Efficiency of Electrochemical Methods of Purification and Control over the Oxide Concentration in Halide Melts: PbCl2
Abstract
1. Introduction
- −
- Escalation of nuclear waste and additional complex processing procedures;
- −
- Changes in the process parameters and possible disruption of the process control;
- −
- Increased corrosion of the reactor materials and decrease in the reactor operation life;
- −
- Contamination of the target products and decline in the process efficiency.
- −
- Portable equipment;
- −
- Relatively cheap consumable electrode materials;
- −
- Possibility of direct measurement both in laboratory and industrial reactors;
- −
- Rapid in-situ multiple analysis;
- −
- Theoretical background of the methods;
- −
- The electrochemical sensor placed directly in the reactor allows for eliminating its depressurization during the analysis.
2. Materials and Methods
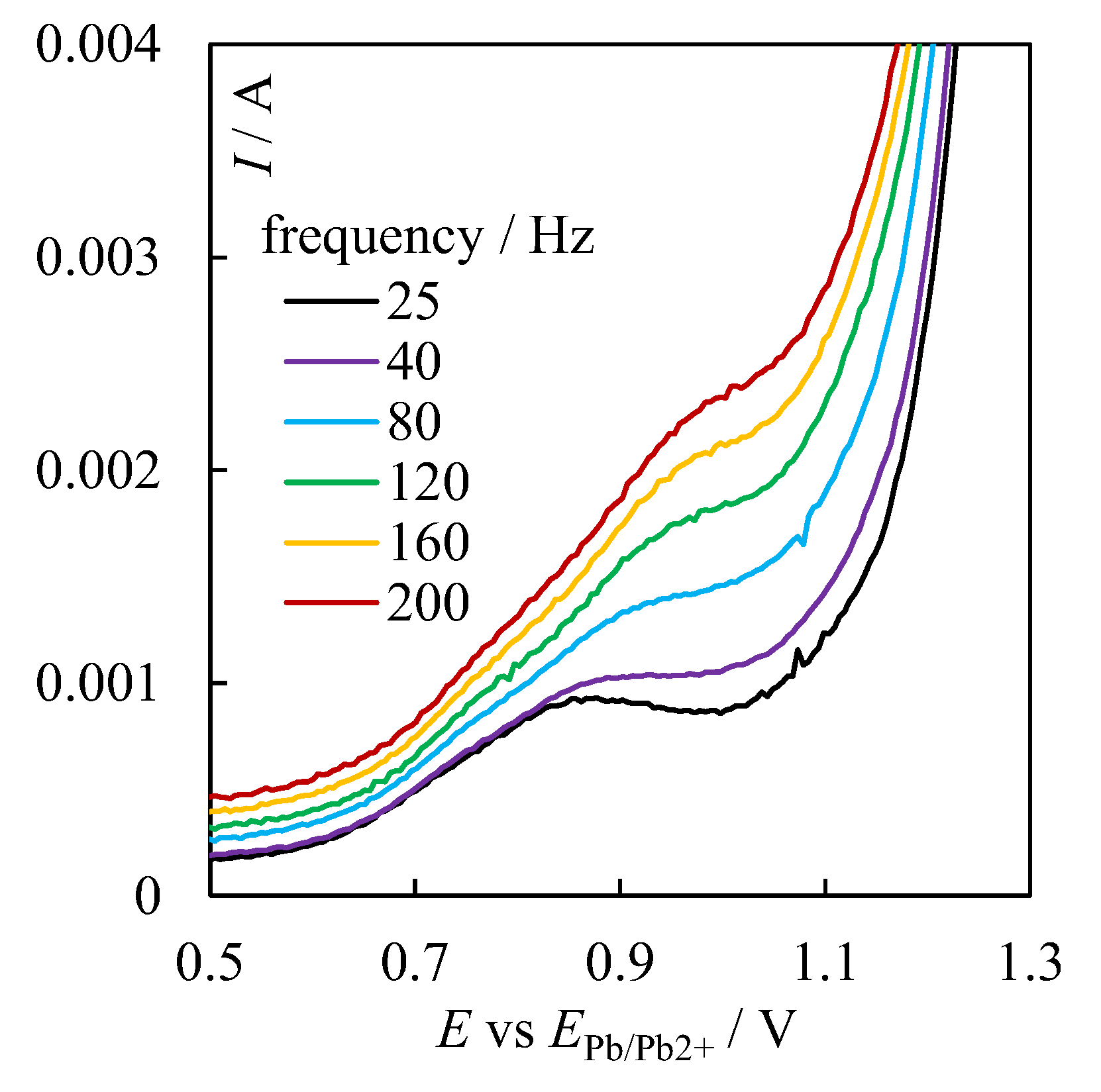
3. Results
- −
- Dissolution of oxygen formed at the anode in the melt;
- −
- Oxidation of oxygen-containing anions with relatively strong bonds [65] only at potentials more positive than 1.0 V;
- −
- Side reduction of oxygen dissolved in the melt at the cathode.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaikov, Y.; Batukhtin, V.; Shurov, N.; Suzdaltsev, A. High-Temperature Electrochemistry of Calcium. Electrochem. Mat. Techn. 2022, 1, 20221007. [Google Scholar] [CrossRef]
- Stulov, Y.; Dolmatov, V.; Dubrovskiy, A.; Kuznetsov, S. Electrochemical Synthesis of Functional Coatings and Nanomaterials in Molten Salts and Their Application. Coatings 2023, 13, 352. [Google Scholar] [CrossRef]
- Kushkhov, K.; Kardanova, R.; Kholkina, A. Peculiarities of Holmium and Iron Triad Ions Co-Reduction: Formation of HoxNiy (HoxCoy, HoxFey) Intermetallic Compounds in Chloride Melts. Processes 2022, 10, 1723. [Google Scholar] [CrossRef]
- Zeng, Y.; Cui, G.; Wu, W.; Xu, C.; Huang, J.; Wang, J.; Yang, Z. Numerical Simulation Study on Flow Heat Transfer and Stress Distribution of Shell-and-Tube Superheater in Molten Salt Solar Thermal Power Station. Processes 2022, 10, 1003. [Google Scholar] [CrossRef]
- Redkin, A.; Korzun, I.; Reznitskikh, O.; Yaroslavtseva, T.; Zaikov, Y.; Kumkov, S. Heat of Fusion of Halide Salts and their Eutectics. J. Therm. Anal. Calorim. 2018, 131, 2021–2026. [Google Scholar] [CrossRef]
- Carotti, F.; Wu, H.; Scarlat, R.O. Characterization of a Thermodynamic Reference Electrode for Molten LiF–BeF2 (FLiBe). J. Electrochem. Soc. 2017, 164, H854–H861. [Google Scholar] [CrossRef]
- Ignatiev, V.V.; Merzlyakov, V.A.; Subbotin, V.G.; Panov, A.V. Experimental Study of Physical Properties of Molten Salts Containing Fluorides of Sodium, Lithium and Beryllium Difluoride. At. Energy 2006, 101, 364–372. [Google Scholar] [CrossRef]
- Alekseev, P.N.; Gagarinskii, A.Y.; Kalugin, M.A.; Kukharkin, N.E.; Semchenkov, Y.M.; Sidorenko, V.A.; Subbotin, S.A.; Teplov, P.S.; Fomichenko, P.A.; Asmolov, V.G. On a Strategy for the Development of Nuclear Power in Russia. At. Energy 2019, 126, 207–219. [Google Scholar] [CrossRef]
- Zaikov, Y.P.; Shishkin, V.Y.; Potapov, A.M.; Dedyukhin, A.E.; Kovrov, V.A.; Kholkina, A.S.; Volkovich, V.A.; Polovov, I.B. Research and Development of the Pyrochemical Processing for the Mixed Nitride Uranium-Plutonium Fuel. J. Phys. Conf. Ser. 2020, 1475, 012027. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Cho, D.; Bang, S. Quantitative Cost-Benefit Analysis of Direct Disposal and Pyroprocessing in Korea’s Nuclear Fuel Cycle. Sustainability 2021, 13, 7789. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Lee, J. Highly Enhanced Reduction of Rare Earth Oxides in Simulated Oxide Fuel in Li2O–LiCl Salt Using Lithium Metal. J. Nucl. Mat. 2018, 511, 367–374. [Google Scholar] [CrossRef]
- Sakamura, Y.; Murakami, T.; Tada, K.; Kitawaki, S. Electrowinning of U–Pu onto Inert Solid Cathode in LiCl–KCl Eutectic Melts Containing UCl3 and PuCl3. J. Nucl. Mat. 2018, 502, 270–275. [Google Scholar] [CrossRef]
- Pitchaiah, K.C.; Sujatha, K.; Deepitha, J.; Ghosh, S.; Sivaraman, N. Recovery of Uranium and Plutonium from Pyrochemical Salt Matrix Using Supercritical Fluid Extraction. J. Supercrit. Fluids 2019, 147, 194–204. [Google Scholar] [CrossRef]
- Kim, S.; Ko, W.; Bang, S. Analysis of Unit Process Cost for an Engineering-Scale Pyroprocess Facility Using a Process Costing Method in Korea. Energies 2015, 8, 8775–8797. [Google Scholar] [CrossRef]
- Williamson, M.A.; Willit, J.L. Pyroprocessing Flowsheets for Recycling Used Nuclear Fuel. Nucl. Eng. Technol. 2011, 43, 329–333. [Google Scholar] [CrossRef]
- Karfidov, E.A.; Zaikov, Y.P.; Nikitina, E.V.; Seliverstov, K.E.; Dub, A.V. High-Temperature Passivation of the Surface of Candidate Materials for MSR by Adding Oxygen Ions to FLiNaK Salt. Materials 2022, 15, 5174. [Google Scholar] [CrossRef]
- Arkhipov, S.P.; Zaikov, Y.P.; Arkhipov, P.A.; Mullabaev, A.R. Interaction between Iron Fluoride and Molten FLiBe. Processes 2022, 10, 2742. [Google Scholar] [CrossRef]
- Swain, L.; Ghosh, S.; Pakhui, G.; Prabhakara Reddy, B. Redox Behavior of Moisture in LiCl–KCl Eutectic Melts: A Cyclic Voltammetry Study. Nucl. Technol. 2021, 207, 119–146. [Google Scholar] [CrossRef]
- Tian, W. Grand Challenges in Advanced Nuclear Reactor Design. Front. Nucl. Eng. 2022, 1, 1000754. [Google Scholar] [CrossRef]
- Redkin, A.; Il’ina, E.; Pershina, S.; Mushnikov, P.; Stankus, S.; Agazhanov, A.; Zaikov, Y.; Kholkina, A.; Artamonov, A. Thermal Properties of Li2BeF4 near Melting Point. Thermo 2022, 2, 107–115. [Google Scholar] [CrossRef]
- Salyulev, A.B.; Moskalenko, N.I.; Shishkin, V.Y.; Zaikov, Y.P. Selective Evaporation of the Components of Molten (LiCl–KCl)eut–BaCl2–SrCl2–NdCl3 Mixtures at Low Pressures. Rus. Met. 2021, 2021, 151–158. [Google Scholar] [CrossRef]
- Laitinen, H.A.; Ferguson, W.S.; Osteryoung, R.A. Preparation of Pure Fused Lithium Chloride-Potassium Eutectic Solvent. J. Electrochem. Soc. 1957, 104, 516. [Google Scholar] [CrossRef]
- Suzdaltsev, A.V.; Filatov, A.A.; Nikolaev, A.Y.; Pankratov, A.A.; Molchanova, N.G.; Zaikov, Y.P. Extraction of Scandium and Zirconium from Their Oxides during the Electrolysis of Oxide−Fluoride Melts. Rus. Met. 2018, 2018, 133–138. [Google Scholar] [CrossRef]
- Chernyshev, A.A.; Arkhipov, S.P.; Apisarov, A.P.; Shmygalev, A.S.; Isakov, A.V.; Zaikov, Y.P. Rhenium Electrodeposition and Its Electrochemical Behavior in Molten KF–KBF4–B2O3–KReO4. Materials 2022, 15, 8679. [Google Scholar] [CrossRef]
- Shishkin, A.V.; Shishkin, V.Y.; Pankratov, A.A.; Burdina, A.A.; Zaikov, Y.P. Electrochemical Reduction of La2O3, Nd2O3, and CeO2 in LiCl–Li2O Melt. Materials 2022, 15, 3963. [Google Scholar] [CrossRef]
- Nikolaev, A.Y.; Mullabaev, A.R.; Suzdaltsev, A.V.; Kovrov, V.A.; Kholkina, A.S.; Shishkin, V.Y.; Zaikov, Y.P. Purification of Alkali-Metal Chlorides by Zone Recrystallization for the Use in Pyrochemical Processing of Spent Nuclear Fuel. At. Energy 2022, 131, 195–201. [Google Scholar] [CrossRef]
- Suzdaltsev, A. Silicon Electrodeposition for Microelectronics and Distributed Energy: A Mini-Review. Electrochem 2022, 3, 760–768. [Google Scholar] [CrossRef]
- Kosov, A.V.; Semerikova, O.L.; Vakarin, S.V.; Grishenkova, O.V.; Vorob’ev, A.S.; Khudorozhkova, A.O.; Zaikov, Y.P. Ionic Equilibria in Polytungstate Melts. Processes 2022, 10, 2658. [Google Scholar] [CrossRef]
- Rudenko, A.; Redkin, A.; Il’ina, E.; Pershina, S.; Mushnikov, P.; Zaikov, Y.; Kumkov, S.; Liu, Y.; Shi, W. Thermal Conductivity of FLiNaK in a Molten State. Materials 2022, 15, 5603. [Google Scholar] [CrossRef]
- Gevel, T.; Zhuk, S.; Leonova, N.; Leonova, A.; Trofimov, A.; Suzdaltsev, A.; Zaikov, Y. Electrochemical Synthesis of Nano-Sized Silicon from KCl–K2SiF6 Melts for Powerful Lithium-Ion Batteries. Appl. Sci. 2021, 11, 10927. [Google Scholar] [CrossRef]
- Zheng, J.; Sahoo, S.K.; Aono, T. Recent Progress on Mass Spectrometric Analysis of Artificial Radionuclides in Environmental Samples Collected in Japan. Nucl. Anal. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Suárez-Oubiña, C.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; Moreda-Piñeiro, A. Exploiting Dynamic Reaction Cell Technology for Removal of Spectral Interferences in the Assessment of Ag, Cu, Ti, and Zn by Inductively Coupled Plasma Mass Spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2022, 187, 106330. [Google Scholar] [CrossRef]
- Panchuk, V.; Petrov, Y.; Semenov, V.; Kirsanov, D. Quantification of Elements in Spent Nuclear Fuel Using Intrinsic Radioactivity for Sample Excitation and Chemometric Data Processing. Anal. Chim. Acta 2023, 1239, 340694. [Google Scholar] [CrossRef]
- Santos, J.S.; Teixeira, L.S.G.; Santos, W.N.L.; Lemos, V.A.; Sérgio, J.M.G.; Ferreira, L.C. Uranium Determination Using Atomic Spectrometric Techniques: An Overview. Anal. Chim. Acta 2010, 674, 143–156. [Google Scholar] [CrossRef]
- Mullabaev, A.R.; Kovrov, V.A.; Kholkina, A.S.; Zaikov, Y.P. Anode Processes on Pt and Ceramic Anodes in Chloride and Oxide-Chloride Melts. Nucl. Eng. Techn. 2022, 54, 965–974. [Google Scholar] [CrossRef]
- Guo, X.; Sun, Z.; Sietsma, J.; Blanpain, B.; Guo, M.; Yang, Y. Quantitative Study on Dissolution Behavior of Nd2O3 in Fluoride Melts. Ind. Eng. Chem. Res. 2018, 57, 1380–1388. [Google Scholar] [CrossRef]
- Nikolaev, A.Y.; Pavlenko, O.B.; Suzdaltsev, A.V.; Zaikov, Y.P. Electrochemical Sensor for Monitoring the Alumina Dissolution and Concentration in a Cryolite-Alumina Melt. J. Electrochem. Soc. 2020, 167, 126511. [Google Scholar] [CrossRef]
- Senanu, S.; Ratvik, A.P.; Gudbrandsen, H.; Martinez, A.M.; Store, A.; Gebarowski, W. Dissolution and Online Monitoring of Nd and Pr Oxides in NdF3–PrF3–LiF Electrolytes. Metals 2021, 11, 326. [Google Scholar] [CrossRef]
- Peng, H.; Huang, W.; Xie, L.; Li, Q. Solubility and Precipitation Investigations of UO2 in LiF–BeF2 Molten Salt. J. Nucl. Mat. 2020, 531, 152004. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Choi, I.-K.; Hur, J.-M.; Kang, D.-S.; Shin, H.-S.; Jeong, S.M. In Situ Electrochemical Measurement of O2− Concentration in Molten Li2O/LiCl During Uranium Oxide Reduction Process. Electrochem. Solid-State Lett. 2012, 15, E11–E13. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, S.; Zhang, C.; Faulkner, E.; Alnajjar, N.; Okabe, P.; Horvath, D.C.; Simpson, M.F. Square Wave Voltammetry for Real Time Analysis of Minor Metal Ion Concentrations in Molten Salt Reactor Fuel. J. Nucl. Mat. 2019, 527, 151791. [Google Scholar] [CrossRef]
- Pershin, P.S.; Suzdaltsev, A.V.; Zaikov, Y.P. Dissolution of Al2O3 in KF–AlF3. Rus. Met. 2021, 2021, 213–218. [Google Scholar] [CrossRef]
- Cvetkovic, V.S.; Feldhaus, D.; Vukicevic, N.M.; Milicevic-Neumann, K.; Barudžija, T.S.; Friedrich, B.; Jovicevic, J.N. Influence of Rare Earth Oxide Concentration on Electrochemical Co-Deposition of Nd and Pr from NdF3–PrF3–LiF Based Melts. Metals 2022, 12, 1204. [Google Scholar] [CrossRef]
- Valtseva, A.I.; Pershin, P.S.; Kalyakin, A.S.; Volkov, A.N.; Suzdaltsev, A.V.; Zaikov, Y.P. Development of Oxygen Sensor for Pyrochemical Reactors of Spent Nuclear Fuel Reprocessing. J. Phys. Conf. Ser. 2020, 1565, 012050. [Google Scholar] [CrossRef]
- Suzdaltsev, A.V.; Nikolaev, A.Y.; Pavlenko, O.B.; Zaikov, Y.P. Monitoring Alumina Content in Cryolite-Alumina melt. IOP Conf. Ser. Mat. Sci. Eng. 2020, 918, 012108. [Google Scholar] [CrossRef]
- Shen, M.; Peng, H.; Ge, M.; Zuo, Y.; Xie, L. Use of Square Wave Voltammeter for Online Monitoring of O2- Concentration in Molten Fluorides at 600 °C. J. Electroanal. Chem. 2015, 748, 34–39. [Google Scholar] [CrossRef]
- Nibedita, S.; Satendra, K.; Maji, S.; Chandra, M.; Venkatesh, P.; Jain, A. Electrochemical and Spectroscopic Analysis of Thermochemical Conversion of UO2 to UCl3 Using AlCl3 and Al in LiCl–KCl Eutectic. Progr. Nucl. Energy 2022, 153, 104429. [Google Scholar] [CrossRef]
- Song, Y.; Shen, M.; Zhao, S.; Tang, R.; Xie, L.; Qian, Y. Interactions Between Oxide and LiF–BeF2–ZrF4–UF4 System through Electrochemical Techniques. J. Electrochem. Soc. 2021, 168, 036513. [Google Scholar] [CrossRef]
- Pershin, P.; Khalimullina, Y.; Arkhipov, P.; Zaikov, Y. The Electrodeposition of Lead in LiCl–KCl–PbCl2 and LiCl–KCl–PbCl2–PbO Melts. J. Electrochem. Soc. 2014, 161, D824–D830. [Google Scholar] [CrossRef]
- Haarberg, G.M.; Owe, L.-E.; Qin, B.; Wang, J.; Tunold, R. Electrodeposition of Lead from Chloride Melts. ECS Trans. 2012, 50, 215–219. [Google Scholar] [CrossRef]
- Arkhipov, P.A.; Zaikov, Y.P.; Khalimullina, Y.R.; Arkhipov, S.P. Electrochemical Production of Bismuth in the KCl–PbCl2 Melt. Materials 2021, 14, 5653. [Google Scholar] [CrossRef]
- Zhu, Z.-L.; Liu, H.; Chen, J.-S.; Kong, H.; Xu, L.; Hua, Z.-S.; Zhao, Z. Electrochemical Behavior and Electrolytic Preparation of Lead in Eutectic NaCl−KCl Melts. Trans. Nonferrous Met. Soc. China 2020, 30, 2568–2576. [Google Scholar] [CrossRef]
- Pershin, P.S.; Kataev, A.A.; Shurov, N.I.; Arkhipov, P.A.; Zaikov, Y.P. Dissolution Rate of Lead(II) Oxide in an Equimolar KCl-PbCl2 Melt. Rus. J. Non-Ferr. Met. 2013, 54, 195–200. [Google Scholar] [CrossRef]
- Zhitkov, A.; Potapov, A.; Karimov, K.; Shishkin, V.; Dedyukhin, A.; Zaykov, Y. Interaction Between UN and CdCl2 in Molten LiCl–KCl Eutectic. I. Experiment at 773 K. Nucl. Eng. Techn. 2020, 52, 123–134. [Google Scholar] [CrossRef]
- Nikolaev, A.Y.; Suzdaltsev, A.V.; Zaikov, Y.P. High Temperature Corrosion of ZrN Powder in Molten LiCl with Additions of PbCl2, KCl, Li2O, H2O, and LiOH. J. Electrochem. Soc. 2019, 166, C147–C152. [Google Scholar] [CrossRef]
- Shchepin, A.S.; Koshcheev, A.M.; Kalenova, M.Y.; Melnikova, I.M. SNF Processing Electrochemical Operations: Liquid-Metal and Salt Medium Purification. Izv. Vuzov. Yad. Energ. 2021, 4, 53–65. (In Russian) [Google Scholar] [CrossRef]
- Kizub, P.A.; Blokhin, A.I.; Blokhin, P.A.; Mitenkova, E.F.; Mosunova, N.A.; Kovrov, V.A.; Shishkin, A.V.; Zaikov, Y.P.; Rakhmanova, O.R. Criticality Analysis of Pyrochemical Reprocessing Apparatuses for Mixed Uranium-Plutonium Nitride Spent Nuclear Fuel Using the MCU-FR and MCNP Program Codes. Nucl. Eng. Techn. 2023. [Google Scholar] [CrossRef]
- Swain, N.; Soni, I.; Kumar, P.; Kudur Jayaprakash, G. Electrochemical Reduction and Voltammetric Sensing of Lindane at the Carbon (Glassy and Pencil) Electrodes. Electrochemistry 2022, 3, 248–258. [Google Scholar] [CrossRef]
- Nikolaev, A.Y.; Pavlenko, O.B.; Suzdaltsev, A.V.; Zaikov, Y.P.; Vykhoteds, V.B.; Kurennykh, T.E. Reduction of ZrO2 During the SNF Pyroprocessing. J. Electrochem. Soc. 2021, 168, 036506. [Google Scholar] [CrossRef]
- Volkov, V.N.; Vykhodets, V.B.; Golubkov, I.K.; Klotsman, S.M.; Lerkh, P.V.; Pavlov, V.A. Accurate Light Ion Beam Monitoring by Backscattering. Nucl. Instrum. Methods Phys. Res. 1983, 205, 73. [Google Scholar] [CrossRef]
- Vykhodets, V.B.; Kurennykh, T.E.; Vykhodets, E.V. Disks of Oxygen Vacancies on the Surface of TiO2 Nanoparticles. Appl. Sci. 2022, 12, 11963. [Google Scholar] [CrossRef]
- Suzdaltsev, A.V.; Nikolaev, A.Y.; Zaikov, Y.P. Towards the Stability of Low-Temperature Aluminum Electrolysis. J. Electrochem. Soc. 2021, 168, 046521. [Google Scholar] [CrossRef]
- Mohamedi, M.; Børresen, B.; Haarberg, G.M.; Tunold, R. Anodic Behavior of Carbon Electrodes in CaO–CaCl2 Melts at 1123 K. J. Electrochem. Soc. 1999, 146, 1472–1477. [Google Scholar] [CrossRef]
- Roine, A. HSC Chemistry® 9.0; Outotec: Pori, Finland, 2018; Available online: www.outotec.com/HSC (accessed on 1 January 2021).
- Zakiryanova, I.D.; Arkhipov, P.A.; Zakiryanov, D.O. Reaction Mechanism of Lead (II) Oxide with a PbCl2–CsCl Melt According to Raman Spectroscopic Data. J. Appl. Spectrosc. 2016, 82, 920–924. [Google Scholar] [CrossRef]

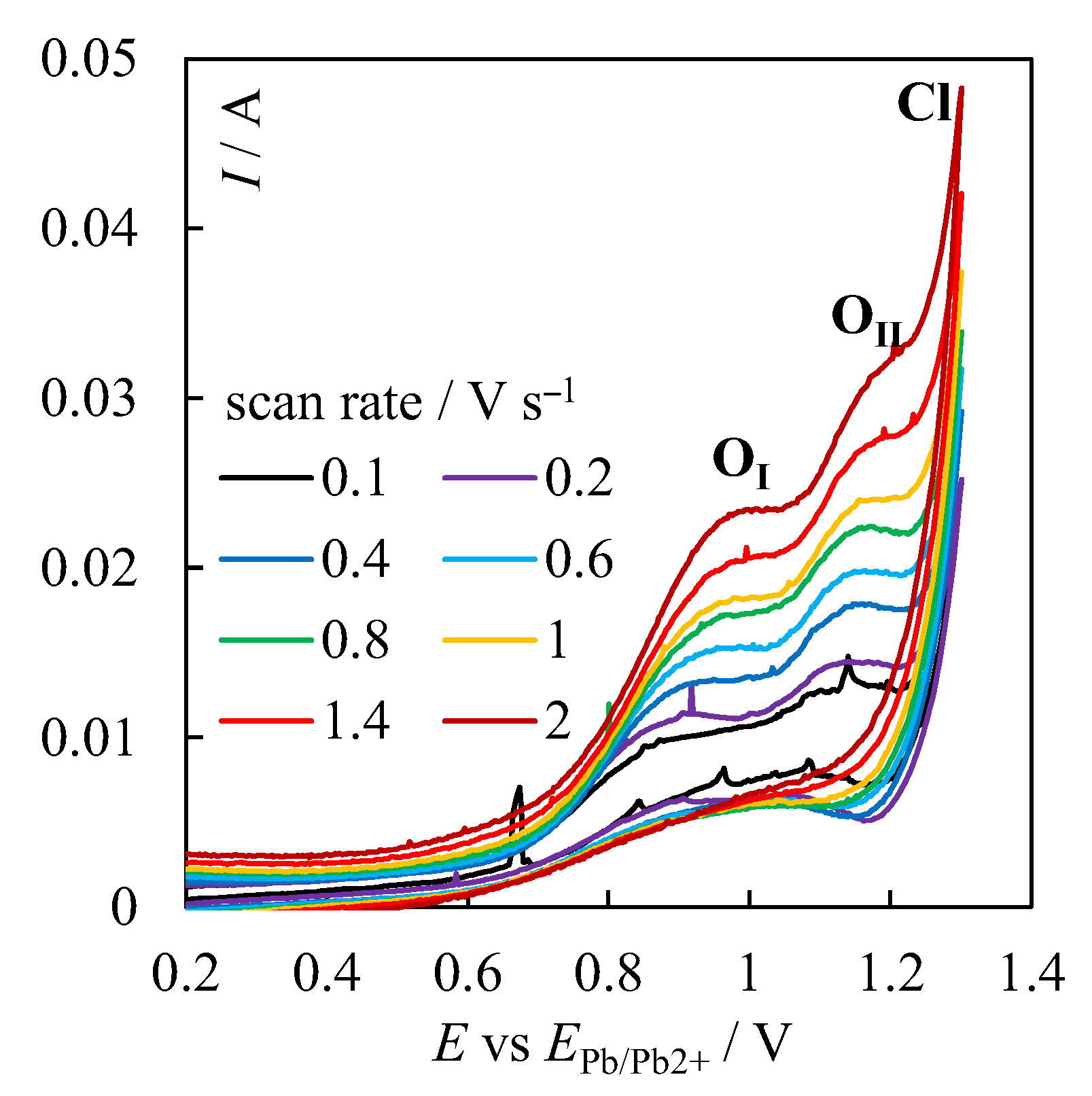
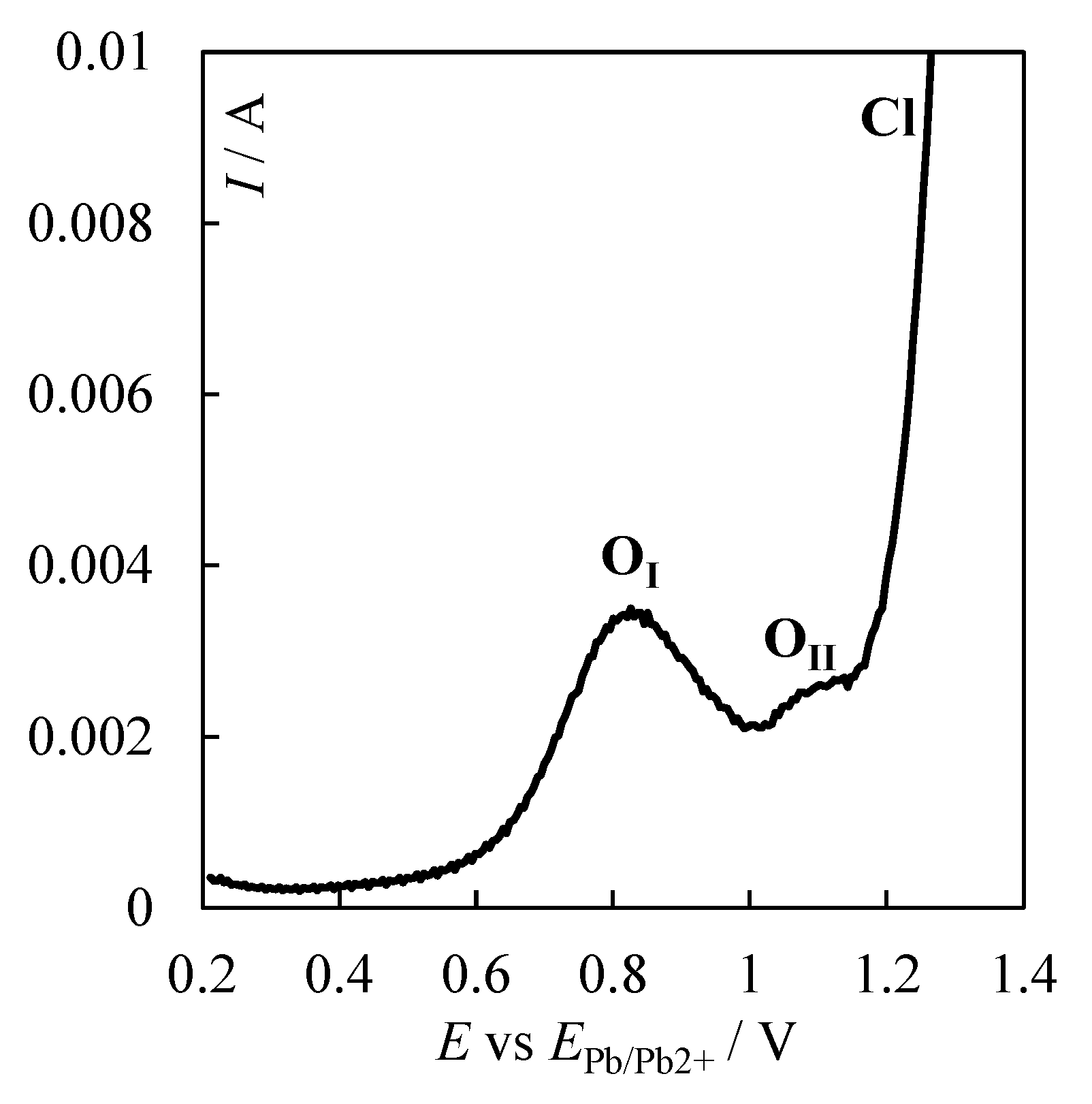
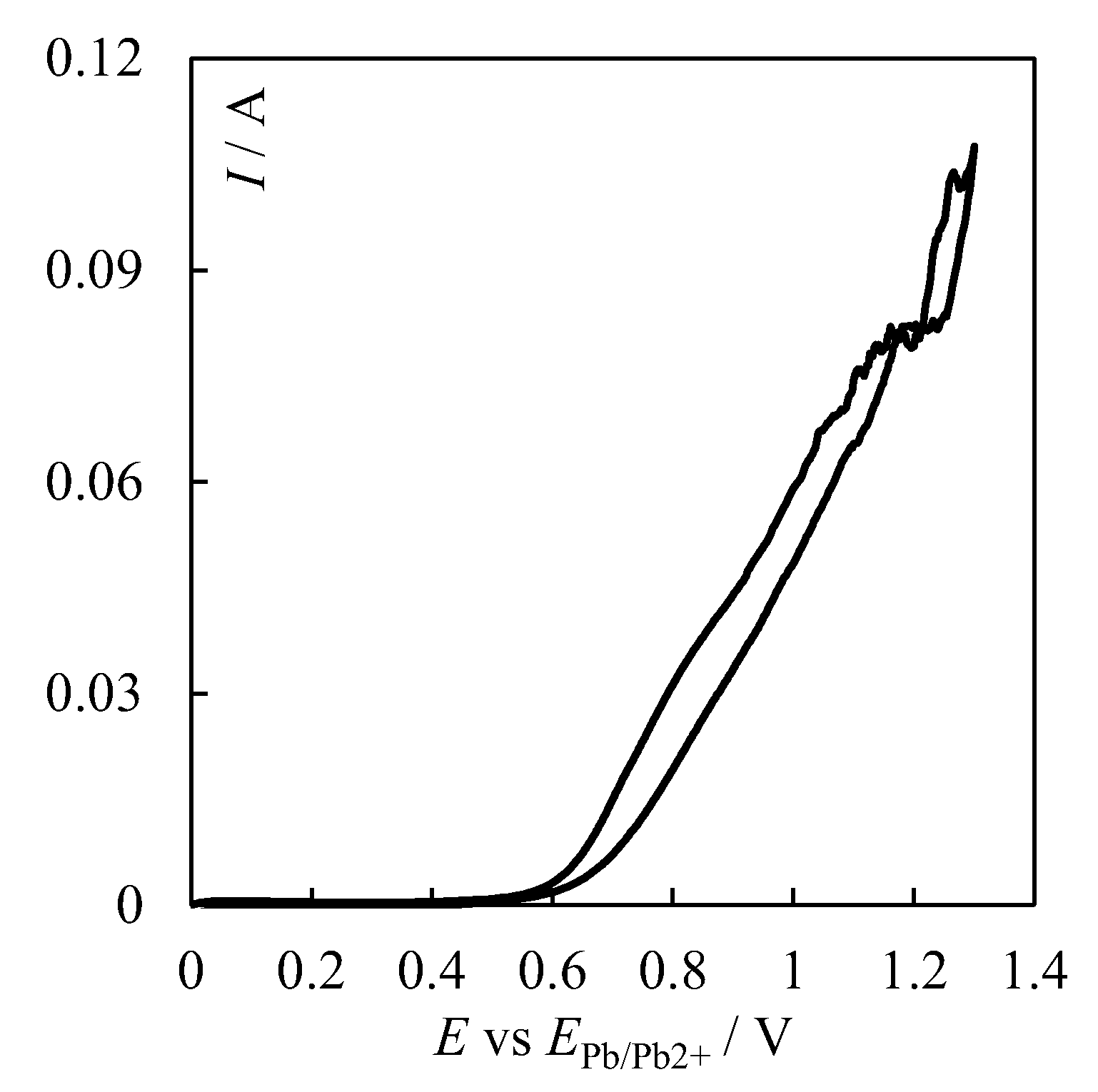
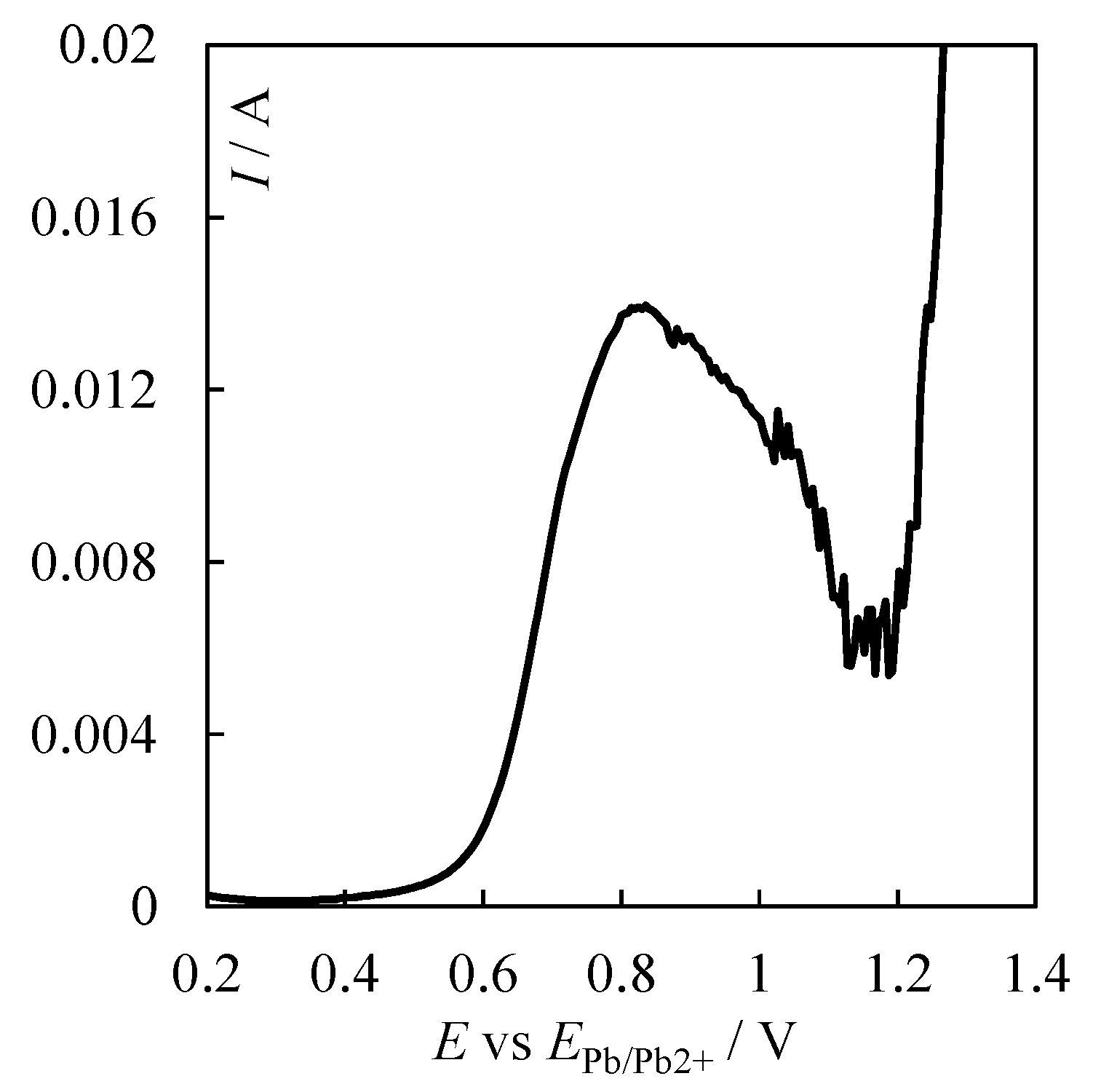
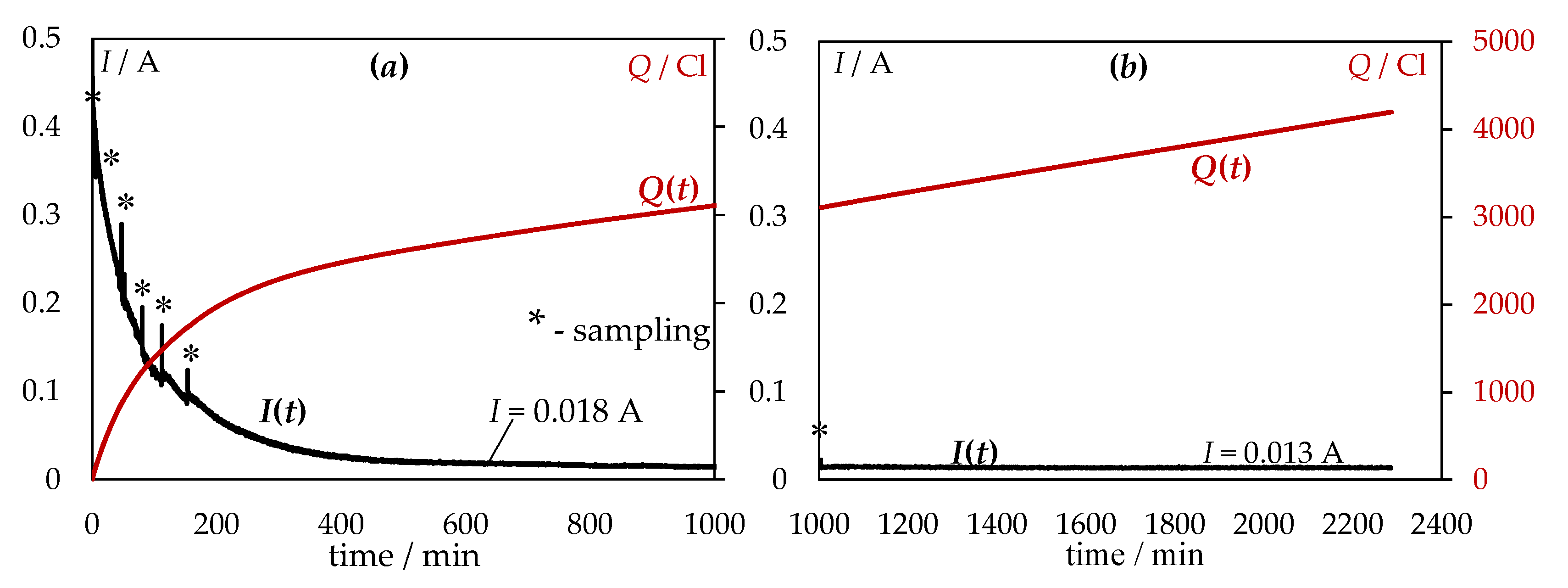
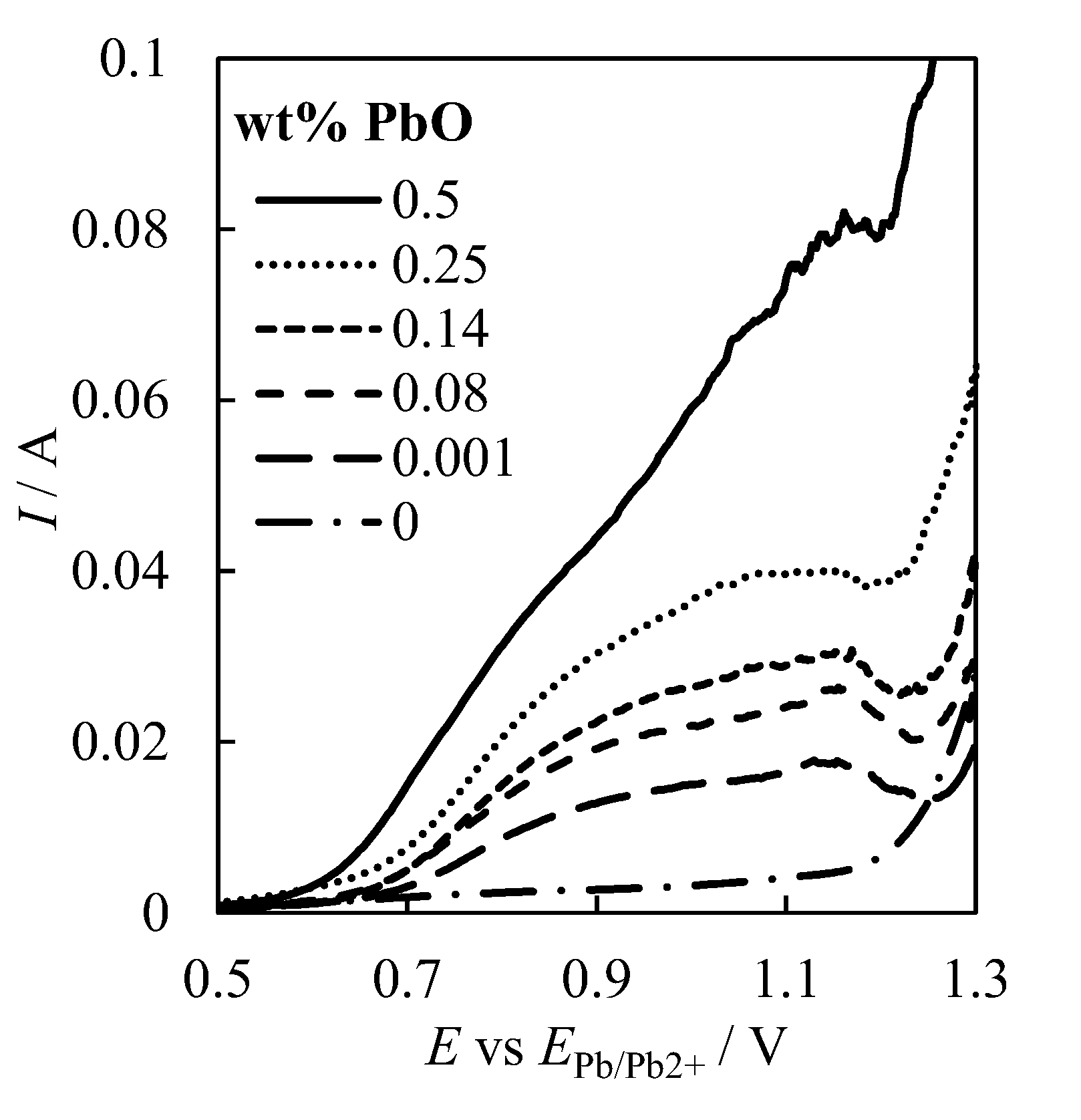

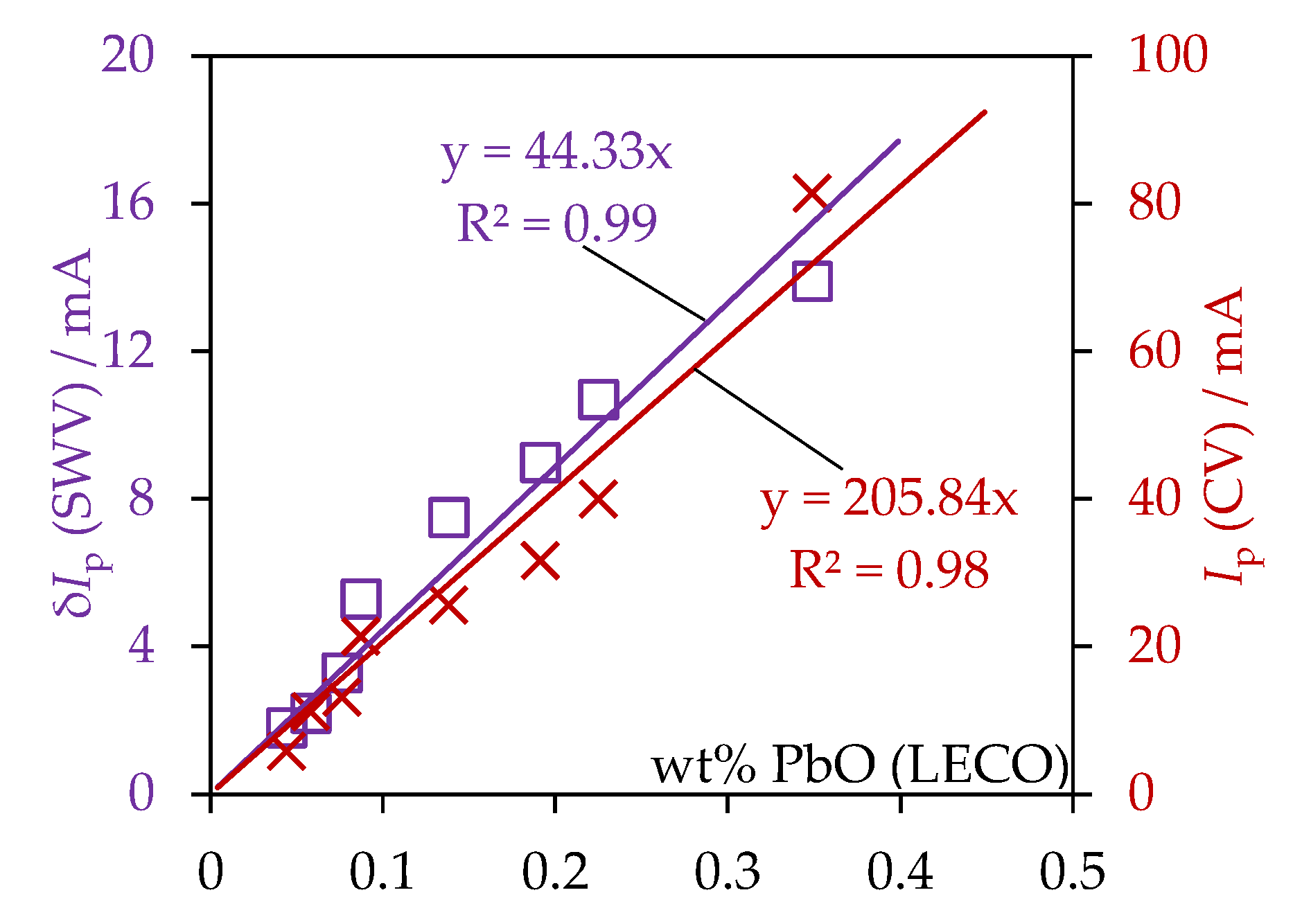
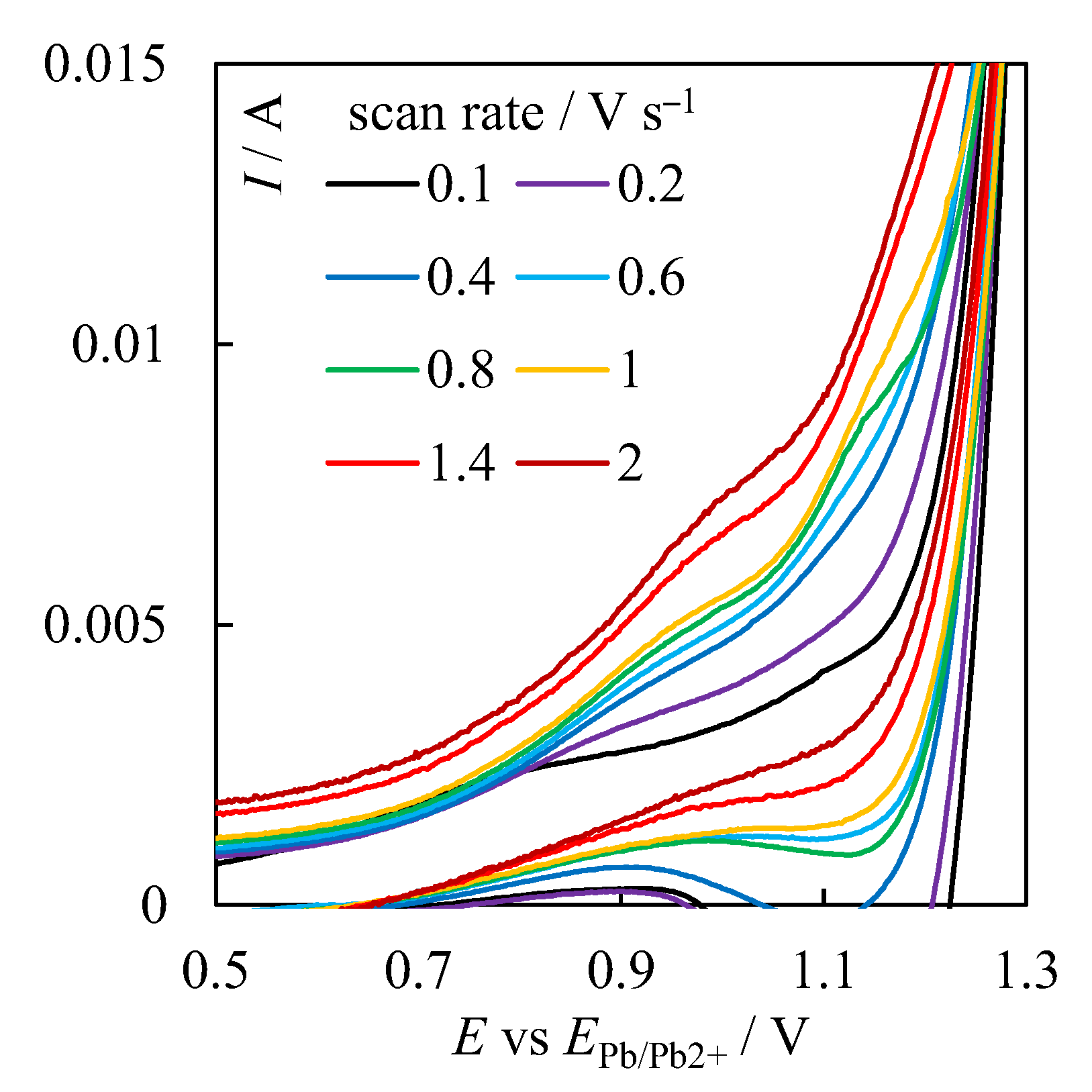
| № | Set Amount of PbO, wt% (Expected) | Electrochemical Analysis | Concentration of PbO, wt%, according to the Analytical Data | Concentration of O, wt% | ||
|---|---|---|---|---|---|---|
| * Ip/mA | ** δIp/mA | O Analysis | NMA | O Analysis | ||
| - | 0 (PbCl2 salt) | - | - | 0.067 ± 0.012 | - | 0.011 ± 0.001 |
| - | 0 (PbCl2 melted in air) | - | - | 0.096 ± 0.011 | - | 0.015 ± 0.001 |
| 1 | 0 (PbCl2 melted in Ar) | 13.1 ± 0.4 | 3.3 ± 0.2 | 0.076 ± 0.014 | 0.14 ± 0.02 | 0.012 ± 0.002 |
| 2 | 0.5 | 81.4 ± 0.4 | 13.9 ± 0.1 | 0.349 ± 0.022 | 0.18 ± 0.03 | 0.056 ± 0.003 |
| 3 | 0.25 | 40.0 ± 0.5 | 10.7 ± 0.1 | 0.225 ± 0.019 | 0.11 ± 0.02 | 0.036 ± 0.003 |
| 4 | 0.14 | 31.7 ± 0.3 | 9.0 ± 0.1 | 0.191 ± 0.014 | 0.10 ± 0.02 | 0.030 ± 0.002 |
| 5 | 0.08 | 25.6 ± 0.4 | 7.5 ± 0.2 | 0.138 ± 0.015 | 0.11 ± 0.01 | 0.022 ± 0.002 |
| 6 | 0.001 | 21.4 ± 0.2 | 5.7 ± 0.1 | 0.087 ± 0.013 | 0.08 ± 0.01 | 0.014 ± 0.002 |
| 7 | 0 | 11.2 ± 0.2 | 2.2 ± 0.1 | 0.058 ± 0.012 | 0.07 ± 0.02 | 0.009 ± 0.001 |
| 8 | 0 | 5.8 ± 0.3 | 1.8 ± 0.1 | 0.044 ± 0.012 | 0.06 ± 0.02 | 0.007 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaev, A.; Mullabaev, A.; Suzdaltsev, A.; Zaikov, Y.P. Efficiency of Electrochemical Methods of Purification and Control over the Oxide Concentration in Halide Melts: PbCl2. Processes 2023, 11, 636. https://doi.org/10.3390/pr11020636
Nikolaev A, Mullabaev A, Suzdaltsev A, Zaikov YP. Efficiency of Electrochemical Methods of Purification and Control over the Oxide Concentration in Halide Melts: PbCl2. Processes. 2023; 11(2):636. https://doi.org/10.3390/pr11020636
Chicago/Turabian StyleNikolaev, Andrey, Albert Mullabaev, Andrey Suzdaltsev, and Yuriy P. Zaikov. 2023. "Efficiency of Electrochemical Methods of Purification and Control over the Oxide Concentration in Halide Melts: PbCl2" Processes 11, no. 2: 636. https://doi.org/10.3390/pr11020636
APA StyleNikolaev, A., Mullabaev, A., Suzdaltsev, A., & Zaikov, Y. P. (2023). Efficiency of Electrochemical Methods of Purification and Control over the Oxide Concentration in Halide Melts: PbCl2. Processes, 11(2), 636. https://doi.org/10.3390/pr11020636







