0.4 b | 32.3 ± 0.2 d | 38.3 ± 0.2 c | 13.4 ± 1 a | ND | ND | ND | ND | ND | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harizi, N.; Madureira, J.; Zouari, A.; Ayadi, M.A.; Cabo Verde, S.; Boudhrioua, N. Effects of Spray Drying, Freeze Drying and Gamma Irradiation on the Antioxidant Activities of Camel and Cow Milk Fractions. Processes 2023, 11, 897. https://doi.org/10.3390/pr11030897
Harizi N, Madureira J, Zouari A, Ayadi MA, Cabo Verde S, Boudhrioua N. Effects of Spray Drying, Freeze Drying and Gamma Irradiation on the Antioxidant Activities of Camel and Cow Milk Fractions. Processes. 2023; 11(3):897. https://doi.org/10.3390/pr11030897
Chicago/Turabian StyleHarizi, Nouha, Joana Madureira, Ahmed Zouari, Mohamed Ali Ayadi, Sandra Cabo Verde, and Nourhène Boudhrioua. 2023. "Effects of Spray Drying, Freeze Drying and Gamma Irradiation on the Antioxidant Activities of Camel and Cow Milk Fractions" Processes 11, no. 3: 897. https://doi.org/10.3390/pr11030897
APA StyleHarizi, N., Madureira, J., Zouari, A., Ayadi, M. A., Cabo Verde, S., & Boudhrioua, N. (2023). Effects of Spray Drying, Freeze Drying and Gamma Irradiation on the Antioxidant Activities of Camel and Cow Milk Fractions. Processes, 11(3), 897. https://doi.org/10.3390/pr11030897









