Abstract
Sargassum is a brown macroalga that has become a general environmental problem in the Mexican Caribbean. Despite the negative effects on the beaches, the algae contain compounds of biotechnological and agronomic interest. The possibility of using sargassum as a substrate under liquid media fermentation (LMF) processes will allow the obtention of bioactive compounds. In this research, five species of Sargassum from the Puerto Morelos region were collected from the beach. The samples were divided into natural Sargassum and washed Sargassum, and the total phenolic compounds (TPC), flavonoids (F), and antioxidant capacity (AOxC) were determined. Once the material was characterized, it was fermented in the LMF process using the Aspergillus niger strain, where the obtained extracts were analyzed. Three holopelagic and one benthic species were identified. The proximal analysis of the seaweed in natural and washed conditions shows adequate carbon–nitrogen ratio values for use as a substrate for microbial degradation. Comparing the fermented extracts with fresh Sargassum, the analyses showed a TPC increase for washed Sargassum fermentation and a TPC decrease for natural Sargassum fermentation; the flavonoid content reached 8-fold higher in the washed Sargassum fermentation. An average AOxC of 57% was achieved during the washed Sargassum LMF process, with a maximum of 69% of ABTS inhibition. Considering these results, Sargassum can be used as a substrate in LMF processes to obtain bioactive compounds.
1. Introduction
Sargassum is a type of floating brown macroalgae of the genus Sargassum, which from 2011 to date has become a tourist, economic, ecological, and health problem due to massive arrivals in the Mexican Caribbean [1,2]. Beaches covered with Sargassum makes the sea access difficult, as they decompose and produce unpleasant odors, generally due to the generation of ammonium and sulfuric acid [1]. The use and exploitation of Sargassum has been sought in different economical areas, and the efforts to prevent it from reaching the beaches has been arduous [3,4]. However, these efforts are still not enough to solve the problem, because the excess of nutrients in the water, the temperature of the water, and the change in sea currents have favored the reproduction and growth of Sargassum at a higher speed [5]. Sargassum contains, among other substances, a large number of bioactive compounds with antioxidant power, which can be used to generate different profitable products that are environment friendly [2]. The composition of the species of Sargassum depends to a large extent on various factors such as the geographical origin of the algae, the season, or even the part of the plant analyzed, as well as the extraction methods [6]. The dry base chemical composition of Sargassum contains 14–44% ash, 4–68% carbohydrates, 9–20% proteins, and 0.5–3.9% lipids [7]. The main energy content of the seaweed is presented in carbohydrates, which mainly contain polysaccharides. The principal families of carbohydrates found in Sargassum spp. are alginates, fucose-containing sulfated polysaccharides (generally fucoidans, laminarin, and mannitol), as well as cellulose [6]. It has been shown that under the biorefinery concept, high-value compounds derived from Sargassum can be obtained, such as antioxidant bioactive compounds [8], and other bioproducts, such as alginates, bioethanol [9,10], biogas, and biofertilizers [11], because some components of the algae represent a limitation in the direct use of sargassum biomass [12]. Therefore, it will be possible to potentially use Sargassum as a substrate in liquid fermentation for the production into high-value products such as antioxidant compounds through microbial treatments.
Within microorganisms, the fungus Aspergillus niger is one of the most abundant strains in the environment, with an enzymatic machinery that allows it to degrade various plant materials [13]. This microorganism is highly used in industrial fermentation processes for the production of citric acid, α-amylase, cellulases, and pectinases, among other compounds [14]. Fermentation is a biological process by which complex molecules can be converted into simpler molecules. Liquid fermentation is the noblest microbial biotechnological processes, which is used for the production of various metabolites of commercial interest such as antioxidants, antibiotics, and enzymes, among others. The efficiency of fermentation depends on using the appropriate substrate, which can be combined with nutrients such as glucose, yeast extract, and mineral salts, among others [15,16], to increase productivity.
The objective of this work was to use Sargassum as a source of carbon and energy in liquid fermentation, and to compare the material cleaning effects in terms of the production of antioxidant compounds. The hypothesis was derived from the idea that the enzymatic machinery and adaptability of the A. niger M4 strain could use natural Sargassum as a substrate and increase the amount of antioxidant compounds in the fermentation broth.
2. Materials and Methods
Collection of study material
The algae used in this study were brown macroalgae of the genus Sargassum, which were collected during the month of July 2019, in the municipality of Puerto Morelos, Quintana Roo, México. For the material identification, fresh algae were collected on the shore of the beach, cleaned of impurities, and washed with purified water. Subsequently, they were placed in hermetic bags with distilled water and stored on ice for their transfer. The algae were kept frozen until analysis. For the fermentation processes and biochemical analyses, the algae collected on the beach were sun-dried and transferred into dark polyethylene (PE) bags for their transportation to the laboratory. All analysis and determinations were conducted in the Fermentations and Biomolecules Lab in the Food Science and Technology Department of Antonio Narro Autonomous Agrarian University. The dry material from the PE bags was divided into two batches: one batch was washed with rainwater to remove salt excess and foreign residues from the algae, then sun-dried for a second time (material considered as washed Sargassum—WS). The second batch was taken directly from the PE bags and used as is (material considered as natural Sargassum—NS). Both batches were macerated and sieved, until particle sizes between 0.5 and 1 mm in diameter were obtained. Sieved material was used as a source of substrate in LMF.
Identification of the collected species
The collected fresh algae were examined in the laboratory, dissected, and observed in the stereoscope to identify each species. The species were classified according to differential morphological characteristics between each one, with the help of taxonomic keys of algae found in the literature and what has been reported on morphological and molecular studies related to species found in the same collection site [17,18,19,20].
Biochemical characterization of Sargassum
The biochemical characterization of Sargassum biomass was determined in terms of humidity content, crude fat, ash, crude fiber, and total protein according to the methods of the AOAC [21]. Reducing sugars [22], total polyphenols [23], and flavonoids [24] were also determined. The content of polysaccharides (fucoidan, glucan, and galactan) were determined according to Aparicio [9] by quantitative acid hydrolysis using sulfuric acid at 72% (v/v).
Quantification of total phenols
Phenolic compounds were evaluated based on the method described by Waterman and Mole [23]. For the complete sample of both WS and NS, a 1:4 fresh homogenization was performed in distilled water. Aliquots of 0.400 mL of fresh material and fermented material were placed into a testing tube, and then 0.15 mL of Folin-Ciocalteu reagent (SIGMA-ALDRICH, St. Louis, MO, USA) and 0.05 mL of 20% sodium carbonate were added. Subsequently, 3.6 mL of distilled water was added, incubating in the dark for 30 min. Finally, mixtures were added into a microplate well and the absorbance at 760 nm was read in a microplate reader (BIOBASE EL-10A). A gallic acid standard curve (0.1% w/v) (SIGMA-ALDRICH) was constructed. The results were expressed as milligrams of gallic acid equivalent per gram of dry sargassum (mg GAE/g ds).
Flavonoid quantification
Flavonoid quantification was performed using the colorimetric method of aluminum chloride [24]. An aliquot of 100 μL of fermented material was taken and placed into testing tubes, then 100 μL of AlCl3 (Jalmek Scientific) at 10% and 100 μL of CH3CO2K (Jalmek Scientific) were added. Mixtures were settled down for 30 min, transferred into microplate wells, and read at 415 nm in a microplate reader (BIOBASE EL-10A). A calibration curve was constructed using quercetin (0.1% w/v) (SIGMA-ALDRICH) as standard. The results were expressed as milligrams of quercetin equivalent per gram of dry Sargassum (mg QE/g ds).
Microorganism and Liquid Fermentation process
For the fermentation processes, the Aspergillus niger M4 strain (code KY825168.1) from the Fermentations and Biomolecules Lab located in the Food Science and Technology Department at Antonio Narro Autonomous Agrarian University was used. The strain was preserved at −20 °C in a glycerol–skim milk cryoprotectant. Reactivation was performed on potato dextrose agar (PDA) and incubated at 30 °C for 8 days. The culture of the strain was kept in Petri dishes with PDA at 4 °C until use.
The fermentation process was evaluated in liquid systems, using WS and NS as two different treatments. Modified Czapek–Dox medium was used as culture broth for the fermentations: yeast extract (Bioxon) (7.63 g/L); KH2PO4 (Jalmek Scientific) (3.04 g/L); MgSO4 (Jalmek Scientific) (1.52 g/L); KCl (Jalmek Scientific) (1.52 g/L). For both systems, a C/N ratio of 4.45 was kept.
For LMF, 50 mL of medium was placed in Erlenmeyer flasks with a capacity of 250 mL and 5 g of Sargassum (washed and natural) was added as a carbon source. A concentration of 1 × 106 spores of Aspergillus niger M4 was inoculated per milliliter of culture media. The flasks were incubated in an Innova® orbital shaker at 150 rpm and temperature of 30 °C. The samples during the kinetics were obtained following the three-step methodology of Rodríguez-Luna [25]. The final filtrate was used as a crude fermented extract of liquid medium (CFE-LM) and was used for further analysis.
For each LMF system, a completely randomized design was developed, with a kinetic study of 240 h, sampling every 24 h. Each reactor was taken as an experimental sample, and each experimental time was performed in triplicate, for a total of 30 reactors in each system.
Fungal Biomass Quantification
For the LMF system, the biomass obtained in the first filtration (sargassum + microbial cells) was used for the indirect determination of fungal growth. The results were expressed as milligram of fungal biomass per gram of dry Sargassum (mg/g ds).
Microbial growth rate
The specific growth rate (μ) was obtained through the modeling of the experimental data with the logistic growth equation [15] (Equation (1)).
where X is the biomass density (mg/g ds), μmax is the maximum specific growth rate (h−1), and K is the equilibrium level of X for which dx/dt = 0 for X > 0.
Antioxidant capacity in the fermented broth
The analysis of the ABTS test [2,2′azino-bis (3-ethylbenzothiazolin-6-sulfonic acid)] (Thermo Scientific, Waltham, MA, USA) was performed by the spectrophotometric method of [26], for both WS and NS systems. The ABTS cation at a concentration of 7 mM in water was mixed with potassium persulfate (Jalmek Scientific, San Nicolás de los Garza, Mexico) until reaching 2.45 mM (final concentration). The mixture was left in the dark for 16 h for the radical formation. Once the ABTS·+ radical was formed, it was diluted in ethanol until an absorbance of 0.7 ± 0.01 at 754 nm was obtained. For the determination a microplate technique was used, placing in the well 3 μL of fermented material and 297 μL of ABTS-ethanol reagent. The mixture was allowed to react for 7 min, and it was read at 754 nm in a microplate reader (BIOBASE EL-10A). A calibration curve with Trolox as standard (ChemCruz) was used. The results were expressed as Trolox equivalent antioxidant capacity per gram of dry Sargassum (TEAC/g ds).
Data analysis
The data were processed with the statistical software Minitab (version 17.1.0), where an analysis of variance (ANOVA) and a Tukey comparison test of means (p ≤ 0.05) were performed. For all the adjustments of the experimental data to the logistic model, the Excel Solver tool was used, seeking to minimize the squared errors between the experimental values and the model. The results were represented as the mean of the data ± standard deviation, performed from three replicates per sample.
3. Results
3.1. Sargassum Identification
In the collected material (Figure 1), three holopelagic species (they stay floating in the water) were identified, which correspond to the species Sargassum fluitans III, Sargassum natans I, and Sargassum natans VIII. A benthic species (they are attached to a substrate) was also identified as Sargassum buxifolium, and one more where the collected material did not allow its complete identification (Sargassum spp.). The presence or absence of spines in the cauloid (stem or axes of the thallus) was one of the main morphological characteristics that helped to distinguish one species from another, in addition to the structures that make up the thallus (body of the alga).

Figure 1.
In the image you can see the different species of Sargassum that were identified. (A) Sargassum fluitans III representative morphological characteristics of the species (thallus with spines). (B) Sargassum natans I with vesicles with projections of phyllodes or with spine. (C) Sargassum natans VIII hydroids attached to the cauloid; vesicles and phyllodes can be observed. (D) Sargassum buxifolium benthic species. (E) Sargassum spp., unidentified species, and (F) dehydrated species.
Sargassum fluitans type III predominated with 80% in the collected material: the thalli (structures that make up the algae) had fronds (cauloid with phyllodes and vesicles) up to 15 cm long and were abundant; the cauloid (thallus axes, stipe, or branches) had spines and foliar phyllodes (leaves or laminae), the latter with a serrulate margin, acute apex, darkened and thickened rib, and slightly asymmetrical base, with a small pedicel; the phyllodes were between 1–3.5 cm long and 3–4 mm wide. It also had smooth oval vesicles (floaters, pneumatocyst, bladders, or aerocyst) without phyllodes or spine projections; the vesicles were alternated in the cauloid and had a small pedicel, a cryptostome, and an absent receptacle (Figure 1A).
Sargassum natans I was present in 16% of the collected material. The species had alternate fronds 6–8 cm long and cauloid without spines, linear leaf phyllodes with toothed margins, an acute apex, a slightly asymmetrical base, and slightly defined vein, in addition to phyllodes 3.5–4 cm long and 1–2.8 mm wide. The species had spherical and abundant vesicles, with spine-like projections or phyllodes projections and had a small pedicel, a cryptostome, and an absent receptacle (Figure 1B).
Sargassum natans VIII was present in 1% of the material collected. The species had fronds 8–10 cm long; a cauloid without spines; simple foliar phyllodes, finely serrulate, 2–3 cm long and 3–6 mm wide; a slightly asymmetrical base; an acute apex; a darkened and thickened vein; and the phyllodes had hydroids. The species had spherical vesicles, rarely with a spine or projection of phyllodes, but some had attached hydroids. In addition, the vesicles had a small pedicel, a cryptostome, and an absent receptacle (Figure 1C).
Sargassum buxifolium was present in 1% of the collected material, and had a spineless cauloid, abundant foliar phyllodes 2–4 cm long and 10–12 mm wide, a symmetrical base and smooth or finely serrulate margins, a slightly acute apex, a darkened and thickened vein, a phylloid with a short pedicel, cryptostomes near the margins which were randomly scattered, spherical or ovoid vesicles, a small pedicel, and an absent receptacle (Figure 1D).
The species that could not be identified (Sargassum spp.) was found in 2% of the collected material, and this material had fronds 10–14 cm long, a cauloid with spines, abundant phyllodes with toothed margins, an obtuse apex, an asymmetrical base, and a slightly defined vein. The phyllodes were between 1.5–2 cm long and 2–5 mm wide. The species had abundant flattened vesicles and a small pedicel, the receptacle could be seen, and the cryptostome was absent (Figure 1E).
Figure 1F shows the species as they are commonly found stranded on the beach after days of being exposed to the sun; the morphology difference between species can be observed even when they are completely dehydrated.
3.2. Sargassum Characterization
Being a macroalgae, Sargassum has a varied chemical composition, with diverse biological properties. The physicochemical characterization carried out (Table 1) shows us the possibility of establishing a fermentation process using Sargassum residues as a substrate to obtain energy. In the case of the Sargassum used in this project, the characterization shows considerable values of total sugars (carbon source) and protein (nitrogen source), both for the WS and for the NS.

Table 1.
Wet-based characterization of samples of washed Sargassum and natural Sargassum used as raw material for LMF.
3.3. Microbial Growth Rate
The evaluations of the growth of the Aspergillus niger M4 strain in LMF using WS and NS are shown in Figure 2. The microbial strain was able to grow in both Sargassum conditions evaluated in LMF. The fermentation carried out using LMF shows a short exponential phase between 24 and 48 h in both conditions (WS and NS) and to subsequently have a rate of cell death after the maximum of 48 h (13.2 ± 0.89 mg/g ds) for LMF-WS and at 96 h (15.2 ± 0.15 mg/g ds) for LMF-NS. For fungal growth, the WS increases the growth rate (μmax = 0.2186 h−1) compared to the use of NS (μmax = 0.0717 h−1). It should be noted that after 120 h of fermentation in the LMF-WS and LMF-NS systems there is a decrease in biomass. During the kinetic study in the LMF systems, an increase in the viscosity of the medium was observed after 24 h, and the sand residues containing the NS began to accumulate at the bottom of the medium.
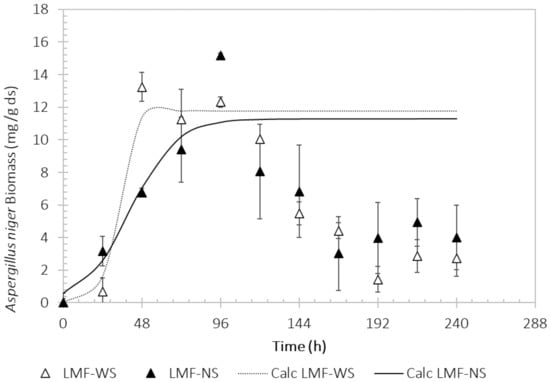
Figure 2.
Growth evaluation of the Aspergillus niger M4 strain using washed Sargassum (light color) and natural Sargassum (solid color) as substrate. The lines represent the data calculated using the logistic growth model.
3.4. Total Polyphenol Concentrations
Total phenolic compounds were considered as the source for the accumulation of bioactive compounds, such as phenolic acids, flavonoids, and tannins. Figure 3 shows the variation in the content of phenolic compounds during the fermentation process. It was observed that in WS the TPC increases in the first 48 h, varying in the following hours during the kinetic study. On the other side, the TPC in the NS system has a slight increase in the first 24 h, then having a tendency of decreasing as time progresses. In the fermentation with WS there was a higher concentration of TPC (8.11 ± 0.44 mg GAE/g ds at 48 h) compared to the fermentation with NS (5.52 ± 1.13 mg GAE/g ds at 24 h).
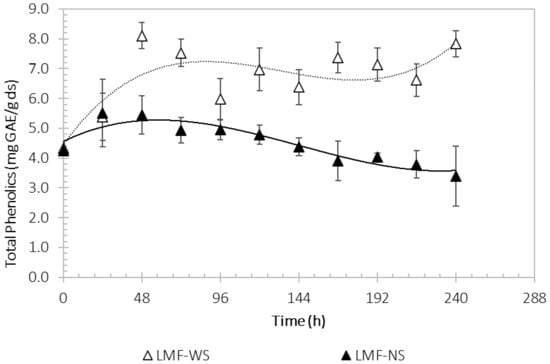
Figure 3.
Process kinetics of total phenolic compounds by Aspergillus niger M4, using washed Sargassum (light color) and natural Sargassum (solid color) as substrate.
3.5. Flavonoid Concentration
Figure 4 shows the flavonoid content during the fermentation kinetics with Sargassum. The maximum accumulation of flavonoids (36.2 ± 0.4 mg QE/g ds) occurred at 216 h in the fermentation broth obtained with WS. For the NS process, the highest value was reached at 144 h, with 8.5 ± 1.7 mg QE/g ds. The adaptation of the microorganism (lag phase) to the initial fermentation conditions in both systems, WS and NS, ended at 24 h, meaning a good adaptability to the substrate. In the fermentation with WS at 120 h, a slight decrease in the flavonoid content was seen to later increase with time and showed the maximum at 216 h, contrary to what happened in the fermentation with NS where the stationary and decrease phases began at 120 h. During the process time the content of flavonoids varied for both systems, but it was more variable in the fermentation with WS, indicating there are fungal metabolic processes.
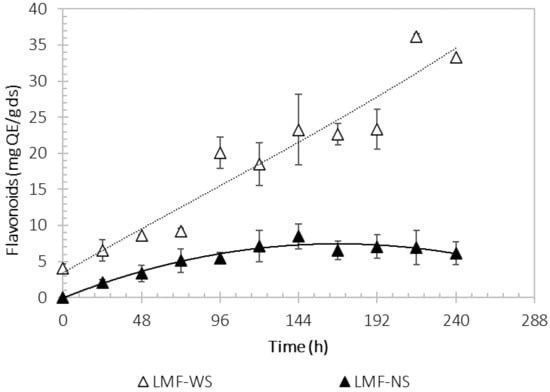
Figure 4.
Kinetics of flavonoid production by Aspergillus niger M4 in a liquid fermentation process, using washed Sargassum (light color) and natural Sargassum (solid color) as substrate.
3.6. Polysaccharides
Polysaccharide content was represented by fucoidan, glucan, and galactan from the raw material, where their values were 71.78, 107.44, and 51.62 mg/g ds, respectively, for WS and 70.86, 104.11, and 55.90 mg/g ds for NS. During the fermentation process these three characteristic structural polysaccharides of sargassum were monitored and are shown in Figure 5. Fucoidan is the polysaccharide that increased its presence the most over time; for WS there was a maximum value of 175.5 ± 20.1 mg/g ds at 72 h, which subsequently decreased to zero at 144 h and remained so until 240 h. In the case of NS, the maximum was also present at 72 h, but with a value of 77.6 ± 0.5 mg/g ds, showing no significant statistical difference between 24 and 48 h. The second polysaccharide that was present in greater quantity was the glucan with 77.7 ± 2.5 mg/g ds for WS and 57.0 ± 1.1 mg/g ds for NS, both values obtained at 24 h of fermentation. Galactan showed the same value (34.4 ± 0.1 mg/g ds) for both WS and NS.
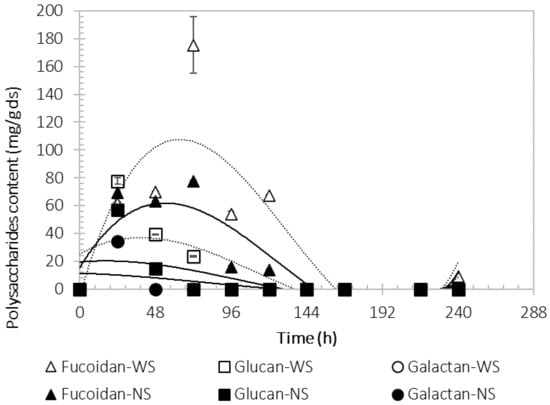
Figure 5.
Polysaccharide content profile during Aspergillus niger M4 Sargassum fermentation process, using washed Sargassum (light color) and natural Sargassum (solid color) as substrate.
3.7. ABTS Antioxidant Capacity
Figure 6 shows the variation in the antioxidant capacity (AOxC) during the fermentation process. AOxC values ranged from 42 to 69% for WS and from 15 to 47% for NS. The AOxC for WS was 1.3 times higher at time 144 h compared to time 0 h. In this figure, it can be seen that when WS was used, the AOxC percentage increased after 24 h (60% of ABTS inhibition); however, the highest AOxC (69% of ABTS inhibition) occurred 144 h after the fermentation process, and from that hour there was a decrease in AOxC. Meanwhile, in the fermentation where NS was used, the AOxC decreased at 24 and 48 h (25% and 15% of ABTS inhibition, respectively), and subsequently increased the antioxidant capacity after 96 h when the maximum AOxC was reached (47% of ABTS inhibition).
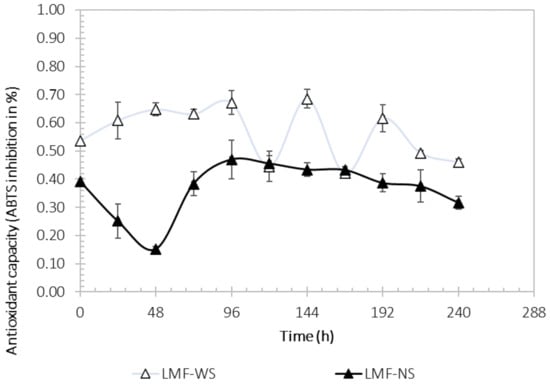
Figure 6.
Antioxidant capacity during Aspergillus niger M4 Sargassum fermentation process, using washed Sargassum (light color) and natural Sargassum (solid color) as substrate.
4. Discussion
In different collections from the Caribbean Sea and the Gulf of Mexico, Camacho [27] identified through morphological and molecular analysis different species of sargassum, among which they reported Sargassum fluitans in the Mexican Caribbean. They described this species morphologically, where they mentioned that it had branches (cauloid) with leaves (phyllodes) and vesicles, with lanceolate, flat, and serrated leaves, which is similar to what could be observed in the collected material for this work. Mendoza-Becerril [19] found in Puerto Morelos, Mexico, in collections from April 2018 to March 2019, the species Sargassum natans type I, Sargassum natans type VIII, and Sargassum fluitans type III. On the other hand, various authors have reported the presence of Sargassum natans type I and type VIII in Puerto Morelos from 1986 to date, in the months of March to September and December. The presence of Sargassum fluitans type III has also been reported from 1971 to 2019 in Puerto Morelos, Mexico, during the different months of the year.
Among the main differences reported to identify one species of sargassum from another is the type of life, the absence of a spine or phyllode projection in the floaters, and the presence or absence of spines in the cauloid, as well as the morphology of the phyllodes and the presence or absence of reproductive structures [28]. It has also been reported that the highest concentration of biomass worldwide in places where the arrivals are massive belongs to the species of Sargassum fluitans type III, Sargassum natans I, and Sargassum natans VIII [29,30]. Schell [20] describes Sargassum fluitans III, Sargassum natans I, and Sargassum natans VIII with similar characteristics to this study, however, they describe hydroids attached to the cauloid and vesicles of Sargassum fluitans and Sargassum natans VIII, unlike what was observed in the species of this work, where only hydroids attached to Sargassum natans VIII were observed. Govindarajan [17], in collections between 2015 and 2018, also report Sargassum fluitans III, Sargassum natans I, and Sargassum natans VIII, where they distinguish the species by the presence or absence of spines in the cauloid and the projections of phyllodes or spines in the vesicles. This coincides with this work, where these characteristics were the main morphotypes for the identification of each of the species. They also observed hydroids attached to Sargassum natans and Sargassum fluitans.
The composition of the material plays a very important role in the development of microbiological processes, due to the use of Sargassum as a source of carbon and energy during the bioprocess. Various studies mention alginate as the main compound in the cells of Sargassum species, with values between 15–25%, followed by fucoidans (3–12%) [6]. Brown algae, and in particular fucals (to which the Sargassum species belong), are known for their high content of polyphenols [6]. These polyphenols or phenolic compounds are mainly composed of phlorotannins, oligomers and polymers of phloroglucinol, reporting a content of polyphenols with a value of around 5–15% [6,31]. The presence of fucoidans and the phenolic compounds in WS and NS samples gives the possibility of generating antioxidant molecules with low molecular weight through the fungal degradation process. The number and positions of the hydroxyl groups and the nature of the substitutions on the aromatic rings give phenolic compounds the ability to quench free radicals [32]. Ouattara [33] reports the presence of sterols, hydrolysable phenols, catechin, and saponins for aqueous samples of Sargassum fluitans and Sargassum natans.
Previous studies have reported that combinations of Sargassum natans and Sargassum fluitans reached compositions of 57.3% carbohydrates, 15.4% protein, 7.15% fiber, 96.5 mg/100 g of phosphorus, 775 mg/100 g of flavonoids, 122.5 mg/100 g of tannins, and 80 mg/100 g of phenols [34]. In this study, 20% fiber was quantified for WS and 22% for NS, while the phenolic compounds were slightly similar to those reported in the literature, with 12.50% for WS and 9.60% for NS.
Derived from the physicochemical characterization of WS and NS, it can be determined that both elements can be used as a substrate during a fermentation process in liquid medium. Davis [12] mentioned that the Sargassum species have the potential to produce a wide range of biochemical, nutraceutical, and pharmaceutical products with high value, but they consider that it is still necessary to further characterize the species, in addition to considering the events of arrivals of Sargassum, the type of species, and the seasonal collection period.
The content of free compounds of easy metabolic assimilation homogenized in the medium favored the maximum microbial growth rate within the first 48 h. The subsequent decrease in the biomass content could be due to the possible accumulation of various compounds that are related to the viscosity of the medium, which decreases the metabolism rate of the microorganism [35]. This change in the media could cause homogenization problems, which, added to the formation of mycelial pellets, hindered the transfer of oxygen and the removal of carbon dioxide in the system. In fermentative kinetic studies, Bizukojc and Gonciarz [36], using Aspergillus terreus, observed cell death processes, because oxygen passed only those pellets with diameters less than 1400 μm. The effect of washing the material can eliminate different toxic compounds but also reduce the availability of some sugars that are easily assimilated by the microorganism, so that the adaptation to the culture media and the degradation of Sargassum polysaccharides is delayed [31]. The polysaccharides, as well as the phenolic compounds present in Sargassum, are associated with growth, while the accumulation of flavonoids occurs after maximum growth. This could be because the fungus hydrolyzed the compounds in the WS fermentation to obtain energy for its metabolic activities. When optimizing the culture medium to meet the needs of mycelium development, conditions can be favored to maximize the yields of the final product, prolonging the stationary phase.
The use of the fermentation process can favor the accumulation of bioactive compounds, as demonstrated in a study where they used cocoa beans as a substrate subjected to a fermentation process. In that study, they reported maximum accumulation values between 22.58 and 50.23 mg per gram of substrate (mg/g S) regarding the accumulation of phenolic compounds [37]. These values are similar to those obtained in the present work, where WS and NS were used in the fermentation, and as already mentioned, the highest values of phenolic compounds were obtained with WS. Naringenin production studies by Aspergillus niger IB-56 [38] found that the maximum accumulation of flavonoids occurred 24 h after the fermentation process. They mentioned that the increase in the concentration of flavonoids was related to the decrease in the concentration of naringin and reducing sugars in the medium, where the enzymes of the fungus hydrolyzed the flavonoid sugars to be used as a carbon source for cell development and maintenance. Unlike this study, the same pattern was slightly observed in the fermentation using WS and much less in the NS process, because the flavonoid accumulation did not match with the biomass growth. On the other hand, the TPCs presented changes associated with microbial growth, so it is suggested that they served as the carbon source for the microorganism. The same behavior was observed with the content of polysaccharides, which being the main carbon source, their monomeric components increased in the first hours of fermentation.
Among these polysaccharides, fucoidans belongs to a complex group of macromolecules characterized as fucose-containing sulfated polysaccharides, with a wide range of important biological properties such as antioxidant capacity, among others [39]. Hifney [40] improved the antioxidant properties of the fucoidans and alginates of the seaweed Sirophysalis trinodis (formerly Cystoseira trinosis) by subjecting it to a fungal fermentation process with different fungi. Before fucoidan and alginate extraction, the different fungi used by Hifney [40] produced fucoidanase, thus reducing the molecular weight of fucoidan, unlike the non-fermented control. The fungal fermentation also induced significant increases in the fucose and fucoidan sulfate contents, in addition to observing that when using Aspergillus niger in the fermentation process Hifney [40] induced the production of fucoidanase; however, it was with other fungi that a higher percentage of fucose was obtained. In comparison with this investigation, it was possible to observe that at 72 h using Aspergillus niger M4 the content of fucoidans increased.
The antioxidant capacity could be improved by the fermentation process due to its association with the increase in bioactive compounds, such as antioxidant polysaccharides, phenolic compounds, and flavonoids, among others. Cuellar Alvarez [41] evaluated the effect of fermentation time on the phenolic content and the antioxidant potential in Copoazú (Theobroma grandiflorum) grains. Carrying out a 10-day kinetic study where they evaluated different compounds every 48 h, they observed that the maximum accumulation of phenolic compounds and antioxidant activity occurred on day 6 of fermentation, and from that day there was a decrease. This could also be observed in the present investigation where NS was used. Since the maximum accumulation was on day 6, and from the 6th day there was a decrease in antioxidant capacity, these authors recommend not extending the fermentation after 6 days, since they consider that the fermentation process negatively influences the content of bioactive substances.
On the other hand, Zhao [42] mentioned that microorganisms are capable of modifying antioxidant compounds during the fermentation process, because this can lead to the structural rupture of plant cell walls, which induces the synthesis and release of several bioactive compounds. In addition, it was mentioned that microorganisms such as Aspergillus niger, among others, give rise to a series of reactions that result in greater antioxidant activity, which is related to the increase in phenolic compounds that are converted to form released enzymes produced by microorganisms. In the case of this research, the increase in phenolic compounds, flavonoids, fucoidans, and many others that were not quantified during the fermentation process resulted in a greater antioxidant capacity at certain hours of the kinetics.
5. Conclusions
The liquid fermentation process using unwashed and washed Sargassum as carbon and energy source is useful for obtaining metabolites, which are excreted into the culture medium, such as phenolic compounds with antioxidant power. The use of washed Sargassum in liquid fermentation with Aspergillus niger M4 could be used for release and recovery of the different phenolic compounds. Liquid fermentation with Sargassum serves as a guide to improve fermentation processes with Aspergillus niger to increase the concentration of metabolites with commercial interest.
The next step in the research would be to find another way to dry the macroalgae on a large scale to avoid exposure to the sun, increase the volume of Sargassum used, as well as explore solid- or semi-solid-state fermentation, with the intention of generating the least amount of waste, although these residues can be used as an additional substrate to reduce the use of peatmoss in horticultural crops.
From this research it can be concluded that the treatment of Sargassum by a fermentation process allows the release of phenolic compounds of biotechnological interest, giving added value to the waste that reaches the Mexican Caribbean Sea.
Author Contributions
Conceptualization, A.R.-O.; methodology, A.R.-O., S.G.-M. and J.A.G.-F.; software, A.R.-O.; validation, A.B.-M., R.M.R.-J. and J.A.G.-F.; formal analysis, A.R.-O. and A.V.C.-R.; investigation, R.M.P.-C.; resources, A.R.-O. and J.A.G.-F.; data curation, A.V.C.-R.; writing—original draft preparation, R.M.P.-C.; writing—review and editing, A.R.-O. and A.V.C.-R.; visualization, A.R.-O.; supervision, A.R.-O.; project administration, A.R.-O.; funding acquisition, A.R.-O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Autónoma Agraria Antonio Narro, grant number 30-38111-425204001-2289.
Data Availability Statement
Data are unavailable due to privacy statements from the funder institution.
Acknowledgments
Student Rosa Maria Paredes Camacho acknowledges CONACYT for the grant during her doctorate studies.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R. Massive influx of pelagic Sargassum spp. on the coasts of the Mexican Caribbean 2014–2020: Challenges and opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Leal-Bautista, R.M.; Tapia-Tussel, R.; Alzate-Gaviria, L. Usos potenciales del sargazo. Rev. Acad. Méxicana Cienc. 2020, 71, 52–57. [Google Scholar]
- Martínez-González, G. Sargassum: The atypical irruption of an ancient ecosystem. Salud Pública México 2019, 61, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Sentíes, A.; Dreckmann, K.M. Lista actualizada de las macroalgas de Tabasco, México. Acta Bot. Venez. 2013, 36, 109–118. [Google Scholar]
- Marx, U.C.; Roles, J.; Hankamer, B. Sargassum blooms in the Atlantic Ocean–From a burden to an asset. Algal Res. 2021, 54, 102–188. [Google Scholar] [CrossRef]
- Devault, D.A.; Pierre, R.; Marfaing, H.; Dolique, F.; Lopez, P.-J. Sargassum contamination and consequences for downstream uses: A review. J. Appl. Phycol. 2021, 33, 567–602. [Google Scholar] [CrossRef]
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Nyvall-Collén, P.; Bourgougnon, N. Enzymatic recovery of metabolites from seaweeds: Potential applications. Adv. Bot. Res. 2014, 7, 1279–1320. [Google Scholar]
- Shobharani, P.; Nanishankar, V.; Halami, P.; Sachindra, N. Antioxidant and anticoagulant activity of polyphenol and polysaccharides from fermented Sargassum sp. Int. J. Biol. Macromol. 2014, 65, 542–548. [Google Scholar] [CrossRef]
- Aparicio, E.; Rodríguez-Jasso, R.M.; Pinales-Márquez, C.D.; Loredo-Treviño, A.; Robledo-Olivo, A.; Aguilar, C.N.; Kostas, E.T.; Ruiz, H.A. High-pressure technology for Sargassum spp. biomass pretreatment and fractionation in the third generation of bioethanol production. Bioresour. Technol. 2021, 329, 124935. [Google Scholar] [CrossRef]
- Ardalan, Y.; Jazini, M.; Karimi, K. Sargassum angustifolium brown macroalga as a high potential substrate for alginate and ethanol production with minimal nutrient requirement. Algal Res. 2018, 36, 29–36. [Google Scholar] [CrossRef]
- Thompson, T.; Young, B.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Lin, X.; Wang, J.; Liu, Y.; Tao, H.; Zhou, X. Asperpyrone-Type Bis-Naphtho-γ-Pyrones with COX-2–Inhibitory Activities from Marine-Derived Fungus Aspergillus niger. Molecules 2016, 21, 941. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.A.S.; Oliveira, M.d.C.F.d.; Pimenta, A.T.; Uchôa, P.K. Aspergillus niger: A hundred years of contribution to the natural products chemistry. J. Braz. Chem. Soc. 2019, 30, 2029–2059. [Google Scholar] [CrossRef]
- Charles-Rodríguez, V.; Guerrero-Mata, A.; Martínez-Vázquez, G.; Cruz-Hernández, M.A.; Belmares-Cerda, R.E.; Robledo, A. Bioreactor Analysis for the Corn-Cob Valorization in the Xylanase Production. Waste Biomass Valorization 2018, 9, 995–1001. [Google Scholar] [CrossRef]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind. Crops Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef]
- Govindarajan, A.F.; Cooney, L.; Whittaker, K.; Bloch, D.; Burdorf, R.M.; Canning, S.; Carter, C.; Cellan, S.M.; Eriksson, F.A.A.; Freyer, H.; et al. The distribution and mitochondrial genotype of the hydroid Aglaophenia latecarinata is correlated with its pelagic Sargassum substrate type in the tropical and subtropical western Atlantic Ocean. PeerJ 2019, 7, e7814. [Google Scholar] [CrossRef]
- Godínez-Ortega, J.L.; Cuatlán-Cortés, J.V.; López-Bautista, J.M.; van Tussenbroek, B.I. A Natural History of Floating Sargassum Species (Sargasso) from Mexico; IntechOpen: London, UK, 2021. [Google Scholar]
- Mendoza-Becerril, M.A.; Serviere-Zaragoza, E.; Mazariegos-Villarreal, A.; Rivera-Perez, C.; Calder, D.R.; Vázquez-Delfín, E.F.; Freile-Pelegrín, Y.; Agüero, J.; Robledo, D. Epibiont hydroids on beachcast Sargassum in the Mexican Caribbean. PeerJ 2020, 8, e9795. [Google Scholar] [CrossRef]
- Schell, J.; Goodwin, D.; Siuda, A. Recent Sargassum Inundation Events in the Caribbean: Shipboard Observations Reveal Dominance of a Previously Rare Form. Oceanography 2015, 28, 8–10. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: London, UK, 1994. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar] [CrossRef]
- Rodríguez-Luna, D.; Ruiz, H.A.; González-Morales, S.; Sandoval-Rangel, A.; Cabrera de la Fuente, M.; Charles-Rodríguez, A.V.; Robledo-Olivo, A. Recovery of melon residues (Cucumis melo) to produce lignocellulolytic enzymes. Biomass Convers. Biorefinery 2020, 12, 5915–5922. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Camacho, O.; Mattio, L.; Draisma, S.; Fredericq, S.; Diaz-Pulido, G. Morphological and molecular assessment of Sargassum (Fucales, Phaeophyceae) from Caribbean Colombia, including the proposal of Sargassum giganteum sp. nov., Sargassum schnetteri comb. nov. and Sargassum section Cladophyllum sect. nov. Syst. Biodivers. 2015, 13, 105–130. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.; Graham, C.; Vera, E.; Escalante-Mancera, E.; Álvarez-Filip, L.; van Tussenbroek, B.I. Temporal changes in the composition and biomass of beached pelagic Sargassum species in the Mexican Caribbean. Aquat. Bot. 2020, 167, 103275. [Google Scholar] [CrossRef]
- Robledo, D.; Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Vásquez-Elizondo, R.M.; Qui-Minet, Z.N.; Salazar-Garibay, A. Challenges and Opportunities in Relation to Sargassum Events Along the Caribbean Sea. Front. Mar. Sci. 2021, 8, 699664. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Hernandez-Vargas, G.; Iqbal, H.M.N.; Barceló, D.; Parra-Saldívar, R. Bioremediation potential of Sargassum sp. biomass to tackle pollution in coastal ecosystems: Circular economy approach. Sci. Total Environ. 2020, 715, 136978. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability; IntechOpen: London, UK, 2017. [Google Scholar]
- Ouattara, A.; Kpan, W.B.; Komoe, K.; Ouattara, D.; Kone, M.W. The acute toxicity of Sargassum fuiltans (Børgesen) Børgesen and Sargassum natans (Børgesen) Børgesen on some rats of wistar stock. J. Anim. Plant Sci. 2021, 47, 9. [Google Scholar]
- Oyesiku, O.O.; Egunyomi, A. Identification and chemical studies of pelagic masses of Sargassum natans (Linnaeus) Gaillon and S. fluitans (Borgessen) Borgesen (brown algae), found offshore in Ondo State, Nigeria. Afr. J. Biotechnol. 2014, 13, 1188–1193. [Google Scholar]
- Reyes-Ocampo, I.; González-Brambila, M.; López-Isunza, F. Un análisis del metabolismo de Aspergillus niger creciendo sobre un sustrato sólido. Rev. Mex. Ing. Química 2013, 12, 41–56. [Google Scholar]
- Bizukojc, M.; Gonciarz, J. Influence of oxygen on lovastatin biosynthesis by Aspergillus terreus ATCC 20542 quantitatively studied on the level of individual pellets. Bioprocess Biosyst. Eng. 2015, 38, 1251–1266. [Google Scholar] [CrossRef]
- Zapata Bustamante, S.; Tamayo Tenorio, A.; Alberto Rojano, B. Efecto de la fermentación sobre la actividad antioxidante de diferentes clones de cacao Colombia. Rev. Cuba. Plantas Med. 2013, 18, 391–404. [Google Scholar]
- Rubio, M.C.; Alurralde, T.; Suárez, S.; Navarro, A. Producción de naringenina por Aspergillus niger IB-56. Boletín Micológico 2011, 26, 23–27. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.S.; Dantas-Santos, N.; Camara, R.B.G.; Cordeiro, S.L.; Pereira Costa, M.S.S.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M.; Gomaa, M. Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018, 269, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Cuellar Alvarez, L.; Cuellar Alvarez, N.; Galeano Garcia, P.; Suárez Salazar, J.C. Effect of fermentation time on phenolic content and antioxidant potential in Cupuassu (Theobroma grandiflorum (Willd. ex Spreng.) K. Schum.) beans. Acta Agronómica 2017, 66, 473–479. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).