Abstract
The study is devoted to the creation of a dataset of protein structural motifs of the 3β-corner type. The relevance and importance of creating a dataset of 3β-corners is determined by the fact that this structure can be an embryo or a ready-made structural block in the process of protein folding, and can also act as an independent object of research in the field of structural biology. The dataset also contains 3β-corner-like structures that are geometrically similar to 3β-corners. The dataset consists of 45,896 structures. For each motif, its characteristics are presented: the name of the protein in which the 3β-corner is recognized, the method and resolution of the protein structure, the coordinates of localization in the protein, the secondary structure of the amino acid sequence, the gyration radius, the solvent-accessible area, and the composition of the elements of the secondary structure. The dataset will allow a comprehensive study of structures on a large scale and advance the understanding of the features and patterns of their structural organization.
1. Introduction
The relevance of the study of the structural motifs of protein molecules follows from the great interest of researchers in these structures. Structural motifs have unique stackings of secondary structure elements sequentially running along the polypeptide chain, forming compact spatial structures. The uniqueness of the structures and their ability to act as nuclei in the process of protein folding or be used as starting structures for searching for possible polypeptide chain folds in protein structure modeling makes structural motifs an object of study for many scientific teams [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21]. Such studies are carried out on separate samples of structures that have a small volume. Therefore, the development and creation of datasets for structural motifs is extremely important and relevant. The availability of datasets makes it possible to conduct a wide range of studies on a large variety of structures, including all the possible motifs of certain types of protein molecules registered in the PDB protein data bank (https://www.rcsb.org/) [22]. And this, in turn, will help to advance the understanding of the features of the studied structural motifs. It should also be noted that the creation of a dataset provides the opportunity to conduct research on a large set of data, which will increase the reliability of the results obtained.
The purpose of this work is to create a dataset of one of the types of structural motifs that is often encountered and has a unique arrangement in its space structure, the 3β-corner. The 3β-corner structural motif was first described in 1992 [23]. 3β-corner can be represented as a triple-stranded β-sheet folded on to itself so that the two β-hairpins are packed approximately orthogonally in different layers, and the central strand bends by ~90° in a right-handed direction when passing from one β-layer to the other. All the 3β-corners observed in proteins, when viewed from their concave surfaces, can be considered as Z-like folded β-sheets [23].
It has been established that the 3β-corner structural motif is often found in both homologous and non-homologous proteins and domains. There are well known small proteins consisting only of 3β-corners and short irregular regions adjacent to 3β-corners [24]. All this suggests that a given motif can take on its own unique structure as such and can be a core around which other sections can be built.
At the same time, 3β-corners are found in various proteins. While the overall folding of the polypeptide chain remains unchanged, the following characteristics can differ:
- lengths of β-strands;
- lengths and conformations of the irregular sections connecting them (turns, loops, bends);
- as well as the conformations of amino acid residues that ensure the bending of the polypeptide chain by approximately ~90° and its transition from one β-layer to another with the formation of a right-handed superhelix [23,25,26] (Figure 1).
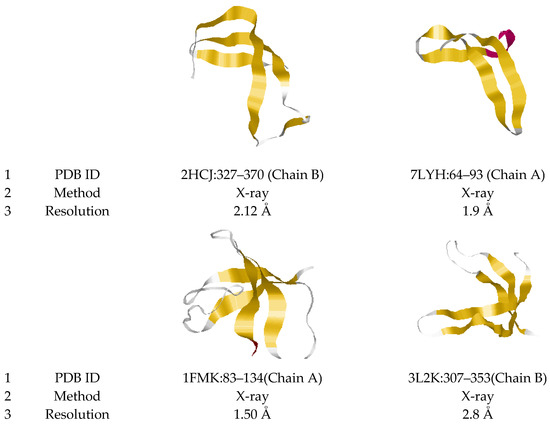 Figure 1. Ribbon model representations of 3β-corners. Examples of the tape model of the motif are presented. 3β-corners were extracted from elongation factor Tu 1 (2HCJ, E. coli), replicase polyprotein 1ab (7LYH, Coronavirus 2), tyrosine-protein kinase c-SRC (1FMK, Homo sapiens), enoyl-ACP reductase (1NHG, Plasmodium falciparum), EhpF (3L2K, Pantoea agglomerans) and peptidyl-prolyl cis-trans isomerase A (3K0M, Homo sapiens) proteins. The coordinates of the 3β-corner in each protein (1), the experimental method for obtaining the structure (2) and the analysis resolution (less than 2 Å) are indicated (3). Secondary structure elements are highlighted by color: β-stands (yellow), helix (red) and coil (white).
Figure 1. Ribbon model representations of 3β-corners. Examples of the tape model of the motif are presented. 3β-corners were extracted from elongation factor Tu 1 (2HCJ, E. coli), replicase polyprotein 1ab (7LYH, Coronavirus 2), tyrosine-protein kinase c-SRC (1FMK, Homo sapiens), enoyl-ACP reductase (1NHG, Plasmodium falciparum), EhpF (3L2K, Pantoea agglomerans) and peptidyl-prolyl cis-trans isomerase A (3K0M, Homo sapiens) proteins. The coordinates of the 3β-corner in each protein (1), the experimental method for obtaining the structure (2) and the analysis resolution (less than 2 Å) are indicated (3). Secondary structure elements are highlighted by color: β-stands (yellow), helix (red) and coil (white).
Figure 1 shows examples of different structural variants of 3β-corners. The most common variants in nature are represented by four β-stands and three short stretches of 2–5 amino acid residue connectors (coils and turns), as shown for structure 7LYH:64–93 (Chain A). Structures containing various “loops” in connectors also meet the requirements for attributing the motif to the 3β-corner: relatively long unstructured sequences, as in structures 2HCJ:327–370 (Chain B) and 1FMK: 83–134 (Chain A).
The dataset also includes protein motifs we called 3β-corner-like structures. These motifs are similar in geometry to 3β-corners because they can be represented as two β-hairpins packed approximately orthogonally in different layers. Yet, these motifs contain irregular regions of various lengths and conformations between β-strands. The lengths of the connections between the β-strands can significantly exceed the allowed length limits of the 3β-corners folding. Further, these irregular regions may contain helices of various lengths and different types (α- and 310-helices), and additional β-strands can occur between two β-hairpins. Yet, these motifs captured our attention because they have the main component of a 3β-corner (two β-hairpins packed approximately orthogonally in different layers, forming a hydrophobic core). This type of motifs seems to be very interesting, as it is similar to 3β-corners, while demonstrating a large variety of polypeptide chain folding. Figure 2 illustrates examples of the described motifs; the structures 1NHG:97–160 (Chain B) and 3K0M:3–66 (Chain A) have long unstructured sequences containing α-helices between β-strands.
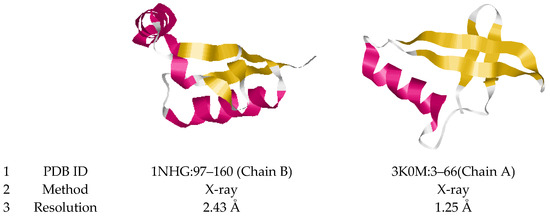
Figure 2.
Ribbon model representations of 3β-corner-like motifs. Examples of the tape model of the motif are presented. They were extracted from enoyl-ACP reductase (1NHG, Plasmodium falciparum) and peptidyl-prolyl cis-trans isomerase A (3K0M, Homo sapiens) proteins. The coordinates of the motif in each protein (1), the experimental method for obtaining the structure (2), and the analysis resolution are indicated (3). Secondary structure elements are highlighted by color: β-stands (yellow), α-helix (red) and coil (white).
Previously, in [27], devoted to the study of the stability of 3β-corners, using a molecular dynamics experiment, we showed the autonomous stability of this structural motif in an aqueous medium, that is, outside the protein globule. In this study, the molecular dynamics experiments were performed under a whole protein molecule holding the motif under consideration and 3β-corners exhibited outside of the protein environment. During the experiments, changes of the geometric characteristics of 3β-corners were analyzed. The experiment involved 330 structural motifs of the 3β-corner type, recognized and segmented in protein structures annotated in the PDB protein structure bank (https://www.rcsb.org/, accessed on 20 September 2022). During the molecular dynamics experiment, the 3β-corner structures showed the preservation of the main geometric characteristics: standard deviation (RMSD), gyration radius (Rg), solvent accessible area (SASA), number of hydrogen bonds and others. The work showed the stability of conformational patterns as a result of comparative analysis of the change in values of angles φ and ψ plotted on the Ramachandran map. The stability of contacts (hydrogen bonds and hydrophobic interactions) between β-strands of the studied structural motif of the 3β-corner type was also shown. Analysis of the results of the molecular dynamics experiment showed that all the studied 3β-corners, which have unique and compact packing of the polypeptide chain, retain their spatial topology. This result displays in advance that this structure can be an embryo or a ready-made structural block in the process of protein folding. Further, the results obtained indicate that the 3β-corner can act as an independent object for study in the field of theoretical and experimental structural biology.
2. Materials and Methods
Recognition and segmentation of 3β-corners was performed using the 3D-Blast public search service (http://3d-blast.life.nctu.edu.tw/dbsas.php, accessed on 20 September 2022). This service allows finding molecular structures similar in their geometric properties and topology. Using a set of 330 structures collected and verified by experts [27] as a sample set with the 3D-Blast service, we obtained 45,896 structures with topology similar to the samples. The 3D-Blast service also provides a structural similarity metric called E-value for each of the found structures. We have defined the E-value threshold as 10−9 in order to form the target dataset. Table 1 below shows the distribution of the found structures amounts by the ranges of E-value.

Table 1.
Distribution of recognized protein motifs by E-value metrics buckets.
Using the model, 45,896 structures were selected: 14,995 3β-corners and 30,901 3β-corner-like structures.
Structures with the following characteristics were selected for the set of 3β-corners:
- length of structures from 20 to 64 amino acid residues;
- length of connections between β-strands in β-hairpins vary to 10 amino acid residues;
- the length of the irregular section that forms the bend of the polypeptide chain by approximately ~90° and its transition from one β-layer to another varies from 0 to 4 amino acid residues;
- constrictions may contain “loops”, taking the form of turns, loops, bends, no more than one helix turn (α- and 310-).
- Motifs selected as 3β-corner-like structures are characterized by:
- lengths of structures from 20 to 64 amino acid residues;
- unlimited (within the framework of the structure) lengths of irregular sections between β-strands in β-hairpins, as well as an unlimited length of the irregular section bending the polypeptide chain by approximately ~90° and transiting from one β-layer to another;
- connections can take any shape, contain all possible elements of the secondary structure—β-strand, helices (α- and 310-).
Supplementary Materials File S1 contain all 45,896 structures in MS Excel 2007+ file format (*.xlsx). For each motif, the following are indicated: the name of the protein in which the structure is recognized; method and quality of protein structure determination; coordinates of localization in the protein (chain, amino acid residues number of the beginning and end of the structure); lengths of β-strands; length and structure of amino acid sequences between β-strands; gyration radius; solvent-accessible surface area; torsion angles; and the number of hydrogen bonds. The presented characteristics make it possible to study in detail the features of each structure, and also, by obtaining samples of structures according to the given parameters, to study the patterns of the structural organization of motifs.
3. Results
A dataset of 3β-corner and 3β-corner-like structures has been created, which contains 45,896 protein motives. These structures were recognized and selected from protein structures annotated in the PDB protein structure bank. Protein structures are resolved by known experimental methods, including cryoelectron microscopy (EM), X-rays, nuclear magnetic resonance (NMR) and a combination thereof (Table 2).

Table 2.
Methods of protein 3D-structure determination experiments used to recognize and segment 3β- corners and 3β-corner-like structures.
As expected, the predominant part of the structures was experimentally obtained by X-ray diffraction and cryoelectron microscopy. The distribution of the proportions of the methods presented in Table 2 reflects that, in general, for the protein structures contained in the data bank.
The dataset structures are characterized by different qualities of resolution (Figure 3).
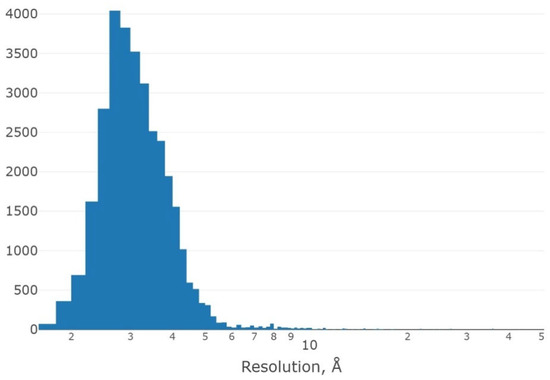
Figure 3.
Histogram of the distribution of the number of protein structures containing motifs of the dataset, depending on the resolution quality (logarithmic scale).
Figure 3 shows a histogram of the distribution of protein structures containing selected structural motifs, depending on the resolution of the determination. We see that the main part of protein structures is characterized by the quality of determination from 2 Å to 5 Å; only a small part of the proteins are solved with a resolution of more than 5.5 Å. The maximum is localized at resolutions around 3 Å. Thus, the dataset as a whole is characterized by a good quality of determination of protein structures, from which the structural motifs of 3β-corners were selected.
The created dataset contains a huge number of structures meeting the criteria of 3β-corner and about a twice as large amount of 3β-corner-like structures. These structures have common topology (contain two β-hairpins placed orthogonally in different layers), while 3β-corner-like structures do not meet short connection criteria (see criteria list in Methods above).
Structural analysis shows significant differences in the secondary structure between 3β-corners and 3β-corner-like structures.
3β-corners contain strictly three β-strands or four β-strands, with a short (up to 4 a.a.) connection between the 2nd and 3rd β-strands. The connections between strands in β-hairpins can contain short loops of various forms that can be recognized as a single turn of α-helix or 310-helix, with no more than one such turn per connection.
Figure 4 shows the distribution of the motifs by the amount of helix turns in them. There are only about 12% of the 3β-corner set that contain at least a single helix turn in their connections. 3β-corner-like structures can contain longer helices, loops and turns. More than 50% of the 3β-corner-like structures contain at least a single turn of the helix (α-helix or 310-helix), while the total amount of helix turns (two types combined) in the 3β-corner-like structure can reach 8.
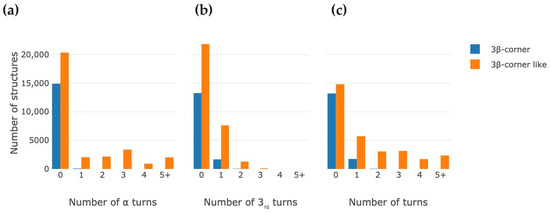
Figure 4.
Distribution histograms of the number of found motifs depending on the amount of helix coils in them. (a,b) Histograms show distribution based on separate α-helix turns and 310-helix turns respectively. (c) Histogram shows distribution of motifs depending on total amounts of helix turns in motif.
It is also noticeable that the rate of occurrence of the 310-helix turns is significantly higher in 3β-corners, while both types of helix turns have almost the same occurrence in 3β-corner-like structures. Further, longer helices in 3β-corner-like structures are α-helices: most of the 310-helices in these motifs have one or two turns, while a significant amount of 3β-corner-like structures (about 25%) contain from two to five α-helix turns.
While 3β-corners contain strictly 3–4 β-strands by definition, 3β-corner-like structures may contain more than four β-strands in their structures. While three or four β-strands form a structure’s basic geometry, longer connections can also contain additional short β-strands. Table 3 contains the distribution of the motifs’ percentage based on the number of β-strands they contain. It is clear that the absolute majority of 3β-corners and 3β-corner-like structures contain four β-strands, while about 6% of the 3β-corner-like structures contain at least (and commonly) one additional β-strand in their structure. The occurrence of structures with only three β-strands in both sets does not exceed 1%.

Table 3.
Distribution of 3β-corners and 3β-corner-like structures depending on number of β-strands in their secondary structure.
Figure 5 shows a histogram of the distribution motifs in the created dataset depending on the length. For the segmentation of motifs, selection criteria were determined; the main characteristics of the motif’s geometry are the length of the motif, the lengths of secondary structural elements and the distance between them, torsion angles, etc. It should be noted that the range of structure lengths were limited from 20 to 64 amino acid residues. Therefore, structures containing less than 20 amino acid residues and more than 64 amino acid residues were not included in the dataset by definition.
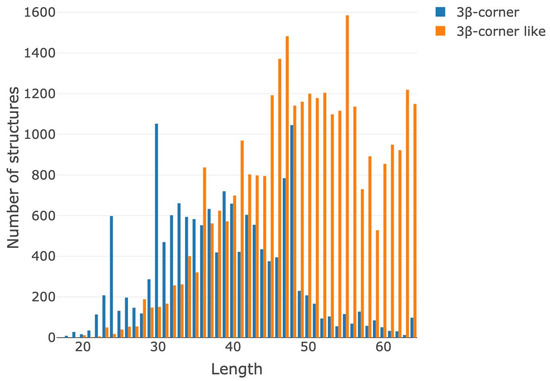
Figure 5.
Histogram of the distribution of the number of found motifs depending on the lengths (number of amino acid residues) of the structures.
Most of the structures contain 30–50 amino acid residues. The histogram distinctly shows that 3β-corner-like structures are significantly longer than 3β-corner (30–62 residues). This result is obvious because 3β-corner-like structures have no limitations for connections. The local maximums for 3β-corner-like structures’ length distributions are in intervals of 46–48 and about 56 residues (Figure 5).
The membership of studied motifs in taxonomic groups is shown in Figure 6.
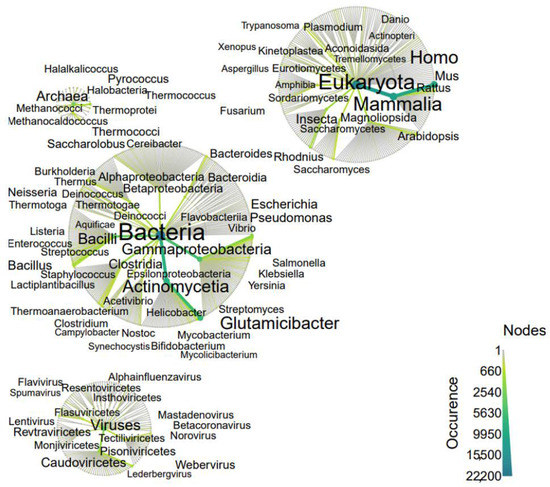
Figure 6.
Origin of proteins in which 3β-corners have been identified. The taxonomic community is revealed by a heat tree map, showing the taxonomic context from higher ranks in the center to lower ranks in peripheries (kingdom to class). The abundance of species (occurrence) can be quantified and visualized by color and size of the nodes and edges. Graph components, such as the size and color of text, nodes and edges, allow for the quantitative representation of multiple statistics simultaneously. Each node represents a taxon, and the edges determine where the taxon fits within the overall taxonomic hierarchy.
As shown in Figure 6, proteins containing the studied structural motifs are most represented in the kingdom of bacteria and eukaryotes, including mammals and viruses.
Figure 6 shows the distributions of the values of two parameters that characterize the geometry of the motif under study: the gyration radius (Rg, nm) and the solvent-accessible area (SASA, Å2).
As Figure 7a shows, the radius of gyrations for 3β-corners and 3β-corner-like structures is up to 25 nm. The 3β-corners distribution is two-modal: the first maximum is about 16 nm, while the second is 18 nm. The 3β-corner-like structures’ maximum is on 17 nm. The solvent accessible surface area for 3β-corners is in the range of 1000–8000 Å2, while the 3β-corner-like structures have bigger SASA values (1000–9000 Å2) with a maximum of about 6000 Å2. 3β-corners have two maximums at 4200 Å2 and 6000 Å2 (Figure 6b).
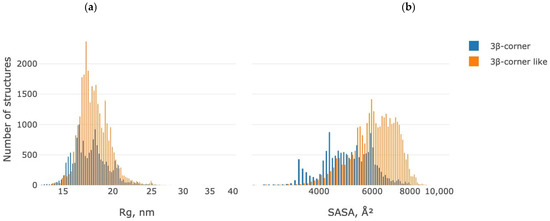
Figure 7.
Distribution of gyration radius (Rg, nm) (a) and solvent accessible area (SASA, Å2) values (b) for the dataset under study. Blue—3β-corner, orange—3β-corner-like structures.
Proteins whose three-dimensional structure contains the studied 3β-corner motif are diverse both in terms of localization in the cell and in terms of their molecular function and belonging to biological processes (Figure 8).
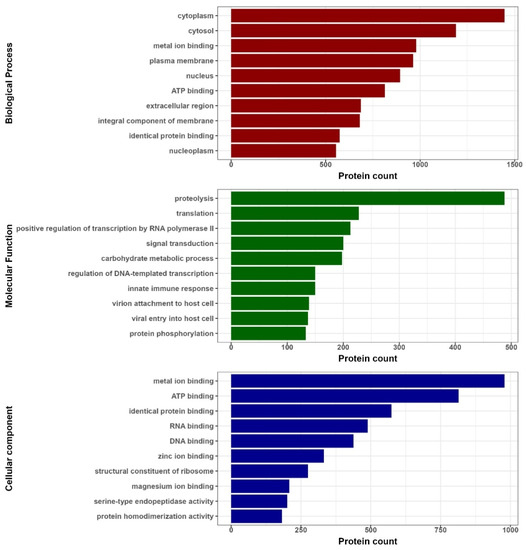
Figure 8.
Biological functions of proteins in which any of the researched motifs were identified. Red bars correspond to biological processes, green bars correspond to molecular functions (MF) and blue bars correspond to cell compartments (CC).
In the set of structures, the share of cytosolic proteins is about 37%, nuclear proteins—20%, membrane proteins—23%, extracellular proteins—10%.
In terms of molecular function, the largest in number are proteins belonging to the group of proteolytic enzymes (7%), participants in the regulation of translation (3%) and transcription (5%), signal transduction (3%), and participants in the regulation of carbohydrate metabolism (3%), regulation of the innate immune response (2%), viral proteins (4%) and participants in the post-translational modification of proteins (2%).
By biological processes, the proteins of our dataset are involved in the binding of metals (22%), ATP (11.5%), proteins (14%), RNA and DNA (7% and 6%, respectively) and ribosome structural proteins (7%).
4. Discussion
In this study, we draw the reader’s attention to the 3β-corner structural motif, which is widely found in nature and can be found in proteins of almost all known taxonomic groups. The analysis of the dataset for the motif reflects its structural heterogeneity, which is due to the “loops” of unstructured regions (coil). In such areas, the presence of helical elements (α- and 310-) is acceptable. At the same time, the general topology of the motif remains unchanged and contains, as a rule, three or four β-strands. If the structure contains three β-strands, one of the strands is bent and transits to another layer. In the case of structure formation by four β-strands, an orthogonal orientation of two β-hairpins is observed. The basic geometry and compactness of 3β-corners is unchanged, despite the differences in conformation and the length of constrictions between β-strands, the length (number of amino acid residues) of the irregular region that forms the bend of the polypeptide chain by approximately 90° and its transition from one β-layer to another, as well as the lengths of the β-strands themselves. The general topology of the 3β-corner structural motif is provided by contacts between β-strands. The structures of 3β-corners are characterized by a high proportion of hydrophobic amino acid residues and contacts between β-strands. 3β-corner-like structures commonly contain a greater number of β-strands conditioned by turns or short coils separating long β-strands. The unlimited length of the irregular regions between β-strands in β-hairpins, as well as the irregular region that ensures the bending of the polypeptide chain and its transition from one β-layer to another, in 3β-corner-like structures leads to an increase in the total length of such structures. The length of 3β-corner-like structures on average exceeds the length of 3β-corners due to the presence of different conformations of short sections of the polypeptide chain that break the main β-strands and determine the topology of 3β-corner-like structures.
Among parameters characterizing the geometry of the structural motif, two more parameters can also be distinguished: the gyration radius and the area accessible to the solvent. We showed the most typical values of Gyration radius and SASA characterized by its maximums of distribution. Earlier in our work, we discussed the biological role of the 3β-corner structural motif in proteins of various origins [28]. Proteins that embed the studied structural motif of the 3β-corner are diverse in terms of localization in the cell, as well as their molecular function and association with biological processes. We also note in this study that the motif is found in proteins that regulate almost all vital processes in cell ontogeny, including translation, transcription, signaling, metabolism and modification of proteins after synthesis.
The created dataset also contains 3β-corner-like structures. These protein motifs contain similar geometries: two β-hairpins in different plains are positioned almost orthogonally. These motifs differ from 3β-corners, as they can contain more that 3–4 β-strands; coils between β-strands can have a variety of lengths and conformations (e.g., helices). These characteristics are out of the 3β-corner definition, but we found these structures may be of interest to researches by their similarity to 3β-corners and their difference in structural organization. We also described and characterized these structures.
Thus, the dataset provides valuable information about the 3β-corner motif and 3β-corner-like structure; the list and abstract are available in the Supplementary Materials and allow you to conduct research on a large variety of structures and gain the necessary knowledge for research teams.
5. Conclusions
The 3β-corner is one of the known and actively studied types of structural motifs in protein molecules. This structure is often found in homologous and non-homologous proteins and has a unique and compact spatial fold. It is stable outside the protein globule. All this speaks in favor of the fact that the 3β-corner structural motif can be an embryo or a ready-made structural block in the process of protein folding, and can also act as an independent object of research in the field of structural biology. 3β-corners, as well as other structural motifs known to the scientific world, are considered as the nuclei of protein folding and starting structures for searching for possible folding of the polypeptide chain when modeling the structure of proteins.
We hope that the dataset of 3β-corner structures we prepared will be useful to research teams in the study of these structures and will help to obtain the necessary knowledge to understand the features and regularities of the structural organization of 3β-corners.
The dataset also contains 3β-corner-like structures that are similar in geometry and topology to the 3β-corner, while having some specific features in their structural organization. These motifs are not currently considered as structural motifs of protein molecules, but as candidates for being germinal structures in the process of protein folding. Yet, it is possible that the created dataset including a large number of 3β-corner-like structures recognized and selected will allow researchers to conduct a deep and comprehensive study of these structures.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pr11020368/s1, File S1 Dataset of 3β-corner and 3β-corner-like structures.
Author Contributions
Conceptualization, A.L.K., L.I.K. and V.R.R.; methodology, K.S.N., D.V.P. and V.R.R.; software, K.S.N., A.A.S. and D.V.P.; validation, K.S.N. and D.V.P.; investigation, V.R.R. and D.V.P.; data curation, A.L.K. and L.I.K.; writing—original draft preparation, A.L.K., L.I.K. and K.S.N.; writing—review and editing, V.R.R. and K.A.M.; visualization, K.S.N., K.A.M. and D.V.P. All authors have read and agreed to the published version of the manuscript.
Funding
The work was conducted in the framework of the Russian Federation Fundamental Research Program for the long-term period for 2021–2030 (No. 122092200056-9).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to A.V. Efimov for reading the manuscript and helpful remarks. Simulations have been performed using the equipment of the shared research facilities of HPC computing resources at Lomonosov Moscow State University and the Joint Supercomputer Center of the Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hollingsworth, S.A.; Karplus, P.A. A fresh look at the Ramachandran plot and the occurrence of standard structures in proteins. Biomol. Concepts 2010, 1, 271–283. [Google Scholar] [CrossRef]
- Chino, M.; Maglio, O.; Nastri, F.; Pavone, V.; DeGrado, W.F.; Lombardi, A. Artificial diiron enzymes with a de novo designed four-helix bundle structure. Eur. J. Inorg. Chem. 2015, 21, 3371–3390. [Google Scholar] [CrossRef]
- Chino, M.; Leone, L.; Maglio, O.; Lombardi, A. Designing Covalently Linked Heterodimeric Four-Helix Bundles. Methods Enzymol. 2016, 580, 471–499. [Google Scholar] [CrossRef]
- Adamian, L.I.; Liang, J. Helix-helix packing and interfacial pairwise interactions of residues in membrane proteins. J. Mol. Biol. 2001, 311, 891–907. [Google Scholar] [CrossRef]
- Trovato, A.; Seno, F. A new perspective on analysis of helix-helix packing preferences in globular proteins. Proteins Struct. Funct. Bioinform. 2004, 55, 1014–1022. [Google Scholar] [CrossRef]
- Efimov, A.V. Standard structures in proteins. Prog. Biophys. Mol. Biol. 1993, 60, 201–239. [Google Scholar] [CrossRef]
- Efimov, A.V. Favoured structural motifs in globular proteins. Structure 1994, 2, 999–1002. [Google Scholar] [CrossRef]
- Efimov, A.V. Structure of a-a-hairpins with short connections. Protein Eng. 1991, 4, 245–250. [Google Scholar]
- Levitt, M.; Chothia, C. Structural patterns in globular proteins. Nature 1976, 261, 552–558. [Google Scholar]
- Edwards, M.S.; Sternberg, J.E.; Thornton, J.M. Structural and sequence patterns in the loops of βαβ units. Protein Eng. 1987, 1, 173–181. [Google Scholar] [CrossRef]
- Chothia, C.; Levitt, M.; Richardson, D. Structure of proteins: Packing of α-helices and pleated sheets. Proc. Natl. Acad. Sci. USA 1977, 74, 4130–4134. [Google Scholar] [CrossRef] [PubMed]
- Schulz, G.E. The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta 2002, 1565, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Tamm, L.K.; Hong, H.; Liang, B. Folding and assembly of beta-barrel membrane proteins. Biochim. Biophys. Acta 2004, 1666, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Hagan, C.L.; Silhavy, T.J.; Kahne, D. β-Barrel membrane protein assembly by the Bam complex. Annu. Rev. Biochem. 2011, 80, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Taldaev, A.; Rudnev, V.; Kulikova, L.; Nikolsky, K.; Efimov, A.; Malsagova, K.; Kaysheva, A. Molecular Dynamics Study of Citrullinated Proteins Associated with the Development of Rheumatoid Arthritis. Proteomes 2022, 10, 8. [Google Scholar] [CrossRef]
- Wetlaufer, D.B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc. Natl. Acad. Sci. USA 1973, 70, 697–701. [Google Scholar] [CrossRef]
- Lin, S.I.; Tsai, J.; Nussinov, R. A study of 4-helix bundles–investigating protein folding via similar architectural motifs in protein cores and in subunit interfaces. J. Mol. Biol. 1995, 248, 151–161. [Google Scholar]
- Walther, D.; Eisenhaber, F.; Argos, P. Principles of helix-helix packing in proteins: The helical lattice superposition model. Mol. Biol. 1996, 255, 536–553. [Google Scholar] [CrossRef]
- Efimov, A.V. Super-secondary structures and modeling of protein folds. In Methods in Molecular Biology; Kister, A.E., Ed.; Humana Press: Clifton, NJ, USA, 2013; Volume 932. [Google Scholar]
- Fairman, J.W.; Noinaj, N.; Buchanan, S.K. The structural biology of β-barrel membrane proteins: A summary of recent reports. Curr. Opin. Struct. Biol. 2011, 21, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, J.R.; Nastri, F.; Maglio, O.; Pavone, V.; Lombardi, A.; De Grado, W.F. Artificial diiron proteins: From structure to function. Pept. Sci. 2005, 80, 264–278. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar]
- Efimov, A.V. A novel super-secondary structure of β-proteins. A triple-strand corner. FEBS Lett. 1992, 298, 261–265. [Google Scholar] [PubMed]
- Efimov, A.V. A structural tree for proteins containing 3β-corners. FEBS Lett. 1997, 407, 37–41. [Google Scholar] [PubMed]
- Chothia, C.; Janin, J. Orthogonal packing of beta-pleated sheets in proteins. Biochemistry 1982, 21, 3955–3965. [Google Scholar]
- Boshkova, E.A.; Efimov, A. Structures closed into cycles in proteins containing 3β-corners. Biochemistry 2010, 75, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, V.R.; Nikolsky, K.S.; Petrovsky, D.V.; Kulikova, L.I.; Kargatov, A.M.; Malsagova, K.A.; Stepanov, A.A.; Kopylov, A.T.; Kaysheva, A.L.; Efimov, A.V. 3β-Corner Stability by Comparative Molecular Dynamics Simulations. Int. J. Mol. Sci. 2022, 23, 11674. [Google Scholar] [CrossRef]
- Rudnev, V.R.; Petrovsky, D.V.; Nikolsky, K.S.; Kulikova, L.I.; Stepanov, A.A.; Malsagova, K.A.; Kaysheva, A.L.; Efimov, A.V. Biological Role of the 3β-Corner Structural Motif in Proteins. Processes 2022, 10, 2159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).