Progresses of the Influencing Factors and Detection Methods of Domoic Acid
Abstract
1. Introduction
2. The Discovery of DA and Its Physicochemical Properties
3. Factors Affecting DA Production
3.1. Species and Life Stages of Algae
3.2. Nutrients Supply
3.3. Trace Metals
3.4. Temperature and Irradiance
3.5. Bacteria
3.6. Other Factors
- (1)
- Clay and Germanic acid: In early studies, Yu et al. found that both clay minerals and Germanic acid could inhibit the production of DA by P. multilocularis. Germanic acid completely inhibited the production of DA when the Ge/Si ratio was 35 [85,86]. The reason may be that the high concentration of clay affects the photosynthesis and nutritional environment of cells and thus affects the production of DA, while Germanic acid may destroy or even interrupt the normal silicification in cells, destroying the respiration, nucleic acid synthesis, and protein compounds of algae, thus inhibiting DA production.
- (2)
- pH: Lundholm et al. found that DA produced by P. multilocularis in the late exponential period significantly increased with the increase in pH (9.3~9.8) in the laboratory [87]. In the field observation, the water body of P. multilocularis usually had higher pH (about 9 or even 10), which suggested that this water body might be polluted with lots of DA. The effect of pH on DA production may be realized by affecting enzyme process, carbon content, metal toxicity, or bacterial structure. pH in natural water is not easy to regulate and mainly depends on the water’s ecosystem self-healing. If it is a specific area, such as fish ponds, there are relevant methods of equilibrium pH. Therefore, pH can affect the production of DA, but whether DA will affect pH, in turn, needs further research.
- (3)
- pCO2: Increasing of pCO2 can promote the production of DA in two different Pseudo-nitzschia, especially under phosphorus [88] or silicon deficieny [89]. Even if the nutrients are enough, the intracellular DA production of some algae, such as P. multilocularis, increased due to the increased pCO2 [90]. The expression of intracellular DA synthesis gene upregulated with the increased pCO2 [57]. With global warming and ocean acidification, it is of great practical significance to study the impact of pCO2 on algae.
- (4)
- Predator: Several studies have shown that the toxicity of toxic diatoms such as P. seriata increased under the direct or indirect existence of calanoid copepods, indicating that toxic diatoms may resist predation by producing poison [91]. For zooplankton, there was no obvious selection tendency in the predation of toxic and nontoxic diatoms, and the predation of toxic diatoms had no obvious effect on itself. Therefore, zooplankton is more likely to act as a carrier to realize the transfer or transformation of DA indirectly in the marine food web by predating toxic diatoms [92,93].
| Factors | Effects on DA Production | Related Species | Reference |
|---|---|---|---|
| Biotic factors | |||
| Strains/Species | DA production varies in different strains | P. australis | [64] |
| Life stages | Produce lots of DA in stationary phase while noting on exponential growth phase | P. multilocularis | [62] |
| Intracellular DA production increased from the exponential growth period to stationary period | P. vulgaris and P. australis | [33,63] | |
| Bacteria | Effectively promote the production of DA | P. multiseries | [80,82] |
| Predator | DA production increased up to 3300% when exposed to grazing copepodites | P. seriata | [94] |
| DA production induced in nontoxic species | P. obtusa | [94] | |
| Abiotic factors | |||
| Irradiance | DA production increases with increasing irradiance | P. australis | [77] |
| Temperature | DA production was below the detection limit at temperatures <20 °C, but increased exponentially from 23 to 30 °C | P. australis | [78] |
| The intracellular DA production at 27 °C was much lower than that at 18 °C | P. multilocularis | [79] | |
| pH | Elevated pH induced production of domoic acid DA production in the late exponential growth period increased significantly with pH (9.3–9.8) in the laboratory | P. multilocularis | [87] |
| pCO2 | Increase due to the increased pCO2 | P. multilocularis | [89] |
| Nitrogen | Higher DA production when grown on NO3− or NH4+ than on urea during exponential growth | P. cuspidata | [95] |
| Highest DA production on urea and NO3− | P. multiseries | [72] | |
| DA production on urea >than on NO3−, NH4+ | P. multiseries | [96] | |
| Highest DA production on glutamate and NH4+ | P. australis | [72] | |
| Silicon (Si) | DA production increased when stressed by Si limitation during the stationary phase | P. seriata | [64] |
| Copper (Cu) | increased due to the excessive copper during exponential growth period | P. multilocularis and P. australis | [74] |
| Phosphorus (P) | DA synthesis was significantly upregulated under phosphorus restriction | P.multiseries | [57,65] |
| Iron (Fe) | Increased due to the lack of iron or excessive copper during exponential growth period | P. multiseries and P. australis | [74] |
| Intracellular DA production increased, accompanied by increase in iron | P. multilocularis | [14] | |
| Lithium (Li) | Significantly promote DA production | P.multiseries | [76] |
| Salinity | DA production rates varied significantly with salinity; they were low and similar at salinities of 5–15 (2.56–3.12 ng mL−1 day−1) and increased with increasing salinity, highest in 35 | P. pungens | [97] |
| Clay (halloysite) | Inhibit the production of DA | Psuedonitzschia pungens f. multiseries | [85] |
| Germanic acid | Completely inhibit the production of DA when the Ge/Si ratio was 35 | P. pungens | [86] |
4. Detection Methods of DA
4.1. Bioassay Methods
4.2. High-Performance Liquid Chromatography (HPLC)
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. Other Detection Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Lilley, M.; Shutler, J.; Lowe, C.; Artioli, Y.; Torres, R.; Berdalet, E.; Tyler, C.R. Assessing risks and mitigating impacts of harmful algal blooms on mariculture and marine fisheries. Rev. Aquacult. 2020, 12, 1663–1688. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful algal blooms: Causes; impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Hynie, I.; Hockin, J.; Wright, J.; Iverson, F. Panel discussion: Evidence that domoic acid was the cause of the 1987 outbreak. Can. Dis. Wkly. Rep. 1990, 16, 37–40. [Google Scholar]
- Tian, D.; Zhang, G. Toxic effects of domoic acid on Caenorhabditis elegans and the underlying mechanism. Int. J. Biol. 2019, 11, v11n3p1. [Google Scholar] [CrossRef]
- Miller, M.A.; Moriarty, M.E.; Duignan, P.J.; Zabka, T.S.; Dodd, E.; Batac, F.I.; Young, C.; Reed, A.; Harris, M.D.; Greenwald, K.; et al. Clinical signs and pathology associated with domoic acid toxicosis in southern sea otters (Enhydra lutris nereis). Front. Mar. Sci. 2021, 8, 585501. [Google Scholar] [CrossRef]
- Radad, K.; Moldzio, R.; Al-Shraim, M.; Al-Emam, A.; Rausch, W.D. Long-term neurotoxic effects of domoic acid on primary dopaminergic neurons. Toxicol. Vitr. 2018, 52, 279–285. [Google Scholar] [CrossRef]
- Brodie, E.C.; Gulland, F.M.D.; Greig, D.J.; Hunter, M.; Jaakola, J.; Leger, J.S.; Leighfield, T.A.; Dolah, F.M.V. Domoic acid causes reproductive failure in california sea lions (Zalophus californianus). Mar. Mammal. Sci. 2006, 22, 700–707. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A.; Frame, E.R.; Colegrove, K.M.; Nance, S.; Baugh, K.A.; Wiedenhoft, H.; Gulland, F.M.D. Clinical signs and histopathology associated with domoic acid poisoning in northern fur seals (Callorhinus ursinus) and comparison of toxin detection methods. Harmful Algae 2010, 9, 374–383. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, H.; Guo, M.; Peng, J.; Zhai, Y.; Tan, Z. First observation of domoic acid and its isomers in shellfish samples from Shandong Province, China. J. Oceanol. Limnol. 2022, 40, 2231–2241. [Google Scholar] [CrossRef]
- Dursun, F.; Yurdun, T.; Ünlü, S. The First Observation of Domoic Acid in Plankton Net Samples from the Sea of Marmara, Turkey. Bull. Environ. Contam. Toxicol. 2016, 96, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.E.R.; Ujević, I.; Mahfouz, C.; Fakhri, M.; Roje-Busatto, R.; Jemaa, S.; Nazlić, N. Occurrence of domoic acid and cyclic imines in marine biota from Lebanon-Eastern Mediterranean Sea. Sci. Total Environ. 2021, 755, 142542. [Google Scholar] [CrossRef] [PubMed]
- Zabaglo, K.; Chrapusta, E.; Bober, B.; Kaminski, A.; Adamski, M.; Bialczyk, J. Environmental roles and biological activity of domoic acid: A review. Algal Res. 2016, 13, 94–101. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Takemoto, T.; Daigo, K. Constituents of Chondria armata and their pharmacological effect. Chem. Pharm. Bull. 1958, 6, 578–580. [Google Scholar] [CrossRef]
- Bates, S.S.; Bird, C.J.; Freitas, A.S.W.d.; Foxall, R.; Gilgan, M.; Hanic, L.A.; Johnson, G.R.; McCulloch, A.W.; Odense, P.; Pocklington, R.; et al. Pennate diatom Nitzschia pungens as the primary source of domoic acid, a toxin in shellfish from Eastern Prince Edward Island, Canada. Can. J. Fish Aquat. Sci. 1989, 46, 1203–1215. [Google Scholar] [CrossRef]
- Grattan, L.M.; Boushey, C.J.; Liang, Y.; Lefebvre, K.A.; Castellon, L.J.; Roberts, K.A.; Toben, A.C.; Morris, J.G. Repeated dietary exposure to low levels of domoic acid and problems with everyday memory: Research to public health outreach. Toxins 2018, 10, 103. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Robertson, A. Domoic acid and human exposure risks: A review. Toxicon 2010, 56, 218–230. [Google Scholar] [CrossRef]
- Sahraoui, I.; Bates, S.S.; Bouchouicha, D.; Mabrouk, H.H.; Hlaili, A.S. Toxicity of Pseudo-nitzschia populations from Bizerte Lagoon; Tunisia; southwest Mediterranean; and first report of domoic acid production by P. brasiliana. Diatom Res. 2011, 26, 293–303. [Google Scholar] [CrossRef]
- Li, A. Occurrence of Pseudo-nitzschia species and associated domoic acid production along the Guangdong coast, South China Sea. Harmful Algae 2020, 98, 101899–101916. [Google Scholar]
- Yang, Y. The Comparative Proteomic Study of Potential Toxic Pseudonitzschia (Bacillariophyta)-Inverstigation of Toxin-Related Proteins. Master’s Thesis, Xiamen University, Fujian, China, 2011. (In Chinese). [Google Scholar]
- Kotaki, Y.; Lundholm, N.; Onodera, H.; Kobayashi, K.; Bajarias, F.F.A.; Furio, E.F.; Iwataki, M.; Fukuyo, Y.; Kodama, M. Wide distribution of Nitzschia navisvaringica; A new domoic acid-producing benthic diatom found in Vietnam. Fish Sci. 2004, 70, 28–32. [Google Scholar] [CrossRef]
- Buck, K. Autecology of the diatom Pseudonitzschia australis, a domoic acid producer, from Monterey Bay, California. Mar. Ecol. Prog. Ser. 1992, 84, 293–302. [Google Scholar] [CrossRef]
- Wekell, J.C.; Gauglitz, E.J., Jr.; Bamett, H.J.; Hatfield, C.L.; Simons, D.; Ayres, D. Occurrence of domoic acid in Washington state razor clams (Siliqua patula) during 1991–1993. Nat. Toxins 1994, 2, 197–205. [Google Scholar] [CrossRef] [PubMed]
- O’Dea, S.N.; Flewelling, L.J.; Wolny, J.; Brame, J.; Henschen, K.; Scott, P.; Hubbard, K.A.; Wren, J.; Jones, C.; Knight, C.; et al. Florida’s first shellfish closure due to domoic acid. In Seventh Symposium on Harmful Algae in the U.S.; Sarasota, FL, USA, 2013; p. 103. [Google Scholar]
- Wright, J.L.; Boyd, R.K.; Freitas, A.D.; Falk, M.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; McCulloch, A.W.; McInnes, A.G.; Odense, P.; et al. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can. J. Chem. 1989, 67, 481–490. [Google Scholar] [CrossRef]
- Dickey, R.W.; Fryxell, G.A.; Granade, H.R.; Roelke, D. Detection of the marine toxins okadaic acid and domoic acid in shellfish and phytoplankton in the Gulf of Mexico. Toxicon 1992, 30, 355–359. [Google Scholar] [CrossRef]
- Vale, P.; Sampayo, M.A.; Quilliam, M. DSP complex toxin profiles relation with Dinophysis spp. occurrence and domoic acid confirmation by LC-MS in Portuguese bivalves. In Harmful Algae; Reguera, B., Blanco, J., Fernández, M.L., Wyatt, T., Eds.; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Vigo, Espagne, 1998; pp. 503–506. [Google Scholar]
- Míguez, A.; Fernandez, M.L.; Fraga, S. First Detection of Domoic Acid in Galicia (NW Spain) Harmful and Toxic Algal Blooms; Yasumoto, T., Oshima, Y., Fukuro, Y., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1996; pp. 143–145. [Google Scholar]
- Rhodes, L.; White, D.; Syhre, M.; Atkinson, M. Pseudonitzschia Species Isolated from New Zealand Coastal Waters: Domoic Acid Production In Vitro and Links with Shellfish Toxicity Seventh International Conference on Toxic Phytoplankton; Yasumoto, T., Oshima, Y., Fukuyo, Y., Eds.; UNESCO: Sendai, Japan, 1995; pp. 155–158. [Google Scholar]
- Kotaki, Y.; Koike, K.; Yoshida, M.; Chu, V.T.; Huyen, N.T.M.; Hoi, N.C.; Fukuyo, Y.; Kodama, M. Domoic acid production in Nitzschia sp. (Bacillariophyceae) isolated from a shrimp-culture pond in Do Son, Vietnam. J. Phycol. 2000, 36, 1057–1060. [Google Scholar] [CrossRef]
- Amzil, Z.; Fresnel, J.; Gal, D.L.; Billard, C. Domoic acid accumulation in French shellfish in relation to toxic species of Pseudo-nitzschia multiseries and P. pseudodelicatissima. Toxicon 2001, 39, 1245–1251. [Google Scholar] [CrossRef]
- Cusack, C.K.; Bates, S.S.; Quilliam, M.A.; Patching, J.W.; Raine, R. Confirmation of domoic acid production by Pseudo-nitzschia australis (Bacillariophyceae) isolated from Irish waters. J. Phycol. 2002, 38, 1106–1112. [Google Scholar] [CrossRef]
- Gallacher, S.; Howard, G.; Hess, P.; Mac Donald, E.; Kelly, M.C.; Bates, L.A.; Brown, N.; MacKenzie, M.; Gillibrand, P.; Turrell, W.L. The Occurrence of Amnesic Shellfish Poisons in Shellfish from Scottish Waters Harmful Algal Blooms 2000; Hallegraff, G.M., Blackburn, S.I., Bolch, J.C., Lewis, R.J., Eds.; IOC of UNESCO: Paris, France, 2001; pp. 30–33. [Google Scholar]
- Sarno, D.; Dahlmann, J. Production of domoic acid in another species of Pseudo-nitzschia: P. multistriata in the Gulf of Naples (Mediterranean Sea). Harmful Algae News 2000, 21, 5. [Google Scholar]
- Chen, X.; Wang, C.; Hu, J.; Lu, B. Determination of domoic acid in water and aquatic animals by high performance liquid chromatography. J. Hyg. Res. 2001, 30, 247–248. [Google Scholar]
- Li, D.Z.; Zhu, W.J.; Song, W.B.; Lin, B.C. Capillary electrophoretic analysis of amnesic shellfish toxin-domoic acid. Chin. J. Chromatogr. 2002, 20, 125–128. [Google Scholar]
- Kaniou-Grigoriadou, I.; Mouratidou, T.; Katikou, P. Investigation on the presence of domoic acid in Greek shellfish. Harmful Algae 2005, 4, 717–723. [Google Scholar] [CrossRef]
- Louw, D.; Doucette, G.; Lundholm, N. Morphology and toxicity of Pseudo-nitzschia species in the northern Benguela Upwelling System. Harmful Algae 2018, 75, 118–128. [Google Scholar] [CrossRef]
- Bajarias, F.F.A.; Kotaki, Y.; Relox, J.R., Jr.; Romero, M.; Kodama, M. Screening of diatoms producing domoic acid and its derivatives in the Philippines. Coast. Mar. Sci. 2006, 30, 121–129. [Google Scholar]
- Iverson, F.; Truelove, J. Toxicology and seafood toxins: Domoic acid. Neurogastroenterol. Motil. 2010, 2, 334–339. [Google Scholar] [CrossRef]
- Benlahcen, R.l.; Lundholm, N.; Goux, D.; Véron, B.; Sagou, R.; Taleb, H.; Nhhala, H.; Er-Raioui, H. Pseudo-nitzschia Peragallo (Bacillariophyceae) diversity and domoic acid accumulation in tuberculate cockles and sweet clams in M’diq Bay, Morocco. Acta Bot. Croat. 2013, 72, 35–47. [Google Scholar]
- Bouchouicha-Smida, D.; Lundholm, N.; Sahraoui, I.; Lambert, C.; Mabrouk, H.H.; Hlaili, A.S. Detection of domoic acid in Mytilus galloprovincialis and Ostrea edulis linked to the presence of Nitzschia bizertensis in Bizerte Lagoon (SW Mediterranean). Estuar. Coast. Shelf Sci. 2015, 165, 270–278. [Google Scholar] [CrossRef]
- Takata, Y.; Sato, S.; Dao, V.; Montojo, U.; Kamolsiripichaiporn, S.; Kotaki, Y.; Fukuyo, Y.; Kodama, M. Occurrence of domoic acid in tropical bivalves. Fish Sci. 2009, 75, 473–480. [Google Scholar] [CrossRef]
- Alvarez, G.; Uribe, E.; Quijano-Scheggia, S.; López-Rivera, A.; Mariño, C.; Blanco, J. Domoic acid production by Pseudo-nitzschia australis and Pseudo-nitzschia calliantha isolated from North Chile. Harmful Algae 2009, 8, 938–945. [Google Scholar] [CrossRef]
- Peteva, Z.V.; Georgieva, S.; Stancheva, M.; Makedonski, L. Recreational angler exposure to domoic acid via consumption of contaminated shellfish from the Black Sea, Bulgaria: A preliminary study. Arch. Balk. Med. Union 2017, 52, 291–297. [Google Scholar]
- Malhi, N.; Turnbull, A.; Tan, J.; Kiermeier, A.; Nimmagadda, R.; McLeod, C. A national survey of marine biotoxins in wild-caught abalone in Australia. J. Food Prot. 2014, 77, 1960–1967. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.L.; Kotaki, Y.; Lundholm, N.; Thoha, H.; Ogawa, H.; Relox, J.; Terada, R.; Takeda, S.; Takata, Y.; Haraguchi, K.; et al. Unique amnesic shellfish toxin composition found in the South East Asian diatom Nitzschia navis-varingica. Harmful Algae 2011, 10, 456–462. [Google Scholar] [CrossRef]
- Thoha, H.; Kotaki, Y.; Panggabean, L.; Lundholm, N.; Ogawa, H.; Lim, P.T.; Takata, Y.; Kodama, M.; Fukuyo, Y. Screening of diatoms that produce ASP toxins in Southernmost Asian waters. Coast. Mar. Sci. 2012, 35, 34–38. [Google Scholar]
- Smida, D.B.; Lundholm, N.; Kooistra, W.H.C.F.; Sahraoui, I.; Ruggiero, M.V.; Kotaki, Y.; Ellegaard, M.; Lambert, C.; Mabrouk, H.H.; Hlaili, A.S. Morphology and molecular phylogeny of Nitzschia bizertensis sp. nov.-A new domoic acid-producer. Harmful Algae 2014, 32, 49–63. [Google Scholar] [CrossRef]
- Tan, S.N.; Teng, S.T.; Lim, H.C.; Kotaki, Y.; Bates, S.S.; Leaw, C.P.; Lim, P.T. Diatom Nitzschia navis-varingica (Bacillariophyceae) and its domoic acid production from the mangrove environments of Malaysia. Harmful Algae 2016, 60, 139–149. [Google Scholar] [CrossRef]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Cheng, W.; Lu, J.; Zheng, Y. Research Advance in neurotoxic mechanism of domoic acid. Asian J. Ecotoxicol. 2013, 8, 1–6. [Google Scholar]
- Saeed, A.F.; Awan, S.A.; Ling, S.; Wang, R.; Wang, S. Domoic acid: Attributes, exposure risks, innovative detection techniques and therapeutics. Algal Res. 2017, 24, 97–110. [Google Scholar] [CrossRef]
- Munday, R.; Holland, P.T.; McNabb, P.; Selwood, A.I.; Rhodes, L.L. Comparative toxicity to mice of domoic acid and isodomoic acids A, B and C. Toxicon 2008, 52, 954–956. [Google Scholar] [CrossRef]
- Sawant, P.M.; Tyndall, J.D.; Holland, P.T.; Peake, B.M.; Mountfort, D.O.; Kerr, D.S. In vivo seizure induction and affinity studies of domoic acid and isodomoic acids-D, -E and -F. Neuropharmacology 2010, 59, 129–138. [Google Scholar] [CrossRef]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Obornik, M.; Smith, G.J.; et al. Biosynthesis of the neurotoxin domoic acid in a bloom-forming diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef]
- Chen, J.F.; Huang, W.J.; Xu, N.; Xie, L.C.; Qi, Y.Z. Domoic acid producing diatom genus Pseudo-nitzschia peragallo: A review. Mar. Sci. 2003, 27, 13–17. (In Chinese) [Google Scholar]
- Turk Dermastia, T.; Dall’Ara, S.; Dolenc, J.; Mozetič, P. Toxicity of the diatom genus Pseudo-nitzschia (Bacillariophyceae): Insights from toxicity tests and genetic screening in the Northern Adriatic Sea. Toxins 2022, 14, 60. [Google Scholar] [CrossRef] [PubMed]
- Rowland-Pilgrim, S.; Swan, S.C.; O’Neill, A.; Johnson, S.; Coates, L.; Stubbs, P.; Dean, K.; Parks, R.; Harrison, K.; Alves, M.T.; et al. Variability of Amnesic Shellfish Toxin and Pseudo-nitzschia occurrence in bivalve molluscs and water samples-Analysis of ten years of the official control monitoring programme. Harmful Algae 2019, 87, 101623. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Murray, J.S.; Boundy, M.J.; Balci, M.; Bowers, H.A.; Smith, K.F.; Wood, D.T.H.; Rhodes, L.L. Update of the planktonic diatom genus Pseudo-nitzschia in Aotearoa New Zealand coastal waters: Genetic diversity and toxin production. Toxins 2021, 13, 637. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.S. Ecophysiology and metabolism of ASP toxin production. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer-Verlag: Berlin, Germany, 1998; pp. 405–426. [Google Scholar]
- Pan, Y.L.; Parsons, M.; Busman, M.B.; Moeller, P.; Doucette, G. Pseudo-nitzschia sp. cf. pseudodelicatissima-a confirmed producer of domoic acid from the northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2001, 220, 83–92. [Google Scholar] [CrossRef]
- Fehling, J.; Green, D.H.; Davidson, K.; Bolch, C.J.; Bates, S.S. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) in scottish waters. J. Phycol. 2004, 40, 622–630. [Google Scholar] [CrossRef]
- Pan, Y.; Bates, S.S.; Cembella, A.D. Environmental stress and domoic acid production by Pseudo-nitzschia: A physiological perspective. Neurogastroenterol. Motil. 2010, 6, 127–135. [Google Scholar]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) species; domoic acid and amnesic shellfish poisoning: Revisiting previous paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Fehling, J.; Davidson, K.; Bolch, C.J.; Bates, S.S. Growth and domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceae) under phosphate and silicate limitation. J. Phycol. 2004, 40, 674–683. [Google Scholar] [CrossRef]
- Pan, Y.; Subba Rao, D.V.; Mann, K.H. Changes in domoic acid production and cellular chemical composition of the toxigenic diatom Pseudo-nitzschia multiseries under phosphate limitation. J. Phycol. 1996, 32, 371–381. [Google Scholar] [CrossRef]
- Auro, M.E. Nitrogen Dynamics and Toxicity of the Pennate Diatom Pseudo-Nitzschia Cuspidata: A Field and Laboratory Study; San Francisco State University: San Francisco, CA, USA, 2007; p. 91. [Google Scholar]
- Howard, M.; Cochlan, W.P.; Ladizinsky, N.; Kudela, R.M. Nitrogenous preference of toxigenic Pseudo-nitzschia australis (Bacillariophyceae) from field and laboratory experiments. Harmful Algae 2007, 6, 206–217. [Google Scholar] [CrossRef]
- Martin-Jézéquel, V.; Calu, G.; Candela, L.; Amzil, Z.; Jauffrais, T.; Séchet, V.; Weigel, P. Effects of Organic and Inorganic Nitrogen on the Growth and Production of Domoic Acid by Pseudo-nitzschia multiseries and P. australis (Bacillariophyceae) in Culture. Mar. Drugs 2015, 13, 7067–7086. [Google Scholar] [CrossRef]
- Rue, E.; Bruland, K. Domoic acid binds iron and copper: A possible role for the toxin produced by the marine diatom Pseudo-nitzschia. Mar. Chem. 2001, 76, 127–134. [Google Scholar] [CrossRef]
- Maldonado, M.T.; Hughes, M.P.; Rue, E.L.; Wells, M.L. The effect of Fe and Cu on growth and domoic acid production by Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 2002, 47, 515–526. [Google Scholar] [CrossRef]
- Shahzad, B.; Mughal, M.N.; Tanveer, M.; Gupta, D.; Abbas, G. Is lithium biologically an important or toxic element to living organisms? An overview. Environ. Sci. Pollut. R. 2017, 24, 103–115. [Google Scholar] [CrossRef]
- Rao, D.V.S.; Pan, Y.; Mukhida, K. Production of domoic acid by Pseudo-nitzschia multiseries Hasle, affected by lithium. Mar. Ecol. 1998, 19, 31–36. [Google Scholar] [CrossRef]
- Thorel, M.; Fauchot, J.; Morelle, J.; Raimbault, V.; Le Roy, B.; Miossec, C.; Kientz-Bouchart, V.; Claquin, P. Interactive effects of irradiance and temperature on growth and domoic acid production of the toxic diatom Pseudo-nitzschia australis (Bacillariophyceae). Harmful Algae 2014, 39, 232–241. [Google Scholar] [CrossRef]
- Zhu, Z.; Qu, P.; Fu, F.; Tennenbaum, N.; Tatters, A.O.; Hutchins, D.A. Understanding the blob bloom: Warming increases toxicity and abundance of the harmful bloom diatom Pseudo-nitzschia in California coastal waters. Harmful Algae 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Amato, A.; Lüdeking, A.; Kooistra, W.H.C.F. Intracellular domoic acid production in Pseudo-nitzschia multistriata isolated from the Gulf of Naples (Tyrrhenian Sea, Italy). Toxicon 2010, 55, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Fehling, J.; Davidson, K.; Bates, S.S. Growth dynamics of non-toxic Pseudo-nitzschia delicatissima and toxic P. seriata (Bacillariophyceae) under simulated spring and summer photoperiods. Harmful Algae 2005, 4, 763–769. [Google Scholar] [CrossRef]
- Bates, S.S.; Douglas, D.J.; Doucette, G.J.; Léger, C. Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo-nitzschia multiseries. Nat. Toxins 1995, 3, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takata, Y.; Kodama, M. Direct contact between Pseudo-nitzschia multiseries and bacteria is necessary for the diatom to produce a high level of domoic acid. Fish Sci. 2009, 75, 771–776. [Google Scholar] [CrossRef]
- Guannel, M.L.; Horner-Devine, M.C.; Rocap, G. Bacterial community composition differs with species and toxigenicity of the diatom Pseudo-nitzschia. Aquat. Microb. Ecol. 2011, 64, 117–133. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P. Link between domoic acid production and cell physiology after exchange of bacterial communities between toxic Pseudo-nitzschia multiseries and non-toxic Pseudo-nitzschia delicatissima. Mar. Drugs 2014, 12, 3587–3607. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.M.; Rao, D.V.S. Impact of halloysite on growth of Psuedonitzschia pungens f. multiseries and production of algal toxin. Oceanol. Limnol. Sin. 1998, 29, 47–52. [Google Scholar]
- Yu, Z.M.; Rao, D.V.S. Effects of Germanium on the growth and the production of microcystins in Pseudo-nitzschia multiseries. Chin. Sci. Bull. 1998, 43, 2311–2315. (In Chinese) [Google Scholar]
- Lundholm, N.; Hansen, P.J.; Kotaki, Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar. Ecol. Prog. Ser. 2004, 273, 1–15. [Google Scholar] [CrossRef]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F.-X. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Tatters, A.O.; Fu, F.X.; Hutchins, D.A.; Brett, N. High CO2 and Silicate Limitation Synergistically Increase the Toxicity of Pseudo-nitzschia fraudulenta. PLoS ONE 2012, 7, e32116. [Google Scholar] [CrossRef] [PubMed]
- Tatters, A.; Schnetzer, A.; Xu, K.; Walworth, N.; Fu, F.; Spackeen, J.; Sipler, R.; Bertrand, E.; McQuaid, J.; Allen, A.; et al. Interactive effects of temperature, CO2 and nitrogen source on a coastal California diatom assemblage. J. Plankton Res. 2018, 40, 151–164. [Google Scholar] [CrossRef]
- Tammilehto, A.; Nielsen, T.; Krock, B.; Møller, E.; Lundholm, N. Induction of domoic acid production in the toxic diatom Pseudo-nitzschia seriata by calanoid copepods. Aquat. Toxicol. 2015, 159, 52–61. [Google Scholar] [CrossRef]
- Leandro, L.; Teegarden, G.; Roth, P.; Wang, Z.; Doucette, G. The copepod Calanus finmarchicus: A potential vector for trophic transfer of the marine algal biotoxin; domoic acid. J. Exp. Mar. Biol. Ecol. 2010, 382, 88–95. [Google Scholar] [CrossRef]
- Maneiro, I.; Iglesias González, P.; Guisande, C.; Riveiro, I.; Barreiro Felpeto, A.; Zervoudaki, S.; Granéli, E. Fate of domoic acid ingested by the copepod Acartia clausi. Mar. Biol. 2005, 148, 123–130. [Google Scholar] [CrossRef]
- Harðardóttir, S.; Pančić, M.; Tammilehto, A.; Krock, B.; Møller, E.F.; Nielsen, T.G.; Lundholm, N. Dangerous Relations in the Arctic Marine Food Web: Interactions between Toxin Producing Pseudo-nitzschia Diatoms and Calanus Copepodites. Mar. Drugs 2015, 13, 3809–3835. [Google Scholar] [CrossRef]
- Auro, M.; Cochlan, W. Nitrogen Utilization and Toxin Production by Two Diatoms of the Pseudo-nitzschia pseudodelicatissima Complex: P. cuspidata and P. fryxelliana. J. Phycol. 2013, 49, 156–169. [Google Scholar] [CrossRef]
- Radan, R.L.; Cochlan, W.P. Differential toxin response of Pseudo-nitzschia multiseries as a function of nitrogen speciation in batch and continuous cultures, and during a natural assemblage experiment. Harmful Algae 2018, 73, 12–29. [Google Scholar] [CrossRef]
- Pednekar, S.M.; Bates, S.S.; Kerkar, V.; Matondkar, S.G.P. Environmental Factors Affecting the Distribution of Pseudo-nitzschia in two monsoonal estuaries of Western India and effects of salinity on growth and domoic acid production by P. pungens. Estuar. Coast. 2018, 41, 1448–1462. [Google Scholar] [CrossRef]
- Botana, L. The Mouse Bioassay as a Universal Detector. In Seafood and Freshwater Toxins; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Dolah, F.M.; Finley, E.L.; Haynes, B.; Doucette, G.J.; Moeller, P.; Ramsdell, J. Development of rapid and sensitive high throughput pharmacologic assays for marine phycotoxins. Nat. Toxins 1994, 2, 189–196. [Google Scholar] [CrossRef]
- Vera-Avila, L.; Marín-Pérez, D.; Covarrubias-Herrera, R. Trace level determination of domoic acid in seawater by Off-line/on-line Solid-phase extraction coupled to HPLC-UV. J. Mex. Chem. Soc. 2011, 55, 65–71. [Google Scholar]
- Furey, A.; Lehane, M.; Gillman, M.; Fernandez-Puente, P.; James, K.J. Determination of domoic acid in shellfish by liquid chromatography with electrospray ionization and multiple tandem mass spectrometry. J. Chromatogr. A 2001, 938, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Maucher-Fuquay, J.; Fire, S.E.; Mikulski, C.M.; Haynes, B.; Doucette, G.J.; Ramsdell, J.S. Optimization of solid-phase extraction and liquid chromatography-tandem mass spectrometry for the determination of domoic acid in seawater, phytoplankton, and mammalian fluids and tissues. Anal. Chim. Acta. 2012, 715, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Beach, D.G.; Walsh, C.M.; Cantrell, P.; Rourke, W.; O’Brien, S.; Reeves, K.; McCarron, P. Laser ablation electrospray ionization high resolution mass spectrometry for regulatory screening of domoic acid in shellfish. Rapid Commun. Mass Spectrom. 2016, 30, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, D.; Hong, Z. A rapid LC-HRMS method for the determination of domoic acid in urine using a Self-Assembly Pipette Tip Solid-Phase extraction. Toxins 2016, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Liu, B.; Wu, T.; Chi, T.; Su, M. Development of a sensitive Enzyme-Linked Immunosorbent Assay for the determination of domoic acid in shellfish. J. Agric. Food Chem. 2004, 52, 5334–5339. [Google Scholar] [CrossRef]
- Zhang, X.W.; Zhang, Z.X. Quantification of domoic acid in shellfish samples by capillary electrophoresis-based enzyme immunoassay with electrochemical detection. Toxicon 2012, 59, 626–632. [Google Scholar] [CrossRef]
- Tsao, Z.J.; Liao, Y.C.; Liu, B.H.; Su, C.C.; Yu, F.Y. Development of a monoclonal antibody against domoic acid and its application in Enzyme-Linked Immunosorbent Assay and colloidal gold immunostrip. J. Agric. Food Chem. 2007, 55, 4921–4927. [Google Scholar] [CrossRef]
- Kvasnicka, F.; Sevcik, R.; Voldrich, M. Determination of domoic acid by on-line coupled capillary isotachophoresis with capillary zone electrophoresis. J. Chromatogr. A 2006, 1113, 255–258. [Google Scholar] [CrossRef]
- Müller, C.; Glamuzina, B.; Pozniak, I.; Weber, K.; Cialla, D.; Popp, J.; Cîntă Pînzaru, S. Amnesic shellfish poisoning biotoxin detection in seawater using pure or amino-functionalized Ag nanoparticles and SERS. Talanta 2014, 130, 108–115. [Google Scholar] [CrossRef]
- Di Paola, D.; Abbate, J.M.; Iaria, C.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Environmental Risk Assessment of Dexamethasone Sodium Phosphate and Tocilizumab Mixture in Zebrafish Early Life Stage (Danio rerio). Toxics 2022, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Arangia, A.; Crupi, R.; Cuzzocrea, S.; Spanò, N.; Gugliandolo, E.; Peritore, A.F. Impact of Mycotoxin Contaminations on Aquatic Organisms: Toxic Effect of Aflatoxin B1 and Fumonisin B1 Mixture. Toxins 2022, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; McGovern, E.; McMahon, T.; Morris, S.; Stobo, L.A.; Brown, N.A.; Gallacher, S.; McEvoy, J.D.G.; Kennedy, G.; Young, P.B.; et al. LC-UV and LC-MS methods for the determination of domoic acid. Trends Anal. Chem. 2005, 24, 358–367. [Google Scholar] [CrossRef]
- Garthwaite, I.; Ross, K.M.; Miles, C.O.; Hansen, R.P.; Towers, N.R. Polyclonal antibodies to domoic acid; and their use in immunoassays for domoic acid in sea water and shellfish. Nat. Toxins 2015, 6, 93–104. [Google Scholar] [CrossRef]
- Kawatsu, K.; Hamano, Y.; Noguchi, T. Production and characterization of a monoclonal antibody against domoic acid and its application to enzyme immunoassay. Toxicon 1999, 37, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Kleivdal, H.; Kristiansen, S.-I.; Nilsen, M.; Goksøyr, A.; Briggs, L.; Holland, P.; McNabb, P. Determination of domoic acid toxins in shellfish by Biosense ASP ELISA-A direct competitive enzyme-linked immunosorbent assay: Collaborative study. J. AOAC Int. 2007, 90, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Litaker, R.; Stewart, T.; Eberhart, B.-T.; Wekell, J.; Trainer, V.; Kudela, R.; Miller, P.; Roberts, A.; Hertz, C.; Johnson, T.; et al. Rapid Enzyme-linked Immunosorbent Assay for Detection of the Algal Toxin Domoic Acid. J. Shellfish. Res. 2008, 27, 1301–1310. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Cheng, J.; Wang, Q.; Wang, W. Establishment of indirect ELISA to detect domoic acid. Acta Sci. Circumstantiae 2014, 34, 404–408. (In Chinese) [Google Scholar]
- Wang, Q.; Chen, J.; Gao, L.; Dong, Y.; Lei, X. Development of Direct Competitive Enzyme-Linked Immunosorbent Assay for the Determination of Domoic Acid. Environ. Sci. 2012, 33, 647–651. (In Chinese) [Google Scholar]
- Fraga, M.; Vilarino, N.; Louzao, M.C.; Rodriguez, P.; Campbell, K.; Elliott, C.T.; Botana, L.M. Multidetection of paralytic, diarrheic, and amnesic shellfish toxins by an inhibition immunoassay using a microsphere-flow cytometry system. Anal. Chem. 2013, 85, 7794–7802. [Google Scholar] [CrossRef]
- Abdul Rahman, M.S.; Jayasundera, K.; Mukhopadhyay, S.C. A low cost novel sensing system for detection of dangerous marine biotoxins in seafood. Sensor. Actuat. B Chem. 2009, 137, 67–75. [Google Scholar]
- McGrath, T.F.; Andersson, K.; Campbell, K.; Fodey, T.L.; Elliott, C.T. Development of a rapid low cost fluorescent biosensor for the detection of food contaminants. Biosens. Bioelectron. 2013, 41, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yakes, B.; Buijs, J.; Elliott, C.; Campbell, K. Surface plasmon resonance biosensing: Approaches for screening and characterising antibodies for food diagnostics. Talanta 2016, 156–157, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Abdul Keyon, A.; Guijt, R.; Gaspar, A.; Kazarian, A.; Nesterenko, P.; Bolch, C.; Breadmore, M. Capillary electrophoresis for the analysis of paralytic shellfish poisoning toxins in shellfish: Comparison of detection methods. Electrophoresis 2014, 35, 1496–1503. [Google Scholar] [CrossRef]
- Boissonneault, K.R.; Henningsen, B.M.; Bates, S.S.; Robertson, D.L.; Milton, S.; Pelletier, J.; Hogan, D.A.; Housman, D.E. Gene expression studies for the analysis of domoic acid production in the marine diatom Pseudo-nitzschia multiseries. BMC Mol. Biol. 2013, 14, 25–43. [Google Scholar] [CrossRef]
- Di Dato, V.; Musacchia, F.; Petrosino, G.; Patil, S.; Montresor, M.; Sanges, R.; Ferrante, M.I. Transcriptome sequencing of three Pseudo-nitzschia species reveals comparable gene sets and the presence of Nitric Oxide Synthase genes in diatoms. Sci. Rep. 2015, 5, 12329. [Google Scholar] [CrossRef]
- Lewis, N.I.; Bates, S.S.; Quilliam, M.A. Production of domoic acid from large-scale cultures of Pseudo-nitzschia multiseries: A feasibility study. Harmful Algae 2018, 79, 58–63. [Google Scholar] [CrossRef]
- Lahvis, G.P. What California sea lions exposed to domoic acid might teach us about autism: Lessons for predictive and preventive medicine. EPMA J. 2017, 8, 229–235. [Google Scholar] [CrossRef]
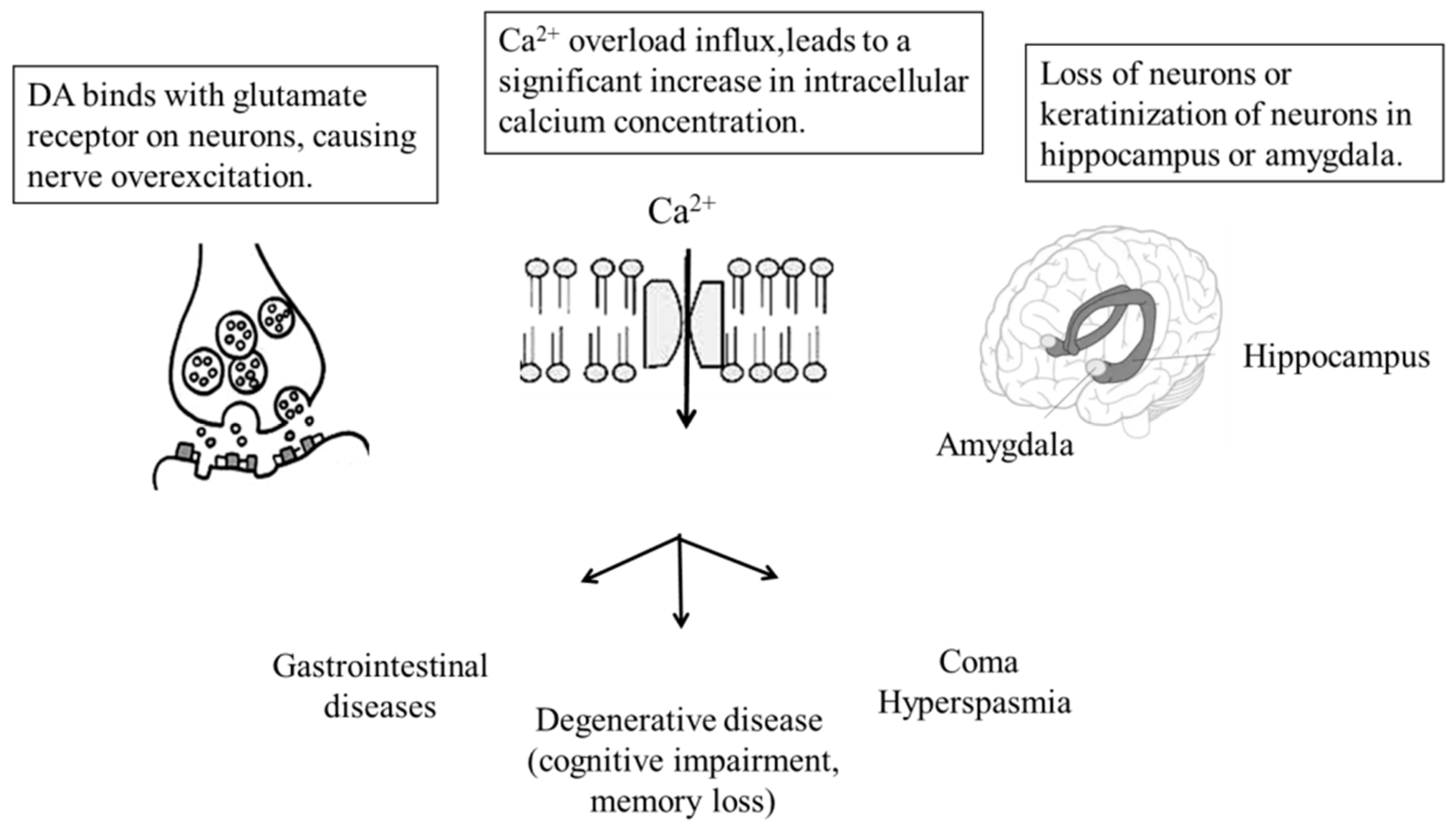

| Country | Sampling Date | Sampling Area | Specimen | References |
|---|---|---|---|---|
| Japan | 1957 | Tropical and sub-tropical waters | Chrondria armata | [15] |
| Natori estuary, Ishigaki Island, Okinawa | [22] | |||
| American | 1961 | Capitola, California | ||
| 1991 | Monterey Bay, California Washington State beaches | Pseudo-nitzschia australis | [23,24] | |
| Florida (Gulf of Mexico) | [25] | |||
| Canada | 1987 | Estuaries on the eastern coast of Prince Edward Island | Pseudo-Nitzschia pungens | [16,26] |
| Mexico | 1992 | Gulf of Mexico | Nitzschia pungens f. multiseries | [27] |
| Portugal | 1995 | Not mentioned | Not mentioned | [28] |
| Spain | 1996 | Ria de Vigo, Galicia | Pseudo-nitzschia multiseries | [29] |
| New Zealand | 1992/1993 | Not mentioned | [30] | |
| Vietnam | 1997 | Do Son | Nitzschia navis-varingica | [31] |
| France | 1998 | CoÃtes d’Armor (English Channel) | P. pseudodelicatissima | [32] |
| Ireland | 1999 | Southwest Ireland | Pseudo-nitzschia australis | [33] |
| Scotland | 1999 | wild and cultivated molluscs waters in Scottish | Pseudo-nitzschia maximus (mainly) | [34] |
| Italy | 2000 | the Gulf of Naples (Mediterranean Sea) | Pseudo-nitzschia multiseries | [35] |
| China | 2001 | The Bohai Sea and the lakes rivers polluted by algae in the South | Pseudo-nitzschia simulans | [36,37] |
| Greece | 2002 | Greek coasts along Thermaikos Gulf | genus Pseudo-nitzschia (P. pungens f.pungens, P. pseudodelicatissima) | [38] |
| Namibia | 2004 | Inshore and offshore stations | P. australis and P. pungens | [39] |
| Philippines | 2004 | Manila Bay, San Pedro Bay, South Sulawesi | Pseudo-Nitzschia pungens | [40] |
| Croatia | 2006 | the Croatian coast of the Adriatic Sea | Pseudo-nitzschia spp. | [41] |
| Morroco | 2007 | M’diq Bay, west Mediterranean coast of Morocco | P. multistriata, P.cuspidata, P. galaxiae, P. multiseries, P. pseudodelicatissima, P. pungens var. aveirensis, P. Calliantha, P. fraudulenta. | [42] |
| Tunisia | 2008 | Bizerte Lagoon | [43] | |
| Thailand | 2006 | 12°38′ N, 100°53′ E | Pseudo-nitzschia multiseries | [44] |
| Chile | 2004–2006 | Bahı’a Inglesa (27°7′ S, 70°52′ W) and Bahı’a Tongoy (30°15′ S, 71°20′ W) | Pseudo-nitzschia species (P. Australis, P. calliantha, P. subfraudulenta) | [45] |
| Turkey | 2010 | Sea of Marmara | P. delicatissima, P. fraudulenta, and P. pungens | [11] |
| Bulgaria | 2011 | North Black Sea | Pseudo-nitzschia | [46] |
| Australia | 2012 | Tasmania, Victoria, South Australia, Western Australia, New South Wales | Pseudo-nitzschia delicatissima, P. multiseries, and P. australis | [47] |
| Indonesia | 2010 | Panyula in South Sulawesi, Jakarta Bay, Lampung Bay, and Sangihe Island | Pseudo-nitzschia strains | [48,49] |
| Tunisia | 2014 | Bizerte Lagoon | Nitzschia bizertensis sp. nov. | [50] |
| Malaysia | 2015 | Johor, Negeri Sembilan, Kelantan | Nitzschia navis-varingica | [51] |
| Methods | Detection Limitation | Merits | Limitations | Reference |
|---|---|---|---|---|
| Bioassay | ||||
| Mouse bioassay | >20 μg·g−1 | Universal detection, easy to perform, cheap | Ethical pressure; poor repeatability; interference of extracts and salts; long operation time and inability to distinguish toxins types; high detection limit; error % high | [98] |
| Receptor bioassay | 0.001 ng·g−1 | Sensitive | Difficult to obtain the receptor | [99] |
| HPLC | Needs standards, needs toxicology information for each toxin | |||
| HPLC-UV | 20 ng·mL−1 | High versatility, high sensitivity, easy to use, simple maintenance, low equipment cost, can detect a large number of samples | Low sensitivity to compounds with poor UV absorption | [9] |
| HPLC-UV&SPE | 0.04 ng·mL−1 | High sensitivity, automatic analysis, suitable precision (<5%) | Special instrument, professional operation | [100] |
| HPLC-FLD | 0.2 ng·mL−1 (1.5 pg·mL−1 for seawater) | High sensitivity, automatic analysis, less clutter interference | Most of the derivatization reagents are expensive and unstable, and the reagent’s deterioration may lead to toxin’s incomplete derivatization | [11] |
| HPLC-MS/MS | 0.02 ng·mL−1 | No need for derivative reagent and toxin standard, wide detection range, high sensitivity, fast speed, and the operation is simple | The equipment requirements are high, and can not be used for a large number of grass-roots day-to-day monitoring | [9] |
| HPLC/ESI-IT-MS | 0.02 ng·mL−1 | High sensitivity, high selectivity, can carry on the mass examination. Can provide chemical structure information | High requirements for sample pretreatment | [101] |
| LC-MS | <1 pg·mL−1 | Allows quantification sensitive | Slow, complex, expensive, needs standards | [102] |
| LAESI-HRMS | 0.24 μg·g−1 | Realized high-throughput screening or quantitation of DA in a variety of shellfish matrices | Low accuracy, suit to screening than direct quantitation | [103] |
| LC-HRMS | 0.12 ng·mL−1 | Less solvent consumption, low cost, the absence of the evaporation step, and short time requirement. | High requirements for pH, the number of aspirating/dispensing cycles, and the type and volume of eluent | [104] |
| ELISA | ||||
| ELISA | 0.02 ng·mL−1 | High sensitivity, easy to use | Unable to detect all individuals. Expensive DA standards, professional microplate instruments, small molecular weight of DA, difficulties in preparation of immune antigen | [105] |
| CEEIA | 0.02 ng·mL−1 | Rapid detection and high sensitivity | [106] | |
| ICS | 5 ng·mL−1 | Fast, sensitive, quantitative, easy to use | Need enough toxin to obtain antibody Expensive | [107] |
| Other methods | ||||
| cITP-CZE | 1.5 ng·mL−1 | Simple to use, low cost, and portability. High sensitivity | High requirement for pH value, poor repeatability | [108] |
| SERS | 0.1 μg·mL−1 (in pure water) 0.01μg·mL−1 (in seawater) | Lower limit of detection, rapid detection of DA in different situations | Sensitivity and accuracy are far less than those of HPLC and ELISA | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, A.; Zhang, H.; Yang, Y.; Jiang, Z. Progresses of the Influencing Factors and Detection Methods of Domoic Acid. Processes 2023, 11, 592. https://doi.org/10.3390/pr11020592
Yang A, Zhang H, Yang Y, Jiang Z. Progresses of the Influencing Factors and Detection Methods of Domoic Acid. Processes. 2023; 11(2):592. https://doi.org/10.3390/pr11020592
Chicago/Turabian StyleYang, Aoao, Haiguang Zhang, Yu Yang, and Zhaoyu Jiang. 2023. "Progresses of the Influencing Factors and Detection Methods of Domoic Acid" Processes 11, no. 2: 592. https://doi.org/10.3390/pr11020592
APA StyleYang, A., Zhang, H., Yang, Y., & Jiang, Z. (2023). Progresses of the Influencing Factors and Detection Methods of Domoic Acid. Processes, 11(2), 592. https://doi.org/10.3390/pr11020592





