Abstract
The biosynthesis of citric acid (CA) and its derivatives is of great interest due to its wide range of applications in various manufacturing sectors. The fungus Aspergillus niger is mainly used for the commercial production of CA, using sucrose and molasses as the primary carbon sources. Since the 1960s, intensive research has been underway to introduce Yarrowia lipolytica yeast as an alternative to traditional fungal technology. This review discusses the practical uses of CA and its derivatives. Also, the challenges and developments that have led to efficient and green CA synthesis technologies using Y. lipolytica are outlined. The nutrient medium requirements and the use of various carbon sources, encompassing pure substrates and industry, agriculture, and food waste are considered. Additionally, the choice and improvement of strain producers, including efficient mutagenesis, genetic modification, and screening methods, are discussed.
1. Introduction
Citric acid (CA) and its derivatives, including salts and esters, are in high demand across various manufacturing sectors. According to the Global Citric Acid Market Outlook (2023) [1], the world market for CA reached a volume of approximately 2.59 million tons in 2022, and is projected to increase to 3.29 million tons by 2028. About 70% of global CA production is dedicated to the food industry, while 12% is allocated to pharmacological preparations, and 18% is used in technical applications [2].
Currently, the fungus Aspergillus niger is the most commonly used producer for commercial CA production, using sucrose and molasses as the main carbon sources. The fungus A. niger offers several advantages. These include ease of cultivation, high concentration, and specific rate of CA production, as well as refined nutrient media and cultivation conditions. The drawbacks of using fungal CA technology involve the reality that molasses typically contain around 44–54% fermentable sugars, 20% non-sugar substances, and 8–12% mineral compounds, including a considerable number of heavy metals. As a result, the maximum product yield (YCA) generally falls within 50–70% of the molasses consumed. It is necessary to treat the molasses with potassium hexacyanoferrate or other complexing compounds to eliminate excessive trace elements. This production of CA poses environmental hazards due to the accumulation of substantial solid and liquid waste, resulting in high disposal costs. Additionally, the A. niger production process emits an air mixture containing spores, which are potent allergens causing respiratory illnesses like aspergillosis [3,4].
Since the 1960s, researchers have been working intensively to introduce Yarrowia lipolytica yeast as an alternative to traditional fungal technology. Y. lipolytica yeast can produce CA from various carbon sources. In addition, Y. lipolytica is more tolerant to low pH, resulting in significant cost reductions. Y. lipolytica yeast exhibits a high tolerance towards high concentrations of carbon sources and impurities present within substrates. Additionally, Y. lipolytica yeast cultivation processes can be easily scaled and automated [3,4,5,6,7]. Y. lipolytica yeast, as well as products based on its synthesis, are generally recognized as safe (GRAS) and can be used in food, medicine, and pharmacology [5].
This review summarizes past and present challenges and advances in citric acid production using Y. lipolytica yeast. This review presents key developments in the research of wild strains of Y. lipolytica that produce CA. It covers their origins, performance on different carbon sources, and the potential of newly discovered strains that require further evaluation. In addition, the review examines effective techniques in mutagenesis and screening, and adaptive laboratory evolution, as well as methods for metabolic engineering of CA producers. In general, these crucial topics have not yet been sufficiently studied.
2. Properties and Applications
Citric acid (CA) exists in two forms: an anhydrous form and a monohydrate form (food grade). Table 1 outlines the main characteristics of the two forms.

Table 1.
Physicochemical properties of CA (based on [3,8,9]).
Anhydrous CA is found in citrus fruits, mahogany and cotton leaves, and wild pomegranates [4,9]. Maintaining a plasma citrate concentration of 100–150 micromoles per liter is vital for various normal physiological processes in humans and animals [10].
CA monohydrate (a food additive E330) is used to enhance the taste, to adjust the acidity, and to improve the efficacy of antimicrobial agents and preservation properties [3,11,12]. CA monohydrate is an ingredient in pharmaceuticals, cosmetics, and fragrances. The reaction of CA with bicarbonates, releasing CO2, is used to improve the solubility of poorly soluble drugs [13]. The composition containing CA is also used to remove metal oxides from the surface of ferrous and non-ferrous metals. CA helps to prevent the boiler fouling [11]). CA is used as a feed additive in agriculture, livestock, and aquaculture [14]. It contributes to the remediation of soil contaminated with heavy metals [12]. CA is also used in electroplating, photography, textiles, etc. [3,9].
Tributyl citrate, the known ester of citric acid, is a safe and non-toxic plasticizer, mostly used in the production of polyvinyl chloride, food films, cellulose resins, synthetic rubber, toys, and flexographic inks. Tributyl citrate is also used as a stabilizer for resins in cosmetics [15].
CA salts are also in great demand in various manufacturing sectors. The most in-demand representative of CA salts is tri-substituted sodium citrate. It is utilized in the chemical, metallurgical, food, medicine, and agriculture industries. It substitutes for hazardous polyphosphates in synthetic detergents, does not corrode metals, and decomposes into carbon dioxide and water in wastewater treatment systems [16,17].
Sodium salts of CA with varying degrees of substitution can be obtained without the need for isolating CA from the culture broth. Titration of the culture broth with NaOH to pH 4.6 produces an equimolar mixture of monosodium citrate and divalent sodium citrate, whereas titration to pH 8.3 yields tri-substituted sodium citrate [18].
3. History
The species Y. lipolytica has approximately twenty synonyms, of which Candida lipolytica is the most prevalent [19].
The yeast C. lipolytica was initially characterized by Harrison in 1928 [9]. Until the discovery of sexual reproduction, this yeast species was classified as an imperfect fungus. Afterward, it was reclassified as a perfect fungus and received various species names. Initially, it was known as Endomycopsis lipolytica (1970), then as Saccharomycopsis lipolytica (1972), and since 1980 as Y. lipolytica [20]. In order to prevent confusion in this review, the organism will be referred to as it is named in the relevant literature source.
The first publications on the production of CA by C. lipolytica yeast came from Japan and the USSR. Tabuchi et al. (1969) reported that the C. lipolytica No. 228 wild strain could produce 11–34 g/L of CA from glucose, acetic acid, n-butyric acid, oleic acid, fish oil, linseed oil, and soybean oil [21]. This strain prospered from petroleum hydrocarbons, yielding 62.5 g/L of total citric acid from 60 g/L, with a CA/ICA ratio of 2:1 [22]. During that time, Finogenova and colleagues conducted research on the production of organic acids by various strains of C. lipolytica grown on n-alkanes. Their research showed that the growth limitation by mineral components resulted in CA production [23,24,25].
Illarionova and Suetina (1984) [26] noted that there was considerable scientific and practical interest in the production of CA from non-food substrates between 1968 and 1981. The authors reported that 115 patents had been granted for producing CA from n-alkanes using C. lipolytica yeast. The majority of these patents were filed by Japan, the USA, France, Germany, the GDR, and the USSR. Japan’s Kyowa Hakko Kogyo Co. and Taked Chemical Industries LTD were the front-runners in developing CA from n-alkanes.
In the UK, Pfizer played a crucial role in advancing the production of CA. The one-step continuous process with C. lipolytica growing on a mixture of n-paraffins for 304 h was innovative at the time [27].
In France, the Institut Français du Pétrole and the Ecole Nationale Supérieure d’Agronomie et des Industries Alimentaires were responsible for all scientific publications and patents related to the production of CA from n-alkanes [28,29,30].
In the GDR, the Institut für Technische Chemie (Leipzig) carried out the work [31,32].
Since the 1980s, the number of papers and patents on CA biosynthesis from n-alkanes has decreased, and researchers have shifted their focus to new substrates, which will be discussed in the following sections of this review.
4. Factors Affecting Citric Acid Production
Y. lipolytica yeast cannot produce citric acid (CA) in a complete nutrient medium. The principal condition of CA overproduction is the limitation of yeast growth by mineral components (nitrogen, phosphorus, sulfur, or magnesium) at a simultaneous excess of a carbon source. As microbial cells require large amounts of nitrogen for growth, the studies are typically conducted using nitrogen limitation. Y. lipolytica yeast produces CA and isocitric acid (ICA) at the same time. The CA/ICA ratio varies depending on the carbon source used. CA is predominantly produced in the medium with glucose and glycerol, while approximately equal amounts of CA and ICA are formed with vegetable oils and n-alkanes. ICA is produced when grown on ethanol. The excretion of CA and ICA into the medium commences only after the exhaustion of nitrogen from the medium, and following the transition of the culture from the logarithmic growth phase to the growth retardation phase. The stationary phase cells remain viable, continuing to synthesize the acids until complete depletion of the carbon source [4,20,33,34,35,36,37,38,39,40].
Y. lipolytica yeast can produce CA from different carbon sources with varying degrees of efficiency. Figure 1 presents the maximal yields (YCA) obtained from different substrates: rapeseed oil [41], n-paraffines [42], sunflower oil [43], raw glycerol [44], extract of Jerusalem artichoke tubers [45], ethanol [46], glucose [47], the mixture of glucose and acetate [48], inulin [49], glucose hydrol [50], the mixture of glucose and oleic acid [51], the mixture of glycerol and olive mills [52], sucrose [53], pure glycerol [54], the mixtue of glucose and olive mills [55], xylose [56], galactose [57], expired “waste” glucose [58], aspen waste [46], grape must [59], carrot juice [60], waste cooking oil [61], fructose [62], the mixture of fructose and whey [59], waste bread hydrolysate [63].These values were found among the wild, mutant, or genetically modified strains of Y. lipolytica.
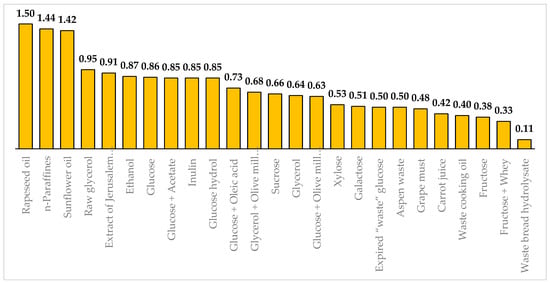
Figure 1.
Maximal citric acid production yields (in g/g) on various carbon sources. The yields are displayed at the top of the bars.
Y. lipolytica yeast gives maximum yields from n-paraffins (1.44 g/g) [42] and vegetable oils (1.42–1.5 g/g) [41,43]. High yields can be obtained using glucose (0.86 g/g) [47] and ethanol (0.87 g/g) [46] as carbon sources. Y. lipolytica yeast can utilize industrial waste. For instance, the mutant strain Y. lipolytica NG40/UV7 can produce CA with a yield of 0.95 g/g from glycerol-containing waste from the biodiesel industry [44].
Y. lipolytica yeast can utilize plant sources and waste from the food industry. For instance, the mutant strain of Y. lipolytica A-101-1.14 can produce CA with a yield of 0.95 g/g from starch-derived glucose hydrol [50]. The wild strain Y. lipolytica ACA-YC 5033 can utilize the combination of olive mill wastewater with glucose or glycerol and produce CA with a yield of 0.63–0.69 g/g [52,55]. Ra et al. [45] achieved a yield of 0.91 g/g using Jerusalem artichoke tuber extract. Recombinant strains of Y. lipolytica can utilize sucrose [53,64] and inulin [49]. Y. lipolytica can use aspen waste [46], xylose [56], galactose [57], fructose [62], whey [59], grape must [59], carrot juice [60], waste cooking oil [61], waste bread hydrolysate [63], sunflower waste cooking oil [65], and straw hydrolysate [66]. Diamantopoulou et al. [58] converted the expired “waste” glucose to CA with a yield of 0.5 g/g. Promising carbon sources for large-scale CA production include low-cost corn syrups and corn steep liquor derived from the saccharification of corn starch [67,68]. Gao et al. [69] used corn stover treated with glycerol-assisted instantaneous catapult steam explosion to produce CA and mannitol. Although industrial and agricultural wastes are affordable carbon sources, making them economically preferable raw materials, their processing is challenging and arduous. These wastes can contain trace metals that can inhibit yeast growth and CA synthesis. Researchers often employ chemical pretreatment of substrates and use distilled water instead of tap water when working with waste materials [36].
High yields can be obtained using a dual-substrate medium. Venter et al. [70] found that acetate enhanced CA production; its addition to sunflower oil resulted in a 37-fold increase in CA production and a 2-fold increase in the CA/ICA ratio in Y. lipolytica UOFS Y-1701. Robak et al. [48] found that the addition of acetate to the glucose medium improved the specific production rate, yield, and productivity of CA biosynthesis by Y. lipolytica.
It should be noted that studies comparing the effect of different carbon sources on citrate production are limited. Rywinska et al. [71] found that pure glycerol was a superior substrate compared with crude glycerol and glucose. Celik et al. [72] reported that sunflower oil was superior to glucose and glycerol. When comparing rapeseed oil, glucose, glycerol, ethanol, glycerol-containing biodiesel industry waste, and glucose-containing aspen waste, it was shown that rapeseed oil, ethanol, and crude glycerol allowed high CA production (100–140 g/L), whereas aspen waste resulted in a low CA concentration (31.2 g/L) [46].
The choice of the optimum initial carbon source concentration depends on the strain, the composition of the nutrient medium, and the cultivation conditions. Usually, the optimal yields are obtained at initial carbon concentrations of 50–250 g/L [73], with the exception of ethanol (0.1–1.0 g/L) [46].
Y. lipolytica yeast requires nitrogen, phosphorus, sulfur, potassium, magnesium, calcium, and trace elements in its nutrient medium. The ideal dosage of these elements is dependent on the physiological characteristics of the strain and the carbon source [36,73].
Y. lipolytica yeast can use both inorganic salts, such as NH4Cl and (NH4)2SO4, and organic compounds, such as yeast extract and peptone, as nitrogen sources for CA production. It is crucial to select an appropriate nitrogen concentration that guarantees enough cell density in the culture medium. In general, Y. lipolytica yeast grown on pure substrates such as glucose and glycerol requires between 630 and 1200 mg/L of nitrogen [73]. Kamzolova et al. [74] found that the volumetric productivity (QCA) was very high (1.11 g/L·h) at a nitrogen concentration of 1200 mg/L. However, waste with a high nitrogen content can be utilized without adding nitrogen to the medium. Ra et al. [45] reported that CA production from Jerusalem artichoke tuber extract requires no nitrogen source. Moreover, Carsanba et al. [63] observed similar results with waste bread hydrolysate. Da Silva et al. [75] showed that the addition of ammonium sulfate to the culture medium shifts the metabolic pathway to isocitrate acid production in Y. lipolytica grown on crude glycerol. However, it was necessary to add nitrogen and magnesium to accumulate CA from waste cooking oil [61].
The carbon-to-nitrogen (C/N) ratio plays a critical role in the regulation of CA biosynthesis. Carsanba et al. [76] studied the effect of initial C/N ratios (167, 367, and 567) on CA production by Y. lipolytica K57 grown on glucose. The best C/N ratio was found to be 367, which resulted in the highest CA concentration, yield (YCA), and CA production rate (qCA). Levinson et al. [77] found that the best CA/ICA ratios in Y. lipolytica grown on glycerol were achieved between C/N ratios of 343 and 686. Another study found that Y. lipolytica W29 could produce CA from high fructose syrup at a C/N mass ratio of 75, but, after 15 days at a C/N ratio of 150, CA production stopped while lipid accumulation increased [68].
Y. lipolytica yeast uses KH2PO4, K2HPO4, and Na2HPO4 as sources of potassium and phosphorus. The concentrations vary from 100 to 2400 mg/L [33,48,74,75].
For intensive production of CA, Y. lipolytica yeast require specific microelements. These microelements can either activate or inhibit enzymes involved in the metabolism of Y. lipolytica. To activate alcohol dehydrogenase when Y. lipolytica is grown on ethanol, it is necessary to maintain a Zn2+ concentration of at least 1 mg/g of cells [78]. Y. lipolytica also requires iron. Under iron deficiency, most of the acetyl-CoA does not participate in the TCA cycle but undergoes condensation to form ethyl acetate [79]. In contrast, the increased iron concentration leads to activation of the TCA cycle, respiratory chain, and oxidative phosphorylation in Y. lipolytica [80]. However, the substantial increase in Fe2+ ion concentration has minimal effect on yeast growth and total amounts of acids. But, it alters the balance between these acids, favoring the formation of ICA [81,82]. The positive effect of copper ions on the metabolism of Y. lipolytica is well studied. The addition of 2.5–5 mg/L Cu2+ to Y. lipolytica yeast grown on glycerol leads to increased production of erythritol, CA, and α-ketoglutaric acid [83]. In addition, CA production is stimulated by Mn2+ [84]. Researchers often use tap water rather than a microelement solution [48,53,56,85].
Y. lipolytica is unable to synthesize the thiamine molecule, i.e., the pyrimidine moiety, and requires thiamine to be added to the medium. In the absence of thiamine, the yeast produces pyruvic acid and α-ketoglutaric acid rather than CA [86,87,88,89,90,91]. A sufficient quantity of thiamine (100–200 µg/L) should be added to the growth medium. Yeast extract can replace thiamine [86,87,88,89,90,91]. However, an excessive amount is not recommended because nitrogen is also present, not just vitamins.
Information regarding the impact of growth temperature on CA production is limited. Morgunov et al. [54] found that Y. lipolytica grown on glycerol exhibited optimal CA production at temperatures between 28 and 30 °C, with the yield declining even if the cultivation temperature shifted by just 2 °C in either direction. Moeller et al. [92] determined that Y. lipolytica growth was ideal within the 30–34 °C temperature range. However, it was found that CA production reached its maximum levels only at 30 °C. Arslan et al. [93] introduced the Y. lipolytica B9 strain that is adapted to low temperatures. This strain successfully produced 33.3 g/L of CA, even at 20 °C.
The pH medium level is a critical factor affecting Y. lipolytica yeast metabolism. Yeast grows at pH values between 2.5 and 11.5, although CA production only occurs within a pH range of 4.5 to 6.0 [73]. Zhang et al. [94] found that the wild strain Y. lipolytica W 29 produced CA at a neutral pH while generating lipids at an acidic pH. The authors have proposed that this pH-dependent mechanism is impacted by CA transport, rather than alterations in enzyme expression for acid production and lipid synthesis. Gao et al. [69] found the shift from CA production at pH 5.5 to mannitol synthesis at pH 3.5 in Y. lipolytica CGMCC 2.1506 yeast grown on corn stover enzymatic hydrolysate. Another strain, Y. lipolytica H222, grown on glycerol, simultaneously produced CA (19.1 g/L), mannitol (18.1 g/L), arabidol (2.3 g/L), and erythritol (6.7 g/L) at pH 3.5, but at pH 5.5 it produced 42.5 g/L CA without polyol accumulation [95]. In spite of this, some genetically modified strains have a higher pH tolerance. Mirończuk et al. [96] found that strains overexpressing GUT1 and/or GUT2 have the ability to produce substantial amounts of CA from glycerol at pH 3. This capability to synthesize metabolites at a low pH has significant industrial value since it reduces production costs, prevents bacterial contamination, and maintains aseptic conditions. Another genetically modified strain exhibited higher invertase at a pH range of 6.0–6.8, thus increasing the production of CA from sucrose to 127–140 g/L. However, at a pH of 5.0, the yield dropped to 87 g/L [64].
The production of CA by Y. lipolytica yeast is affected by aeration, namely the oxygen saturation level (pO2) in the culture medium. The appropriate pO2 level is dependent upon the carbon source. For the biosynthesis of CA from n-paraffins, it is necessary to maintain optimum pO2 levels between 70 and 90% saturation [23]. To promote CA production from vegetable oils and ethanol, it is essential to maintain high levels of aeration, typically at 50–60% saturation [41,97]. In order to achieve the highest levels of CA production using other carbon sources, it is important to maintain a minimum pO2 value of 20% saturation [72]. Liu et al. [51] recommended increasing the oxygen saturation level by introducing oxygen vectors such as oleic acid. Bellou et al. [98] noted that the pO2 value, rather than the type of carbon source or the nitrogen concentration in the medium, had an effect on the morphology of Y. lipolytica. Their research showed that, at low or zero pO2, mycelial and/or pseudomycelial forms were more prevalent than yeast-like forms. Another important oxygenation factor is the initial volumetric oxygen mass transfer coefficient (kLa), which is sensitive to operating conditions such as stirring speed, specific air flow rate, and cell density. Ferreira et al. [99] showed a 7.8-fold increase in CA production by increasing the initial kLa from 7 h−1 to 55 h−1.
Different types of bioreactors have been constructed to obtain an adequate volumetric oxygen mass transfer coefficient without high aeration rates. These bioreactors can provide significant cost savings during operations. It was observed that, using airlift and pressurized bioreactors, Y. lipolytica W29 could produce 14 g/L and 6 g/L of CA, respectively [100].
In summary, the essential conditions for CA synthesis by the Y. lipolytica yeast include nitrogen limitation, carbon excess, temperature control between 28 and 30 °C, pH control of the medium to about 4.5 to 5.5, and adequate aeration.
5. Wild-Type CA Producers
Table 2 presents efficient methods for the production of citric acid (CA) by well-known wild strains of Y. lipolytica. The strains under consideration were sourced from various countries, including Poland (A-101), the United States (ATCC 76598, ATCC 8661, and ATCC 20346), China (SWJ-1b), Germany (H 222, DSM 8218, and DSM 3286), Greece (ACA-DC 50109 [LGAM S(7)1], ACA-DC 5031, ACA-DC 5033, LMBF-46), France (W29), Russia (VKM Y-2373), and Turkey (K 57).

Table 2.
Citric acid production by wild strains of Y. lipolytica.
Wild-type strains of Y. lipolytica exhibit their universal capability to produce CA through various carbon sources and cultivation techniques, such as flask, batch, fed-batch, repeated-batch, and repeated-fed-batch. For instance, the wild strain Y. lipolytica A-101 from Poland can produce over 65 g/L of CA from glucose and glycerol [71], and more than 90 g/L of CA from glucose hydrolysates [50] in batch mode.
The wild strain Y. lipolytica ACA-DC 50109 (Y. lipolytica LGAM S(7)1), selected at the Laboratory of General and Agricultural Microbiology of the Agricultural University of Athens, was one of the first strains to be investigated for its ability to produce CA from biodiesel waste. This strain can produce 35.1 g/L CA with a yield of 0.42 g/g on crude glycerol [102]. The strain Y. lipolytica LGAM S(7)1 can produce 82 g/L CA with a yield of 0.50 g/g from expired “waste” glucose [58]. Additionally, this strain was tested with a mixture of crude glycerol and olive mill effluent—an agro-industrial waste known for its difficulty in processing. The purpose of this experiment was to reduce the potential of the crude glycerol by blending it with the olive mill effluent and replacing some or all of the tap water used in the process. By using two substrates, the Y. lipolytica LGAM S(7)1 yeast produced 30.3 g/L CA with a yield (YCA) of 0.62 g/g in flasks [103]. Later, the authors enhanced the CA production process up to 52.0 g/L, with a yield (YCA) of 0.64 g/g, using another wild strain, Y. lipolytica ACA-YC 5033. This strain effectively eliminated the noxious phenolic compounds from olive mill wastewater (around 51% in weight) [107]. Another strain, Y. lipolytica ACA-DC 5029, grew well on a mixture of crude glycerol and olive mill effluent and produced up to 79.0 g/L CA with a yield (YCA) of ~0.46 g/g, but accumulated erythritol in significant amounts (66.0 g/L) [106].
The French wild strain Y. lipolytica W29 can produce more than 100 g/L CA from glucose and glycerol [30]. In addition to these carbon sources, Y. lipolytica W29 produced CA from untreated crude glycerol [117], as well as from inexpensive, year-round nutrient sources obtained during the corn wet milling process, such as food-grade corn syrups and corn steep liquor [68]. However, when cultivated in glucose-enriched olive mill wastewater, the strain produced only 15.8 g/L CA with a yield (YCA) of 0.46 g/g [116]. Using this particular strain, it was shown that the control of reactive oxygen species, particularly via alternative oxidase activity, is essential for regulating the balance between citrate and lipid fluxes [118].
The wild strain Y. lipolytica H222 from Germany has served as the foundation for creating certain recombinant strains that overproduce CA. Furthermore, it is an invaluable model for evaluating the impact of various cultivation methods on CA biosynthesis. Moeller et al. (2010) examined CA biosynthesis in batch, fed-batch, repeated batch, and repeated fed-batch using the aforementioned strain [112]. The authors showed that using a repeated fed-batch process with Y. lipolytica H222 grown on glucose gave the best results. In each 72 h cycle, 100 g/L of CA was produced, with a yield (YCA) ranging from 0.51 to 0.65 g/g and a selectivity of 94%. The repeated fed-batch mode allowed for a 32% increase in CA production compared with batch fermentation.
The wild strain Y. lipolytica K 57, selected at the Department of Food Technology, Faculty of Engineering, University of Ankara (Turkey), is recognized as one of the leading producers of CA in fructose, whey, and grape must media [59,62].
In our studies, we use the wild strain Y. lipolytica 704 (Y. lipolytica VKM Y-2373). This strain was originally obtained in 1963 from activated sludge from the Angarsk oil refinery effluent treatment plant in the USSR. It can produce significant amounts of CA from crude glycerol [44] and glucose [74]. The biomass, a by-product of CA production, possesses a significant quantity of essential amino acids and unsaturated fatty acids, making it suitable for application in the areas of food biotechnology and agriculture [74].
In recent years, there have been reports of various pioneering strains that produce considerable amounts of CA. One such strain is the wild strain Y. lipolytica LMBF Y-46, which was isolated from gilt-head (sea) bream (Sparus aurata) fish and cultivated with glycerol. This strain achieved over 100 g/L CA, which is among the highest reported values [113]. Moreover, in Germany, the novel strain Y. lipolytica DSM 8218 was isolated from a diesel tank. This microbe can grow on fuel, so its suitability for growth on crude glycerol, containing microbial inhibitors and diesel production residues, should be investigated. This particular strain showed a faster growth rate on impure glycerol than on pure glycerol due to its consumption of acetic acid as a secondary substrate [111].
There are a number of publications on wild strains of Y. lipolytica D 1805, Y. lipolytica NRRL Y-7576, and Y. lipolytica NRRL Y-1095 that are able to promote active acid production during long-term cultivation (500–700 h) [47,108,114].
It should be noted that wild strains of Y. lipolytica produce CA and ICA simultaneously in virtually all carbon sources studied. This has led research teams to focus on the development of strains with predominant CA production.
6. Mutagenesis and Selection
Many research teams used physical and chemical mutagens to produce mutant strains with increased CA synthesis and decreased ICA content. Exposure to UV radiation leads to the excitation of electrons in DNA molecules, resulting in the creation of additional bonds between adjacent pyrimidine bases on one or both chains of DNA, thus forming dimers. This process causes transitions, transversions, frame shifts, and deletions. UV radiation has a wavelength range of 200–400 nm. N-methyl-N-nitro-N-nitrosoguanidine (NTG) and N-nitroso-N-methylurea (NMM) are common chemical mutagens. They induce alkylation and oxidation of nucleotide bases. This leads to mutations [9].
The conventional method of selecting mutant CA producers generally involves the following steps [119]:
- (1)
- Isolation of monoclones from the parent strain;
- (2)
- Treatment of clones by physical action or chemical mutagen;
- (3)
- Streaking of treated clones on agar plates and selection of variants weakly growing on citrate (Cit−) or acetate (Ace−);
- (4)
- Evaluation of the acid-forming activity of the Cit− or Ace− variants by zones of dissolution of chalk on agar medium;
- (5)
- Cultivation of the selected variants under standard conditions on liquid medium and selection of the most active mutant strain;
- (6)
- Conservation of the active mutant strain of CA production.
Table 3 presents efficient methods for the production of CA by well-known mutant strains of Y. lipolytica.

Table 3.
Citric acid production by mutant strains of Y. lipolytica.
The first mutants with minimal amounts of ICA in n-paraffin medium were obtained in Japan. A wild strain of C. lipolytica ATCC20114 was treated with NTG (250 µg/mL) for thirty minutes. The mutant variants were then screened for lack of growth in citrate media or sensitivity to fluoroacetate. The use of fluoroacetate is necessary because it inhibits the TCA cycle. Fluoroacetate undergoes enzymatic conversion to the toxic metabolite fluorocitrate, which inhibits aconitase and shifts the metabolism towards predominantly synthesizing CA. Two mutants were selected: K-20 (unable to grow on citrate), which produced 91 g/L CA with a selectivity of 85%, and the fluoroacetate-sensitive mutant S-22, which produced 106 g/L CA with a selectivity of 97%. In contrast, the parental strain C. lipolytica ATCC20114 can produce CA with low selectivity (60%) [124].
The Polish team successfully generated a series of effective mutants by exposing the wild strain Y. lipolytica A-101 to UV irradiation and NTG. The positive variants were selected by their inability to grow on media containing acetate (Wratislavia 1.31, AWG7, A-101.-1.22) or citrate (A-101.-1.14) and by prolonged cultivation in a chemostat. These mutants have the ability to produce CA from different carbon sources. For example, the UV citrate-negative mutant Y. lipolytica A-101-1.14 can produce 94 g/L of CA from glucose hydrolysates with a selectivity of 94%, a product yield (YCA) of 0.85 g/g, and a productivity (QCA) of 1.25 g/L·h [50]. In addition, the mutants show remarkable stability during continuous cultivation. For example, the double UV acetate-negative mutant Wratislavia AWG7 grown on glycerol can produce 97.8 g/L CA with a yield (YCA) of 0.49 g/g and a productivity (QCA) of 0.98 g/L·h for 550 h in the chemostat (using a dilution rate of 0.01 h−1) [121]. A more efficient approach using the 40% repeated batch mode was also developed: the mutant Wratislavia AWG7 produced 154 g/L CA with a product yield (YCA) of 0.78 g/g and a productivity (QCA) of 1.05 g/L·h. The activity of the mutant remained stable for more than 1650 h [122]. These mutants are maintained in the yeast culture collection of the Department of Biotechnology and Food Microbiology, Wroclaw University of Environmental and Life Sciences in Poland.
A highly efficient mutant of C. lipolytica VKPM Y-184 was obtained through exposure of the wild strain Y. lipolytica 704 to NMM. The mutant can produce 217 g/L CA with a selectivity of 97% and yield (YCA) of 1.45 g/g [4].
The mutant strain Y. lipolytica BAFC 3852, obtained through exposure of the wild strain Y. lipolytica NRRL Y-1095 to NTG, lacks the ability to form true mycelium or pseudomycelium, but forms only yeast-like cells. This attribute results in better stirring of the nutritional medium in the bioreactor, thus improving the overall process. This mutant produces 32.1 g/L CA from corn wet milling products [68].
The mutant strain Y. lipolytica N 1, obtained by exposing the wild strain Y. lipolytica 704 to NMM, can produce 120 g/L of CA with a yield (YCA) of 0.87 g/g from ethanol, even under low aeration (pO2 = 20% of saturation) [97]. However, it is required to use an iron-enriched medium to achieve these results.
The combination of chemical mutagens and UV radiation produces remarkable results. For example, the double mutant Y. lipolytica NG40/UV7 shows an impressive ability to produce significant amounts of CA while minimizing ICA levels on a variety of carbon sources. This mutant can produce 175 g/L of CA on rapeseed oil [41], 115 g/L on glycerol [54], and 122.2 g/L on crude glycerol [44].
7. Metabolic Engineering
In recent years, rational metabolic engineering has gradually replaced traditional mutagenesis as a powerful approach to improve strains and processes for CA production. Strategies include activation of key metabolic pathways for assimilation of carbon sources, CA biosynthesis, and its precursors; and inactivation of by-product synthesis pathways and pathways for CA degradation or its involvement in further transformations. A schematic overview of the metabolic pathways of citric acid production from different carbon sources, including well-known sources such as n-alkanes, triglycerides, fatty acids, glucose, glycerol, and fructose, as well as less-studied sources such as galactose, xylose, and inulin, can be found in recent reviews [34,36,37,126,127]. Therefore, only genetic techniques that demonstrate significant effectiveness in CA production by Y. lipolytica are presented in this section.
Table 4 presents efficient methods for the production of CA by well-known genetically modified strains of Y. lipolytica.

Table 4.
Citric acid production by genetically modified strains of Y. lipolytica.
Y. lipolytica yeast is unable to assimilate sucrose as it lacks the enzyme invertase, which is necessary for the hydrolysis of sucrose into glucose and fructose. To address this, recombinant strains of Y. lipolytica were created, which overexpress the SUC2 gene encoding invertase. The recombinant strain Y. lipolytica A101-B56-5, which overexpresses the SUC2 gene from Saccharomyces cerevisiae, exhibited strong growth on sucrose and produced 58.83 g/L CA with a yield (YCA) of 0.65 g/g and a productivity (QCA) of 0.82 g/L·h. Meanwhile, the ICA content was very low (1.26%) [53].
Given the problem of glucose preference, efforts have been made to develop strains that can use both fructose and glucose simultaneously. Expression of the HXK1 gene, which encodes the hexokinase enzyme responsible for fructose phosphorylation, improved fructose utilization, reduces filament formation, and increases CA synthesis [138]. Furthermore, simultaneous expression of the HXK1 gene with the hexose transporter genes Yht1 and Yht6 significantly increased CA production [139].
Recombinant strains that can grow on inulin have been developed. For instance, based on Y. lipolytica SWJ-1b, a recombinant strain was constructed in which the INU1 gene from Kluyveromyces marxianus encoding an extracellular inulinase was expressed. This strain can produce 68.9 g/L CA with a yield (YCA) of 0.69 g/g and a productivity (QCA) of 0.22 g/L·h [134].
To enhance glycerol assimilation, the expression of glycerol-assimilating enzymes, namely glycerol kinase encoded by GUT1 and glycerol-3P-dehydrogenase encoded by GUT2, is necessary. Overexpression of GUT1 significantly increases glycerol assimilation, while a strain with the GUT2 gene expression showed increased CA production [96]. The authors note that GUT2 is crucial for CA production, as the carbon flux in the cell is redirected to the TCA cycle. Recombinant strains with GUT1 and GUT2 exhibit metabolic lability: when grown in glycerol medium, the transformant Y. lipolytica AJD pADUTGut1/2 can produce 92.9 g/L CA at pH 6.0, but, at pH 3, the metabolism shifts towards erythritol accumulation (78 g/L) rather than CA. Rzechonek et al. [129] revealed that transformant Y. lipolytica AJD pADUTGut1/2 can produce CA at pH 3 in aseptic conditions using crude glycerol.
Recently, the rapid development of the CRISPR/Cas9 system has facilitated highly efficient genomic genetic editing in Y. lipolytica. New GoldenMOCS plasmids are appropriate for extrachromosomal overexpression of the GUT1 gene in wild-type strains of Y. lipolytica, resulting in an elevated conversion of glycerol to erythritol and CA [140].
It should be noted that wild strains of the yeast Y. lipolytica cannot grow on galactose. However, a modified Y. lipolytica Y4588 strain that overexpresses four genes encoding Leloir pathway enzymes (galactokinase, galactose-1-P-uridyl transferase, UDP-glucose-4-epimerase, and galactose mutarotase), YlGAL1, YlGAL7, YlGAL10E, and YlGAL10M, can assimilate galactose and produce 29.2 g/L CA with a yield (YCA) of 0.51 g/g, which is comparable to glucose [57].
In medium with xylose, the Y. lipolytica XYL+ transformant, overexpressing the xylose reductase-encoding gene XR and xylitol dehydrogenase-encoding gene XDH from Scheersomyces stipites in addition to the endogenous xylulokinase-encoding gene XK, produced 79.4 g/L CA with a yield (YCA) of 0.53 g/g and a productivity (QCA) of 0.91 g/L·h [56].
Acetyl-CoA and oxaloacetate are two direct substrates for CA synthesis. A number of papers have been devoted to recombinant strains overexpressing the PYC gene encoding pyruvate carboxylase, which converts carboxylate pyruvate to oxaloacetate. A recombinant transformant PG86, harboring a pyruvate carboxylase-encoding PYC gene from Meyerozyma guilliermondii, can produce 101 g/L CA with a yield (YCA) of 0.89 g/g and a productivity (QCA) of 0.42 g/L·h from 120 g/L glucose within 240 h of the fed-batch fermentation. Nonetheless, the by-product content was high, reaching 34.4 g/L, of which 12.4% constituted malate [132]. Another transformant PR32 harboring a pyruvate carboxylase-encoding PYC gene from the marine fungus Penicillium rubens exhibited an 8-fold increase in pyruvate carboxylase activity and a 2.6-fold higher acid-forming capacity. When cultivated in a fed-batch mode using glucose medium, the transformant PR 32 produced 111.1 g/L of CA with a yield (YCA) of 0.93 g/g and a productivity (QCA) of 0.46 g/L·h. However, the by-product content was quite high, at 38.1 g/L, including malate at 10.1% [133].
Biochemical studies indicate that citrate biosynthesis is determined by the high activity of citrate synthase compared with subsequent enzymes [42,54]. However, a recombinant strain, obtained from mutant Wratislavia, overexpressing the CIT 1 and CIT 2 genes encoding citrate synthase had no effect on citrate biosynthesis on glycerol, but caused a 10% and 23% increase in total acid biosynthesis compared with the parental strain, as a consequence of increased isocitrate synthesis [128]. The CIT1 and CIT2 mutants, obtained from the wild strain Y. lipolytica A-101, also did not show increased citrate biosynthesis but produced significant amounts of ICA (approximately 60 g/L) on rapeseed oil [128].
Strains that expressed multicopies of isocitrate lyase demonstrated a more favorable CA to ICA ratio compared with the wild type. The ICA percentage decreased from 10–12% to 3–6% in medium containing glycerol, glucose, and sucrose. In medium containing sunflower oil or hexadecane, the percentage of ICA was reduced from 37–45% to 4–7%. However, the transformant did not impact the overall concentration of citric acids [130]. In contrast, the defective strain Y. lipolytica H222-41(JMP5) Z123 exhibited a 2–5% increase in the proportion of isocitric acid compared with the wild strain Y. lipolytica H222 when grown on glucose or glycerol due to the lack of isocitrate lyase activity [130]. Another transformant, Y. lipolytica H222-S4(p67ICL1) T5, that overexpressed SUC2 and ICL1 produced 140 g/L CA on sucrose with a low ICA content (4%) at pH = 6.8 [64]. The strain was subsequently assessed through repeated fed-batch and fed-batch modes for over 200 h. The most effective process was determined to be repeated fed-batch with a duration of 72 h, producing 57.7–114.5 g/L CA with a yield (YCA) of 0.49–0.64 g/g and a productivity (QCA) of 0.66–1.1 g/L·h. The selectivity of the process, which was initially 90% at the beginning of fermentation, increased to 96.4% after five cycles [131]. The manipulation of the Y. lipolytica SWJ-1b strain, through expression of INU1 and ICL1 genes and deletion of the ACL1 gene, generated transformant № 30, which possesses an enhanced acid-forming capacity (84 g/L CA; YCA = 0.84 g/g and QCA = 0.22 g/L·h) [135]. This strain can assimilate extract from Jerusalem artichoke tubers and produce 68.3 g/L CA with a yield (YCA) of 0.91 g/g. CA has been isolated from culture liquid as crystals with a purity of 96%, with a yield of 67.2% at the isolation stage [45]. A transformant, Y. lipolytica AWG7 INU8, overexpressing the INU1 gene from K. marxianus, can produce 203 g/L of CA from inulin, exhibiting a yield (YCA) of 0.85 g/g and a productivity (QCA) of 0.51 g/L·h during repeated batch mode [49].
Papanikolaou et al. [137] developed a technique to inhibit the TCA cycle by deactivating the PHD1 gene, which encodes 2-methyl-citrate dehydratase. The modification impedes the TCA cycle at the aconitate hydratase level, since 2-methyl-citrate accumulation hinders aconitase, potentially preventing citrate transportation to the mitochondria and ultimately leading to citrate excretion. During nitrogen-limited growth in crude glycerol medium, the modified Y. lipolytica strain (Δphd1) produced 57.7 g/L of CA with a yield of 0.91 g/g, demonstrating a 1.6-fold increase in contrast to the wild-type strain. The recombinant strain also produces considerable amount of CA, even when under excess nitrogen conditions.
Yuzbasheva et al. [136] applied an approach involving the overexpression of genes YlYHM2, which encodes the major mitochondrial citrate transporter, and YlAMPD, encoding adenosine monophosphate deaminase. The obtained recombinant strain can produce 97 g/L CA in glucose-containing medium. This amount is 1.3 times greater than that produced by the wild-type strain. The authors observed that the transformant is unable to assimilate citrate as the only source of carbon.
Therefore, genetic modifications have a significant impact on the production of CA by Y. lipolytica yeast.
8. Adaptive Laboratory Evolution
As an alternative to metabolic engineering, which involves the targeted reorganization of genomes, the method of adaptive laboratory evolution is also utilized for the development of novel strains with desirable characteristics. This approach is not connected to knowledge of metabolic pathways. The process of adaptive laboratory evolution entails prolonged cultivation of microorganisms under specific selective conditions, resulting in hundreds of generations [39,141,142,143]. As noted in the above reviews, the adaptive laboratory evolution usually optimizes parameters such as tolerance to high concentrations of substrates and products, toxic impurities arising from substrates, low pH, and the capacity to assimilate novel carbon sources. Thus, the adaptive laboratory evolution via cell immobilization conducted in shake flasks with an aim to restore the glucose metabolism showed that, after 14 generations of evolution, the mutant strain Y. lipolytica PGC01003 had an increased glucose consumption rate of 0.30 g/L·h in a sugar-rich medium (150 g/L), while the starting strain exhibited poor growth. Furthermore, the productivity of succinic acid in the evolved strain increased by 2.3 times [144]. An adaptive evolution method was utilized to develop a hypertolerant strain, Y. lipolytica, capable of producing succinic acid three times higher than the starting strain in the presence of toxic acetate [145]. The process of adaptive evolution was used effectively to produce a Y. lipolytica strain with greater tolerance to ferulic acid, which is a harmful inhibitor derived from lignocellulose [146]. Comparative transcriptome analysis of the original strain and strain adapted with increasing concentrations of ferulic acid revealed several genes underlying toxicity tolerance enhancements to ferulic acid, and their beneficial contribution was confirmed by metabolic engineering. Daskalaki et al. [147] employed adaptive evolution strategies to enhance lipid accumulation in Y. lipolytica yeast: after 77 generations, a population achieved 44% w/w of lipid, a 30% increase over the starting strain.
Currently, there are no published studies on applying the adaptive laboratory evolution method to produce Y. lipolytica strains with enhanced CA synthesis. Nonetheless, the prolongated experiments conducted in chemostat [121] and variants with recycle [47,108,112,123] may be examined as evolutionary strategies.
9. Conclusions
In summary, the yeast Y. lipolytica is the most extensively researched yeast for CA production, displaying considerable potential for large-scale commercialization. The assimilation of low-cost waste derived from industry, agriculture, and food is a fundamental focus for CA production, aiming to mitigate environmental impact and reduce production expenses. To achieve proficient CA production processes, investigating strain selection and its improvement is imperative. The advancement of robust genetic tools for metabolic engineering of Y. lipolytica presents novel prospects for the creation of proficient strains capable of commercial production of CA in the foreseeable future.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Global Citric Acid Market Outlook. Available online: https://www.expertmarketresearch.com/reports/citric-acid-market (accessed on 13 July 2023).
- Dhillon, G.S.; Brar, S.K.; Verma, M.; Tyagi, R.D. Recent advances in citric acid bio-production and recovery. Food Bioprocess Technol. 2010, 4, 505–529. [Google Scholar] [CrossRef]
- Igliński, B.; Kiełkowska, U.; Piechota, G. Proecological aspects of citric acid technology. Clean Technol. Environ. Policy 2022, 24, 2061–2079. [Google Scholar] [CrossRef]
- Finogenova, T.V.; Morgunov, I.G.; Kamzolova, S.V.; Chernyavskaya, O.G. Organic acid production by the yeast Yarrowia lipolytica: A review of prospects. Appl. Biochem. Microbiol. 2005, 41, 418–425. [Google Scholar] [CrossRef]
- Zinjarde, S.S. Food-related applications of Yarrowia lipolytica. Food Chem. 2014, 152, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.G.; Colen, G.; Takahashi, J.A. Yarrowia lipolytica and its multiple applications in the biotechnological industry. Sci. World J. 2014, 2014, 476207. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mawgoud, A.M.; Markham, K.A.; Palmer, C.M.; Liu, N.; Stephanopoulos, G.; Alper, H.S. Metabolic engineering in the host Yarrowia lipolytica. Metab. Eng. 2018, 50, 192–208. [Google Scholar] [CrossRef]
- Verhoff, F.H.; Bauweleers, H. Citric Acid. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–11. [Google Scholar]
- KKarklinsh, R.J.; Liepinsh, G.K. Microbial Biosynthesis of Citric Acid; Zinatne: Riga, Latvia, 1993; 240p. [Google Scholar]
- Costello, L.C.; Franklin, R.B. Plasma Citrate Homeostasis: How It Is Regulated; And Its Physiological and Clinical Implications. An Important, But Neglected, Relationship in Medicine. HSOA J. Hum. Endocrinol. 2016, 1, 005. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.; Rodrigues, C.; Pandey, A. New perspectives for citric acid production and application. Food Tech. Biotech. 2006, 44, 141–149. [Google Scholar]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 1–9. [Google Scholar] [CrossRef]
- Lambros, M.; Tran, T.; Fei, Q.; Nicolaou, M. Citric Acid: A Multifunctional Pharmaceutical Excipient. Pharmaceutics 2022, 14, 972. [Google Scholar] [CrossRef]
- Shah, S.Z.H.; Afzal, M.; Khan, S.Y.; Hussain, S.M.; Habib, R.Z. Prospects of using citric acid as fish feed supplement. Int. J. Agric. Biol. 2015, 17, 1–8. [Google Scholar]
- Alekseeva, N.A. Review of applicability of citric acid and its derivatives. In Problems, Perspectives of Biotechnology and Biological Research; Publishing House of Altai State Technical University: Biysk, Russia, 2018; pp. 30–33. [Google Scholar]
- Raminya, L.O.; Ozolin, M.Y. Obtaining citrates from fermentation solution of n-alkanes. In Biosynthesis of Oxyacids and Ketoacids by Microorganisms; Zinatne: Riga, Latvia, 1984; pp. 35–42. (In Russian) [Google Scholar]
- Finogenova, T.; Morgunov, I.; Melnikov, V. Harmless polyphosphates. Nauka V Ross. 2009, 6, 11–14. (In Russian) [Google Scholar]
- Aghajanyan, A.Y. Isolation of salts of citric and isocitric acids from the enzyme solution. Chem. J. Armen. 2005, 58, 122–129. [Google Scholar]
- Golubev, W.I. Sensitivity of Yarrowia lipolytica to Wickerhamomyces mycocins. Probl. Med. Mycol. 2020, 22, 26–28. [Google Scholar]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Genetics, Biochemistry and Molecular Biology of Non-Conventional Yeasts in Biotechnology; Wolf, W.K., Ed.; Springer: Berlin, Germany, 1996; Volume 1, pp. 313–388. [Google Scholar]
- Tabuchi, T.; Tanaka, M.; Abe, M. Studies on organic acid fermentation in yeast. Part II. Production of citric acid by Candida lipolytica strain No. 228. J. Agric. Chem. Soc. Jpn. 1969, 43, 154–158. [Google Scholar]
- Abe, M.; Tabuchi, T.; Tanaka, M. Studies on organic acid fermentation in yeast. Part III. Accumulation of isocitric acid in cultures of yeast. J. Agric. Chem. Soc. Jpn. 1970, 44, 493–498. [Google Scholar]
- Finogenova, T.V. Biosynthesis of Organic Acids by Yeast Organisms and Its Regulation. Ph.D. Thesis, USSR Academy of Sciences, Institute of Biochemistry and Physiology of Microorganisms, Pushchino, Russia, 1982. (In Russian). [Google Scholar]
- Finogenova, T.V.; Illarionova, V.I.; Lozinov, A.B. Formation of citric acids by Candida lipolytica yeasts growing on n-alkanes. Mikrobiologiia 1973, 42, 790–794. (In Russian) [Google Scholar]
- Lozinov, A.B.; Finogenova, T.V.; Glazunova, L.M.; Illarionova, V.I. Growth limitation in Candida lipolytica cultures and supersynthesis of metabolites. Mikrobiologiia 1974, 43, 786–790. (In Russian) [Google Scholar]
- Illarionova, V.I.; Suetina, R.L. Review of patent literature on the biosynthesis of citric acid from hydrocarbons. In Biosynthesis of Oxy- and Keto-Acids by Microorganisms; Jacobson, Y.O., Ed.; Zinatne: Riga, Latvia, 1984; pp. 80–82. (In Russian) [Google Scholar]
- Klasson, T.K.; Clausen, E.C.; Gaddy, J.L. Continuous fermentation for the production of citric acid from glucose. Appl. Biochem. Biotechnol. 1989, 20, 491–509. [Google Scholar] [CrossRef]
- Charpentier, J.M.; Glikmans, G.; Maldonado, P. Process for Producing Citric Acid by Fermentation. US Patent No. 3.966.553A, 29 June 1976. [Google Scholar]
- Marchal, R.; Chaudé, O.; Metche, M. Production of citric acid from n-paraffins by Saccharomycopsis lipolytica: Kinetics and balance of the fermentation. Eur. J. Appl. Microbiol. Biotechnol. 1977, 4, 111–123. [Google Scholar] [CrossRef]
- Tréton, B.; Le Dall, M.T.; Heslot, H. Excretion of citric and isocitric acids by the yeast Saccharomycopsis lipolytica. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 67–77. [Google Scholar] [CrossRef]
- Behrens, U.; Weissbrodt, E.; Lehmann, W. Zur kinetik der Citronensäurebildung bei Candida lipolytica. Z. Allg. Mikrobiol. 1978, 18, 549–558. [Google Scholar] [CrossRef]
- Stottmeister, U.; Behrens, U.; Göhler, W. Einfluß des Sauerstoffpartialdrucks auf die Citronensäuresynthese durch Saccharomycopsis lipolytica aus n-Paraffinen. Z. Allg. Mikrobiol. 1981, 21, 677–687. [Google Scholar]
- Aurich, A.; Specht, R.; Müller, R.A.; Stottmeister, U.; Yovkova, V.; Otto, C.; Holz, M.; Barth, G.; Heretsch, P.; Thomas, F.A.; et al. Microbiologically Produced Carboxylic Acids Used as Building Blocks in Organic Synthesis. In Reprogramming Microbial Metabolic Pathways; Wang, X., Chen, J., Quinn, P., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 391–424. [Google Scholar]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef]
- Timoumi, A.; Guillouet, S.E.; Molina-Jouve, C.; Fillaudeau, L.; Gorret, N. Impacts of environmental conditions on product formation and morphology of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2018, 102, 3831–3848. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric Acid Production by Yarrowia lipolytica. In Non-Conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 91–117. [Google Scholar]
- Fickers, P.; Cheng, H.; Sze Ki Lin, C. Sugar Alcohols and Organic Acids Synthesis in Yarrowia lipolytica: Where Are We? Microorganisms 2020, 8, 574. [Google Scholar] [CrossRef]
- Behera, B.C.; Mishra, R.; Mohapatra, S. Microbial citric acid: Production, properties, application, and future perspectives. Food Front. 2021, 2, 62–76. [Google Scholar] [CrossRef]
- Madzak, C. Yarrowia lipolytica strains and their biotechnological applications: How natural biodiversity and metabolic engineering could contribute to cell factories improvement. Fungi 2021, 7, 548. [Google Scholar] [CrossRef]
- Park, Y.K.; Ledesma-Amaro, R. What makes Yarrowia lipolytica well suited for industry? Trends Biotechnol. 2023, 41, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Lunina, J.N.; Morgunov, I.G. Biochemistry of citric acid production from rapeseed oil by Yarrowia lipolytica yeast. J. Am. Oil Chem. Soc. 2011, 88, 1965–1976. [Google Scholar] [CrossRef]
- Wojtatowicz, M.; Marchin, G.L.; Erickson, L.E. Attempts to improve strain A-101 of Yarrowia lipolytica for citric acid production from n-paraffins. Process Biochem. 1993, 28, 453–460. [Google Scholar] [CrossRef]
- Aurich, A.; Förster, A.; Mauersberger, S.; Barth, G.; Stottmeister, U. Citric acid production from renewable resources by Yarrowia lipolytica. Biotechnol. Adv. 2003, 21, 454–455. [Google Scholar]
- Morgunov, I.G.; Kamzolova, S.V. Physiologo-biochemical characteristics of citrate-producing yeast Yarrowia lipolytica grown on glycerol-containing waste of biodiesel industry. Appl. Microbiol. Biotechnol. 2015, 99, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Ra, L.-F.; Wang, Z.-P.; Liu, X.-Y.; Chi, Z.-M. Citric acid production from extract of Jerusalem artichoke tubers by the genetically engineered yeast Yarrowia lipolytica strain 30 and purification of citric acid. Bioprocess Biosyst. Eng. 2013, 36, 1759–1766. [Google Scholar]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. Citric Acid Production by Yarrowia lipolytica Yeast on Different Renewable Raw Materials. Fermentation 2018, 4, 36. [Google Scholar] [CrossRef]
- Enzminger, J.D.; Asenjo, J.A. Use of cell recycle in the aerobic fermentative production of citric acid by yeast. Biotech. Lett. 1986, 8, 7–12. [Google Scholar] [CrossRef]
- Robak, M.; Rymowicz, W.; Filipkowski, P. Effect of sodium acetate on citric acid production from glucose by Yarrowia lipolytica. Electron. J. Pol. Agric. Universities. Ser. Biotechnol. 2007, 10, 22. Available online: http://www.ejpau.media.pl/volume10/issue4/art-22.html (accessed on 25 October 2023).
- Rakicka, M.; Wolniak, J.; Lazar, Z.; Rymowicz, W. Production of high titer of citric acid from inulin. BMC Biotechnol. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wojtatowicz, M.; Rymowicz, W.; Kautola, H. Comparison of different strains of the yeast Yarrowia lipolytica for citric acid production from glucose hydrol. Appl. Biochem. Biotechnol. 1991, 31, 165–174. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Xia, J.; Lv, J.; Wu, Z.; Deng, Y. Improved production of citric acid by Yarrowia lipolytica using oleic acid as the oxygen-vector and co-substrate. Eng. Life Sci. 2016, 16, 424–431. [Google Scholar] [CrossRef]
- Sarris, D.; Tsouko, E.; Kothri, M.; Anagnostou, M.; Karageorgiou, E.; Papanikolaou, S. Upgrading Major Waste Streams Derived from the Biodiesel Industry and Olive Mills via Microbial Bioprocessing with Non-Conventional Yarrowia lipolytica Strains. Fermentation 2023, 9, 251. [Google Scholar] [CrossRef]
- Lazar, Z.; Rossignol, T.; Verbeke, J.; Crutz-Le Coq, A.M.; Nicaud, J.M.; Robak, M. Optimized invertase expression and secretion cassette for improving Yarrowia lipolytica growth on sucrose for industrial applications. J. Ind. Microbiol. Biotechnol. 2013, 40, 1273–1283. [Google Scholar] [CrossRef]
- Morgunov, I.G.; Kamzolova, S.V.; Lunina, J.N. The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [Google Scholar] [CrossRef]
- Sarris, D.; Tsouko, E.; Photiades, A.; Tchakouteu, S.S.; Diamantopoulou, P.; Papanikolaou, S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 2: Citric Acid Production and Circular-Oriented Valorization of Glucose-Enriched Olive Mill Wastewaters Using Novel Yarrowia lipolytica Strains. Microorganisms 2023, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Amaro, R.; Lazar, Z.; Rakicka, M.; Guo, Z.; Fouchard, F.; Coq, A.-M.C.-L.; Nicaud, J.-M. Metabolic engineering of Yarrowia lipolytica to produce chemicals and fuels from xylose. Metab. Eng. 2016, 38, 115–124. [Google Scholar] [CrossRef]
- Lazar, Z.; Gamboa-Melendez, H.; Le Coq, A.M.; Neuveglise, C.; Nicaud, J.M. Awakening the endogenous Leloir pathway for efficient galactose utilization by Yarrowia lipolytica. Biotechnol. Biofuels 2015, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Sarris, D.; Tchakouteu, S.S.; Xenopoulos, E.; Papanikolaou, S. Growth Response of Non-Conventional Yeasts on Sugar-Rich Media: Part 1: High Production of Lipid by Lipomyces starkeyi and Citric Acid by Yarrowia lipolytica. Microorganisms 2023, 11, 1863. [Google Scholar] [CrossRef]
- Yalcin, S.; Bozdemir, M.T.; Ozbas, Z.Y. Utilization of whey and grape must for citric acid production by two Yarrowia lipolytica strains. Food Biotech. 2009, 23, 266–283. [Google Scholar] [CrossRef]
- Urak, S.; Yeniay, O.; Karasu-Yalcin, S. Optimization of citric acid production from a carrot juice-based medium by Yarrowia lipolytica using response surface methodology. Ann. Microbiol. 2015, 65, 639–649. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Xu, J.; Zhang, T.; Deng, Y.; He, J. Citric acid production in Yarrowia lipolytica SWJ-1b yeast when grown on waste cooking oil. Appl. Biochem. Biotechnol. 2015, 175, 2347–2356. [Google Scholar] [CrossRef]
- Yalcin, S.; Bozdemir, M.T.; Ozbas, Y. A comparative study on citric acid production kinetics of two Yarrowia lipolytica strains in two different media. Indian J. Biotechnol. 2009, 8, 408–417. [Google Scholar]
- Carsanba, E.; Agirman, B.; Papanikolaou, S.; Fickers, P.; Erten, H. Valorisation of Waste Bread for the Production of Yeast Biomass by Yarrowia lipolytica Bioreactor Fermentation. Fermentation 2023, 9, 687. [Google Scholar] [CrossRef]
- Förster, A.; Aurich, A.; Mauersberger, S.; Barth, G. Citric acid production from sucrose using a recombinant strain of the yeast Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 75, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Călinoiu, L.F.; Teleky, B.E.; Szabo, K.; Martău, A.G.; Ştefănescu, B.E.; Dulf, F.V.; Vodnar, D.C. Waste cooking oil and crude glycerol as efficient renewable biomass for the production of platform organic chemicals through oleophilic yeast strain of Yarrowia lipolytica. Environ. Technol. Innov. 2022, 28, 102943. [Google Scholar] [CrossRef]
- Liu, X.; Lv, J.; Zhang, T.; Deng, Y. Citric acid production from hydrolysate of pretreated straw cellulose by Yarrowia lipolytica SWJ-1b using batch and fed-batch cultivation. Prep. Biochem. Biotechnol. 2015, 45, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Xu, J.; Xia, J.; Lv, J.; Zhang, T.; Wu, Z.; Deng, Y.; He, J. Citric acid production by Yarrowia lipolytica SWJ-1b using corn steep liquor as a source of organic nitrogen and vitamins. Ind. Crops Prod. 2015, 78, 154–160. [Google Scholar] [CrossRef]
- Cavallo, E.; Nobile, M.; Cerrutti, P.; Foresti, M.L. Exploring the production of citric acid with Yarrowia lipolytica using corn wet milling products as alternative low-cost fermentation media. Biochem. Eng. J. 2020, 155, 107463. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, F.; Li, X.; Mao, G.; Xie, H.; Song, A.; dos Santos, J.C.; Zhang, Z. Tailored production of citric acid and mannitol by Yarrowia lipolytica from corn stover pretreated by glycerol-assisted instant catapult steam explosion. Ind. Crops Prod. 2022, 189, 115820. [Google Scholar] [CrossRef]
- Venter, T.; Kock, J.L.F.; Botes, P.J.; Smit, M.S.; Hugo, A.; Joseph, M. Acetate enhances citric acid production by Yarrowia lipolytica when grown on sunflower oil. Syst. Appl. Microbiol. 2004, 27, 135–138. [Google Scholar] [CrossRef]
- Rywinska, A.; Rymowicz, W.; Zarowska, B.; Skrzypinski, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef]
- Celik, G.; Ucar, F.B.; Akpinar, O.; Corbaci, C. Production of citric and isocitric acid by Yarrowia lipolytica strains grown on different carbon sources. Turk. J. Biochem. 2014, 39, 285–290. [Google Scholar]
- Börekçi, B.S.; Kaban, G.; Kaya, M. Citric acid production of yeasts: An overview. EuroBiotech J. 2021, 5, 79–91. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Lunina, J.N.; Samoilenko, V.A.; Morgunov, I.G. Effect of Nitrogen Concentration on the Biosynthesis of Citric Acid, Protein, and Lipids in the Yeast Yarrowia lipolytica. Biomolecules 2022, 12, 1421. [Google Scholar] [CrossRef]
- Da Silva, L.V.; Tavares, C.B.; Amaral, P.F.F.; Coehlo, M.A.Z. Production of citric acid by Yarrowia lipolytica in different crude oil concentrations and in different nitrogen sources. Chem. Eng. Trans. 2012, 27, 199–204. [Google Scholar]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Screening various Yarrowia lipolytica strains for citric acid production. Yeast 2019, 36, 319–327. [Google Scholar] [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Characterization of Yarrowia lipolytica and related species for citric acid production from glycerol. Enzyme Microb. Technol. 2007, 41, 292–295. [Google Scholar] [CrossRef]
- Finogenova, T.; Kamzolova, S.; Dedyukhina, E.; Shishkanova, N.; Il’Chenko, A.; Morgunov, I.; Chernyavskaya, O.; Sokolov, A. Biosynthesis of citric and isocitric acids from ethanol by mutant Yarrowia lipolytica N 1 under continuous cultivation. Appl. Microbiol. Biotechnol. 2002, 59, 493–500. [Google Scholar]
- Hoffmann, A.; Kupsch, C.; Walther, T.; Löser, C. Synthesis of ethyl acetate from glucose by Kluyveromyces marxianus, Cyberlindnera jadinii and Wickerhamomyces anomalus depending on the induction mode. Eng. Life Sci. 2021, 21, 154–168. [Google Scholar] [CrossRef]
- Il’chenko, A.P.; Chernyavskaya, O.G.; Finogenova, T.V. Ethanol metabolism in the yeasts Yarrowia and Torulopsis: A Review. Appl. Biochem. Microbiol. 2005, 41, 426–432. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Effects of Medium Components on Isocitric Acid Production by Yarrowia lipolytica Yeast. Fermentation 2020, 6, 112. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Effect of Metabolic Regulators and Aeration on Isocitric Acid Synthesis by Yarrowia lipolytica Grown on Ester-Aldehyde Fraction. Fermentation 2021, 7, 283. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rymowicz, W.; Rywinska, A. Mineral supplementation increases erythrose reductase activity in erythritol biosynthesis from glycerol by Yarrowia lipolytica. Appl. Biochem. Biotechnol. 2014, 172, 3069–3078. [Google Scholar] [CrossRef]
- Good, D.W.; Droniuk, R.; Lawford, G.R.; Fein, J.E. Isolation and characterization of a Saccharomycopsis lipolytica mutant showing increased production of citric acid from canola oil. Can. J. Microbiol. 1985, 31, 436–440. [Google Scholar] [CrossRef]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Drogui, P. Optimization of trace elements in purified glycerol for microbial lipid and citric acid production by Yarrowia lipolytica SKY7. Syst. Microbiol. Biomanuf. 2021, 1, 76–89. [Google Scholar] [CrossRef]
- Stottmeister, U.; Aurich, A.; Wilde, H.; Andersch, J.; Schmidt, S.; Sicker, D. White biotechnology for green chemistry: Fermentative 2-oxocarboxylic acids as novel building blocks for subsequent chemical syntheses. J. Ind. Microbiol. Biotech. 2005, 32, 651–664. [Google Scholar] [CrossRef]
- Otto, C.; Yovkova, V.; Barth, G. Overproduction and secretion of α-ketoglutaric acid by microorganisms. Appl. Microbiol. Biotechnol. 2011, 92, 689–695. [Google Scholar] [CrossRef]
- Cybulski, K.; Tomaszewska-Hetman, L.; Rakicka, M.; Juszczyk, P.; Rywińska, A. Production of pyruvic acid from glycerol by Yarrowia lipolytica. Folia Microbiol. 2019, 64, 809–820. [Google Scholar] [CrossRef]
- Rywińska, A.; Tomaszewska-Hetman, L.; Rakicka-Pustułka, M.; Juszczyk, P.; Rymowicz, W. Alpha-Ketoglutaric Acid Production from a Mixture of Glycerol and Rapeseed Oil by Yarrowia lipolytica Using Different Substrate Feeding Strategies. Sustainability 2020, 12, 6109. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Morgunov, I.G. Selection of Producer of α-Ketoglutaric Acid from Ethanol-Containing Wastes and Impact of Cultivation Conditions. Fermentation 2022, 8, 362. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Rywińska, A.; Lazar, Z.; Rymowicz, W. Enhancement of α-Ketoglutaric Acid Production by Yarrowia lipolytica Grown on Mixed Renewable Carbon Sources through Adjustment of Culture Conditions. Catalysts 2023, 13, 14. [Google Scholar] [CrossRef]
- Moeller, L.; Strehlitz, B.; Aurich, A.; Zehnsdorf, A.; Bley, T. Optimization of citric acid production from glucose by Yarrowia lipolytica. Eng. Life Sci. 2007, 7, 504–511. [Google Scholar] [CrossRef]
- Arslan, N.P.; Aydogan, M.N.; Taskin, M. Citric acid production from partly deproteinized whey under non-sterile culture conditions using immobilized cells of lactose—Positive and cold-adapted Yarrowia lipolytica B9. J. Biotechnol. 2016, 231, 32–39. [Google Scholar] [CrossRef]
- Zhang, S.; Jagtap, S.S.; Deewan, A.; Rao, C.V. pH selectively regulates citric acid and lipid production in Yarrowia lipolytica W29 during nitrogen-limited growth on glucose. J. Biotechnol. 2019, 290, 10–15. [Google Scholar] [CrossRef]
- Egermeier, M.; Russmayer, H.; Sauer, M.; Marx, H. Metabolic flexibility of Yarrowia lipolytica growing on glycerol. Front. Microbiol. 2017, 8, 49. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Rzechonek, D.A.; Biegalska, A.; Rakicka, M.; Dobrowolski, A. A novel strain of Yarrowia lipolytica as a platform for value-added product synthesis from glycerol. Biotechnol. Biofuels 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Shishkanova, N.V.; Morgunov, I.G.; Finogenova, T.V. Oxygen requirements for growth and citric acid production of Yarrowia lipolytica. FEMS Yeast Res. 2003, 3, 217–222. [Google Scholar] [CrossRef]
- Bellou, S.; Makri, A.; Triantaphyllidou, I.E.; Papanikolaou, S.; Aggelis, G. Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology 2014, 160, 807–817. [Google Scholar] [CrossRef]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen transfer rate and pH as major operating parameters of citric acid production from glycerol by Yarrowia lipolytica W29 and CBS 2073. Chem. Pap. 2016, 70, 869–876. [Google Scholar] [CrossRef]
- Moeleira, P.; Lopes, M.; Belo, I. Use of Pressurized and Airlift Bioreactors for Citric Acid Production by Yarrowia lipolytica from Crude Glycerol. Fermentation 2022, 8, 700. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Chevalot, I.; Komaitis, M.; Marc, I.; Aggelis, G. Influence of glucose and saturated free-fatty acid mixtures on citric acid and lipid production by Yarrowia lipolytica. Curr. Microbiol. 2006, 52, 134–142. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Muniglia, L.; Chevalot, I.; Aggelis, G.; Marc, I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.-E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of Olive Mill Wastewater into High-Added Value Products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Fakas, S.; Fick, M.; Chevalot, I.; Galiotou-Panayotou, M.; Komaitis, M.; Marc, I.; Aggelis, G. Biotechnological valorisation of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: Production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenergy 2008, 32, 60–71. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Kampisopoulou, E.; Blanchard, F.; Rondags, E.; Gardeli, C.; Koutinas, A.A.; Chevalot, I.; Aggelis, G. Production of secondary metabolites through glycerol fermentation under carbon-excess conditions by the yeasts Yarrowia lipolytica and Rhodosporidium toruloides. Eur. J. Lipid Sci. Technol. 2017, 119, 1600507. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef]
- Sarris, D.; Stoforos, N.G.; Mallouchos, A.; Kookos, I.K.; Koutinas, A.A.; Aggelis, G.; Papanikolaou, S. Production of added-value metabolites by Yarrowia lipolytica growing in olive mill wastewater-based media under aseptic and non-aseptic conditions. Eng. Life Sci. 2017, 17, 695–709. [Google Scholar] [CrossRef]
- Kim, E.K.; Ambriano, J.R.; Roberts, R.S. Vigorous stationary phase fermentation. Biotechnol. Bioeng. 1987, 30, 805–808. [Google Scholar] [CrossRef]
- Anastassiadis, S.; Aivasidis, A.; Wandrey, C. Citric acid production by Candida strains under intracellular nitrogen limitation. Appl. Microbiol. Biotechnol. 2002, 60, 81–87. [Google Scholar]
- Mirbagheri, M.; Nahvi, I.; Emtiazi, G.; Darvishi, F. Enhanced Production of Citric Acid in Yarrowia lipolytica by Triton X-100. Appl. Biochem. Biotechnol. 2011, 165, 1068–1074. [Google Scholar] [CrossRef]
- Giacomobono, R.; Albergo, R.; Valerio, V.; Caporusso, A.; De Bari, I. Modelling of the Citric Acid Production from Crude Glycerol by Wild-Type Yarrowia lipolytica DSM 8218 Using Response Surface Methodology (RSM). Life 2022, 12, 621. [Google Scholar] [CrossRef]
- Moeller, L.; Grünberg, M.; Zehnsdorf, A.; Strehlitz, B.; Bley, T. Biosensor online control of citric acid production from glucose by Yarrowia lipolytica using semicontinuous fermentation. Eng. Life Sci. 2010, 10, 311–320. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Diamantopoulou, P.; Blanchard, F.; Lambrinea, E.; Chevalot, I.; Stoforos, N.G.; Rondags, E. Physiological Characterization of a Novel Wild-Type Yarrowia lipolytica Strain Grown on Glycerol: Effects of Cultivation Conditions and Mode on Polyols and Citric Acid Production. Appl. Sci. 2020, 10, 7373. [Google Scholar] [CrossRef]
- Rane, K.D.; Sims, K.A. Citric acid production by Candida lipolytica Y 1095 in cell recycle and fed-batch fermentors. Biotechnol. Bioeng. 1995, 46, 325–332. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica. Lipid Technol. 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater-based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Qian, X.; Xu, N.; Jing, Y.; Song, M.; Zhou, J.; Dong, W.; Xin, F.; Zhang, W.; Jiang, M.; Ochsenreither, K. Valorization of crude glycerol into citric acid and malic acid by Yarrowia lipolytica. Ind. Eng. Chem. Res. 2020, 59, 17165–17172. [Google Scholar] [CrossRef]
- da Veiga Moreira, J.; Jolicoeur, M.; Schwartz, L.; Peres, S. Fine-tuning mitochondrial activity in Yarrowia lipolytica for citrate overproduction. Sci. Rep. 2021, 11, 878. [Google Scholar] [CrossRef]
- Finogenova, T.V.; Puntus, I.F.; Kamzolova, S.V.; Lunina, I.N.; Monastyrskaia, S.E.; Morgunov, I.G.; Boronin, A.M. Obtaining of the mutant Yarrowia lipolytica strains producing citric acid from glucose. Prikl. Biokhim. Mikrobiol. 2008, 44, 219–224. [Google Scholar]
- Rymowicz, W.; Rywińska, A.; Źarowska, B.; Juszczyk, P. Citric acid production from raw glycerol by acetate mutants of Yarrowia lypolitica. Chem. Pap. 2006, 60, 391–394. [Google Scholar] [CrossRef]
- Rywińska, A.; Juszczyk, P.; Wojtatowicz, M.; Rymowicz, W. Chemostat study of citric acid production from glycerol by Yarrowia lipolytica. J. Biotechnol. 2011, 152, 54–57. [Google Scholar] [CrossRef]
- Rywińska, A.; Rymowicz, W. High-yield production of citric acid by Yarrowia lipolytica on glycerol in repeated-batch bioreactors. J. Ind. Microbiol. Biotechnol. 2010, 37, 431–435. [Google Scholar] [CrossRef]
- Rymowicz, W.; Fatykhova, A.R.; Kamzolova, S.V.; Rywińska, A.; Morgunov, I.G. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [Google Scholar] [CrossRef]
- Akiyama, S.I.; Suzuki, T.; Sumino, Y.; Nakao, Y.; Fukuda, H. Induction and citric acid productivity of fluoroacetate-sensitive mutant strains of Candida lipolytica. Agr. Biol. Chem. 1973, 37, 879–884. [Google Scholar] [CrossRef]
- Yalcin, S.K. Enhancing citric acid production of Yarrowia lipolytica by mutagenesis and using natural media containing carrot juice and celery byproducts. Food Sci. Biotechnol. 2012, 21, 867–874. [Google Scholar] [CrossRef]
- Ledesma-Amaro, R.; Nicaud, J.M. Metabolic Engineering for Expanding the Substrate Range of Yarrowia lipolytica. Trends Biotechnol. 2016, 34, 798–809. [Google Scholar] [CrossRef]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A.P. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb. Cell Fact. 2017, 16, 78. [Google Scholar] [CrossRef]
- Hapeta, P.; Rakicka-Pustułka, M.; Juszczyk, P.; Robak, M.; Rymowicz, W.; Lazar, Z. Overexpression of Citrate Synthase Increases Isocitric Acid Biosynthesis in the Yeast Yarrowia lipolytica. Sustainability 2020, 12, 7364. [Google Scholar] [CrossRef]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Aseptic production of citric and isocitric acid from crude glycerol by genetically modified Yarrowia lipolytica. Bioresour. Technol. 2019, 271, 340–344. [Google Scholar] [CrossRef]
- Förster, A.; Jacobs, K.; Juretzek, T.; Mauersberger, S.; Barth, G. Overexpression of the ICL1 gene changes the product ratio of citric acid production by Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2007, 77, 861–869. [Google Scholar] [CrossRef]
- Moeller, L.; Zehnsdorf, A.; Aurich, A.; Barth, G.; Bley, T.; Strehlitz, B. Citric acid production from sucrose by recombinant Yarrowia lipolytica using semicontinuous fermentation. Eng. Life Sci. 2013, 13, 163–171. [Google Scholar] [CrossRef]
- Tan, M.J.; Chen, X.; Wang, Y.K.; Liu, G.L.; Chi, Z.M. Enhanced citric acid production by a yeast Yarrowia lipolytica over-expressing a pyruvate carboxylase gene. Bioprocess Biosyst. Eng. 2016, 39, 1289–1296. [Google Scholar] [CrossRef]
- Fu, G.Y.; Lu, Y.; Chi, Z.; Liu, G.L.; Zhao, S.F.; Jiang, H.; Chi, Z.M. Cloning and characterization of a pyruvate carboxylase gene from Penicillium rubens and overexpression of the gene in the yeast Yarrowia lipolytica for enhanced citric acid production. Mar. Biotechnol. 2016, 18, 1–14. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chi, Z.; Liu, G.-L.; Wang, F.; Madzak, C.; Chi, Z.-M. Inulin hydrolysis and citric acid production from inulin using the surface-engineered Yarrowia lipolytica displaying inulinase. Metab. Eng. 2010, 12, 469–476. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chi, Z.; Liu, G.-L.; Madzak, C.; Chi, Z.-M. Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar. Biotechnol. 2013, 15, 26–36. [Google Scholar] [CrossRef]
- Yuzbasheva, E.Y.; Agrimi, G.; Yuzbashev, T.V.; Scarcia, P.; Vinogradova, E.B.; Palmieri, L.; Shutov, A.V.; Kosikhina, I.M.; Palmieri, F.; Sineoky, S.P. The mitochondrial citrate carrier in Yarrowia lipolytica: Its identification, characterization and functional significance for the production of citric acid. Metab. Eng. 2019, 54, 264–274. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Beopoulos, A.; Koletti, A.; Thevenieau, F.; Koutinas, A.A.; Nicaud, J.-M.; Aggelis, G. Importance of the methyl-citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica. J. Biotechnol. 2013, 168, 303–314. [Google Scholar] [CrossRef]
- Lazar, Z.; Dulermo, T.; Neuvéglise, C.; Crutz-Le Coq, A.M.; Nicaud, J.M. Hexokinase—A limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab. Eng. 2014, 26, 89–99. [Google Scholar] [CrossRef]
- Hapeta, P.; Szczepańska, P.; Witkowski, T.; Nicaud, J.-M.; Crutz-Le Coq, A.-M.; Lazar, Z. The Role of Hexokinase and Hexose Transporters in Preferential Use of Glucose over Fructose and Downstream Metabolic Pathways in the Yeast Yarrowia lipolytica. Int. J. Mol. Sci. 2021, 22, 9282. [Google Scholar] [CrossRef]
- Egermeier, M.; Sauer, M.; Marx, H. Golden Gate-based metabolic engineering strategy for wild-type strains of Yarrowia lipolytica. FEMS Microbiol. Lett. 2019, 366, fnz022. [Google Scholar] [CrossRef]
- Debabov, V.G. Modern approaches to the creation of industrial microorganism strains. Russ. J. Genet. 2015, 51, 365–376. [Google Scholar] [CrossRef]
- Mavrommati, M.; Daskalaki, A.; Papanikolaou, S.; Aggelis, G. Adaptive laboratory evolution principles and applications in industrial biotechnology. Biotechnol Adv. 2022, 54, 107795. [Google Scholar] [CrossRef]
- Fernandes, T.; Osório, C.; Sousa, M.J.; Franco-Duarte, R. Contributions of Adaptive Laboratory Evolution towards the Enhancement of the Biotechnological Potential of Non-Conventional Yeast Species. J. Fungi 2023, 9, 186. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Li, C.; Lin, C.S.K. Restoring of glucose metabolism of engineered Yarrowia lipolytica for succinic acid production via a simple and efficient adaptive evolution strategy. J. Agric. Food Chem. 2017, 65, 4133–4139. [Google Scholar] [CrossRef]
- Narisetty, V.; Prabhu, A.A.; Bommareddy, R.R.; Cox, R.; Agrawal, D.; Misra, A.; Haider, M.A.; Bhatnagar, A.; Pandey, A.; Kumar, V. Development of Hypertolerant Strain of Yarrowia lipolytica Accumulating Succinic Acid Using High Levels of Acetate. ACS Sustain. Chem. Eng. 2022, 10, 10858–10869. [Google Scholar]
- Wang, Z.; Zhou, L.; Lu, M.; Zhang, Y.; Perveen, S.; Zhou, H.; Wen, Z.; Xu, Z.; Jin, M. Adaptive laboratory evolution of Yarrowia lipolytica improves ferulic acid tolerance. Appl. Microbiol. Biotechnol. 2021, 105, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, A.; Perdikouli, N.; Aggeli, D.; Aggelis, G. Laboratory evolution strategies for improving lipid accumulation in Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2019, 103, 8585–8596. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).