Onion (Allium cepa L.) Yield and Quality Depending on Biostimulants and Nitrogen Fertilization—A Chemometric Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Laboratory Procedures
2.2.1. Preparation of Onion Extracts
2.2.2. Total Phenolic Content
2.2.3. Total Flavonoid Content
2.2.4. DPPH Assay
2.2.5. FRAP Assay
2.2.6. ABTS Assay
2.2.7. Total Solids (Dry Matter)
2.2.8. Crude Fibers
2.2.9. Total Sugars
2.2.10. Titratable Acidity
2.2.11. Total Nitrogen and Protein content
2.3. Chemometric Analysis
3. Results and Discussion
3.1. Bioactive Compound Content, Antioxidant Activity Analysis, and Yield
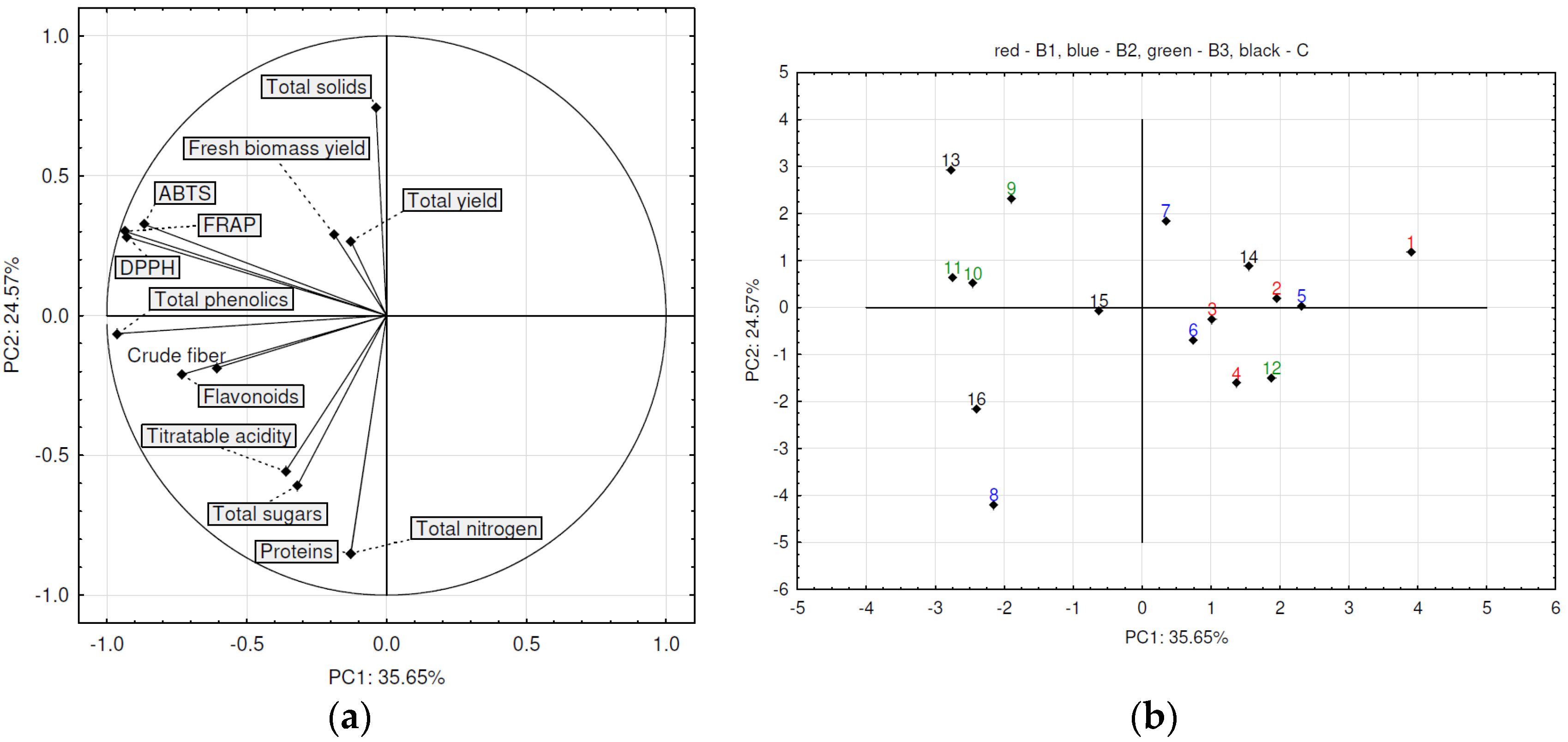
3.2. Classification Analysis
4. Conclusions
- In DS onions, treatments 9 (B3, 64 kg N/ha), 10 (B3, 100 kg N/ha), 11 (B3, 150 kg N/ha), 15 (C, 150 kg N/ha), and 16 (C, 200 kg N/ha) positioned on the negative end of the PC1 axis and generally samples from these treatments have higher values of total phenolic content, FRAP, and DPPH.
- In the negative place of the axis PC2 are positioned as correlated total sugars, titratable acidity, and proteins.
- FS onions treated with 11 (B3, 150 kg N/ha), 15 (C, 150 kg N/ha), and 16 (C, 200 kg N/ha) were placed on the positive end of the PC1 while having higher values of FRAP, DPPH, and ABTS.
- In the positive place of the axis PC1 are positioned treatments 13 (C, 64 kg N/ha), 14 (C, 100 kg N/ha), 15 (C, 150 kg N/ha), and 16 (C, 200 kg N/ha), having the highest values of FRAP, DPPH, and ABTS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Treatment | Biostimulant | Nitrogen Fertilization (kg/ha) | Cultivation Method | Dry Matter (%) | Crude Fiber (g/100g DM) | Total Sugars (g/100g DM) | Titratable Acidity (g/100 g DM) | Total N (g/100 g DM) | Proteins (g/100g DM) | T. Phenolics (mg/100g DM) | Flavonoids (mg/100 g DM) | DPPH (mg/g) | FRAP (mg Fe2+/g) | ABTS (mg/g) | Fresh Biomass Yield (t/ha) | Total Yield (t/ha) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | B1 | 64 | FS | 14.8 | 3.9 | 59.0 | 1.9 | 1.5 | 9.5 | 458.0 | 158.0 | 0.26 | 0.17 | 0.56 | 68.1 | 51.3 |
| 2 | B1 | 64 | DS | 9.4 | 5.5 | 49.6 | 2.7 | 1.6 | 10.1 | 781.7 | 240.5 | 0.35 | 0.24 | 1.0 | 48.0 | 42.8 |
| 3 | B1 | 100 | FS | 15.3 | 3.7 | 68.0 | 1.7 | 1.4 | 10.2 | 454.5 | 158.8 | 0.30 | 0.18 | 0.57 | 64.7 | 49.9 |
| 4 | B1 | 100 | DS | 9.7 | 7.4 | 68.7 | 2.8 | 1.6 | 10.3 | 839.0 | 290.0 | 0.38 | 0.30 | 1.1 | 45.8 | 41.3 |
| 5 | B1 | 150 | FS | 14.2 | 5.3 | 48.7 | 2.3 | 1.9 | 12.2 | 498.9 | 171.9 | 0.24 | 0.16 | 0.53 | 62.6 | 48.2 |
| 6 | B1 | 150 | DS | 9.1 | 7.0 | 60.4 | 3.2 | 1.8 | 11.7 | 928.7 | 291.7 | 0.40 | 0.33 | 1.2 | 60.4 | 54.1 |
| 7 | B1 | 200 | FS | 14.0 | 4.0 | 48.9 | 2.1 | 1.9 | 11.9 | 469.5 | 146.9 | 0.29 | 0.19 | 0.58 | 56.7 | 41.4 |
| 8 | B1 | 200 | DS | 8.8 | 5.6 | 69.2 | 3.6 | 1.8 | 11.8 | 943.6 | 262.8 | 0.42 | 0.33 | 1.2 | 48.2 | 42.8 |
| 9 | B2 | 64 | FS | 14.9 | 4.2 | 66.4 | 1.9 | 1.6 | 10.5 | 458.5 | 190.7 | 0.30 | 0.19 | 0.56 | 52.1 | 38.7 |
| 10 | B2 | 64 | DS | 9.1 | 6.4 | 63.6 | 2.0 | 1.8 | 11.6 | 806.2 | 393.8 | 0.34 | 0.31 | 1.1 | 55.7 | 46.0 |
| 11 | B2 | 100 | FS | 13.4 | 3.9 | 80.6 | 1.9 | 1.7 | 10.6 | 586.8 | 214.4 | 0.38 | 0.27 | 0.68 | 55.4 | 41.4 |
| 12 | B2 | 100 | DS | 8.7 | 8.1 | 55.3 | 2.6 | 1.8 | 11.8 | 977.6 | 388.3 | 0.42 | 0.35 | 1.1 | 49.2 | 43.0 |
| 13 | B2 | 150 | FS | 16.7 | 3.6 | 47.0 | 1.9 | 1.5 | 9.4 | 392.7 | 142.5 | 0.27 | 0.16 | 0.52 | 63.5 | 52.1 |
| 14 | B2 | 150 | DS | 9.6 | 8.3 | 49.3 | 2.7 | 1.7 | 11.2 | 950.2 | 297.0 | 0.44 | 0.38 | 1.2 | 74.4 | 63.9 |
| 15 | B2 | 200 | FS | 13.6 | 4.9 | 63.2 | 2.3 | 2.2 | 13.9 | 522.6 | 171.7 | 0.28 | 0.18 | 0.55 | 59.2 | 47.4 |
| 16 | B2 | 200 | DS | 8.1 | 8.7 | 69.7 | 3.5 | 2.3 | 14.5 | 1109.4 | 592.6 | 0.50 | 0.42 | 1.1 | 51.4 | 45.0 |
| 17 | B3 | 64 | FS | 13.2 | 4.8 | 64.3 | 2.1 | 1.7 | 11.1 | 496.6 | 312.1 | 0.28 | 0.17 | 0.48 | 49.0 | 37.8 |
| 18 | B3 | 64 | DS | 9.5 | 7.2 | 55.3 | 2.7 | 1.5 | 9.4 | 1082.4 | 525.9 | 0.66 | 0.53 | 1.4 | 55.1 | 47.6 |
| 19 | B3 | 100 | FS | 14.9 | 4.7 | 65.6 | 2.0 | 1.5 | 9.5 | 531.0 | 268.9 | 0.36 | 0.24 | 0.60 | 52.1 | 41.1 |
| 20 | B3 | 100 | DS | 9.1 | 8.3 | 61.2 | 3.2 | 1.7 | 10.6 | 1084.5 | 434.4 | 0.64 | 0.53 | 1.5 | 55.2 | 47.4 |
| 21 | B3 | 150 | FS | 14.0 | 4.5 | 53.2 | 2.0 | 1.6 | 10.5 | 596.2 | 264.1 | 0.43 | 0.32 | 0.79 | 56.7 | 44.9 |
| 22 | B3 | 150 | DS | 9.8 | 6.8 | 66.6 | 2.7 | 2.0 | 12.9 | 1083.3 | 419.7 | 0.65 | 0.55 | 1.7 | 66.9 | 56.2 |
| 23 | B3 | 200 | FS | 14.3 | 5.0 | 42.0 | 2.3 | 1.8 | 11.7 | 546.7 | 168.6 | 0.26 | 0.21 | 0.55 | 53.1 | 41.4 |
| 24 | B3 | 200 | DS | 9.7 | 6.6 | 56.1 | 2.9 | 2.2 | 14.3 | 937.9 | 294.0 | 0.35 | 0.31 | 1.0 | 52.9 | 44.5 |
| 25 | C | 64 | FS | 15.4 | 5.1 | 70.1 | 2.3 | 1.6 | 10.0 | 535.1 | 227.2 | 0.46 | 0.37 | 0.80 | 50.8 | 41.7 |
| 26 | C | 64 | DS | 10.2 | 7.3 | 57.6 | 2.8 | 1.4 | 9.2 | 1171.8 | 401.1 | 0.66 | 0.64 | 1.7 | 49.1 | 41.3 |
| 27 | C | 100 | FS | 15.3 | 5.0 | 32.8 | 2.1 | 1.3 | 8.4 | 619.5 | 238.9 | 0.54 | 0.45 | 0.83 | 65.1 | 51.7 |
| 28 | C | 100 | DS | 9.6 | 7.0 | 55.2 | 2.8 | 1.6 | 10.4 | 920.7 | 303.6 | 0.43 | 0.36 | 1.1 | 46.7 | 38.9 |
| 29 | C | 150 | FS | 14.5 | 5.1 | 62.0 | 1.8 | 1.7 | 10.8 | 590.4 | 211.1 | 0.37 | 0.30 | 0.70 | 59.9 | 49.8 |
| 30 | C | 150 | DS | 9.6 | 7.8 | 63.2 | 3.4 | 1.8 | 11.4 | 1034.1 | 328.0 | 0.49 | 0.44 | 1.3 | 55.6 | 46.1 |
| 31 | C | 200 | FS | 15.3 | 5.5 | 49.5 | 2.1 | 1.9 | 12.3 | 587.3 | 231.1 | 0.45 | 0.36 | 0.78 | 58.4 | 48.4 |
| 32 | C | 200 | DS | 9.3 | 8.0 | 67.3 | 3.5 | 2.2 | 13.8 | 1137.4 | 399.4 | 0.59 | 0.48 | 1.5 | 45.2 | 38.0 |
References
- Library, H. Available online: https://helgilibrary.com/indicators/onion-consumption-per-capita/ (accessed on 10 October 2022).
- Liguori, L.; Califano, R.; Albanese, D.; Raimo, F.; Crescitelli, A.; Di Matteo, M. Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. J. Food Qual. 2017, 2017, 687361. [Google Scholar] [CrossRef]
- Griffiths, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Nuutila, A.M.; Puupponen-Pimiä, R.; Aarni, M.; Oksman-Caldentey, K.M. Comparison of antioxidant activities of onion and garlic extracts by inhibition of lipid peroxidation and radical scavenging activity. Food Chem. 2003, 81, 485–493. [Google Scholar] [CrossRef]
- Khalili, S.; Saeidi Asl, M.R.; Khavarpour, M.; Vahdat, S.M.; Mohammadi, M. Comparative study on the effect of extraction solvent on total phenol, flavonoid content, antioxidant and antimicrobial properties of red onion (Allium cepa). J. Food. Meas. Charact. 2022, 16, 3578–3588. [Google Scholar] [CrossRef]
- Lazić, B.; Marković, V.; Đurovka, M.; Ilin, Ž. Vegetable; University of Novi Sad, Faculty of Agriculture: Novi Sad, Serbia, 1998; pp. 1–472. [Google Scholar]
- Geisseler, D.; Ortiz, R.S.; Diaz, J. Nitrogen nutrition and fertilization of onions (Allium cepa L.)—A literature review. Sci. Hortic. 2022, 291, 110591. [Google Scholar] [CrossRef]
- Ottaiano, L.; Di Mola, I.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Biostimulant application under different nitrogen fertilization levels: Assessment of yield, leaf quality, and nitrogen metabolism of tunnel-grown lettuce. Agronomy 2021, 11, 1613. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Akladious, S.A.; Mohamed, H.I. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Sun, H.; Zhang, M.; Li, C. Fulvic acid fertilizer improves garlic yield and soil nutrient status. Gesunde Pflanz. 2022, 74, 685–693. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Vukelić, I.D.; Prokić, L.T.; Racić, G.M.; Pešić, M.B.; Bojović, M.M.; Sierka, E.M.; Kalaji, H.M.; Panković, D.M. Effects of Trichoderma harzianum on photosynthetic characteristics and fruit quality of tomato plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef]
- Szczepanek, M.; Pobereżny, J.; Wszelaczyńska, E.; Gościnna, K. Effect of biostimulants and storage on discoloration potential of carrot. Agronomy 2020, 10, 1894. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and antioxidant responses of lettuce (Lactuca sativa L.) to arbuscular mycorrhiza inoculation and seaweed extract foliar application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Arumugam, A.; Rasheeq, A.A.; Sampathkumar, P. Exploring the microalgae biofertilizer effect on onion cultivation by field experiment. Waste Biomass Valorization 2020, 11, 77–87. [Google Scholar] [CrossRef]
- Suphachai, A.; Takagaki, M.; Chaireag, S.; Sutevee, S.; Inubushi, K. Effect of amount of nitrogen fertilizer on early growth of leafy vegetables in Thailand. J. Trop. Agric. 2006, 50, 127–132. [Google Scholar] [CrossRef]
- Oktem, A.; Oktem, A.G.; Emeklier, H.Y. Effect of nitrogen on yield and some quality parameters of sweet corn. Commun. Soil Sci. Plant Anal. 2010, 41, 832–847. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Cheng, M.; Zhang, F.; Wang, X.; Fan, J.; Wu, L.; Fang, D.; Zou, H.; Xiang, Y. Optimal drip fertigation management improves yield, quality, water and nitrogen use efficiency of greenhouse cucumber. Sci. Hortic. 2019, 243, 357–366. [Google Scholar] [CrossRef]
- Petropoulos, A.S.; Fernandes, Â.; Xyrafis, E.; Polyzos, N.; Antoniadis, V.; Barros, L.; Ferreira, C.F.R.I. The optimization of nitrogen fertilization regulates crop performance and quality of processing tomato (Solanum lycopersicum L. sc. Heinz 3402). Agronomy 2020, 10, 715. [Google Scholar] [CrossRef]
- Lee, J.; Moon, J.; Kim, H.; Ha, I.; Lee, S. Reduced nitrogen, phosphorus, and potassium rates for intermediate-day onion in paddy soil with incorporated rice straw plus manure. HortScience 2011, 46, 470–474. [Google Scholar] [CrossRef]
- Golubkina, N.; Amalfitano, C.; Sekara, A.; Tallarita, A.; Pokluda, R.; Stoleru, V.; Cuciniello, A.; Agafonov, F.A.; Kalisz, A.; Hamburdă, S.B.; et al. Yield and bulb quality of storage onion cultivars as affected by farming system and nitrogen dose. Sci. Hortic. 2022, 293, 110751. [Google Scholar] [CrossRef]
- Randle, M.W. Increasing nitrogen concentration in hydroponic solutions affects onion flavor and bulb quality. J. Amer. Soc. Hort. Sci. 2000, 125, 254–259. [Google Scholar] [CrossRef]
- Amare, G. Review on mineral nutrition of onion (Allium cepa L). Open Biotechol. J. 2020, 14, 134–144. [Google Scholar] [CrossRef]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of vegetal- and seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel- grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef]
- Rostami, M.; Shokouhian, A.; Mohebodini, M. Effect of humic acid, nitrogen concentrations and application method on the morphological, yield and biochemical characteristics of strawberry ‘Paros’. Int. J. Fruit Sci. 2022, 22, 203–214. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Hafez, E.; Geries, L. Effect of nitrogen fertilization and biostimulative compounds on onion productivity. Certec. Agron. Mold. 2018, 1, 75–90. [Google Scholar] [CrossRef]
- Shehata, A.S.; Abdelgawad, F.K.; El-Mogy, M.M. Quality and shelf-life of onion bulbs influenced by biostimulants. Int. J. Veg. Sci. 2017, 23, 362–371. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-Ul-Hye, M.; Khan, R.I.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- Metwally, A.R. Arbuscular mycorrhizal fungi and Trichoderma viride cooperative effect on biochemical, mineral content, and protein pattern of onion plants. J. Basic Microbiol. 2020, 60, 712–721. [Google Scholar] [CrossRef]
- Gupta, S.; Strik, A.W.; Plačková, L.; Kulkarni, G.M.; Doležal, K.; Van Staden, J. Interactive effects of plant growth-promoting rhizobacteria and a seaweed extract on the growth and physiology of Allium cepa L. (onion). J. Plant Physiol. 2021, 262, 153437. [Google Scholar] [CrossRef]
- Gebretsadik, K.; Dechassa, N. Response of onion (Allium cepa L.) to nitrogen fertilizer rates and spacing under rain fed condition at Tahtay Koraro, Ethiopia. Sci. Rep. 2018, 8, 9495. [Google Scholar] [CrossRef]
- Bekele, M.; Mohammed, A. Effects of different levels of nitrogen, phosphorus and potassium fertilization on growth and yield of onion (Allium cepa L.) at Jima, South Wester Ethiopia. J. Nat. Sci. Res. 2018, 8, 1–19. [Google Scholar]
- Kovačević, S.; Lončarević, I.; Pajin, B.; Fišteš, A.; Vasiljević, I.; Lazović, M.; Mrkajić, D.; Banjac, M.K.; Podunavac-Kuzmanović, S. Toward identification of the risk group of food products: Chemometric assessment of heavy metals content in confectionery products. Food Addit. Contam. A 2019, 36, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Šeregelj, V.; Šaponjac, V.T.; Banjac, M.K.; Kovačević, S.; Šovljanski, O.; Ćetković, G.; Čanadanović-Brunet, J.; Jevrić, L.; Podunavac-Kuzmanović, S. From sweet corn by-products to carotenoid-rich encapsulates for food applications. Processes 2022, 10, 1616. [Google Scholar] [CrossRef]
- Jevrić, L.R.; Karadžić, M.Ž.; Podunavac-Kuzmanović, S.O.; Horecki, A.N.T.; Kovačević, S.Z.; Vidović, S.S.; Sumić, Z.M.; Ilin, Ž.M. New guidelines for prediction of antioxidant activity of Lactuca sativa L. varieties based on phytochemicals content and multivariate chemometrics. J. Food Process. Pres. 2018, 42, 13355. [Google Scholar] [CrossRef]
- Kovačević, S.Z.; Tepić, A.N.; Jevrić, L.R.; Podunavac-Kuzmanović, S.O.; Vidović, S.S.; Šumić, Z.M.; Ilin, Ž.M. Chemometric guidelines for selection of cultivation conditions influencing the antioxidant potential of beetroot extracts. Comput. Electron. Agr. 2015, 118, 332–339. [Google Scholar] [CrossRef]
- Vulić, J.; Bibovski, K.; Šeregelj, V.; Kovačević, S.; Banjac, M.K.; Čanadanović-Brunet, J.; Ćetković, G.; Četojević-Simin, D.; Šaponjac, V.T.; Podunavac-Kuzmanović, S. Chemical and biological properties of peach pomace encapsulates: Chemometric modeling. Processes 2022, 10, 642. [Google Scholar] [CrossRef]
- Malbaša, R.; Jevrić, L.; Lončar, E.; Vitas, J.; Podunavac-Kuzmanović, S.; Milanović, S.; Kovačević, S. Chemometric approach to texture profile analysis of kombucha fermented milk products. J. Food Sci. Technol. 2015, 52, 5968–5974. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; Cirillo, V.; Van Oosten, M.J.; Di Stasio, E.; Saiano, K.; Woodrow, P.; Ciarmiello, L.F.; Maggio, A.; Carillo, P. Ascophyllum nodosum based extracts counteract salinity stress in tomato by remodeling leaf nitrogen metabolism. Plants 2021, 10, 1044. [Google Scholar] [CrossRef]
- Parisi, M.; Burato, A.; Pentangelo, A.; Ronga, D. Towards the optimal mineral N fertilization for improving peeled tomato quality grown in Southern Italy. Horticulturae 2022, 8, 697. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Harborne, J.B. Methods of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Vračar, L. Handbook for Quality Control of Fresh and Processed Fruit, Vegetables and Mushrooms, and Refreshing Non-Alcoholic Beverages; Faculty of Technology: Novi Sad, Serbia, 2001; pp. 1–226. [Google Scholar]
- Rulebook of Methods of Sampling and Carry Out the Chemical and Physical Analysis for Quality Control of Fruit and Vegetables Products (“Official Gazette of SFRY”, no. 29/83). Available online: https://www.tehnologijahrane.com (accessed on 3 February 2022).
- ISO 750:1998; Fruit and Vegetable Products—Determination of Titratable Acidity. ISO: Geneva, Switzerland, 1998. Available online: https://www.iso.org/standard/22569.html (accessed on 1 February 2022).
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson: London, UK, 2010; pp. 1–278. [Google Scholar]
- StatSoft Inc.: 2300 East 14th Street, Tulsa, OK, USA. Available online: http://www.statsoft.com/ (accessed on 9 September 2022).
- Wang, Y.; Huang, S.; Liu, R.; Jin, J. Effects of nitrogen application on flavor compounds of cherry tomato fruits. J. Plant Nutr. Soil Sci. 2007, 170, 461–468. [Google Scholar] [CrossRef]
- Mutha, E.R.; Tatiya, U.A.; Surana, J.S. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Future J. Pham. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.A.; Rahman, M.M.; Wardany, A.A.; Dawood, M.F.A.; Mostofa, M.G.; Keya, S.S.; Latef, A.A.H.A.; Tran, L.S.P. Antioxidants and bioactive compounds in licorice root extract potentially contribute to improving growth, bulb quality and yield of onion (Allium cepa). Molecules 2021, 26, 2633. [Google Scholar] [CrossRef]
- El-Gaied, L.F.; El-Heba, G.A.A.; El-Sherif, N.A. Effect of growth hormones on some antioxidant parameters and gene expression in tomato. GM Crop. Food 2013, 4, 67–73. [Google Scholar] [CrossRef]
- Abd-Alrahman, H.; Marzouk, M.N.; El-Sawy, M.S.; Abou-Hussein, D.S. Improving growth, yield, and quality of onion plants by amino and humic acids under sandy soil conditions. Middle East J. Appl. Sci. 2021, 11, 637–648. [Google Scholar] [CrossRef]
- Bayoumi, Y.; Taha, N.; Shalaby, T.; Alshaal, T.; El-Ramady, H. Sulfur promotes biocontrol of purple blotch disease via Trichoderma spp. and enhances the growth, yield and quality of onion. Appl. Soil Ecol. 2019, 134, 15–24. [Google Scholar] [CrossRef]
- Murtic, S.; Oljaca, R.; Smajic-Murtic, M.; Vranac, A.; Koleska, I.; Karic, L. Effects of seaweed extract on the growth, yield and quality of cherry tomato under different growth condition. Acta Agric. Slov. 2018, 111, 315–325. [Google Scholar] [CrossRef]




| Depth (m) | pH | CaCO3 (%) | Humus (%) | N (%) | P2O5 | K2O | |
|---|---|---|---|---|---|---|---|
| KCl | H2O | (mg 100 g−1) | |||||
| 0.3 | 7.54 | 7.35 | 4.89 | 2.43 | 0.13 | 16.78 | 30.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Karadžić Banjac, M.; Kovačević, S.; Daničić, T.; Podunavac-Kuzmanović, S.; Ilin, Ž. Onion (Allium cepa L.) Yield and Quality Depending on Biostimulants and Nitrogen Fertilization—A Chemometric Perspective. Processes 2023, 11, 684. https://doi.org/10.3390/pr11030684
Vojnović Đ, Maksimović I, Tepić Horecki A, Karadžić Banjac M, Kovačević S, Daničić T, Podunavac-Kuzmanović S, Ilin Ž. Onion (Allium cepa L.) Yield and Quality Depending on Biostimulants and Nitrogen Fertilization—A Chemometric Perspective. Processes. 2023; 11(3):684. https://doi.org/10.3390/pr11030684
Chicago/Turabian StyleVojnović, Đorđe, Ivana Maksimović, Aleksandra Tepić Horecki, Milica Karadžić Banjac, Strahinja Kovačević, Tatjana Daničić, Sanja Podunavac-Kuzmanović, and Žarko Ilin. 2023. "Onion (Allium cepa L.) Yield and Quality Depending on Biostimulants and Nitrogen Fertilization—A Chemometric Perspective" Processes 11, no. 3: 684. https://doi.org/10.3390/pr11030684
APA StyleVojnović, Đ., Maksimović, I., Tepić Horecki, A., Karadžić Banjac, M., Kovačević, S., Daničić, T., Podunavac-Kuzmanović, S., & Ilin, Ž. (2023). Onion (Allium cepa L.) Yield and Quality Depending on Biostimulants and Nitrogen Fertilization—A Chemometric Perspective. Processes, 11(3), 684. https://doi.org/10.3390/pr11030684











