Phase Equilibria Simulation of Biomaterial-Hydrogen Binary Systems Using a Simple Empirical Correlation
Abstract
1. Introduction
2. Literature Data and Methods
2.1. Hydrogen Solubility in Biochemicals
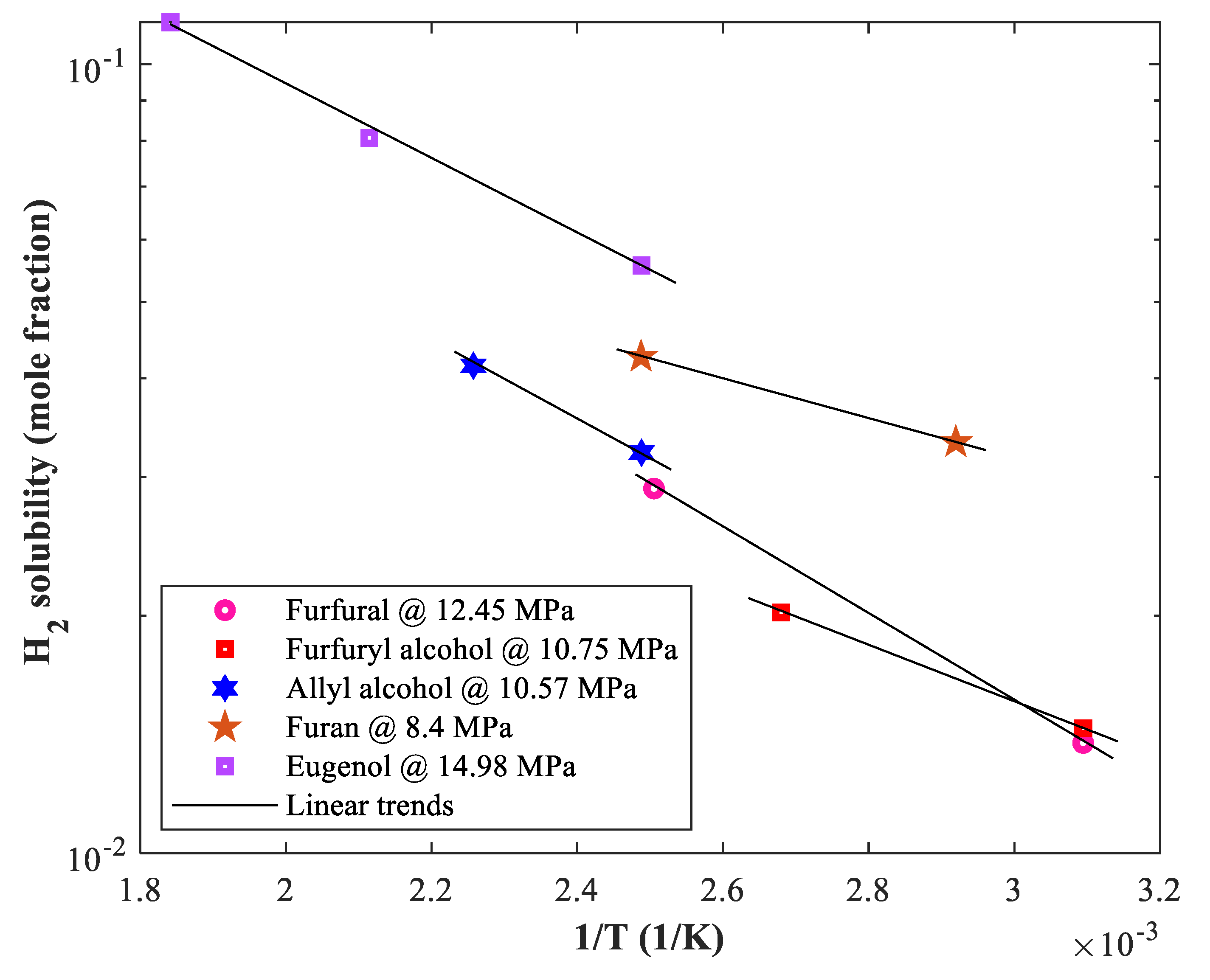
2.2. Arrhenius-Type Correlation
3. Results and Discussion
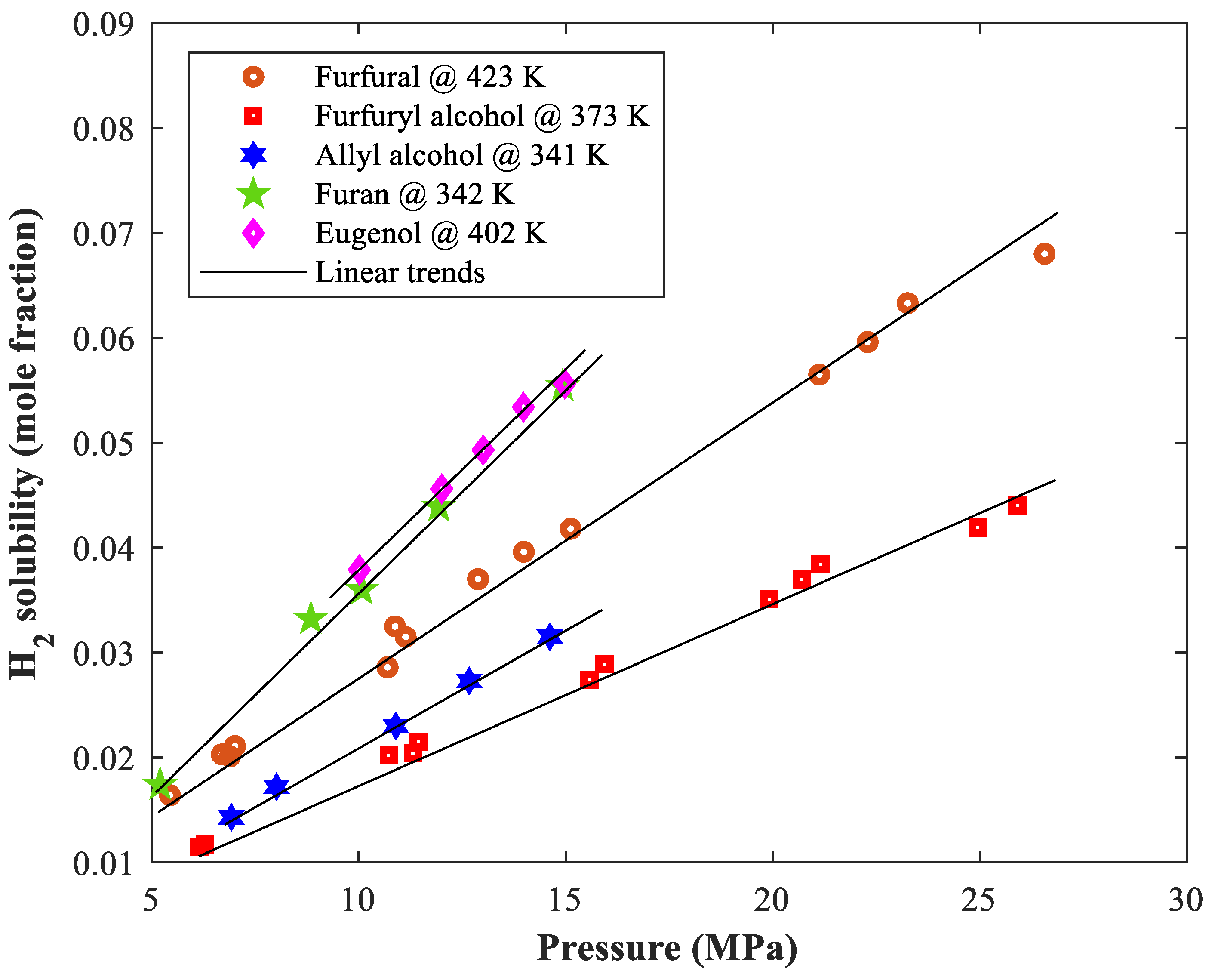
3.1. General Behavior of Biomaterial–Hydrogen Phase Equilibria
3.2. Model Development
3.2.1. Empirical Correlation
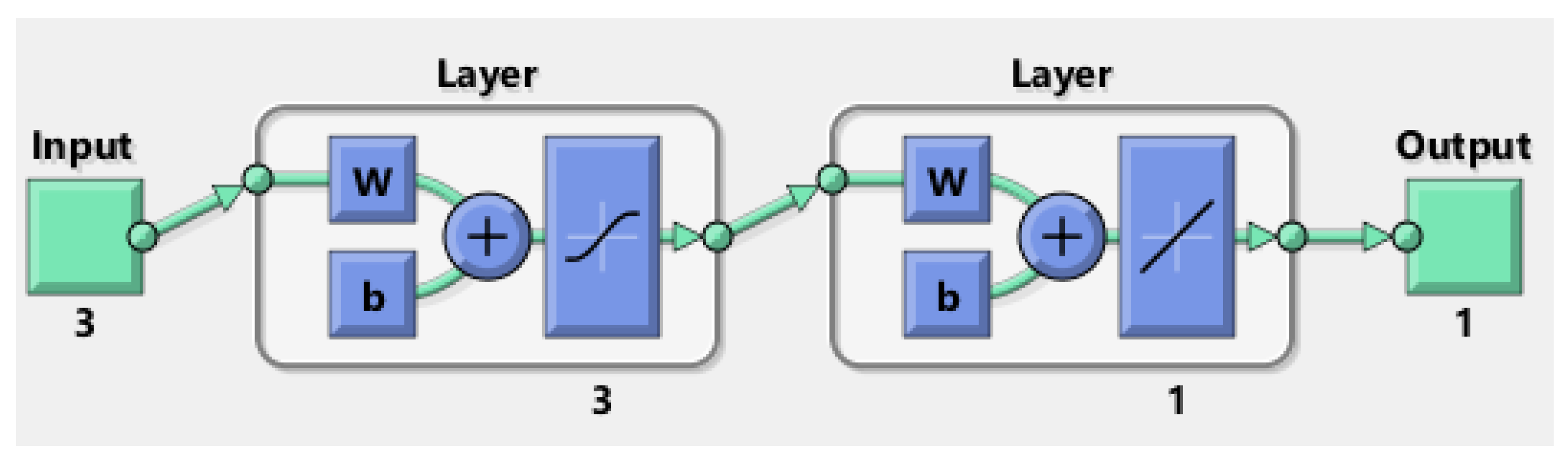
3.2.2. Multilayer Perceptron Artificial Neural Network
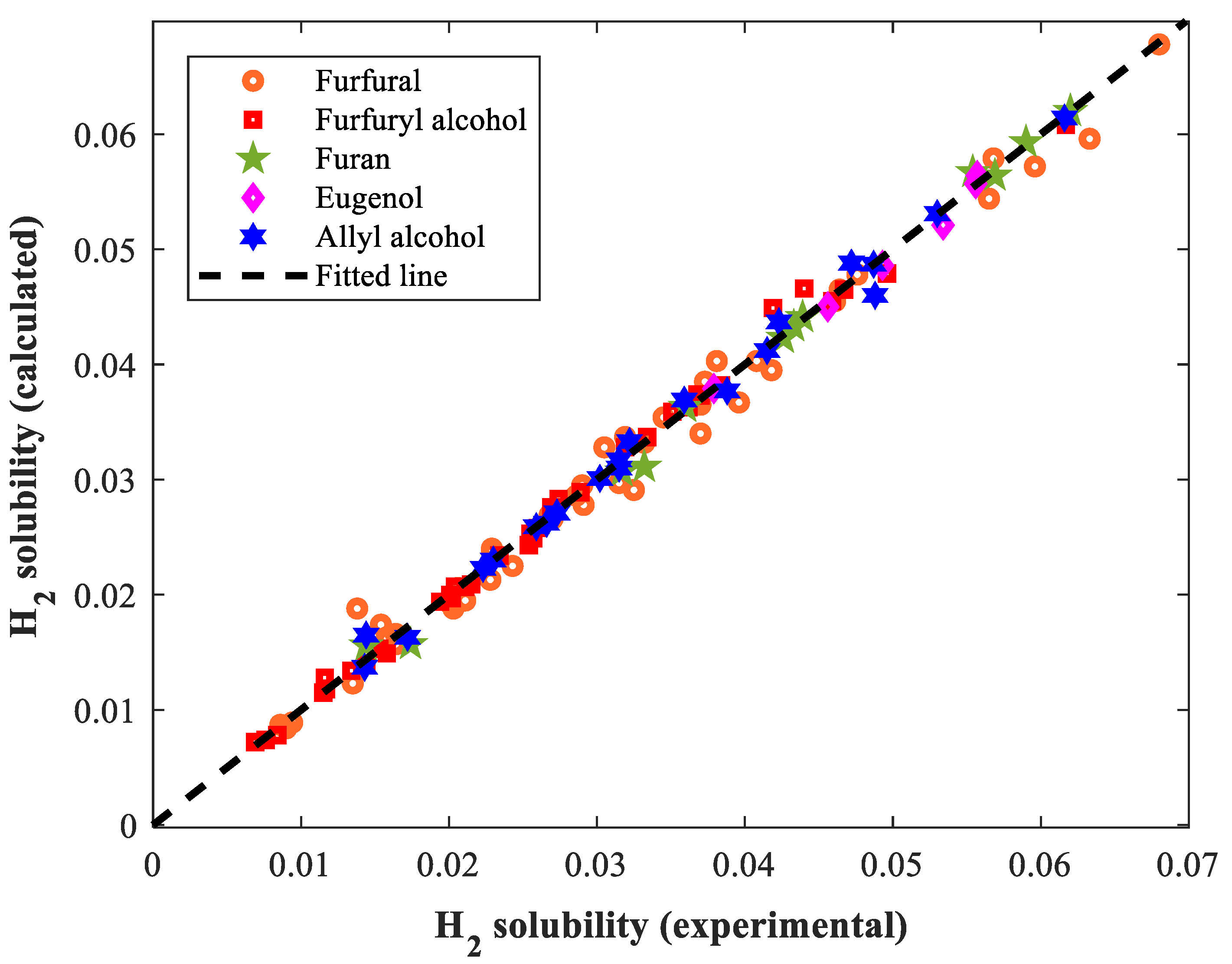
3.3. Comparison between the Empirical Correlation and MLP-ANN
3.4. Comparison between the Empirical Correlation and Equations of State
3.5. Validation by the Literature Data
3.6. Dependency of Biochemical–H2 Equilibrium on Operating Conditions
3.7. Analyzing the Impact of Biomaterial Types on the H2 Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman, A.; Ma, H.; Ozturk, I. Do industrialization, energy importations, and economic progress influence carbon emission in Pakistan. Environ. Sci. Pollut. Res. 2021, 28, 45840–45852. [Google Scholar] [CrossRef] [PubMed]
- Avtar, R.; Tripathi, S.; Aggarwal, A.K.; Kumar, P. Population–urbanization–energy Nexus: A review. Resources 2019, 8, 136. [Google Scholar] [CrossRef]
- Karimi, M.; Zafanelli, L.F.A.S.; Almeida, J.P.P.; Ströher, G.R.; Rodrigues, A.E.; Silva, J.A.C. Novel insights into activated carbon derived from municipal solid waste for CO2 uptake: Synthesis, adsorption isotherms and scale-up. J. Environ. Chem. Eng. 2020, 8, 104069. [Google Scholar] [CrossRef]
- Liang, Y.; Li, J.; Xue, Y.; Tan, T.; Jiang, Z.; He, Y.; Shangguan, W.; Yang, J.; Pan, Y. Benzene decomposition by non-thermal plasma: A detailed mechanism study by synchrotron radiation photoionization mass spectrometry and theoretical calculations. J. Hazard. Mater. 2021, 420, 126584. [Google Scholar] [CrossRef] [PubMed]
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Zhou, P. GVRP considered oil-gas recovery in refined oil distribution: From an environmental perspective. Int. J. Prod. Econ. 2021, 235, 108078. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Q.; Liu, L.; Wu, Y.; Liu, H.; Gu, Z.; Zhu, C. A review of low and zero carbon fuel technologies: Achieving ship carbon reduction targets. Sustain. Energy Technol. Assess. 2022, 54, 102762. [Google Scholar] [CrossRef]
- Liu, L.; Tang, Y.; Liu, D. Investigation of future low-carbon and zero-carbon fuels for marine engines from the view of thermal efficiency. Energy Rep. 2022, 8, 6150–6160. [Google Scholar] [CrossRef]
- Si, Z.; Yang, M.; Yu, Y.; Ding, T. Photovoltaic power forecast based on satellite images considering effects of solar position. Appl. Energy 2021, 302, 117514. [Google Scholar] [CrossRef]
- Li, P.; Yang, M.; Wu, Q. Confidence interval based distributionally robust real-time economic dispatch approach considering wind power accommodation risk. IEEE Trans. Sustain. Energy 2020, 12, 58–69. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Behaien, S.; Vaferi, B. Experimental and modeling analyzing the biogas upgrading in the microchannel: Carbon dioxide capture by seawater enriched with low-cost waste materials. Environ. Technol. Innov. 2022, 27, 102770. [Google Scholar] [CrossRef]
- Aghel, B.; Gouran, A.; Parandi, E.; Jumeh, B.H.; Nodeh, H.R. Production of biodiesel from high acidity waste cooking oil using nano GO@ MgO catalyst in a microreactor. Renew. Energy 2022, 200, 294–302. [Google Scholar] [CrossRef]
- Karimi, M.; Diaz de Tuesta, J.L.; Carmem, C.N.; Gomes, H.T.; Rodrigues, A.E.; Silva, J.A.C. Compost from Municipal Solid Wastes as a Source of Biochar for CO2 Capture. Chem. Eng. Technol. 2020, 43, 1336–1349. [Google Scholar] [CrossRef]
- Karimi, M.; Shirzad, M.; Silva, J.A.C.; Rodrigues, A.E. Biomass/Biochar carbon materials for CO2 capture and sequestration by cyclic adsorption processes: A review and prospects for future directions. J. CO2 Util. 2022, 57, 101890. [Google Scholar] [CrossRef]
- Qureshi, M.S. Phase Equilibria of Bio-Oil Compounds. PhD Dissertation, Aalto University, Espoo, Finland, 2017. [Google Scholar]
- Tun, M.M.; Juchelkova, D.; Win, M.M.; Thu, A.M.; Puchor, T. Biomass energy: An overview of biomass sources, energy potential, and management in Southeast Asian countries. Resources 2019, 8, 81. [Google Scholar] [CrossRef]
- Adams, P.; Bridgwater, T.; Lea-Langton, A.; Ross, A.; Watson, I. Biomass conversion technologies. In Greenhouse Gas Balances of Bioenergy Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 107–139. [Google Scholar]
- Suriapparao, D.V.; Vinu, R. Biomass waste conversion into value-added products via microwave-assisted Co-Pyrolysis platform. Renew. Energy 2021, 170, 400–409. [Google Scholar] [CrossRef]
- Vargas-Moreno, J.M.; Callejón-Ferre, A.J.; Pérez-Alonso, J.; Velázquez-Martí, B. A review of the mathematical models for predicting the heating value of biomass materials. Renew. Sustain. Energy Rev. 2012, 16, 3065–3083. [Google Scholar] [CrossRef]
- Pannone, P.J. Trends in Biomaterials Research; Nova Publishers: Hauppauge, NY, USA, 2007. [Google Scholar]
- Kumar, M.; Sundaram, S.; Gnansounou, E.; Larroche, C.; Thakur, I.S. Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: A review. Bioresour. Technol. 2018, 247, 1059–1068. [Google Scholar] [CrossRef]
- Xie, J.; Liu, X.; Lao, X.; Vaferi, B. Hydrogen solubility in furfural and furfuryl bio-alcohol: Comparison between the reliability of intelligent and thermodynamic models. Int. J. Hydrogen Energy 2021, 46, 36056–36068. [Google Scholar] [CrossRef]
- Alamillo, R.; Tucker, M.; Chia, M.; Pagán-Torres, Y.; Dumesic, J. The selective hydrogenation of biomass-derived 5-hydroxymethylfurfural using heterogeneous catalysts. Green Chem. 2012, 14, 1413–1419. [Google Scholar] [CrossRef]
- Bohre, A.; Hočevar, B.; Grilc, M.; Likozar, B. Selective catalytic decarboxylation of biomass-derived carboxylic acids to bio-based methacrylic acid over hexaaluminate catalysts. Appl. Catal. B Environ. 2019, 256, 117889. [Google Scholar] [CrossRef]
- Nolte, M.W.; Shanks, B.H. A perspective on catalytic strategies for deoxygenation in biomass pyrolysis. Energy Technol. 2017, 5, 7–18. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sanpitakseree, C.; Demir, B.; Ma, K.; Elliott, W.A.; Bai, P.; Johnson, R.L.; Walker, T.W.; Shanks, B.H.; Rioux, R.M. Effects of chloride ions in acid-catalyzed biomass dehydration reactions in polar aprotic solvents. Nat. Commun. 2019, 10, 1132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zijlstra, D.S.; Lahive, C.W.; Deuss, P.J. Combined lignin defunctionalisation and synthesis gas formation by acceptorless dehydrogenative decarbonylation. Green Chem. 2020, 22, 3791–3801. [Google Scholar] [CrossRef]
- Jaatinen, S.; Touronen, J.; Karinen, R.; Uusi-Kyyny, P.; Alopaeus, V. Hydrogen solubility in furfural and 2-propanol: Experiments and modeling. J. Chem. Thermodyn. 2017, 112, 1–6. [Google Scholar] [CrossRef]
- Ivaniš, G.; Žilnik, L.F.; Likozar, B.; Grilc, M. Hydrogen solubility in bio-based furfural and furfuryl alcohol at elevated temperatures and pressures relevant for hydrodeoxygenation. Fuel 2021, 290, 120021. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Touronen, J.; Uusi-Kyyny, P.; Richon, D.; Alopaeus, V. Solubility of hydrogen in bio-oil compounds. J. Chem. Thermodyn. 2016, 102, 406–412. [Google Scholar] [CrossRef]
- Safe, M.; Khazraee, S.M.; Setoodeh, P.; Jahanmiri, A.H. Model reduction and optimization of a reactive dividing wall batch distillation column inspired by response surface methodology and differential evolution. Math. Comput. Model. Dyn. Syst. 2013, 19, 29–50. [Google Scholar] [CrossRef]
- Logan, S.R. The origin and status of the Arrhenius equation. J. Chem. Educ. 1982, 59, 279. [Google Scholar] [CrossRef]
- Hosseini, S.; Vaferi, B. Determination of methanol loss due to vaporization in gas hydrate inhibition process using intelligent connectionist paradigms. Arab. J. Sci. Eng. 2022, 47, 5811–5819. [Google Scholar] [CrossRef]
- Safdari Shadloo, M. Application of support vector machines for accurate prediction of convection heat transfer coefficient of nanofluids through circular pipes. Int. J. Numer. Methods Heat Fluid Flow 2021, 31, 2660–2679. [Google Scholar] [CrossRef]
- Li, Y.; Niu, B.; Zong, G.; Zhao, J.; Zhao, X. Command filter-based adaptive neural finite-time control for stochastic nonlinear systems with time-varying full-state constraints and asymmetric input saturation. Int. J. Syst. Sci. 2022, 53, 199–221. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Xu, N.; Zong, G.; Zhao, X. Reinforcement learning-based decentralized fault tolerant control for constrained interconnected nonlinear systems. Chaos Solitons Fractals 2023, 167, 113034. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, H.; Zhang, L.; Ahmad, A.M.; Xu, N. Decentralized adaptive neural two-bit-triggered control for nonstrict-feedback nonlinear systems with actuator failures. Neurocomputing 2022, 500, 856–867. [Google Scholar] [CrossRef]
- Zheng, Y.; Safdari Shadloo, M.; Nasiri, H.; Maleki, A.; Karimipour, A.; Tlili, I. Prediction of viscosity of biodiesel blends using various artificial model and comparison with empirical correlations. Renew. Energy 2020, 153, 1296–1306. [Google Scholar] [CrossRef]
- Alibak, A.H.; Alizadeh, S.M.; Davodi Monjezi, S.; Alizadeh, A.A.; Alobaid, F.; Aghel, B. Developing a Hybrid Neuro-Fuzzy Method to Predict Carbon Dioxide (CO2) Permeability in Mixed Matrix Membranes Containing SAPO-34 Zeolite. Membranes 2022, 12, 1147. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, C.; Zhou, Y.; Xu, X. Modeling of Vapor-Liquid Equilibrium for Electrolyte Solutions Based on COSMO-RS Interaction. J. Chem. 2022, 2022, 9070055. [Google Scholar] [CrossRef]


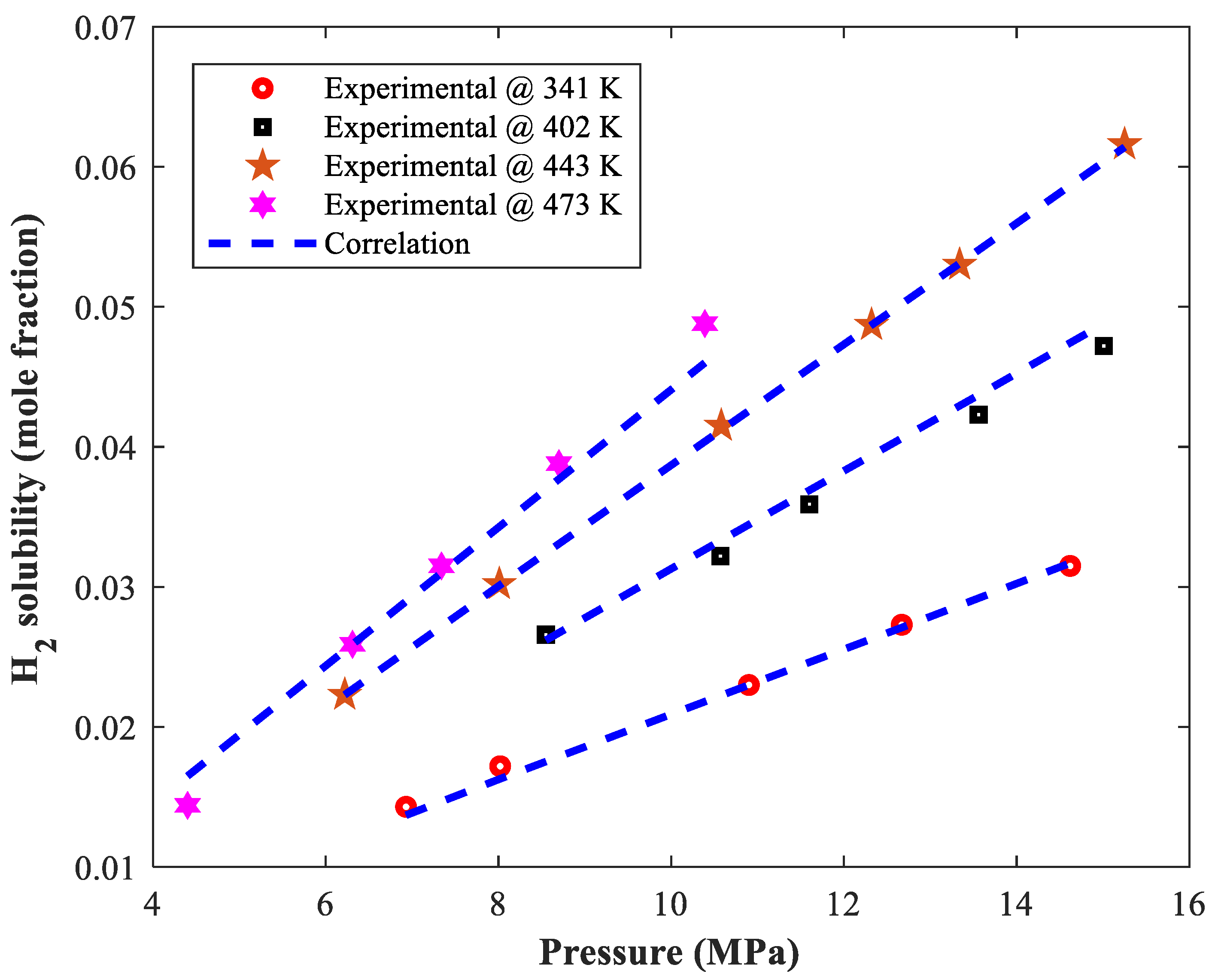
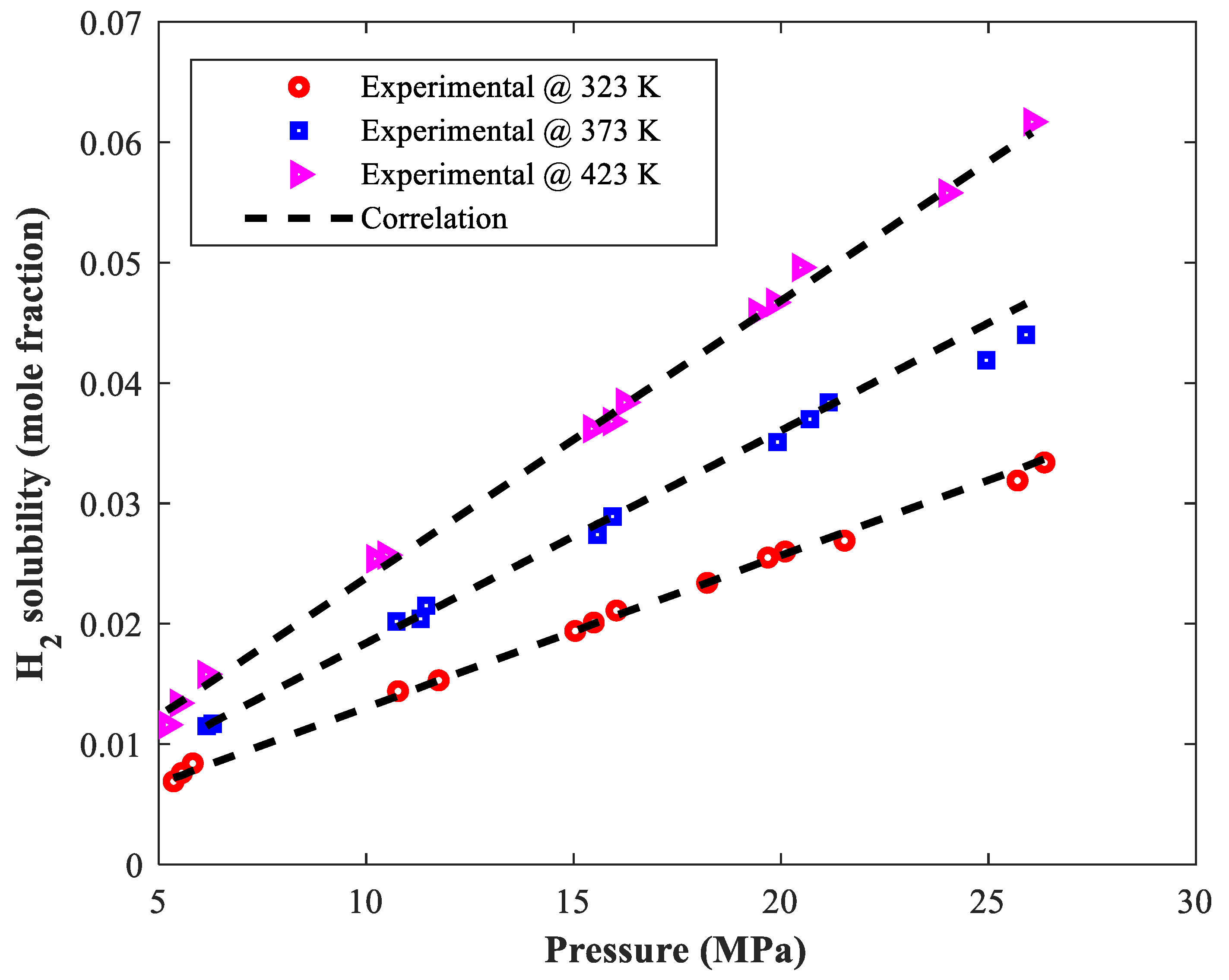

| Binary Mixture | Temperature (K) | Pressure (kPa) | H2 Solubility (Mole Fraction) | Count | Ref. |
|---|---|---|---|---|---|
| Allyl alcohol–Hydrogen | 341–473 | 4400–15,250 | 0.014–0.062 | 21 | [30] |
| Eugenol–Hydrogen | 402–543 | 10,000–14,980 | 0.038–0.113 | 14 | [30] |
| Furan-Hydrogen | 342–402 | 3890–14,930 | 0.014–0.081 | 14 | [30] |
| Furfural–Hydrogen | 323–476 | 6960–12,450 | 0.014–0.038 | 7 | [28] |
| Furfural–Hydrogen | 323–423 | 5111–26,565 | 0.009–0.068 | 39 | [29] |
| Furfuryl alcohol–Hydrogen | 323–423 | 5197–26,348 | 0.007–0.062 | 39 | [29] |
| Hydrogen + | |||
|---|---|---|---|
| Allyl alcohol | −0.0363 | 3.429 × 10−5 | 917.3 |
| Eugenol | 0.0289 | 5.151 × 10−5 | 1071.5 |
| Furan | −0.1121 | 7.556 × 10−5 | 988.4 |
| Furfural | 0.0136 | 1.523 × 10−5 | 769.8 |
| Furfuryl alcohol | 0.0057 | 1.614 × 10−5 | 823.9 |
| MLP-ANN Structure | Overall AARD% | R |
|---|---|---|
| 3-1-1 | 26.81 | 0.84011 |
| 3-2-1 | 15.75 | 0.93326 |
| 3-3-1 | 8.18 | 0.98983 |
| Approach | Overall AARD% | R |
|---|---|---|
| Empirical correlation | 3.02 | 0.99815 |
| MLP-ANN | 8.18 | 0.98983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faress, F.; Pourahmad, A.; Abdollahi, S.A.; Safari, M.H.; Mozhdeh, M.; Alobaid, F.; Aghel, B. Phase Equilibria Simulation of Biomaterial-Hydrogen Binary Systems Using a Simple Empirical Correlation. Processes 2023, 11, 714. https://doi.org/10.3390/pr11030714
Faress F, Pourahmad A, Abdollahi SA, Safari MH, Mozhdeh M, Alobaid F, Aghel B. Phase Equilibria Simulation of Biomaterial-Hydrogen Binary Systems Using a Simple Empirical Correlation. Processes. 2023; 11(3):714. https://doi.org/10.3390/pr11030714
Chicago/Turabian StyleFaress, Fardad, Afham Pourahmad, Seyyed Amirreza Abdollahi, Mohammad Hossein Safari, Mozhgan Mozhdeh, Falah Alobaid, and Babak Aghel. 2023. "Phase Equilibria Simulation of Biomaterial-Hydrogen Binary Systems Using a Simple Empirical Correlation" Processes 11, no. 3: 714. https://doi.org/10.3390/pr11030714
APA StyleFaress, F., Pourahmad, A., Abdollahi, S. A., Safari, M. H., Mozhdeh, M., Alobaid, F., & Aghel, B. (2023). Phase Equilibria Simulation of Biomaterial-Hydrogen Binary Systems Using a Simple Empirical Correlation. Processes, 11(3), 714. https://doi.org/10.3390/pr11030714









