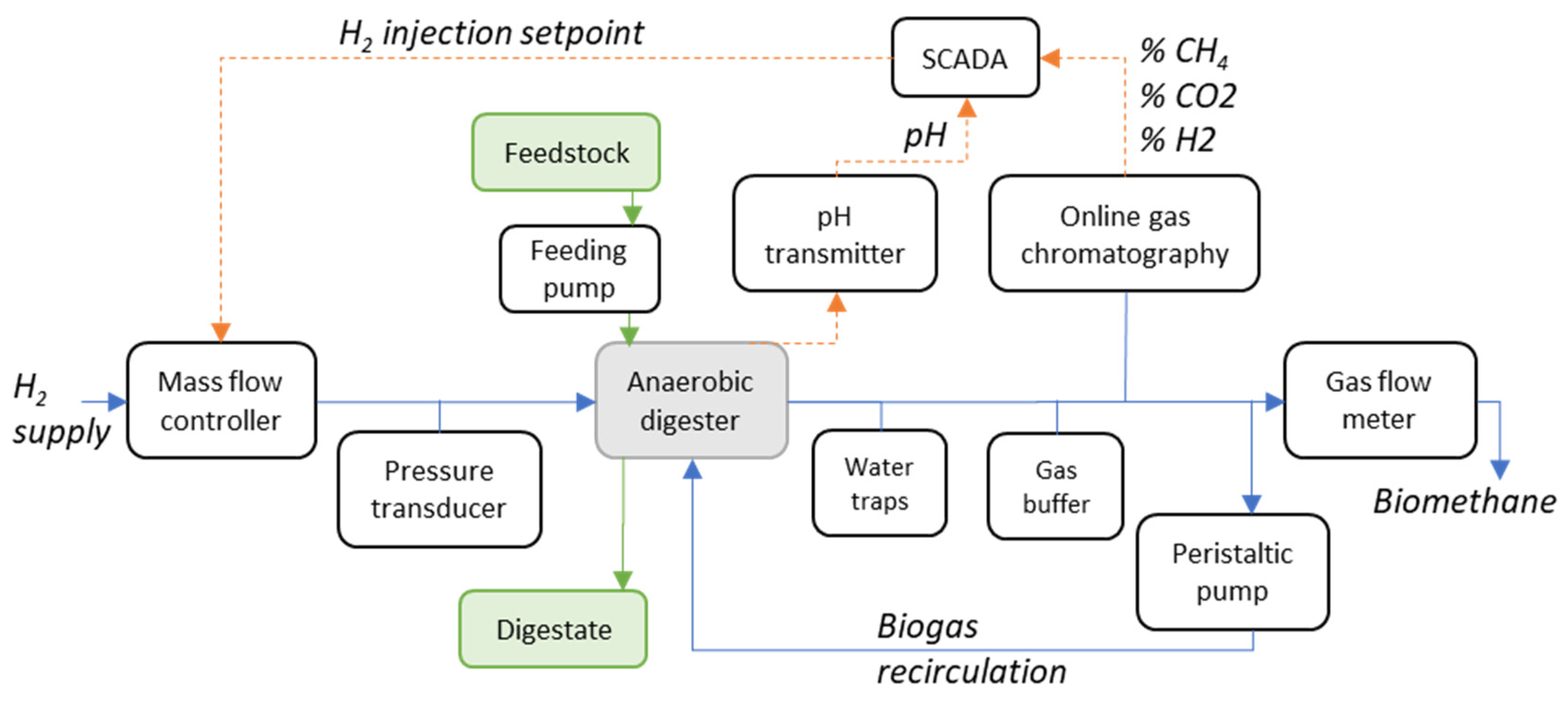
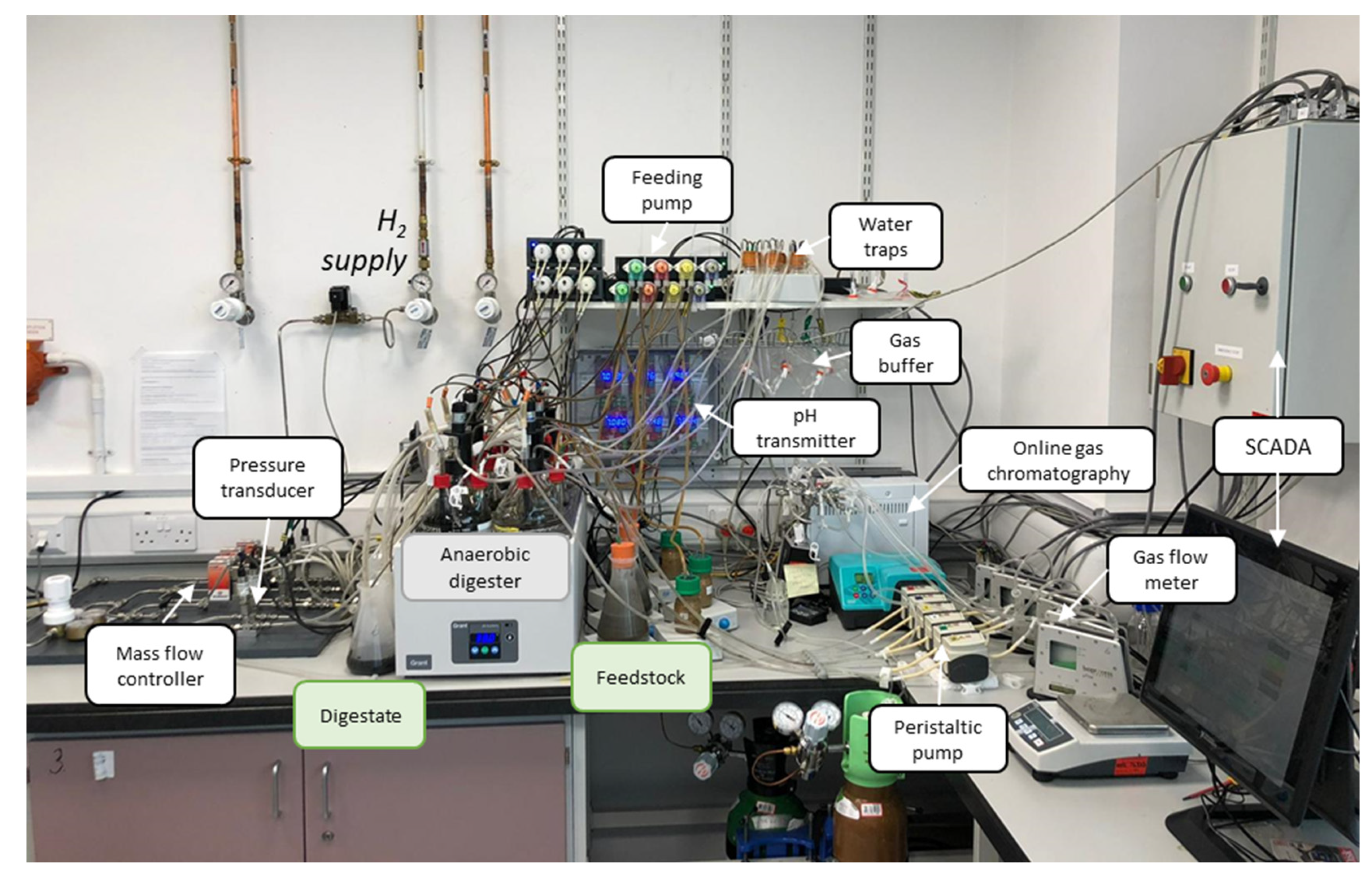
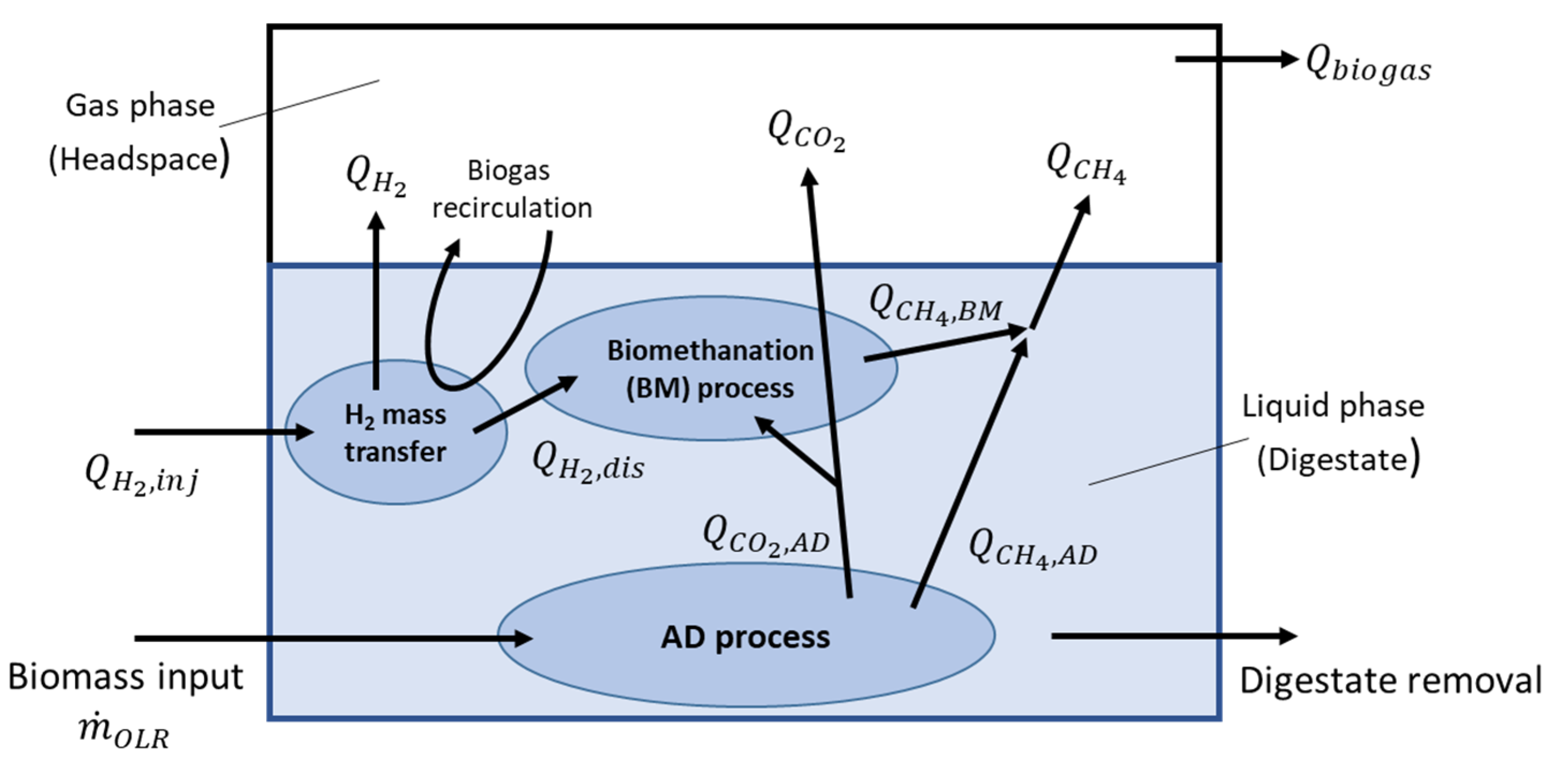
3.1. BMP and Baseline AD Process
The results of the characterisation of the inoculum and feedstock samples are presented in the
Appendix A.1 (
Table A1 and
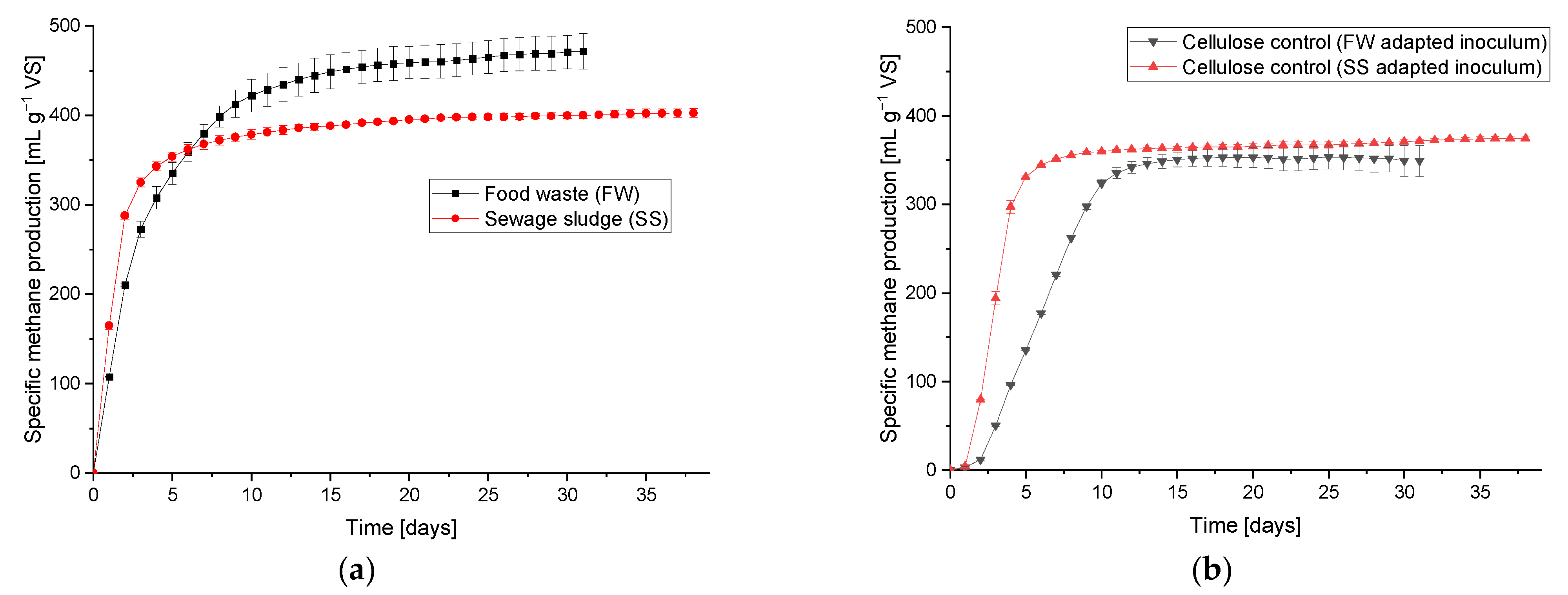
Table A2). The BMP results are shown in
Figure 4. The average methane production of the SS and FW during the BMP test was 0.402 ± 0.005 and 0.471 ± 0.020 L g
−1 VS, respectively. These are within the ranges given in the literature of 0.220–0.460 [
31,
32,
33] and 0.460–0.530 [
34,
35,
36] for SS and FW, respectively. Using the values of the elemental analysis for both feedstocks (
Table A2) and the calculated theoretical methane potential [
37], the BMP of SS and FW resulted in 76.04% and 87.95% of their respective theoretical potentials. The methane potential of the cellulose as a positive control using the two inoculums gave values of 0.370 and 0.374 L g
−1 VS, equivalent to 89.5% and 90.4% of the theoretical values.
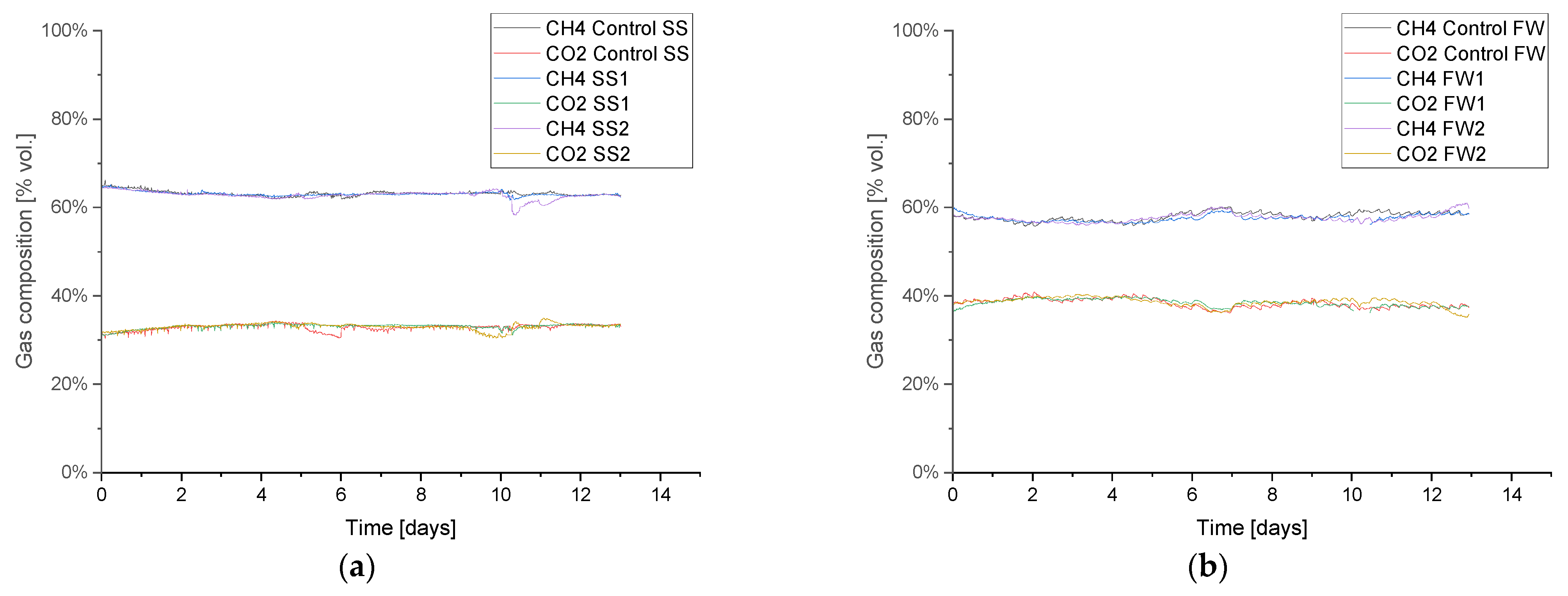
Figure 5 shows the biogas composition from the initial baseline period prior to the biomethanation periods, which was similar in all replicates and validates the repeatability of the experimental approach; further baseline results are given in
Appendix A.1 Table A3. The average methane and carbon dioxide specific yields were 0.24 and 0.12 L g
−1 VS for SS and 0.42 and 0.28 L g
−1 VS for FW, respectively. The baseline AD achieved 58 and 86% of the BMP specific methane yields, which is expected when comparing batch to continuous AD processes [
38]. The lower result for SS can be attributed to its relatively short hydraulic retention time (HRT) (14 days, at OLR 2 g
−1 VS L
−1 day
−1) compared with the FW reactors (~68 days), since, in general, a longer HRT can be associated with a greater degree of feedstock degradation, as shown for FW in [
36]. During the baseline AD testing, the alkalinity ratio (IA/PA) for all reactors was stable, with it being approximately 0.40 (SS) and 0.38 (FW).
3.2. In-Situ Biomethanation Testing
For each experimental period (R1–R3, O1–O3), prior to hydrogen injection, it was ensured that all replicates had a similar baseline in terms of gas composition, gas content, pH, and alkalinity ratio. The hydrogen was then injected into the four biomethanation reactors (FW11, FW2, SS1, and SS2) in accordance with the specific experimental design of each period. The estimated hydrogen injection stoichiometric requirement () was updated before each experimental period based on the carbon dioxide production of the control prior to the start of hydrogen injection. The following sections will present the results for each of these periods, focusing on the results during hydrogen injection.
The volume of data collected, the amount of possible detailed discussion in terms of describing results, and the operational challenges would make a comprehensive discussion of all the experimental data too lengthy. Instead, a single experimental period (R1) and feedstock (SS) will be described in detail to exemplify the data gathered as well as the challenges and complexities faced. Subsequently, average data taken over the whole experimental periods will be used to assess the overall trends and subsequent implications. The average data also include the transient period at the initial hydrogen injection, which generally lasts up to two days; therefore, the average data can be considered a good approximation of the steady state performance of the various experimental conditions. The full dataset, containing the detailed experimental outputs for the six experimental periods for the all the replicates and controls, is available on a separate data repository [
39], and for a more detailed treatment of individual experimental conditions the reader is directed to [
29].
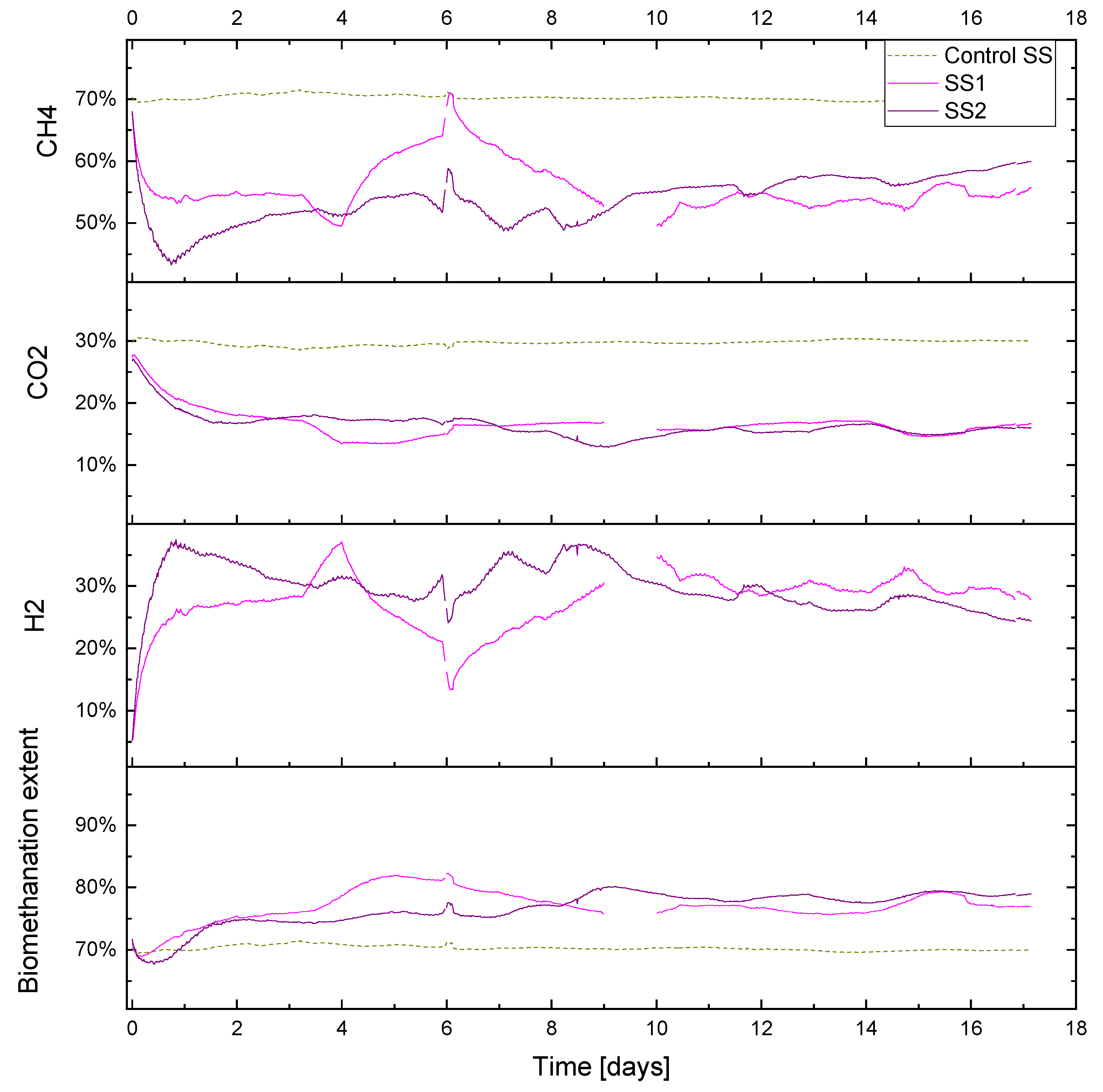
3.2.1. In Situ Biomethanation Dynamics (Example Period R1 with SS)
Period R1 (gas recirculation 12 L L
−1day
−1) was monitored for 17 days, and the average measured OLR during the experiment for control SS, SS1, and SS2 were 1.91, 1.88 and 1.92 g VS. L
−1 day
−1, respectively. Hydrogen injection remained stable at its setpoint value (1.02 mL min
−1, equivalent to approximately 0.86 L L
−1 day
−1), excluding short periods of activation regarding the hydrogen gain loop (i.e.,
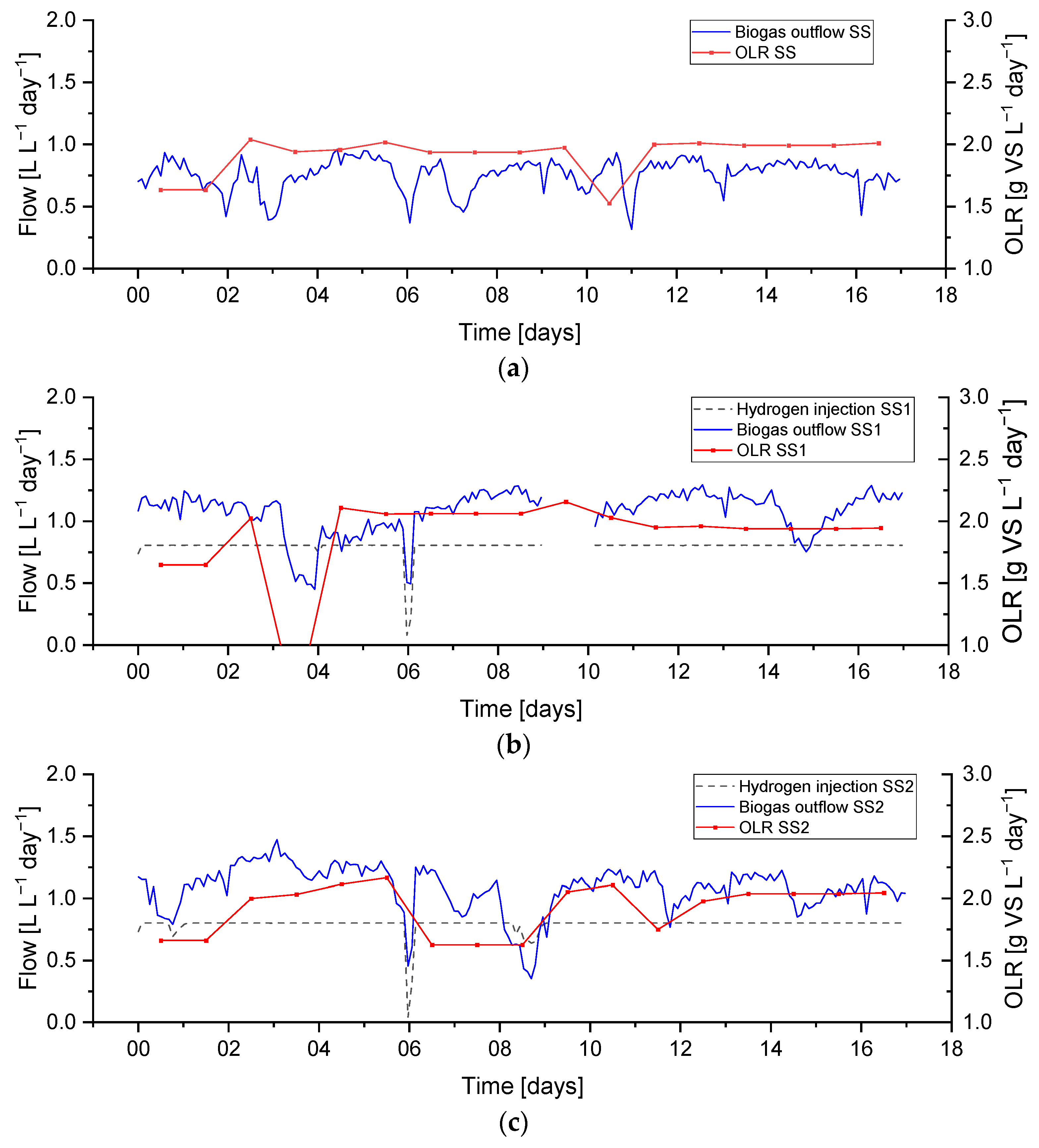
) of the feedback control on days 0 and 9 and a technical problem with the hydrogen injection system on day 6. The biogas output and OLR data for this period are shown in
Figure 6.
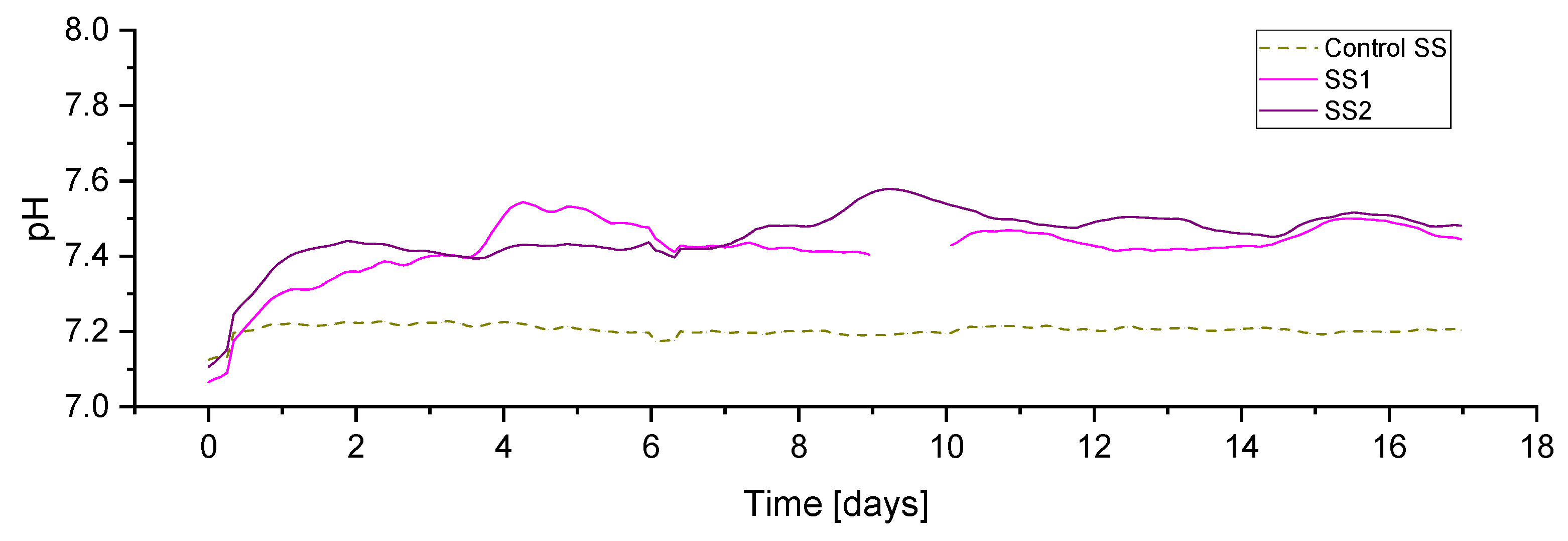
The initial pH, in all reactors, at the beginning of the experiment was around 7.1 (
Figure 7). Indicative of the initiation of hydrogen injection in all experimental periods, the pH in the reactor SS1 and SS2 increased immediately after the addition of hydrogen, which was expected and previously reported due to bicarbonate consumption by the biomethanation reaction and the resulting predominance of the ammonia buffer on the carbonate buffer system [
3]. Both duplicates showed similar pH profiles, with the average values for SS1 and SS2 being 7.41 and 7.44, respectively, compared with 7.20 in the case of the control reactor.
The duplicate reactors showed a similar biomethanation extent, which reached a value of approximately 80%, with the control value being approximately 70% (
Figure 8), while the H
2 conversions were between 50–70%, with an average of 64 and 59% for SS1 and SS2, respectively. The CO
2 content decreased to approximately 15% in both biomethanation reactors and a 30% level in the control reactor. The methane also decreased in the biomethanation reactors, reaching an average of 56 and 54% in SS1 and SS2, respectively, due to the dilution by hydrogen in the headspace, which reached a maximum content of 33 and 36%, respectively.
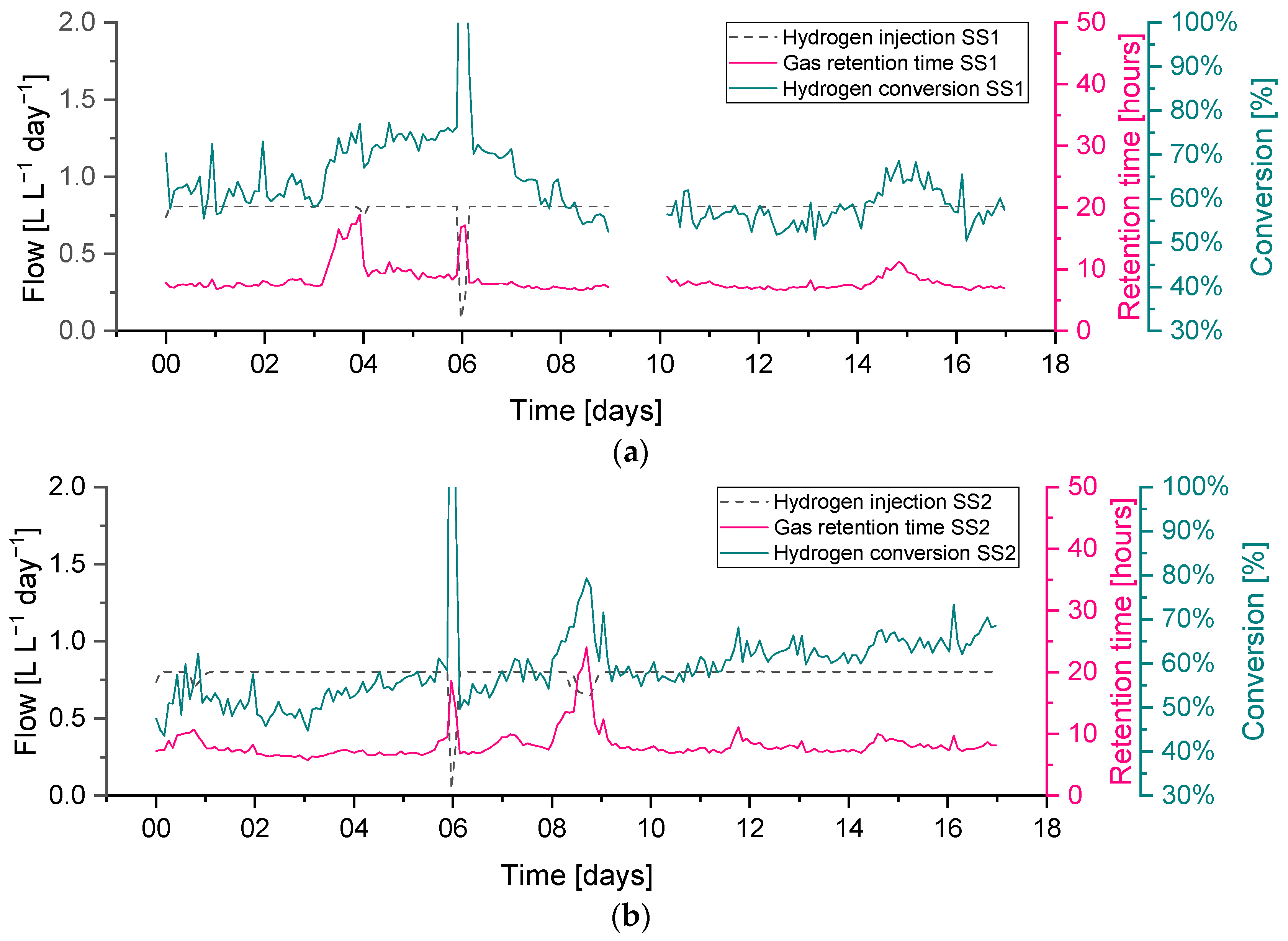
A feedstock feeding pump failure on day 3 on reactor SS1 (the effect on the OLR can be seen in
Figure 6) caused the feeding not to be delivered properly. This feeding failure caused an evident drop in biogas production, while the flow of hydrogen injection remained at its constant setpoint value. The lower biogas production and constant hydrogen injection caused an increase in the hydrogen concentration of up to 37%, while the methane and carbon dioxide concentrations dropped to 50 and 14%, respectively. In addition, the reduction in biogas production resulted in a higher retention time, leading to higher H
2 conversion, evident between days 3–4 in
Figure 9.
Abrupt changes in hydrogen injection also influence the process performance; this can be seen on day 6 in both reactors SS1 and SS2 when hydrogen had to be stopped for approximately five hours due to a technical issue. Biogas outflow immediately follows the change in hydrogen injection, and a similar reduction can be seen in
Figure 6. This leads to a reduction in the gas flow and an increase in the gas residence time RT, which leads to a higher hydrogen conversion. The resulting changes in gas composition can be seen in
Figure 8, where the hydrogen concentration diminishes and methane increase towards the value of the baseline; the carbon dioxide, on the other hand, has a slower response, and its content in the headspace remains more stable due to its increased solubility and residual buffering capacity in the liquid phase.
The hydrogen gain loop (i.e.,
) of the feedback controller was activated on days 0 and 9 on the reactor SS2, as shown as a small drop in the hydrogen injection flow (see
Figure 6). The gas composition of methane and carbon dioxide remained far from the constraints, while the hydrogen was near the upper constraint (40%).
The hydrogen conversion tended to increase from 50% at the beginning to 70% at the end of the experiment in both reactors (
Figure 9). This trend is also confirmed by the gas composition data (
Figure 8), where on the final six days of the experimental period, the methane content was observed to slightly increase in reactor SS2 along with a decrease in the hydrogen content. The H
2 conversion trend can be explained by microbial acclimation and the growth of the hydrogenotrophic population during the experiment. Microbial activity can, in fact, increase the gas–liquid mass transfer rate compared to a purely physical process in abiotic liquid by converting the absorbed gas into the stagnant liquid layer surrounding the gas bubble, thereby increasing the diffusional gradient. This phenomenon is reported in the literature as the microbial enhancement of the gas–liquid mass transfer [
30].
3.2.2. Variation in Biogas Recirculation Rate (Periods R1–R3)
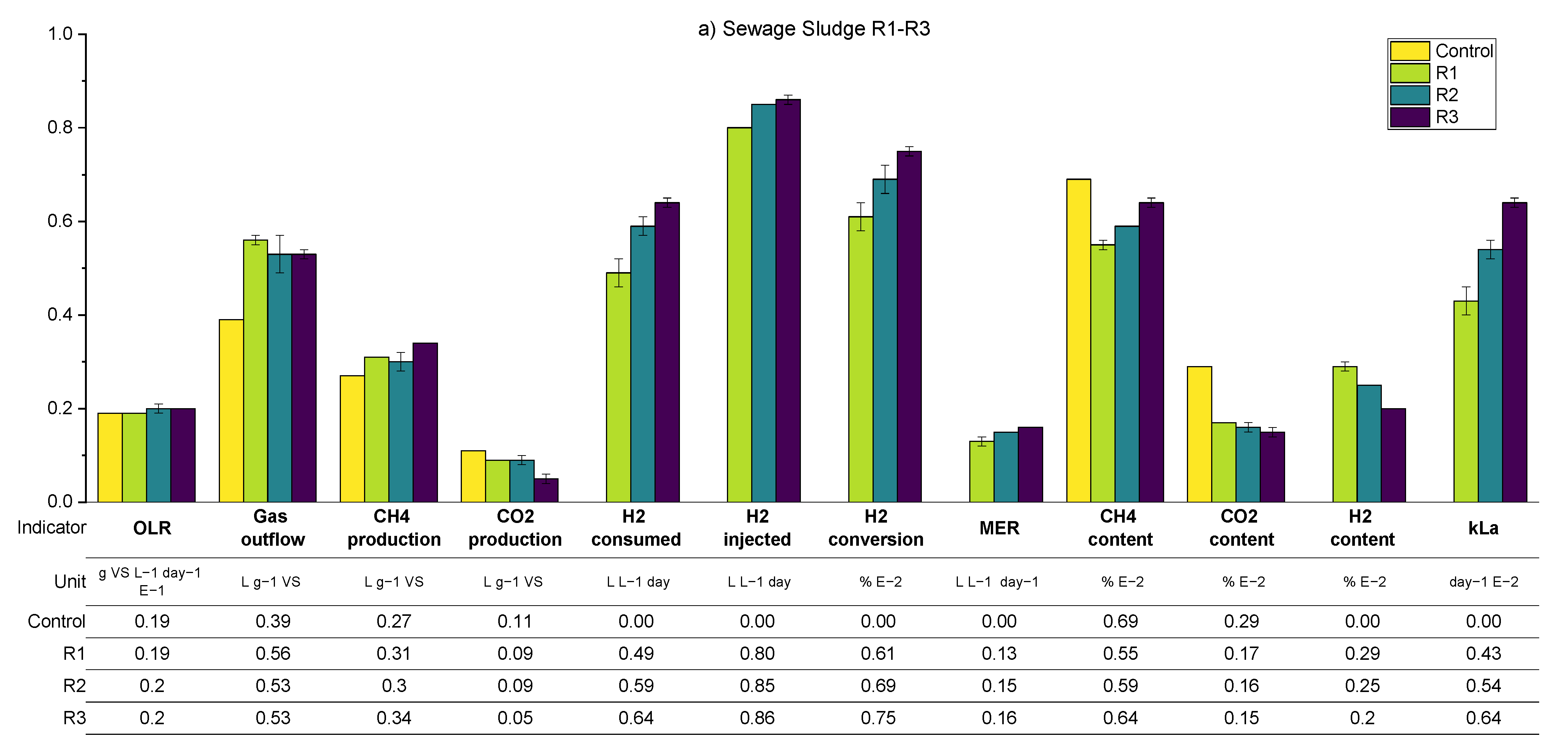
A summary of the average values of the main process parameters across periods R1–R3 (i.e., the variation in the biogas recirculation rate) for both SS and FW can be found in
Figure 10. These data are supplemented by more detailed (per reactor) results presented in the
Appendix A.2 (
Table A4 and
Table A6). During periods R1–R3, the general observations and trends are similar for the SS and FW fed reactors, despite the generally higher all gas flow values in the FW reactors due to the high BMP of FW compared with SS. Consequently, the discussion will focus on SS whilst highlighting deviations from these for the FW reactors.
The general trend is that the increasing recirculation rate improved the hydrogen consumption rate. On average, the hydrogen conversion increased from 0.84 to 1.09 L day
−1 between periods R1–R3 and increased the specific methane production rate by 26% compared with the control reactor. The hydrogen conversion rates constituted with the hydrogen injection rate in all periods were between 60 and 75%, where the highest value was achieved at R3–SS. This conversion rate was lower than that obtained previously [
12] using a UASB reactor and a ceramic sponge diffuser (86.8%), but much larger than that obtained from a large-scale reactor (10–26%) [
15].
Increasing the biogas recirculation rate also improves the methane evolution rate (MER) from 0.12 L L
−1 day
−1 to 0.15 L L
−1 day
−1. In general, the MER of in-situ biomethanation is in the range of 0.08 to 0.39 L L
−1 day
−1 [
40].
An anomaly appears when analysing the consumption of hydrogen and the volume of methane that is produced additional to the control, which should theoretically be 0.25 L CH
4 per L of H
2 consumed. For example, in R1, the ratio of the additional methane enrichment with hydrogen converted was 0.15 L CH
4 L
−1 H
2. In R3, the ratio increased to 0.23 L CH
4 L
−1 H
2 (0.27 for FW). Beyond any undetected experimental error, an explanation for this could be that hydrogen is being consumed for microbial growth [
5,
17]; a H
2:CH
4 ratio above 4 has been suggested to account for microbial biomass growth [
41]. Related to this, the sum of the theoretical consumption of CO
2 by biomethanation (calculated from converted H
2) and the volumetric CO
2 from the output gas in all periods was 35%–39% higher compared to the CO
2 produced in the reactor control. In this case, the extra CO
2 might have come from bicarbonate consumption and reduced final dissolved CO
2, as has been observed previously [
12], or could be the increased biochemical production of carbon dioxide due to differing process conditions between the control reactor and biomethanation reactors (cf. average pH in
Appendix A), meaning that the assumed parity between the background AD process is invalid. The experimental overestimation of hydrogen consumption could explain both of these observations but a thorough examination of all measurements and calculations did not yield an opportunity for such an error.
The average pH values on the SS1 and SS2 increased compared to the pH at the reactor control. The average pH tends to increase along with the increase in the recirculation rate, owing to the increase in hydrogen consumption and therefore reduced carbon dioxide concentration that buffers the digestate pH. The average alkalinity during the experiment in SS1 and SS2 was improved along with the increasing recirculation rates. The average alkalinity ratio (IA/PA) in R1, 2, and 3 were 0.41, 0.35, and 0.28, respectively, with the recommended threshold of ratio being 0.3 [
26]. The average total VFA equally decreased from an average of 2 to 1.2 g L
−1.
As expected, the calculated kLa increased through the experimental periods due to the increased flow rate in the recirculation stream (greater gas holdup in the liquid phase and therefore higher specific area in terms of the bubbles). While this increase in mass transfer allowed for the significant consumption of hydrogen by the reactors, the presence of high concentrations of remaining hydrogen in the biogas outflow indicated that the process was mass transfer limited in all cases.
In the case of FW, despite similar trends, the hydrogen content was observed to be stable at a higher level compared to the equivalent SS reactors. The hydrogen gain loop was activated for the majority of the time during R1–R3, mainly caused by the larger hydrogen injection requirement of the feedstock, but with similar mass transfer characteristics. The methane concentration in the biogas outflow was lower for FW due to dilution with a higher amount of hydrogen but also the lower methane content from the background FW digestion.
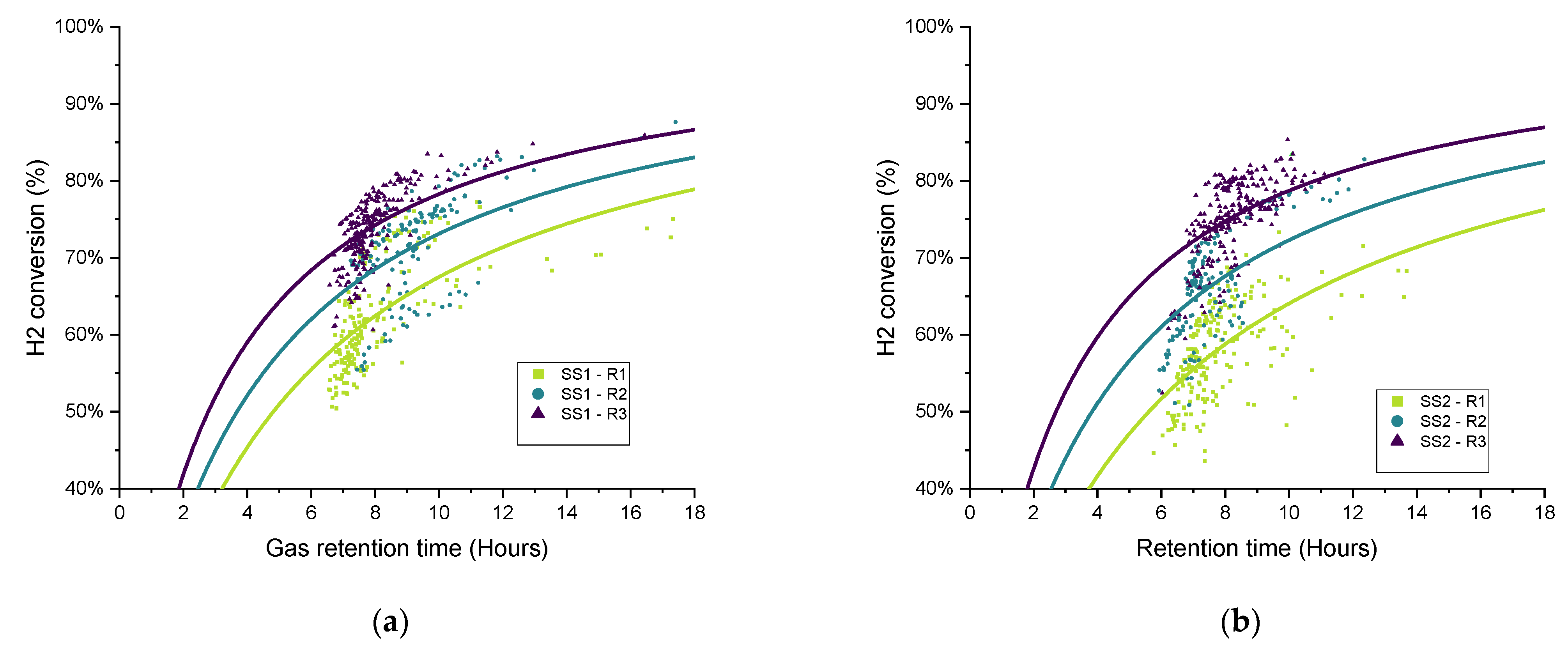
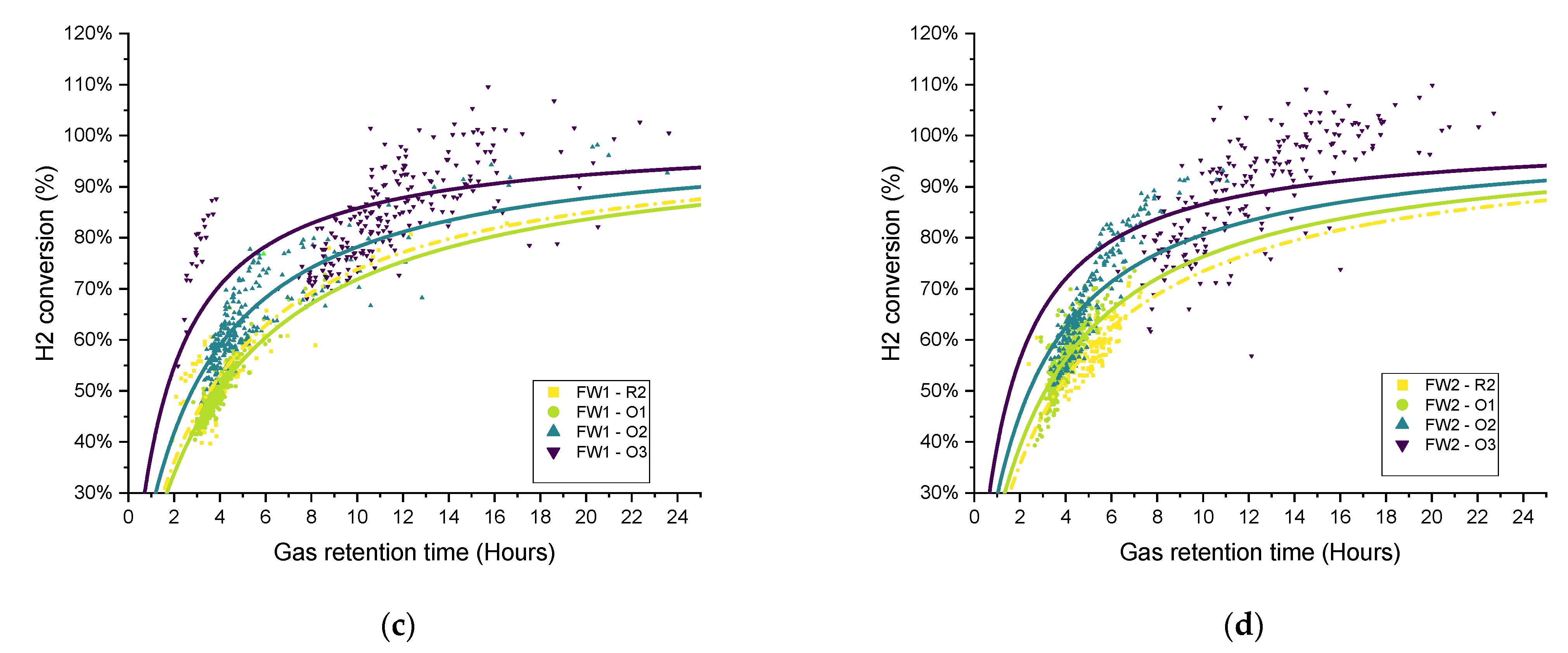
To explore the variations in the gathered data over each operational period, in recognition of the broad variation in the measured data and performance parameters and the difficulty in terms of comparing the different operating conditions, the gas retention time (RT
G) was plotted against the hydrogen conversion for each biomethanation reactor and experimental period, as seen in
Figure 11. Both variables were calculated as two hours average, covering the whole experimental period. Trendlines have been added of the form:
as suggested for a gas–liquid mass transfer limited process [
42] since they offer a good representation of the observed trends, i.e., that the hydrogen conversion eventually appears to saturate with respect to an increased gas retention time (
). The curves were fitted using OriginPro
® to elucidate trends from the highly scattered data.
In general, the data distribution shows that hydrogen conversion increases along with the increase in the recirculation rate since the trendlines are also “ranked” in the graph following the same, from lowest to highest recirculation rates (R1–R3), with this result being previously reported [
17]. There is a certain amount of deviation between the results of duplicates FW1 and FW2, which can also be seen in the hydrogen consumption and
kLa results for FW (
Figure 10).
3.2.3. Further Optimisation of In-Situ Biomethanation (O1–O3)
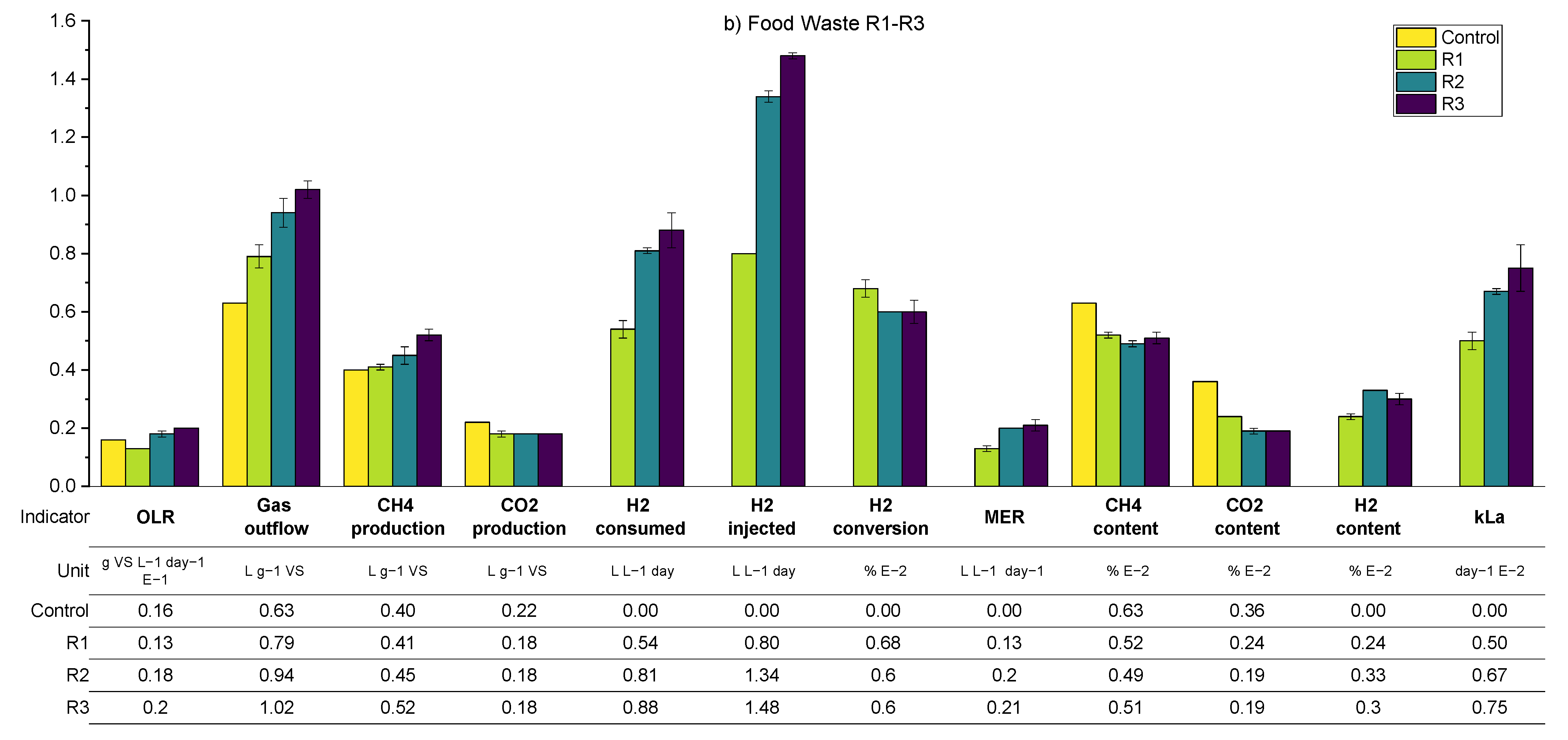
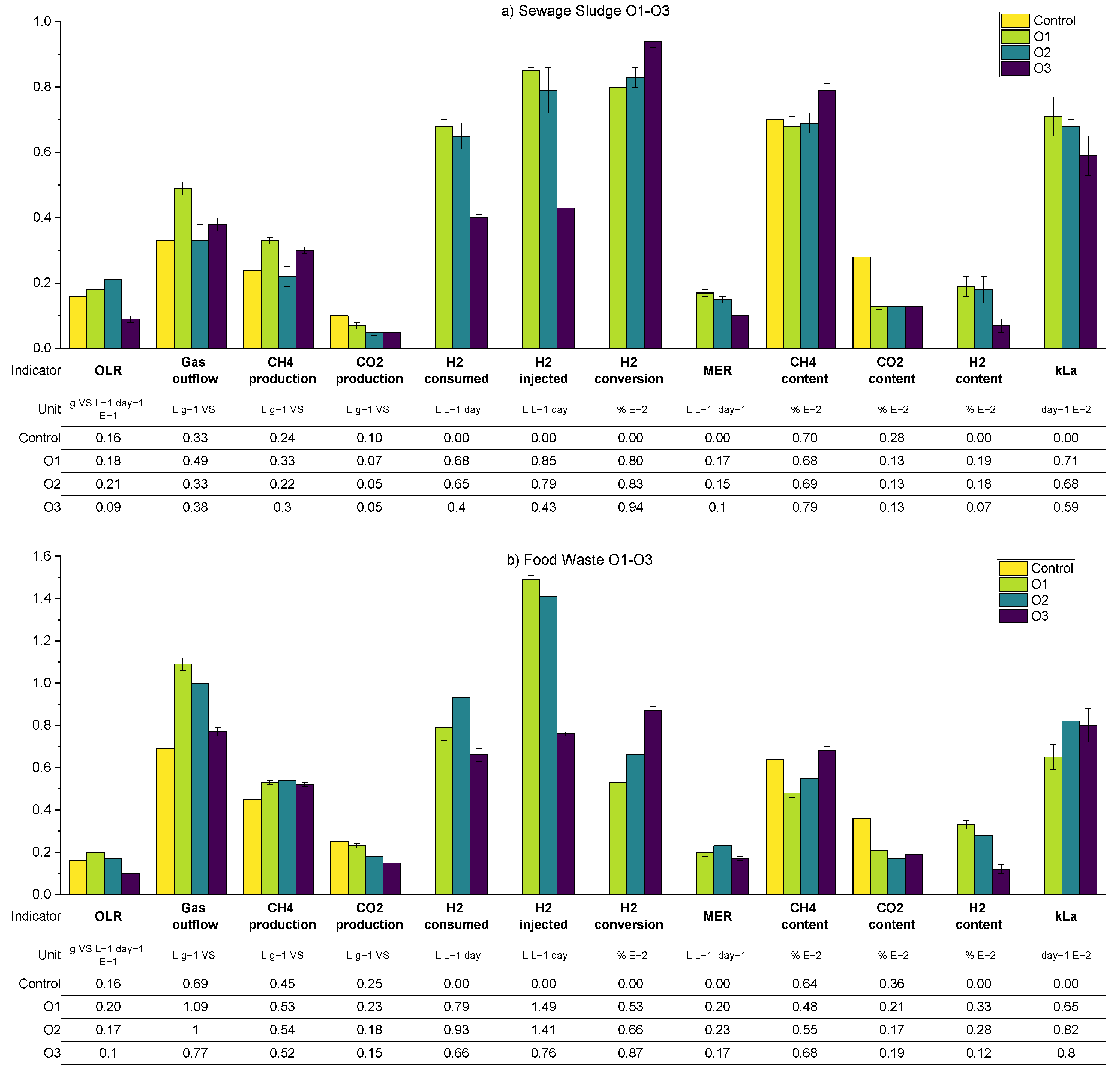
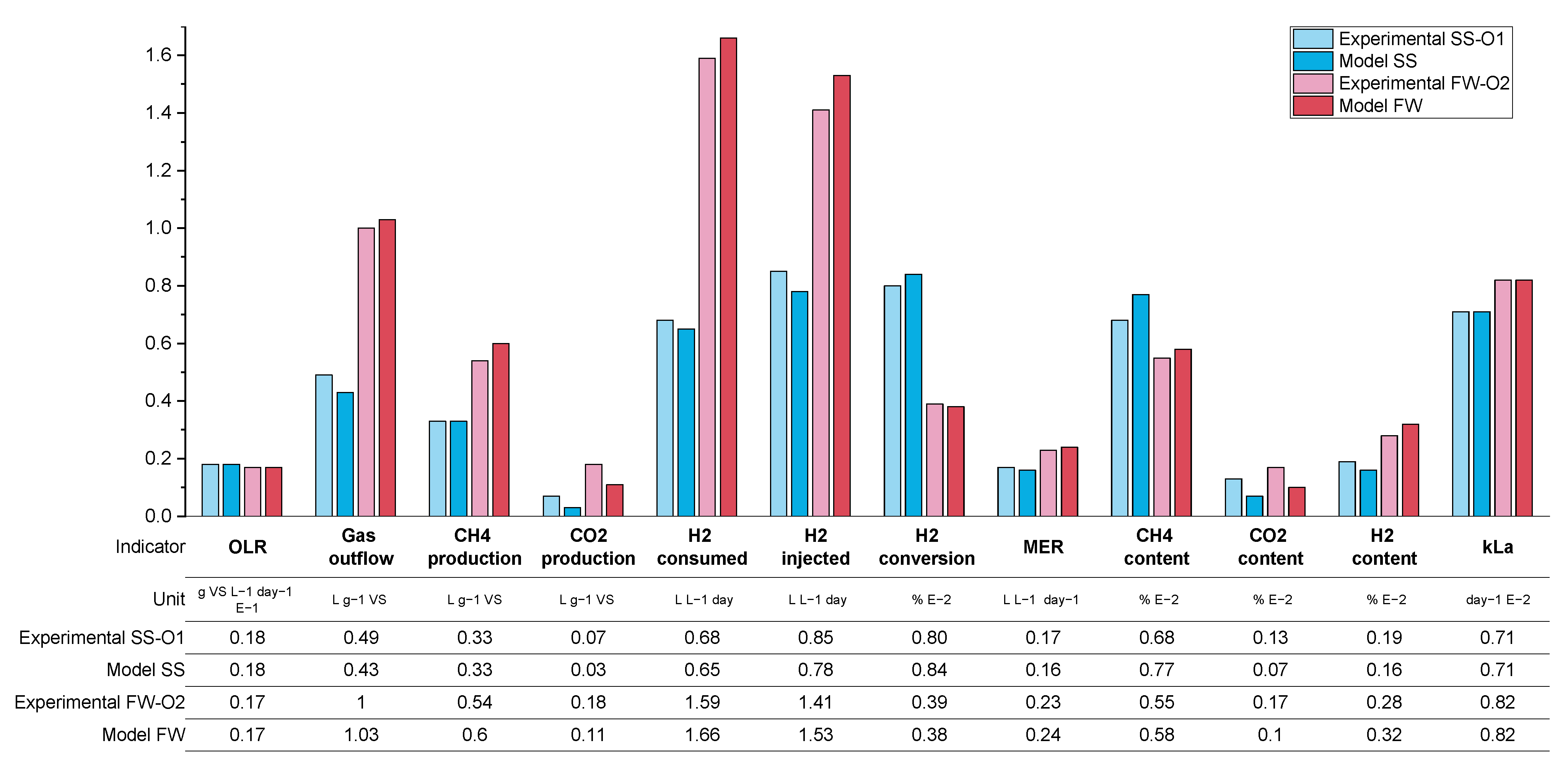
As per the previous results, a summary of the average process parameters for periods O1–O3 for both SS and FW reactors are shown in
Figure 12, with a more detailed breakdown in the
Appendix A.2 (
Table A5 and
Table A7). The expectation for the further optimisation periods was that all three interventions considered, additional sparger on the biogas recirculation line (O1), an increase in the mechanical mixing rate (O2), and a reduction in the OLR (O3), should improve the overall performance of the biomethanation process. The mechanism for this would be through increased mass transfer for the recirculation stream in O1 and both the injection and recirculation stream in O2, while for O3, the improved performance would result from the increased gas retention time due to generally lower biogas production and hydrogen injection.
For the SS reactors in period O1, this prediction was confirmed, by a comparison with the results from R2 (equivalent biogas recirculation rate), through an increase in kLa to 71 day−1 (c.f. 54) and hydrogen conversion to 80% (c.f. 60%). In period O2, however, the results show no improvement from the increase in the mechanical mixing rate, and even a slight, unexplained reduction in performance for SS2, except for the fact that the hydrogen conversion increased in O2 to 83% (c.f. 80% in O1). The periods are difficult to compare since there is a variation in the OLR from 1.8 to 2.0 gVS L−1 day−1 in O1 and O2, respectively. A higher OLR would lead towards shorted gas retention times and a reduction in hydrogen consumption, with other conditions remaining the same.
The best biomethanation performance in terms of desirable output biogas composition (high methane (79%), low carbon dioxide (13%), and hydrogen (7%)) and highest hydrogen conversion (94%) was achieved during O3 with a reduced in the OLR of 0.9 g VS L−1 day−1. This is despite a reduction in the observed kLa, which was expected due to the reduced hydrogen injection rate (in proportion with the reduced OLR) and therefore reduced gas hold-up. This increased performance is traded off against a lower production rate in terms of methane, expressed by a MER of 0.1 L L−1 day−1 (c.f. 0.15 in O2), and, due to the very low OLR used, this is unlikely to be a practical solution for improved biomethanation performance in a real-world scenario.
With regard to the FW experiments, O1–O3, a comparison between O1-FW (with the additional sparger) and R2-FW (with equivalent biogas recirculation rate) shows a slight reduction in hydrogen consumption,
kLa, and the outflowing biogas composition. It is possible that the increased OLR in O1 (2.0 c.f. 1.8 g VS L
−1 day
−1 in R2) is masking any improvements in performance. It was also observed during O1 that there was an increase in the ammonia concentration (from 3.62 to 3.95 gTAN kg
−1) compared with previous experimental runs and, also, for the first time, foaming was detected. There are numerous causes for foaming in AD systems, such as improper mixing, fluctuations in the OLR, and substrate types [
43]. The levels of VFA and alkalinity ratio in the biomethanation reactors were, on average, comparable to the levels in the control reactors, so no indication of biological instability was noted.
The addition of mechanical mixing in O2 did lead to improvements in biomethanation performance compared with R2 in terms of a higher hydrogen conversion (66 c.f. 60%), MER (0.23 cf. 0.20 L L−1 day-), and kLa (82 c.f. 67 day−1), with the trend being similar to that of O2 to R2 for the SS reactors. As per the SS reactors, the best biomethanation performance for FW in terms of hydrogen conversion and desirable output biogas composition was at the reduced OLR in O3.
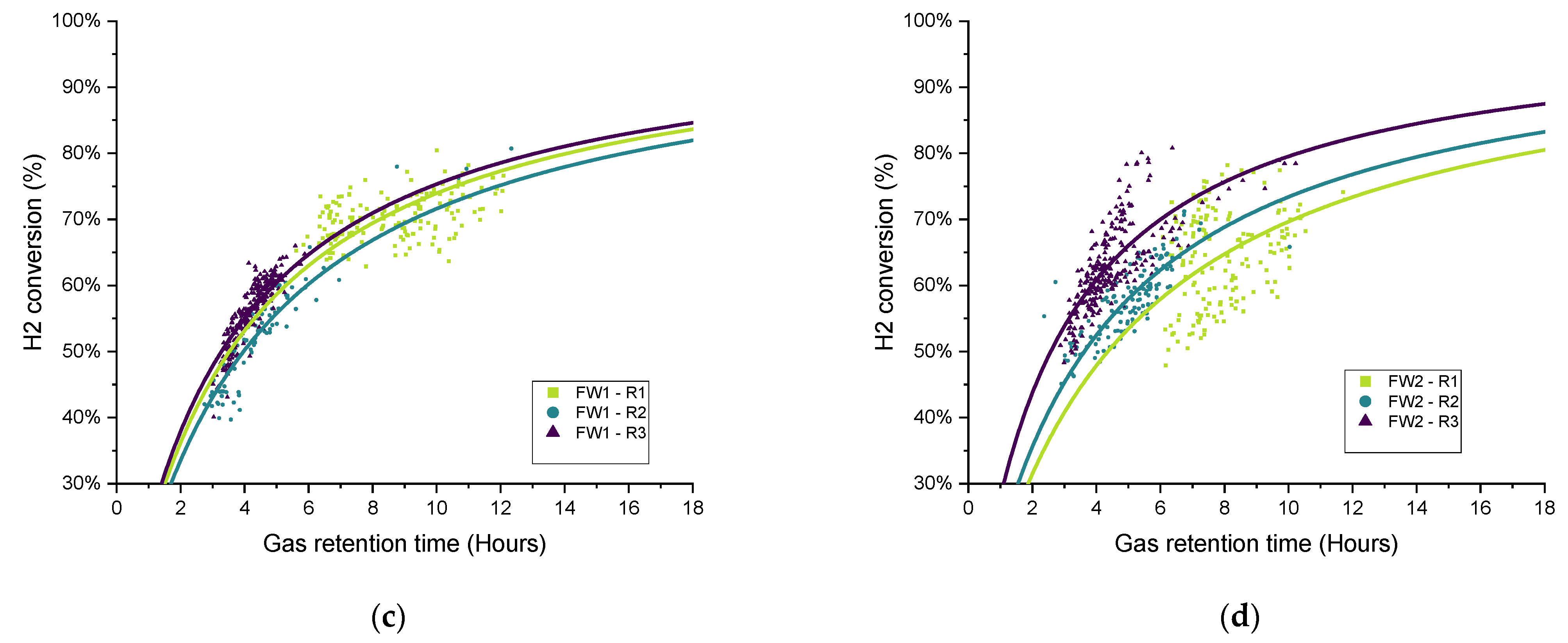
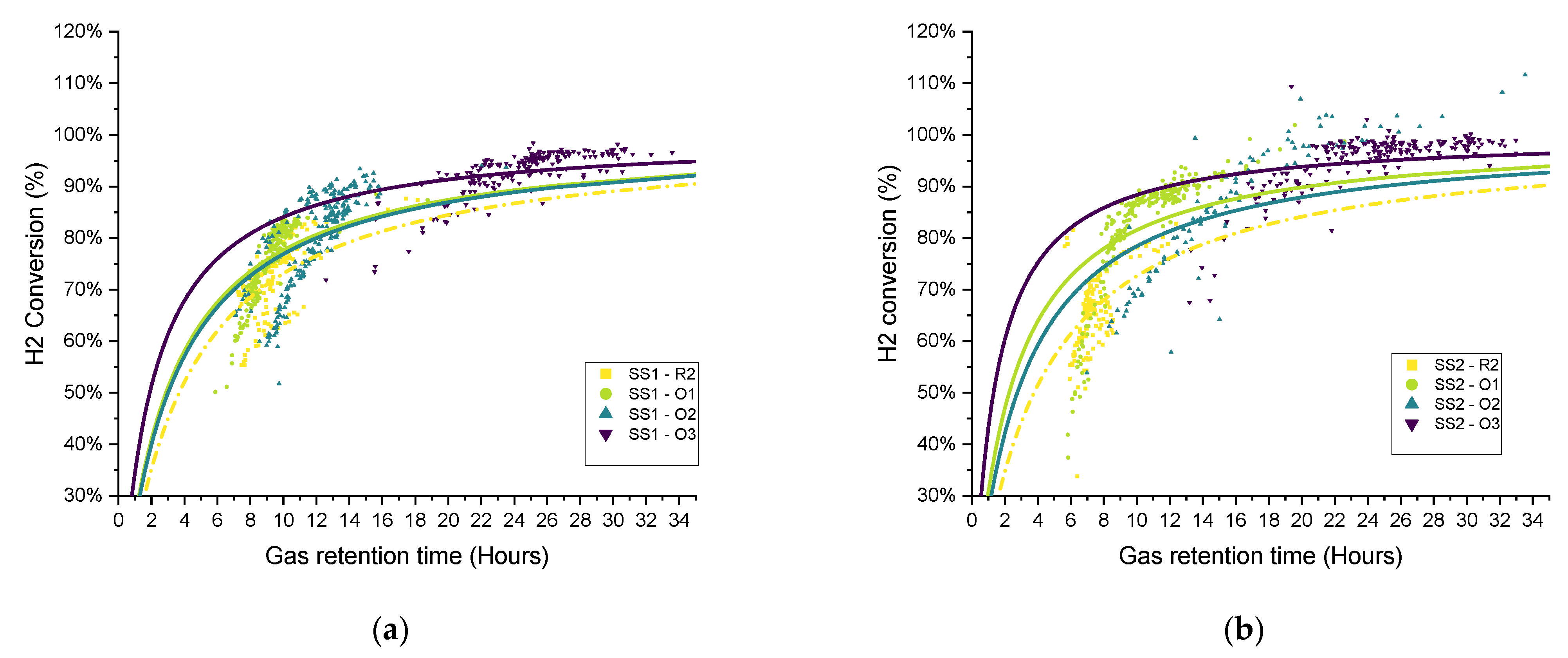
The distribution of the hydrogen conversion as a function of the gas retention time for experimental periods O1–O3 for both SS and FW is shown in
Figure 13. Similar to
Figure 11, the fitted curves were added only for illustration rather than to imply quality of fit. On the whole, the trends observed and discussed above can be confirmed in the scatter plots, in that the biomethanation performance ranked in terms of hydrogen conversion for SS was R2 < O1 ≈ O2 < O3. For FW, O1 (additional sparger) did not see the process gains in one of the duplicates (FW1), and the ranking can be ordered as R2 ≈ O1 < O2 < O3 (as discussed above).
3.3. In-Situ Biomethanation Modelling
The model developed in
Section 2.9 was used to simulate the conditions for the in-situ biomethanation experiments for two periods, O1 for SS and O2 for FW, as these configurations showed the overall best results in terms of biomethanation performance considering a combination of product quality (i.e., a high hydrogen consumption rate and a high/low concentration of methane/carbon dioxide in biogas) and quantity (the methane evolution rate).
Only minimal experimental data were required for the simulation owing to the low model complexity, i.e., no reaction kinetics, inhibition, and biochemical considerations. OLR and hydrogen injection were the model input. Only five parameters were required by the model structure: the specific yields of methane and carbon dioxide produced by the background AD process () from the control reactor, the volumetric gas–liquid mass transfer coefficient () from the biomethanation experimental data, and the reactor and headspace volumes (.
Despite a simplified process model, comparison between the simulated and experimental data show a good fit in terms of reproducing the main experimental average outputs, as shown in
Figure 14. Apart from carbon dioxide specific yield and concentration in biogas, all the main results were fitted with a relative error below 16 and 11% for SS and FW, respectively. Across both feedstocks, carbon dioxide production and content were poorly represented, which can be related back to the discussion in 3.2.2 surrounding the mass balance between the observed hydrogen consumption and carbon dioxide production. Any mechanism that leads to increased observed carbon dioxide production (e.g., release through alkalinity/pH change or changes to the background AD process) was not included in the model, and therefore this experimental anomaly was not replicated.
The qualitative comparison between the modelling and experimental data also validates that the assumptions made during model formulation are also likely to be valid for the experiment. The background AD process appears to be relatively unaffected by the addition of additional hydrogen injection, and the process appears to be mass transfer limited.
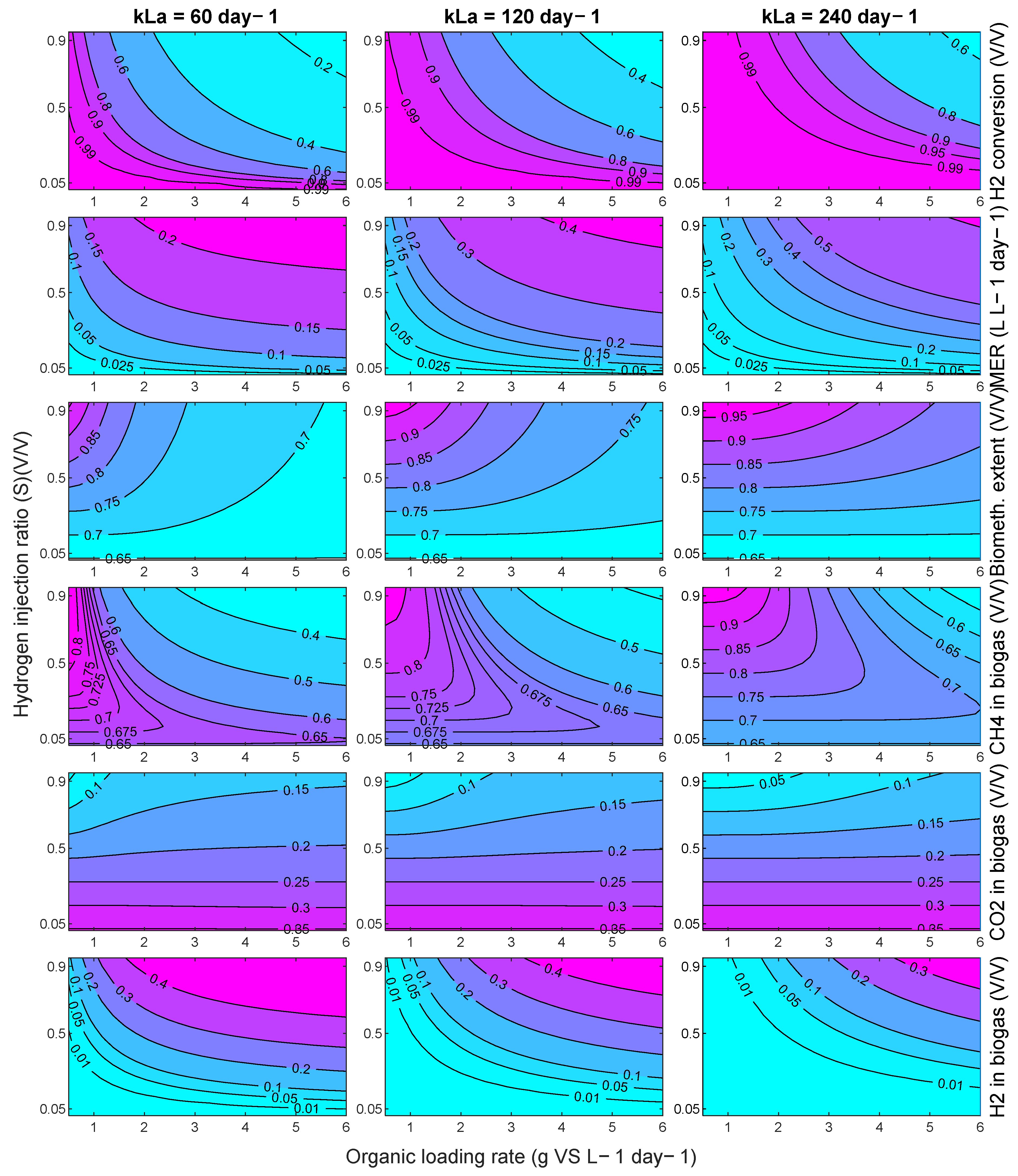
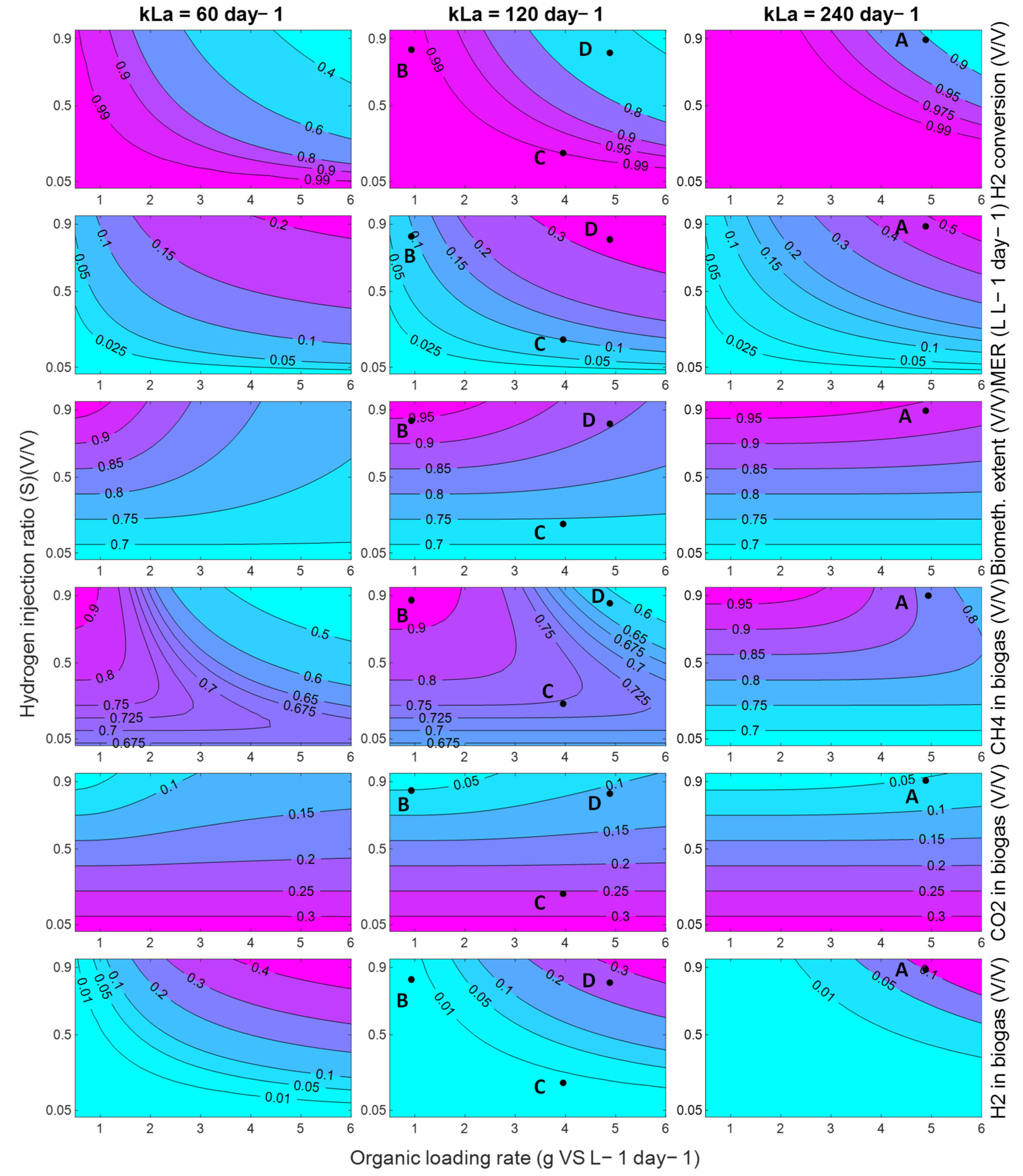
The model can be used more broadly to explore the performance of an in-situ biomethanation system, as long as these founding assumptions hold. For demonstration, process contour maps were generated (
Figure 15) showing the effect of variations in the OLR and hydrogen injection ratio, S (defined as
), on a range of process characteristics and performance indicators, for three levels of mass-transfer: 60 day
−1, corresponding approximately to the conditions explored in this paper, and then 120 and 240 day
−1. The delivery of these improvements in mass transfer characteristics would require the redesign of the equipment, e.g., the use of membranes, increasing the aspect ratio (to increase the bubble path length), or a reduction in bubble size, but, given other works in this area, these values are not considered unrealistic.
Generally speaking, the model predicts that, within the explored conditions, in the best case, the process cannot deliver high performance single reactor in-situ biomethanation in a practical and sensible range in terms of the OLR commonly found in AD systems. For example, even at the highest
kLa explored (240 day
−1), at an OLR of 5 g VS L
−1 day
−1 (Point A,
Figure 15), the process is predicted to produce biogas upgraded to contain ~10% both carbon dioxide and hydrogen with ~80% methane. This gas would require further treatment for most current applications. For use as a biomethane (as a natural gas drop-in replacement), it is likely that some kind of CO
2 removal would be required, either through a further methanation step (e.g., ex situ biomethanation) or through a physical separation. For use as a vehicle fuel (as a CNG drop-in replacement), this gas would likely require hydrogen removal through, e.g., membrane separation.
Depending on the mass transfer capabilities of the system, the available quantity of hydrogen, the targeted product gas application, and/or to match the composition of the product gas with the available downstream purification options, it may be beneficial to tune the in-situ process, which can be facilitated using the contour maps in
Figure 15. The produced process contours can be helpful to explore this. For example, a system with a mass transfer capability of 120 day
−1 may be optimised for ‘near complete’ in-situ biomethanation via operation at an OLR of 1 g VS L
−1 day
−1 (point B), with these conditions being able to produce a high-quality biomethane (~94% CH
4 ~5% CO
2, ~1% H
2) but only at a low productivity (MER) of 0.1 day
−1 and while significantly underutilising the biomass treatment capacity of the system. Another option for the same system may be to accept a low biomethanation extent, but to operate at a reduced stoichiometric ratio (S = 0.25) and an increased OLR (Point C) to partially upgrade the biogas (~75% CH
4 ~24% CO
2, ~1% H
2) whilst minimising hydrogen contamination at a modest value of MER (0.75 L L
−1 day
−1). In another application, it may be better to maximise the consumption of carbon dioxide by applying a higher OLR and stoichiometric ratio (S = 0.9, Point D) to produce a hydrogen-rich biomethane blend (~65% CH
4 ~10% CO
2, ~25% H
2) which, upon CO
2 removal, could be suitable for natural gas grid injection depending on local requirements at an improved MER (0.85 L L
−1day
−1).
For reference, the equivalent contours for a FW fed system are shown in
Appendix A.2 Figure A1. The trends are similar, but the challenge of producing both a high quality and quantity of biomethane product gas from an in-situ system is greater for FW due to its higher biogas production and CO
2 baseline content (and therefore greater hydrogen requirement).