Abstract
Hydroponic production raises economic and environmental issues related to the treatment, recovery or disposal of hydroponic wastewater, which can be rich in eutrophication-related nutrients, nitrogen (N) and phosphorus (P). Little focus has been put on the influence of the growth conditions on the N and P content in hydroponic wastewater, which is of uttermost importance when it is intended to reuse the wastewater for irrigation or other purposes with reduced impact on the environment. This study aimed to optimize an indoor non-recirculating deep-water culture (DWC) hydroponic system for lettuce (Lactuca sativa L. var. crispa) production, in terms of daily light integral (DLI) and volume of nutrient solution (NS) per plant, to maximize both the biomass production and the N and P removal, allowing for the wastewater to meet the criteria established for reusing in irrigation and minimizing the eutrophication impacts. A small-scale DWC hydroponic system with a fluorescent light fixture was built to study lettuce growth indoors for 35 days after transplanting (DAT). A first experiment was conducted under 14, 20 or 23 mol m−2 d−1 DLI and with 1.5 or 2 L of NS per plant. A pronounced inner leaf tip burn was observed, regardless of the volume of NS solution used, related to the unventilated conditions under high radiation. Total biomass was similar in all treatments and N and P removal was higher than 95% and 94%, respectively, at 35 DAT. Lettuces grown in 2 L of NS per plant exhibited higher average biomass. A second experiment was performed under 8, 10 or 12 mol m−2 d−1 DLI and with 2 or 3 L of NS per plant, making it possible to achieve healthy biomass at 35 DAT with higher water and light-use efficiency when compared to the first experiment. A DLI of 10 or 12 mol m−2 d−1 with 2 L of NS per plant and a DLI of 12 mol m−2 d−1 with 3 L of NS per plant made it possible to achieve both the best total biomass production and the highest N and P removal from water. Under those conditions, hydroponic wastewater complied with N and P criteria for reuse in irrigation, showing potential to be used as an alternative resource for agriculture and to minimize negative impacts on the environment.
1. Introduction
Global water demand is expected to grow in the next decades [1] at a higher rate than the population and the economy [2]. Nowadays, agriculture accounts for 70% of all freshwater withdrawals globally, and water scarcity induced by climate change can be a great threat for agriculture [3,4]. Changes in agricultural practices to increase water use efficiency and water reuse can be of uttermost importance to address water scarcity. Agriculture practices such as hydroponics can be used to grow plants with high productivity and quality, no soil dependency, reduced crop duration and less fertilizers, while saving water when compared to conventional agriculture [5,6,7].
Plants can be hydroponically grown using different techniques, the most common being the nutrient film technique (NFT), deep water culture (DWC) and ebb-and-flow systems (e.g., [8]). In DWC, plants grow suspended on a platform on the surface of a nutrient solution stored in a basin or box with air supply. Owing to its simplicity, low operation costs and great yield, DWC has been suggested to be used in the production of vegetables such as lettuce [5]. In fact, lettuce is one of the most common plants grown hydroponically because it is a fast-growing culture, can be matured in one month and its seeds are easily germinated in several media. Hydroponics is usually performed in greenhouses or inside buildings, such as plant factories where little or no solar radiation is available. While in both environments the main requirements for lettuce growth are similar, the challenges to achieve adequate environmental conditions, namely air temperature, light and ventilation can be rather different [9,10].
To produce lettuce hydroponically in buildings, the main challenges are related to the optimization of artificial light quality, intensity and duration and ventilation conditions. Different types of lamps, such as fluorescent, light-emitting diode (LED) and high-pressure sodium lamps, can be used successfully to grow vegetables indoors [11,12]. However, LED lighting has been shown to increase lettuce yield and energy use efficiency when compared to fluorescent lighting [13]. With a proper nutrient solution and light source, the available radiation flux density (photosynthetic photon flux density, PPFD), the photoperiod and the resulting daily light integral (DLI) are key parameters that influence lettuce growth and quality, light-use efficiency (LUE) and water-use efficiency (WUE) in production inside buildings. An optimal DLI for lettuce usually falls between 14 and 18 mol m−2 d−1 but can vary among species. In a study performed by Pennisi et al. [14], a DLI of 14.4 mol m−2 d−1 from LED lighting based on a photoperiod of 16 h d−1 proved to be the best condition for lettuce (cultivar ‘Rebelina’) biomass production in a vertical farm, also maximizing the LUE and WUE. Studying different PPFDs between 150 and 300 μmol m–2 s–1, provided by fluorescent and LED lamps with different red/blue ratios and two photoperiods of 12 and 16 h per day, Zhang et al. [15] concluded that the use of LED with a red/blue ratio of 2.2 with a 16 h per day photoperiod and a PPFD of 250 μmol m–2 s–1 (14.4 mol m−2 d−1 DLI) were the best growth conditions for commercial lettuce (cultivar ‘Ziwei’). Romaine lettuce exhibited high biomass productivity under a DLI ranging from 11.5 to 17.3 mol m−2 d−1 and photoperiods of 16 to 20 h per day in controlled indoor environments [16]. Kelly et al. [17] studied the growth of two lettuce cultivars (‘Rex’ and ’Rouxai’) in 6.9–15.6 mol m−2 d−1 DLI, using a range of 120–270 μmol m–2 s–1 PPFD and photoperiods of 16 to 24 h per day. They found that the biomass, leaf width and number and chlorophyll concentration correlated positively with the DLI, rather than either PPFD or photoperiod alone. However, for a given DLI, they observed a greater production under lower PPFD and higher photoperiod, so the combination of PPFD and photoperiod can affect plant growth. Previous studies have demonstrated that excessive levels of PPFD can decrease the fresh biomass of lettuce, likely due to radiation stress [18]. Potential negative effects of high PPFD and high DLI can lead to low transpiration rates in young lettuce leaves, causing calcium deficiency, which promotes a disorder known as inner tip burn (e.g., [19]). This disorder has been observed under a combination of high DLI and insufficient ventilation, both in greenhouses and inside buildings. Since ventilation can greatly increase the cost of lettuce production, it has been suggested to avoid DLIs over 12 mol m−2 d−1 to prevent lettuce inner tip burn in closed and unventilated environments [20].
One important economic and environmental issue related to hydroponic production is the treatment, recovery or disposal of hydroponic wastewater. Hydroponic wastewater can be recycled to produce a nutrient solution based on a target level of electrical conductivity, but this common practice can significantly reduce productivity due to nutrient imbalances [21] and accumulation of toxic root exudates [22]. Reusing hydroponic wastewater in irrigation raises other concerns, mainly related to the level of salinity and the content of eutrophication-related nutrients, nitrogen and phosphorus (N and P). A high concentration of nitrate (200–300 mg NO3-N L−1) and phosphorus (30–100 mg PO4-P L−1) in hydroponic wastewater has been reported [23], which most certainly renders the wastewater improper for irrigation. For example, FAO guidelines on water quality for irrigation [24] present a range of nitrogen and salinity according to the degree of restriction on use (respectively >30 mg NO3-N L−1 and >3 dS m−1 for severe restriction; 5–30 mg NO3-N L−1 and 0.7–3 dS m−1 for slight and moderate restriction; <5 mg NO3-N L−1 and <0.7 dS m−1 for no restriction). In Portugal, according to 1998 legislation, the recommended limit of N content in water for irrigation is 50 mg NO3 L−1 (11.3 mg NO3-N L−1); the maximum recommended salinity is 1 dS m−1 (total dissolved solids, 640 mg L−1) (DL 236/98) [25]. Recent legislation (DL 119/2019) [26] about the reuse of water from domestic, urban or industrial wastewater treatment set new water quality standards for irrigation, recommending contents of total N and P below 15 mg N L−1 and 5 mg P L−1, respectively. Approaches to treatment, recovery and reuse of nutrients from hydroponic wastewaters have been discussed [27,28]. Additionally, it would be fundamental to grow marketable vegetables hydroponically while taking into consideration the nutrient removal at the end of a growth cycle to allow for hydroponic wastewaters to be reused in irrigation according to the legal standards or other purposes with less impact on the environment. Little focus has been put on the influence of the growth conditions, such as the combination between DLI and volume of nutrient solution per plant, on the N and P content in hydroponic wastewater. This information would be important not only for producers but also for researchers in the field of residual wastewater treatment by hydroponics, which require control assays based on proven optimal growth conditions.
The main goal of the present study was to optimize an indoor/laboratory non-recirculating DWC hydroponic system for lettuce (Lactuca sativa L. var. crispa) production, in terms of DLI and volume of nutrient solution per plant, to maximize both the biomass production and the N and P removal, allowing for the wastewater to meet salinity and eutrophication-related nutrients criteria to be reused in irrigation or other purposes. It is intended to use the findings of this study in the design of an indoor non-recirculating DWC hydroponic system to treat swine wastewater pre-treated by vermifiltration. In a previous study, a recirculating indoor DWC system was used for the treatment of similar wastewaters, raising challenges related to nutrient balance, crop health and productivity as well as water treatment efficiency [29]. The present work aims to provide key information to further investigate those previously raised questions.
2. Materials and Methods
2.1. Hydroponic Setup
A frisee cultivar of leaf lettuce (Lactuca sativa L. var. crispa) was grown indoors, in a 130 m2 laboratory located at the School of Technology and Management of Polytechnic of Leiria, at room temperature (21–22 °C) with no direct solar radiation, by deep-water culture (DWC) hydroponic technique in six 8-litre opaque plastic 49 cm long, 18 cm wide and 14 cm tall containers with narrow bottom, placed side by side on a table (Figure 1). Aeration was ensured by porous glass diffusers fixed at the bottom of each container and fed by a central compressed air line.
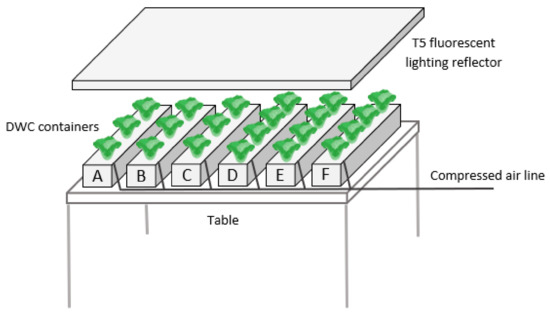
Figure 1.
Schematic representation of the hydroponic setup (not to scale).
Plants were individually placed in 5.5 cm diameter plastic net pots (Bulsø Plastics, Copenhagen, Denmark) that were filled with light expanded clay aggregate (LECA) for root support and placed in holes drilled through 2 cm thick polystyrene boards cut to fit each container. Artificial light for photosynthesis was provided by a horizontally suspended 1190 × 605 × 60 mm Intertek 4,008,920 Reflector holding up to 8 high-output 54 W T5 PRO Pure Light (6500 K) fluorescent tubes. The number of lit tubes and the height above the crops could be changed to optimize photosynthetic photon flux density (PPFD). The daily photoperiod was set to 16 h, controlled by an electric timer socket.
Containers were filled with 6 L of hydroponic nutrient solution (NS). A reference hydroponic NS for lettuce production was used, prepared according to Carvalho et al. [30] with some components replaced by YaraTeraTM CalcinitTM fertilizer (1.1% ammonia nitrogen, 14.4% nitrate nitrogen and 26.5% calcium oxide). The composition of the reference NS is shown in Table 1.

Table 1.
Reference nutrient solution (NS) composition.
2.2. Experiments
In this study, lettuce growth was assessed in two separate batch cycles over 35 days after transplantation (DAT), which is a widely used growth period (e.g., [31]). Young lettuces for hydroponic growth were purchased at a local agricultural supply store (Agriloja, Leiria, Portugal). Prior to transplantation, roots were gently washed of soil with tap water.
In a first batch growth cycle (Experiment 1), six containers were placed side by side under the artificial light source, placed 30 cm above the container’s tops. Three plants were placed in each one of three containers (A–C), and four plants in each one of the other three containers (D–F), as shown in Figure 1. This corresponded to 2 L or 1.5 L of NS per plant, respectively. A previous study [32] used 5–6 L of NS per plant to grow the same cultivar on the same NS, and a low depletion of N and P contents was observed at 35 days of study. Thus, it was estimated that 1.5–2 L of NS per plant would be an adequate starting point according to the objective of the present study.
In a second growth cycle (Experiment 2), six containers were used in the same arrangement as before, this time with the light source 43 cm above the containers and with either three (A–C) or two plants per container (D–F), respectively corresponding to 2 L or 3 L of NS per plant.
Water loss by evapotranspiration was accounted for by periodic additions of tap water.
2.3. Light, Temperature and Crop Growth Monitoring
PPFD was measured at a 10 cm height above the polystyrene board with a quantum sensor (LI-250Q PAR package, LI-COR, Lincoln, NE, USA) and DLI (mol m−2 d−1) determined as PPFD (μmol m−2 s−1) × (photoperiod (h) × 3600 s h−1) × 100,000. Indoor air temperature and air temperature underneath the light source were measured with a K-type thermocouple (HI-935007, Hanna Instruments, Woonsocket, RI, USA). Plants were measured twice a week, with respect to aerial height and diameter, largest leaf length and width, with a tape measure. The number of leaves was counted, considering the leaves longer than 2 cm. Leaves with more than half of their area dead were excluded. Fresh weight of each plant was measured at the beginning and at the end of each experiment, as well as root fresh weight at the end, using a 0.0001 g precision analytical balance (Precisa Gravimetrics 262SMA-FR, Geneva, Switzerland). Dry weight was estimated considering 5% of the fresh weight [33].
2.4. Water Quality and Nutrients Monitoring
Water electrical conductivity (EC) and temperature were measured with a Hanna Instruments HI 98,304 DiST® 4 probe; pH was measured with a Hanna Instruments HI 98,107 pHelp® probe.
Nitrogen and phosphorus parameters were quantified by colorimetric methods using a VARIAN-Cary 50 UV–vis Spectrophotometer: total nitrogen (TN) and nitrate nitrogen (NO3-N) were determined by the brucine method with persulphate digestion for total nitrogen, according to the United States Environment Protection Agency (EPA) method 352.1 [34], ammonia nitrogen (NH3-N) according to the international standard ISO 7150-1:1984 [35] and dissolved phosphorus (PO4-P) according to the Standard Method for the Examination of Water and Wastewater (SMEWW) 4500-P E [36].
2.5. Calculations and Statistical Analysis
Water use efficiency (WUE) was calculated as the ratio between shoot fresh weight increase (considering negligible the initial fresh root weight) and the volume of water consumed by evapotranspiration throughout the experiment and expressed as g FW L−1. Light use efficiency (LUE) was determined according to Pennisi et al. [37] as the ratio between shoot dry weight production per unit of area (g DW m−2) and the total incident light received throughout the experiment (DLI × number of days; mol m−2), and expressed in g DW mol−1. When suitable, means and standard errors of the mean were calculated for each replicate sample. One-way analysis of variance (ANOVA) and Tukey test (H0: no significant difference between two samples; α = 0.05) were used to compare means whose uncertainty distribution could be safely assumed to be normal.
3. Results and Discussion
3.1. Light and Lettuce Growth Parameters
An important limitation of the use of artificial light is the inability to evenly distribute light over the whole study area. PPFD tends to be higher in the center of the illuminated area and lower at the borders. However, this limitation became an opportunity to study the effect of light flow density on hydroponic growth and nutrient uptake by simultaneously monitoring lettuce exposed to different PPFD levels. In Experiment 1, the artificial light source was placed at a height that would provide an average DLI of 14.4 mol m−2 d−1 (PPFD of 250 µmol m−2 s−1 and 16 h of photoperiod) on the outermost containers (A and F), which has been reported to be adequate for lettuce growth [14,15,16]. The resulting light distribution made it possible to obtain an average DLI of 20.2 mol m−2 d−1 (PPFD of 350 µmol m−2 s−1 and 16 h of photoperiod) on containers B and E and 23.0 mol m−2 d−1 (PPFD of 400 µmol m−2 s−1 and 16 h of photoperiod) on containers C and D.
The dry weight (DW) of each plant was determined (estimated as 5% of fresh weight) at the beginning and at the end of the experiment (Table 2), 35 days after transplantation (DAT). Total DW gain per container exhibited no significant differences for plants exposed to different DLI levels and volumes of nutrient solution (NS).

Table 2.
Lettuce dry weight (DW) increase after 35 DAT in Experiment 1. Different superscript letters indicate values with significant difference (ANOVA with Tukey test; p < 0.05).
These results suggest that a DLI level of 14.4 mol m−2 d−1 was not limiting to biomass gain and therefore higher light intensities could be considered redundant in the conditions of this study. The comparison of DW gains of all nine plants from containers A, B and C to all twelve plants from containers D, E and F exhibited a significant difference in average dry biomass (p = 0.0015; ANOVA with Tukey test); thus, a higher biomass per plant was obtained with 2 L of NS. Individual plant size was rather dependent on the amount of nutrients available to each plant, which should be a factor to take into consideration under non-limiting light conditions.
Another relevant observation concerned the plants’ health under different lighting conditions. A pronounced necrosis known as inner leaf tip burn was observed in containers B, C, D and E after 15 DAT and in A and F after 25 DAT (Figure 2). This suggests a combined effect of high DLI and relatively high temperature (26.0 to 27.5 °C at the tips of the leaves, at 21.5 °C ambient temperature), promoting accelerated growth which, in turn, under poor ventilation conditions may have resulted in a deficient young leaf transpiration and consequent calcium deficiency [38,39,40].

Figure 2.
A typical example of inner tip burn observed in lettuces in Experiment 1.
To prevent the occurrence of this disorder in poorly ventilated environments, it has been suggested to avoid DLIs over 12 mol m−2 d−1 when no forced ventilation is provided [20]. To accommodate for that, the artificial light source placement distance was increased, and two middle light tubes were removed in Experiment 2. This resulted in an average DLI of 8.06 mol m−2 d−1 (PPFD 140 µmol m−2 s−1 and 16 h of photoperiod) on containers A and F, 10.4 mol m−2 d−1 (PPFD 180 µmol m−2 s−1 and 16 h of photoperiod) on containers B and E and 12.1 mol m−2 d−1 (PPFD 210 µmol m−2 s−1 and 16 h of photoperiod) on containers C and D. Lower levels of radiation meant temperatures at the tips of the leaves 2 to 3 °C lower than in Experiment 1, i.e., around 24–25 °C (at 22 °C ambient temperature). Biomass results obtained after 35 days of growth exhibited relevant differences from Experiment 1 (Table 3).

Table 3.
Lettuce dry weight (DW) increase after 35 DAT in Experiment 2. Different superscript letters indicate values with significant difference (ANOVA with Tukey test; p < 0.05).
Average DW gain was higher in containers exposed to more photosynthetic light, revealing that a DLI of 8.06 mol m−2 d−1 was insufficient to produce as much lettuce as under 12.1 mol m−2 d−1. Regarding the total DW, only containers B and C achieved biomass gains similar to those obtained in Experiment 1. However, concerning individual plant biomass, 3 L of NS instead of 2 L of NS made it possible to produce larger lettuces under both 12.1 mol m−2 d−1 and 8.06 mol m−2 d−1 DLI. No tip burn disorders were observed under the adjusted lighting conditions.
In addition to dry weight estimation, plants were measured repeatedly during Experiment 1 and Experiment 2 (Figure 3). Concerning the aerial structure height and diameter, no consistent differences were observed between containers in each experiment at 35 DAT, apart from aerial height between containers F and C in Experiment 2, with no apparent relevance. In Experiment 1, the aerial structure growth rate was slightly higher but exhibited a more pronounced decrease from 21 to 25 DAT than in Experiment 2. In Experiment 1, the largest leaf length growth tended to stagnate after reaching around 12 cm at 18 to 21 DAT, and the largest leaf width growth slowed down dramatically after 21 DAT, while in Experiment 2 the growth of the largest leaf only slowed down after 28 DAT, with no significant difference between containers at 35 DAT. A higher growth rate and earlier stagnation in Experiment 1 may reflect a faster metabolism under a higher PPFD and, consequently, faster depletion of growth-limiting nutrients in the medium. The number of leaves in Experiment 1 was significantly different only between containers C and F at 35 DAT, but no clear conclusion can be drawn regarding the influence of DLI or NS per plant. However, the average number of leaves of Experiment 2 (20.6) was significantly lower (p < 0.05) than in Experiment 1 (26.2) at 35 DAT.
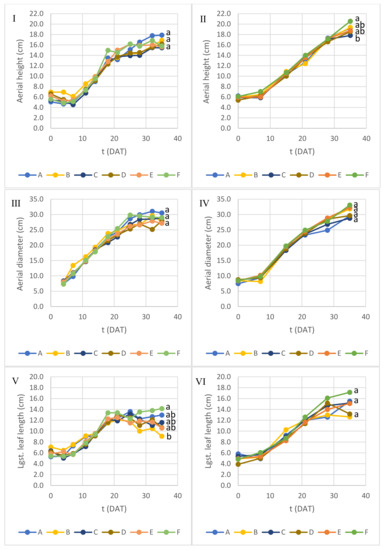
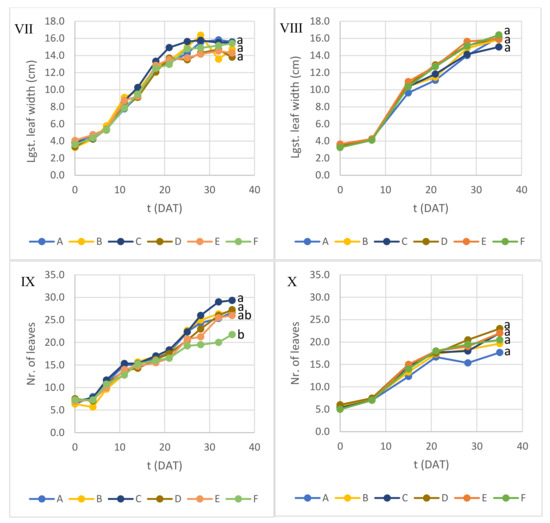
Figure 3.
Lettuce growth parameters over time during (I,III,V,VII,IX) Experiment 1 and (II,IV,VI,VIII,X) Experiment 2. Different letters at 35 DAT indicate average values with significant difference (ANOVA with Tukey test; p < 0.05).
It should be noticed that structure measurement results should be taken cautiously since lettuce leaves had a very elaborate anatomy, compromising the accuracy of superficial measurements. Leaf count also provided results of limited accuracy since the smallest and youngest leaves (<2 cm leaf length) were not considered for counting.
Overall, the results suggest that a DLI between 10 and 12 mol m−2 d−1 from fluorescent lighting can be a good middle-ground solution for faster production of healthy lettuce of marketable size and look on the nutrient solution used in these experiments. Other nutrient media may require different physical conditions, and an initial optimization phase would be advisable for each hydroponic growth installation.
3.2. Water-Use Efficiency (WUE) and Light-Use Efficiency (LUE)
The WUE and LUE results for Experiment 1, calculated over the entire 35 DAT, are presented in Table 4.

Table 4.
WUE and LUE results obtained in Experiment 1.
WUE exhibited no major differences between containers and no correlation with either lighting conditions or volume of NS per plant. However, LUE was higher in containers with a lower DLI (14.4 mol m−2 d−1). These results represent overall biomass gains per mol of photosynthetically active photons; since the biomass gains were similar in all containers at 35 DAT, LUE mainly reflects the overall light use during that period, which was not related to the plants’ physiological state. Measuring or estimating shoot dry weight over shorter periods of time during growth under known lighting conditions could provide more conclusive results.
In Experiment 2, both WUE and LUE exhibited significantly higher values (p < 0.00001 and p = 0.00056, respectively; ANOVA with Tukey test) than in Experiment 1 (Table 5).

Table 5.
WUE and LUE obtained in Experiment 2.
WUE tended to be higher for plants grown as two per container (3L of NS per plant, containers D, E and F) when compared within the same DLI level and, generally, also higher in containers exposed to more photosynthetic light under the same volume of NS per plant. Less plants per container may have caused less water loss by transpiration; on the other hand, a less crowded plant placement in the containers allowed them to grow more freely, increasing their biomass more efficiently. The best WUE of this study, around 70 g FW L−1, was slightly higher than the maximum WUE (60 g FW L−1) observed by Pennisi et al. [37] in indoor cultivation of lettuce under 14 mol m−2 d−1 DLI provided by LED lighting. Comparing with traditional agriculture, WUEs of 4 and 50 g FW L−1 have been reported for lettuce cultivation in open-field and greenhouse, respectively [7].
Conversely, LUE tended to exhibit higher values in containers exposed to less photosynthetic light and same volume of NS per plant and within the same DLI level in those with three plants (2 L of NS per plant) rather than two (3 L of NS per plant). This suggests that over a sufficiently long period it may be possible to achieve a good biomass production even on lower photosynthetic light flows and on less NS per plant. The highest LUE of this study (ca. 0.65 g DW mol−1) was lower than the maximum LUE (1.03 g DW mol−1) observed by Pennisi et al. [37]. A study conducted by Jin et al. [41], which compared lettuce growth in vertical farms with greenhouse and field cultivation, reported an average LUE of 0.55 g DW mol−1 for lettuce cultivated in vertical farms, which was similar to the values obtained in Experiment 2. The same study reported lower LUE for lettuce cultivated in greenhouse (0.39 g DW mol−1) and in open field (0.23 g DW mol−1). A more detailed multivariate study could make it possible to find an optimized balance of light, planting density and water supply for maximized biomass production in the shortest time, while minimizing energy consumption and water use.
3.3. Water Quality and Nutrient Uptake
3.3.1. Water Temperature, pH and Electrical Conductivity (EC)
Water temperature, pH and EC were measured throughout Experiments 1 and 2 for background conditions control. As the hydroponic containers were not thermostatized, water temperature varied in response to ambient air temperature and heat from the fluorescent light tubes—between 19 and 25 °C during Experiment 1 and between 19 and 22 °C during Experiment 2 (Figure 4). Temperature differences between containers, when observed, could not be considered consistent with either DLI or biomass growth.
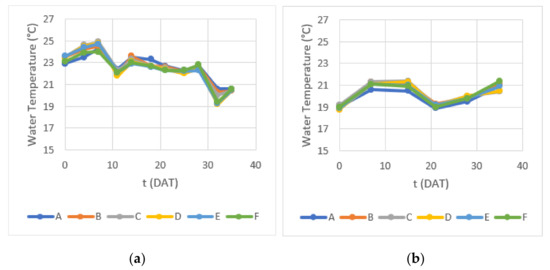
Figure 4.
Water temperature measured during (a) Experiment 1 and (b) Experiment 2.
In this study, it was decided to not correct pH to understand its variation associated with the predicted nutrient removal. pH started between 6.5 and 7.0 in both experiments and exhibited an increase to 7.7–8.1 in the first 21 DAT in Experiment 1, later stabilizing around those values (Figure 5). In Experiment 2, a slower increase in pH was observed and after 28 DAT reached values between 8.2 and 8.4 in containers A, B and F, and between 8.7 and 8.9 in containers C, D and E, ranging from 8.5 to 8.8 at 35 DAT.
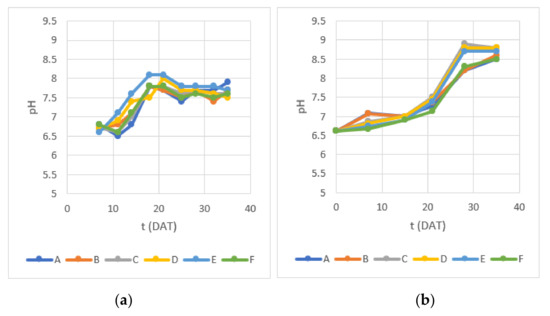
Figure 5.
Water pH measured during (a) Experiment 1 and (b) Experiment 2.
The optimal pH for lettuce production usually falls between 6 and 7 [9,10]. pH increase during lettuce hydroponic growth may be attributed to nutrient depletion and carbonic acid turning into CO2 that evaporates under relatively warm water temperatures. High pH values, mainly in the last 10 days of both experiments, may have become a major limitation of this study, because that may have contributed to limited absorption of iron (Fe), manganese (Mn) and zinc (Zn) [42], with possible negative impacts on photosynthesis [43]. However, no visual signs related to these nutrients’ deficiency [44] were detected. Moreover, while N availability is less sensitive to alkaline pH in the range observed in this study, P availability is expected to slightly decrease because phosphate (HPO4−2) is the main available form in this pH range and may be precipitated as calcium phosphate in aqueous nutrient solutions [45]. According to the Portuguese law, the recommended pH maximum in water for irrigation is 8.4, and the maximum admissible value is 9 (DL 236/98) [25]. A pH correction during the growth period or further treatment to lower pH in the hydroponic wastewater is therefore advisable before it is reused for irrigation; however, the impact of these measures on EC must be assessed as pH correction can increase salts in water.
EC exhibited a general decrease over time in both Experiments 1 and 2, consistent with the uptake of dissolved ionic nutrients by plants (Figure 6).
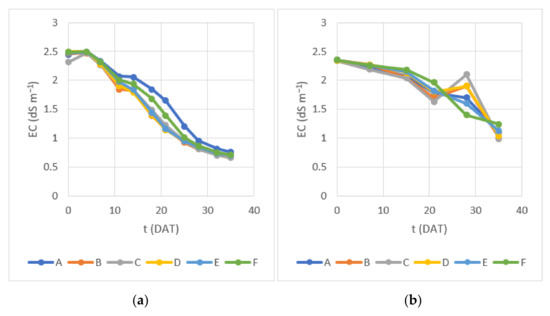
Figure 6.
Water electrical conductivity measured during (a) Experiment 1 and (b) Experiment 2.
Optimal EC for lettuce growth usually falls between 1.2 and 1.8 dS m−1 [9,10]. Andriolo et al. [46] studied lettuce hydroponically grown under increasing EC levels (0.80, 1.93, 2.81, 3.73 and 4.72 dS m−1) and observed a shoot fresh mass increase of 28.5% when EC was raised from 0.80 to 1.93 dS m−1 and a reduction of 16.5% in shoot fresh mass when EC was increased from 1.93 to 4.72 dS m−1. The relatively high values of EC in the beginning of the study of both experiments (2.3–2.5 dS m−1) were below levels that can lead to moderate salinity effects in lettuce [47,48]. EC decrease was generally faster in Experiment 1, especially in containers B through E, consistent with higher DLI values. Final EC values in Experiment 1 were below the maximum of 1 dS m−1, recommended for irrigation by Portuguese law (DL 236/98) [25]; in Experiment 2, EC was measured between ca. 1 dS m−1 (0.99 dS m−1 in container C; 1.0 dS m−1 in container B; 1.04 dS m−1 in container D) and 1.24 dS m−1, which can be considered marginally above the recommended value. A few more days of growth would ensure EC levels consistently below 1 dS m−1 in containers with a higher DLI.
3.3.2. Nitrogen and Phosphorus
Nitrate nitrogen (NO3-N) and dissolved phosphorus (PO4-P) concentration changes over time in Experiment 1 are presented in Figure 7.
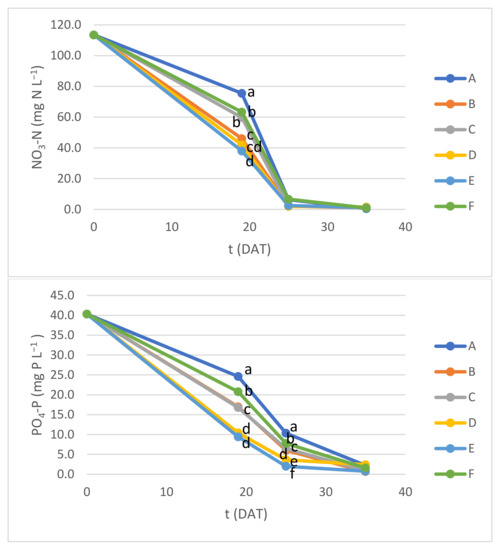
Figure 7.
Nitrate nitrogen (NO3-N) and dissolved phosphorus (PO4-P) concentrations during Experiment 1. Different letters within the same DAT indicate average values with significant difference (ANOVA with Tukey test; p < 0.05).
An increasing removal rate of these nutrients from 0 to 25 DAT and a slower removal from 25 to 35 DAT was observed, consistent with initially accelerating biomass growth, which later became slower due to nutrient depletion. Plant containers exposed to lower DLI (A and F) tended to show slower NO3-N and PO4-P removal than those under higher DLI. Between containers exposed to the same DLI, those with 2 L of NS per plant (A, B and C containers) also tended to exhibit slower nutrient removal than the corresponding ones with 1.5 L of NS per plant (D, E and F containers). This suggests that a larger number of individual plants (less NS per plant) may allow for more efficient nitrogen and phosphorus removal from a given volume of water at earlier stages of growth. However, by the end of 35 DAT, all containers had similar remaining nitrogen and phosphorus levels, suggesting that 2 L of NS per plant was the best compromise between nutrient removal and biomass production per plant, because higher individual biomass was achieved with 2 L of NS per plant. At 35 DAT, the relative removal was at least 95% for total nitrogen, 98% for ammonia nitrogen (NH3-N), 99% for NO3-N and no less than 94% for PO4-P, with no significant difference between containers (Table 6).

Table 6.
Relative nitrogen and phosphorus variation after 35 days, Experiment 1. Data are presented as mean ± SD. Different superscript letters within the same column indicate values with significant difference (ANOVA with Tukey test; p < 0.05).
All nutrient parameters at 35 DAT were compliant with Portuguese law concerning treated wastewater reuse for irrigation (recommended values: TN below 15 mg N L−1, ammonia nitrogen below 10 mg NH4 L−1 (8.2 mg NH3-N L−1) and total phosphorus (TP) below 5 mg P L−1) (DL 119/2019) [26]. In a previous study [32], where the same cultivar was grown on the same nutrient solution, PO4-P corresponded to at least 98% of TP; thus, the estimated TP in all containers of this study still meet the legal criteria. Nutrient removal per gram of dry weight of produced biomass showed results with no clear correlation to volume of NS per plant or DLI, except for NH3-N, where a higher removal per dry weight was observed in containers B and F (Table 7).

Table 7.
Nitrogen and phosphorus variation per dry weight of plant biomass production over 35 days in Experiment 1. Data are presented as mean ± SD. Different superscript letters within the same column indicate values with significant difference (ANOVA with Tukey test; p < 0.05).
Figure 8 shows the changes of nutrient levels over time in Experiment 2. In this second experiment it was also possible to monitor the evolution of NH3-Nlevels during the first three weeks.
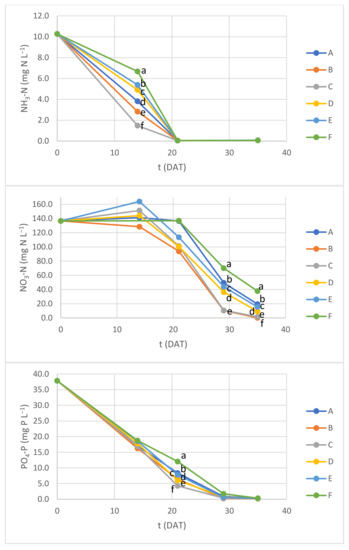
Figure 8.
Ammonia nitrogen (NH3-N), nitrate nitrogen (NO3-N) and dissolved phosphorus (PO4-P) concentrations during Experiment 2. Different letters within the same DAT indicate average values with significant difference (ANOVA with Tukey test; p < 0.05).
Similar to Experiment 1, in Experiment 2 containers that either contained the same volume of NS per plant but were exposed to lower photosynthetic radiation levels, or those under the same radiation intensity but with higher volume of NS per plant exhibited a tendency to have slower nutrient uptake, best noticeable over the first 14 DAT for NH3-N, over the second half of the 35 DAT for NO3-N and between 14 and 21 DAT for PO4-P. A stabilization or increase in NO3-N was detected during the first 14 DAT, which is a behavior observed in previous studies when vegetables are hydroponically grown in conventional inorganic nutrient solutions (e.g., [49]). NO3-N levels result from a balance of two main processes: uptake by plants and nitrification by bacteria, the latter also depending on ammonia levels, which decrease simultaneously due to uptake. A transient net increase in NO3-N suggests that NO3-N production from ammonia by bacterial activity initially prevailed over the uptake of both nitrogen forms by growing plant biomass.
Total nitrogen relative removal in this experiment was lower in containers under lower DLIs and also lower in containers containing 3 L of NS per plant (Table 8). A similar pattern was also observed for NO3-N (with better statistical evidence when comparing the final average values, Figure 8), but not for NH3-N or PO4-P. The lowest relative removal values were 85.1% for total nitrogen, 72% for NO3-N and 99% for both NH3-N and PO4-P.

Table 8.
Relative nitrogen and phosphorus variation after 35 days, Experiment 2. Data are presented as mean ± SD. Different superscript letters within the same column indicate values with significant difference (ANOVA with Tukey test; p < 0.05).
Concerning recommended limits for treated wastewater reuse in irrigation purposes (DL 119/2019) [26], in Experiment 2 phosphorus reached levels below recommended limits after 29 DAT. However, total nitrogen fell below the recommended limits only in containers B, C and D after 35 DAT. Thus, a DLI of 10 or 12 mol m−2 d−1 with 2 L of NS per plant or a DLI of 12 mol m−2 d−1 with 3 L of NS per plant were the best conditions to achieve N and P levels compliant with the recommended contents for wastewater reuse in irrigation under the conditions of this study. Regarding the nutrient removal per gram of dry weight of produced biomass, in Experiment 2 the values tended to be significantly higher in containers under the lowest DLI (8.06 mol m−2 d−1), except for NO3-N, and for total nitrogen, ammonia and phosphorus, higher in containers with higher volume of NS per plant for the same DLI level (Table 9). Seemingly, a slow growth rate under a low DLI level (8.06 mol m−2 d−1) tended to increase the efficiency of nitrogen and phosphorus removal per dry weight of produced plant biomass.

Table 9.
Nitrogen and phosphorus variation per dry weight of plant biomass production over 35 days in Experiment 2. Data are presented as mean ± SD. Different superscript letters within the same column indicate values with significant difference (ANOVA with Tukey test; p < 0.05).
4. Conclusions
In the present study, lettuce growth and eutrophicating nutrient removal were studied in an indoor small-scale batch DWC hydroponic system during 35 days after transplanting (DAT) under different daily light integrals (DLIs), provided by fluorescent light, and different volumes of nutrient solution (NS) per plant. Concerning lettuce growth, DLI levels from 14.4 to 23.0 mol m−2 d−1 and 1.5 or 2 L of NS per plant were sufficient to ensure equal total biomass increase at 35 DAT in all treatments. However, pronounced inner tip burn was observed, presumably as a consequence of excessive light intensity and heat combined with poor ventilation. The distance of the light source was increased to obtain DLIs of 8.06, 10.4 and 12.1 mol m−2 d−1 for the different containers, and a new cycle of growth over 35 DAT was performed with 2 or 3 L of NS per plant. The inner tip burn issue was eliminated for the whole 35 days of growth, water-use (WUE) and light-use efficiency (LUE) were considerably higher under these lower DLI levels, but a DLI of 8.06 mol m−2 d−1 became limiting to lettuce growth on the medium used. Plant measurements showed a lower initial growth rate and later stagnation as well as less leaves produced under these lower DLIs. A greater average biomass per plant was achieved with 3 L of NS per plant, which could be relevant from the point of view of commercial lettuce production.
Hydroponic lettuce growth caused an increase in the pH over time in all treatments to values that however did not exceed the maximum admissible value for irrigation in Portugal. Electrical conductivity was lowered from the initial 2.3–2.5 dS m−1 to around 0.7 dS m−1 under DLIs from 14.4 to 23.0 mol m−2 d−1, and around 1 dS m−1 under DLIs of 10.4 and 12.1 mol m−2 d−1, which is the legally recommended maximum for irrigation purposes in Portugal. Nutrient removal at 35 DAT was at least 95% for total nitrogen (TN), 98% for NH3-N, 99% for NO3-N and 94% for PO4-P under DLIs from 14.4 to 23.0 mol m−2 d−1, with no clear correlation to DLI or volume of NS used (1.5 or 2 L per plant). For DLIs from 8.06 to 12.1 mol m−2 d−1, TN removal correlated with DLI and volume of NS per plant, varying from 85 to 94% (3 L of NS per plant) and from 89 to 97% (2 L of NS per plant), from lower to higher DLI. NO3-N removal exhibited the same pattern, and NH3-N and PO4-P were removed over 99%. Overall, a DLI of either 10 or 12 mol m−2 d−1 with 2 L of NS per plant or DLI of 12 mol m−2 d−1 with 3 L of NS per plant were a good alternative to produce sizeable lettuce with no inner tip burn and high WUE and LUE, and to remove a significant amount of nitrogen and phosphorus, down to levels that were promising for potential use of the hydroponic wastewater in irrigation or other purposes, minimizing its environmental impacts.
This study emphasized the importance of finding the proper DLI and volume of NS per plant in controlled indoor hydroponics, when it is intended to obtain a low-nutrient wastewater, while reducing costs with NS without considerably compromising the production. The developed DWC system and the findings of this study are expected to be useful in optimizing hydroponic nutrient removal from partially treated domestic and agro-industrial wastewater or reclaimed water. Future studies should address changes in other chemical components, pH correction during the growth cycle and at the same time examine the nutritional quality of the produce grown on the low nutrient concentrations observed at the end of the growth cycle.
Author Contributions
Conceptualization, L.M.I.A. and K.I.; methodology, L.M.I.A., K.I., T.R.L., H.P. and J.S.V.; validation, L.M.I.A., K.I., H.P. and J.S.V.; formal analysis, T.R.L., L.M.I.A. and K.I.; investigation, L.M.I.A., K.I. and T.R.L.; writing—original draft preparation, L.M.I.A. and K.I.; writing—review and editing, L.M.I.A., K.I., T.R.L., H.P. and J.S.V.; project administration, L.M.I.A.; funding acquisition, J.S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by LA/P/0045/2020 (ALiCE), UIDB/50020/2020 and UIDP/50020/2020 (LSRE-LCM), funded by national funds through FCT/MCTES (PIDDAC).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Islam, S.M.F. World’s Demand for Food and Water: The Consequences of Climate Change. In Desalination-Challenges and Opportunities; Farahami, M.H.D.A., Vatanpour, V., Taheri, A.H., Eds.; IntechOpen: Rijeka, Croatia, 2019; Chapter 4; ISBN 978-1-78984-739-0. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Marshall, E.; Aillery, M.; Malcolm, S.; Williams, R. Agricultural Production under Climate Change: The Potential Impacts of Shifting Regional Water Balances in the United States. Am. J. Agric. Econ. 2015, 97, 568–588. [Google Scholar] [CrossRef]
- Huang, Z.; Yuan, X.; Liu, X. The key drivers for the changes in global water scarcity: Water withdrawal versus water availability. J. Hydrol. 2021, 601, 126658. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Ahmad Shah, Q.M.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L. var. Longifolia) and comparison with protected soil-based cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar] [CrossRef]
- AlShrouf, A. Hydroponics, Aeroponic and Aquaponic as Compared with Conventional Farming. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Wohlleb, G.M.; Halden, R.U. Comparison of Land, Water, and Energy Requirements of Lettuce Grown Using Hydroponic vs. Conventional Agricultural Methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- El-Kazzaz, K.A.; El-kazzaz, A.A. Soilless Agriculture a New and Advanced Method for Agriculture Development: An Introduction. Agric. Res. Technol. Open Access J. 2017, 3, 555610. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on Hydroponics and the Technologies Associated for Medium- and Small-Scale Operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Rusu, T.; Moraru, P.I.; Mintas, O.S. Influence of environmental and nutritional factors on the development of lettuce (Lactuca sativa L.) microgreens grown in a hydroponic system: A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12427. [Google Scholar] [CrossRef]
- Nagano, S.; Mori, N.; Tomari, Y.; Mitsugi, N.; Deguchi, A.; Kashima, M.; Tezuka, A.; Nagano, A.J.; Usami, H.; Tanabata, T.; et al. Effect of differences in light source environment on transcriptome of leaf lettuce (Lactuca sativa L.) to optimize cultivation conditions. PLoS ONE 2022, 17, e0265994. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Blasioli, S.; Cellini, A.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Resource use efficiency of indoor lettuce (Lactuca sativa L.) cultivation as affected by red:blue ratio provided by LED lighting. Sci. Rep. 2019, 9, 14127. [Google Scholar] [CrossRef]
- Pennisi, G.; Orsini, F.; Landolfo, M.; Pistillo, A.; Crepaldi, A.; Nicola, S.; Fernandez, J.A.; Marcelis, L.F.M.; Gianquinto, G. Optimal photoperiod for indoor cultivation of leafy vegetables and herbs. Eur. J. Hortic. Sci. 2020, 85, 329–338. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Matysiak, B.; Ropelewska, E.; Wrzodak, A.; Kowalski, A.; Kaniszewski, S. Yield and Quality of Romaine Lettuce at Different Daily Light Integral in an Indoor Controlled Environment. Agronomy 2022, 12, 1026. [Google Scholar] [CrossRef]
- Kelly, N.; Choe, D.; Meng, Q.; Runkle, E.S. Promotion of lettuce growth under an increasing daily light integral depends on the combination of the photosynthetic photon flux density and photoperiod. Sci. Hortic. 2020, 272, 109565. [Google Scholar] [CrossRef]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Sago, Y. Effects of light intensity and growth rate on tipburn development and leaf calcium concentration in butterhead lettuce. HortScience 2016, 51, 1087–1091. [Google Scholar] [CrossRef]
- Mattson, N.S. Tipburn of hydroponic lettuce. e-Gro Alert 2015, 4, 1–7. [Google Scholar]
- Miller, A.; Adhikari, R.; Nemali, K. Recycling Nutrient Solution Can Reduce Growth Due to Nutrient Deficiencies in Hydroponic Production. Front. Plant Sci. 2020, 11, 607643. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Verheust, Y.; Bonarrigo, G.; Hulle, S. Closed hydroponic systems: Operational parameters, root exudates occurrence and related water treatment. Rev. Environ. Sci. Biotechnol. 2017, 16, 59–79. [Google Scholar] [CrossRef]
- Gagnon, V.; Maltais-Landry, G.; Puigagut, J.; Chazarenc, F.; Brisson, J. Treatment of hydroponics wastewater using constructed wetlands in winter conditions. Water. Air. Soil Pollut. 2010, 212, 483–490. [Google Scholar] [CrossRef]
- Water Quality for Agriculture. Available online: https://www.fao.org/3/t0234e/t0234e00.htm (accessed on 3 January 2023).
- Diário da República Portuguesa. Decreto-Lei 236/98 de 1 de Agosto, Diário da República n.o 176/1998, Série I-A de 1998-08-01; Diário da República Portuguesa: Lisbon, Portugal, 1998; pp. 3676–3722. [Google Scholar]
- Diário da República Portuguesa. Decreto-Lei 119/2019 de 21 de agosto, Diário da República n.o 159/2019, Série I de 2019-08-21; Diário da República Portuguesa: Lisbon, Portugal, 2019; pp. 21–44. [Google Scholar]
- Richa, A.; Touil, S.; Fizir, M.; Martinez, V. Recent advances and perspectives in the treatment of hydroponic wastewater: A review. Rev. Environ. Sci. Bio Technol. 2020, 19, 945–966. [Google Scholar] [CrossRef]
- Kumar, R.R.; Cho, J.Y. Reuse of hydroponic waste solution. Environ. Sci. Pollut. Res. 2014, 21, 9569–9577. [Google Scholar] [CrossRef] [PubMed]
- Ispolnov, K.; Aires, L.M.I.; Lourenco, N.D.; Vieira, J.S. A Combined Vermifiltration-Hydroponic System for Swine Wastewater Treatment. Appl. Sci. 2021, 11, 64. [Google Scholar] [CrossRef]
- da Silva Cuba Carvalho, R.; Bastos, R.G.; Souza, C.F. Influence of the use of wastewater on nutrient absorption and production of lettuce grown in a hydroponic system. Agric. Water Manag. 2018, 203, 311–321. [Google Scholar] [CrossRef]
- Lei, C.; Engeseth, N.J. Comparison of growth characteristics, functional qualities, and texture of hydroponically grown and soil-grown lettuce. LWT 2021, 150, 111931. [Google Scholar] [CrossRef]
- Santos, O.D. Uso de Água Residual Doméstica para o Cultivo Hidropónico de Alfaces. Master’s Thesis, Escola Superior de Tecnologia e Gestão, Politécnico de Leiria, Leiria, Portugal, 2021. [Google Scholar]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- US EPA. Method 352.1: Nitrogen, Nitrate (Colorimetric, Brucine) by Spectrophotometer; US EPA: Washington, DC, USA, 1971. [Google Scholar]
- ISO Standard 7150-1:1984; Water Quality—Determination of Ammonium—Part 1: Manual Spectrometric Method. International Standard Organization: Geneva, Switzerland, 1984.
- APHA/AWWA/WEF. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA, APHA-AWWA-WEF: Washington, DC, USA, 2005; ISBN 0875532357. [Google Scholar]
- Pennisi, G.; Pistillo, A.; Orsini, F.; Cellini, A.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Crepaldi, A.; Gianquinto, G.; Marcelis, L.F.M. Optimal light intensity for sustainable water and energy use in indoor cultivation of lettuce and basil under red and blue LEDs. Sci. Hortic. 2020, 272, 109508. [Google Scholar] [CrossRef]
- Barta, D.J.; Tibbitts, T.W. Calcium localization and tipburn development in lettuce leaves during early enlargement. J. Am. Soc. Hortic. Sci. 2000, 125, 294–298. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Samarakoon, U.; Palmer, J.; Ling, P.; Altland, J. Effects of electrical conductivity, pH, and foliar application of calcium chloride on yield and tipburn of lactuca sativa grown using the nutrient-film technique. HortScience 2020, 55, 1265–1271. [Google Scholar] [CrossRef]
- Jin, W.; Lopez, D.F.; Heuvelink, E.; Marcelis, L.F.M. Light use efficiency of lettuce cultivation in vertical farms compared with greenhouse and field. Food Energy Secur. 2022, 00, e391. [Google Scholar] [CrossRef]
- Roosta, H.R. Interaction between water alkalinity and nutrient solution ph on the vegetative growth, chlorophyll fluorescence and leaf magnesium, iron, manganese, and zinc concentrations in lettuce. J. Plant Nutr. 2011, 34, 717–731. [Google Scholar] [CrossRef]
- Roosta, H.R.; Estaji, A.; Niknam, F. Effect of iron, zinc and manganese shortage-induced change on photosynthetic pigments, some osmoregulators and chlorophyll fluorescence parameters in lettuce. Photosynthetica 2018, 56, 606–615. [Google Scholar] [CrossRef]
- Yara. Nutrient Deficiencies-Lettuce. 2022. Available online: https://www.yara.co.uk/crop-nutrition/lettuce/nutrient-deficiencies-lettuce/ (accessed on 15 July 2022).
- da Silva Cerozi, B.; Fitzsimmons, K. The effect of pH on phosphorus availability and speciation in an aquaponics nutrient solution. Bioresour. Technol. 2016, 219, 778–781. [Google Scholar] [CrossRef]
- Andriolo, J.L.; da Luz, G.L.; Witter, M.H.; dos Santos Godoi, R.; Barros, G.T.; Bortolotto, O.C. Growth and yield of lettuce plants under salinity. Hortic. Bras. 2005, 23, 931–934. [Google Scholar] [CrossRef]
- Borghesi, E.; Carmassi, G.; Uguccioni, M.C.; Vernieri, P.; Malorgio, F. Effects of Calcium and Salinity Stress on Quality of Lettuce in Soilless Culture. J. Plant Nutr. 2013, 36, 677–690. [Google Scholar] [CrossRef]
- De Pascale, S.; Barbieri, G. Effects of soil salinity from long-term irrigation with saline-sodic water on yield and quality of winter vegetable crops. Sci. Hortic. 1995, 64, 145–157. [Google Scholar] [CrossRef]
- Shinohara, M.; Aoyama, C.; Fujiwara, K.; Watanabe, A.; Ohmori, H.; Uehara, Y.; Takano, M. Microbial mineralization of organic nitrogen into nitrate to allow the use of organic fertilizer in hydroponics. Soil Sci. Plant Nutr. 2011, 57, 190–203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).