Research and Possible Agronomic Applications of C60(OH)24 Adducts with Heavy Metals for Crop Treatment
Abstract
:1. Introduction
2. Synthesis of Me-Adducts
Synthesis Scheme
- As a result of dissolution of 2.9 g. in 30 cm3 0.1 M NaOH solution, an aqueous solution is formed. The pH of the solution is 7.5–8.5 a.u. with the addition of a few drops of 1 M solution of HCl.
- Preparation of 100 cm3 of MeCln solution (Me=Co; Cu; Mn; Zn; Gd; Tb) with a concentration of 55 g/dm3 (CoCl2) ÷ 93 g/dm3 (TbCl3) at a solution pH~3.5 4.0 a.u. in order to avoid hydrolysis of MeCln (by adding a few drops of 1 M HCl solution).
- Adding an aqueous solution of MeCln drop by drop to an aqueous solution of . A loose amorphous colored precipitate is formed. Standing of the resulting solution with the formed precipitate for 24 h. Filtration of the resulting heterogeneous mixture.
- Three-time washing of the sediment with methanol for 50 cm3 of solvent. Final drying of adducts in a vacuum drying cabinet (P ≤ 0.1 mm Hg) at a temperature of ~50 °C for 90 min.
- As a result, we synthesized gram amounts of colored crystal hydrates of Me-adducts: with masses 3.8 g () ÷ 4.1 g ), which corresponds to a practical yield of 65–72% of the theoretically possible.
3. Identification of Me-Adducts
- IR spectra of adducts in KBr tablets were obtained on the Shimadzu FTIR-8400S spectrometer (Shimadzu, Kyoto, Japan) in the range of wave numbers = 400 ÷ 4000 cm−1;
- Electronic absorption spectra were obtained on the SPECORD M32 spectrophotometer (Analytik Jena, Jena, Germany) in the wavelength range λ = 200 ÷ 1000 nm (comparison solution–distilled water);
- Complex thermal analysis of adducts was carried out on a NETZSCH TG 209 F1 Libra analyzer (NETZSCH, Selb, Germany) in the temperature range of 30 ÷ 1100 °C in an air atmosphere with a heating rate of 5 K·min−1;
- For high-performance liquid chromatography, a Shimadzu LC-20 Prominence device with spectrophotometric detection at λ = 270 nm was used, equipped with a “Phenomenex® column NH2” (Phenomenex, Torrance, CA, USA) (150 mm × 2.0 mm, 5 microns, 100 A), input volume 2 × 10−2 cm3, input speed 0.2 mL·min−1, eluent—acetonitrile/0.1% aqueous solution of acetic acid (5/95);
- Elemental composition analysis was carried out by scanning VEGA 3 TESCAN 4th generation electron microscope in EssenceTM EDS software, using a fully integrated energy dispersion spectrometer (on a VEGA3 TESCAN electron microscope);
- Additionally, elemental analysis for the sulfur content of light atoms was carried out using a PerkinElmer PE 2400 CHN analyzer (PerkinElmer, Waltham, MA, USA);
- Dynamic light scattering in aqueous solutions of adducts was carried out, using Zetasizer Nano ZS (Malvern Panalytical, Malvern, UK);
- Solubility of adducts in aqueous solutions was studied using a standard shaker thermostat with additional temperature stabilization (∆T = 0.05 K, saturation time—8 h, shaking frequency ν = 2 Hz, solution concentrations were determined spectrophotometrically by absorption spectra at λ = 270 nm).
3.1. Elemental Analysis
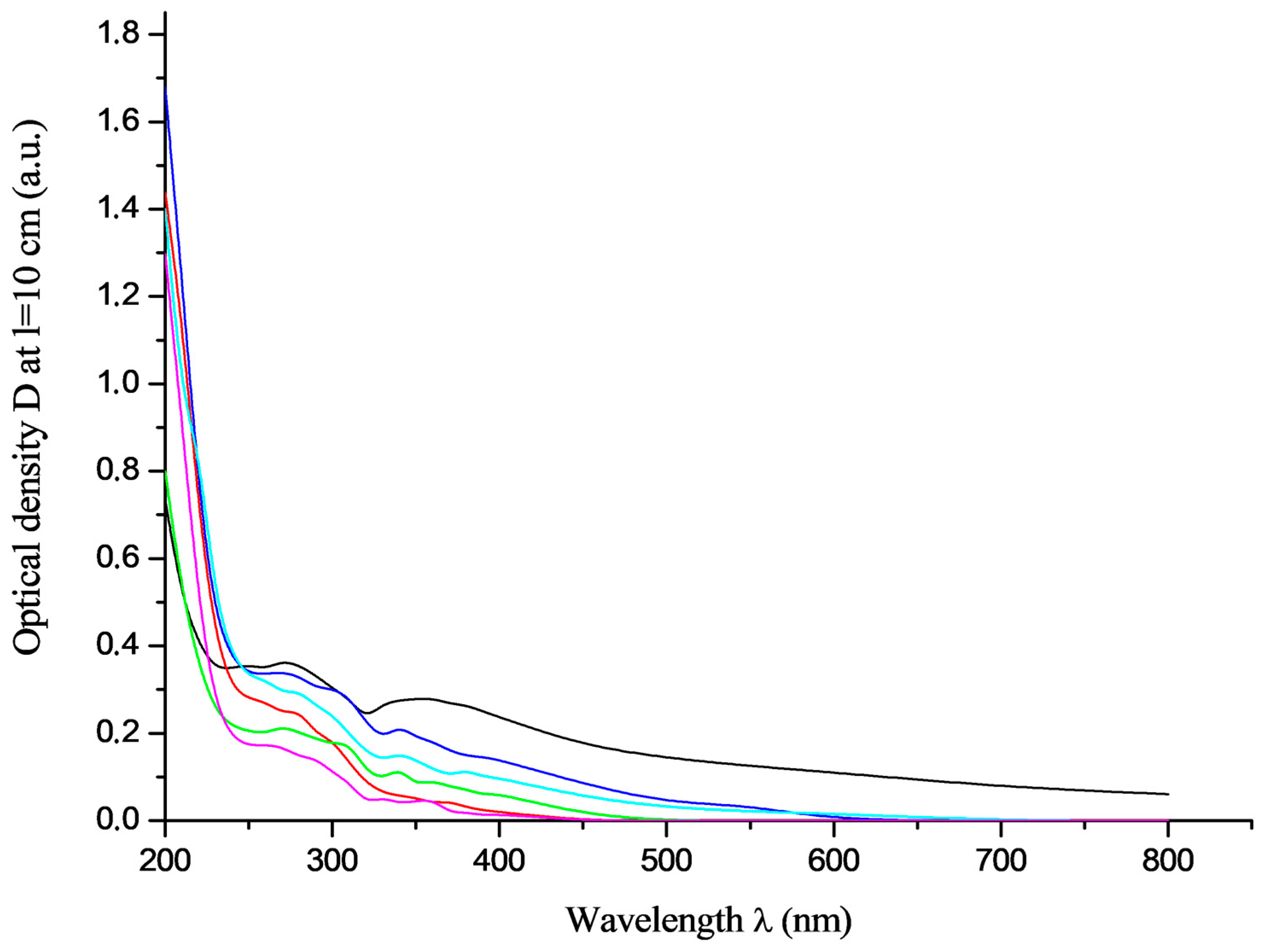
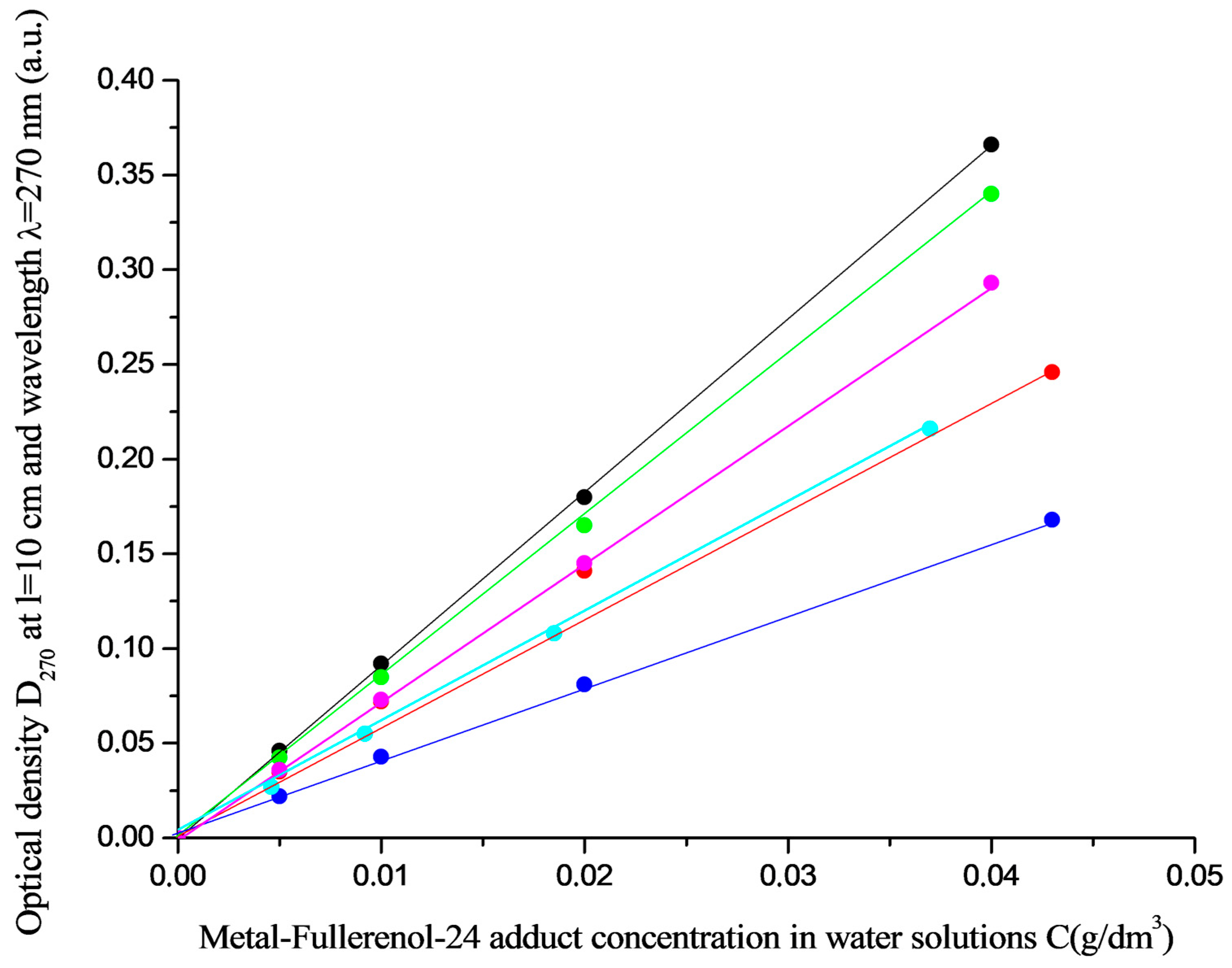
3.2. Electronic Spectroscopy
3.3. Infrared Spectroscopy
3.4. Complex Thermal Analysis
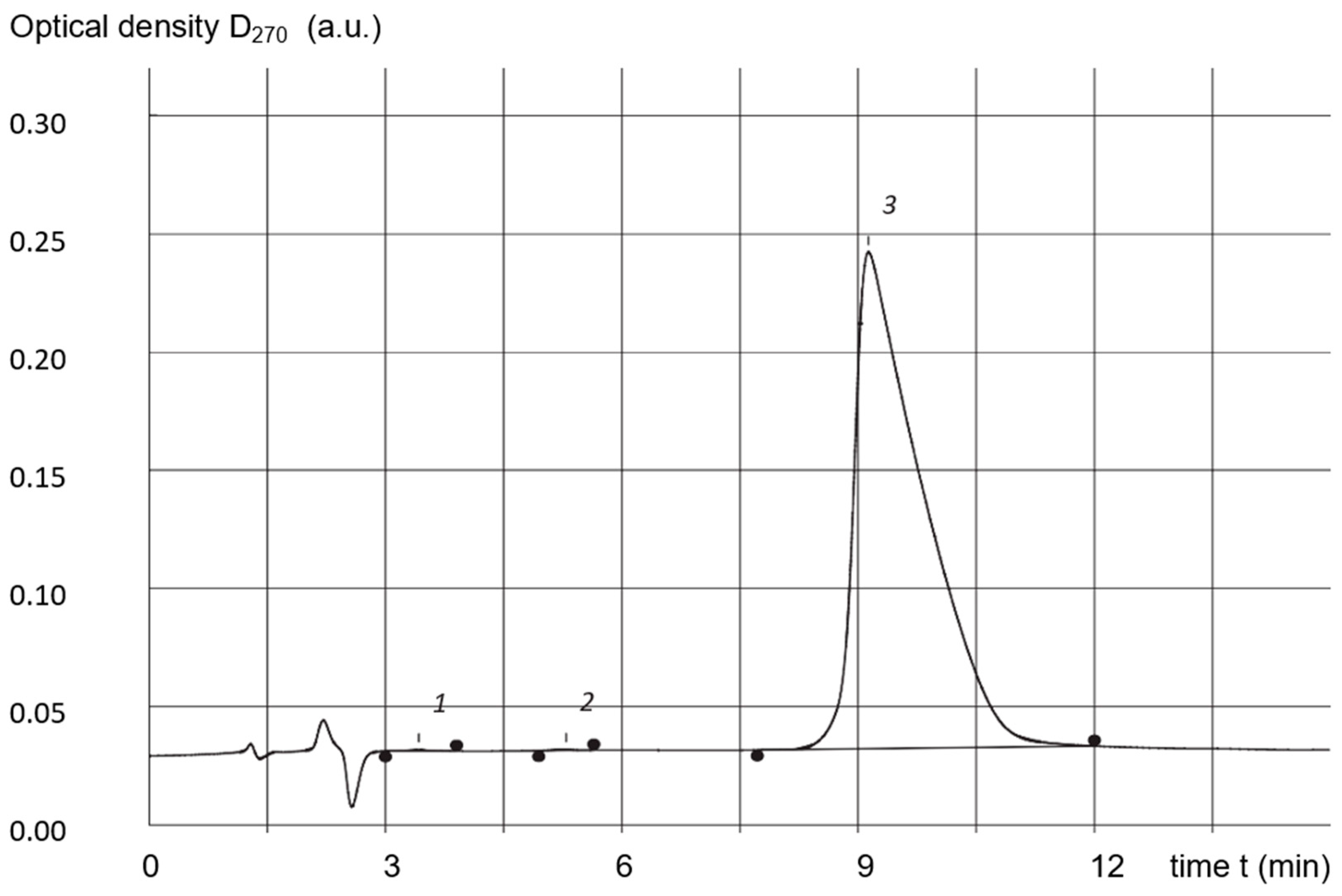
3.5. High-Performance Liquid-Phase Chromatography (HPLC)
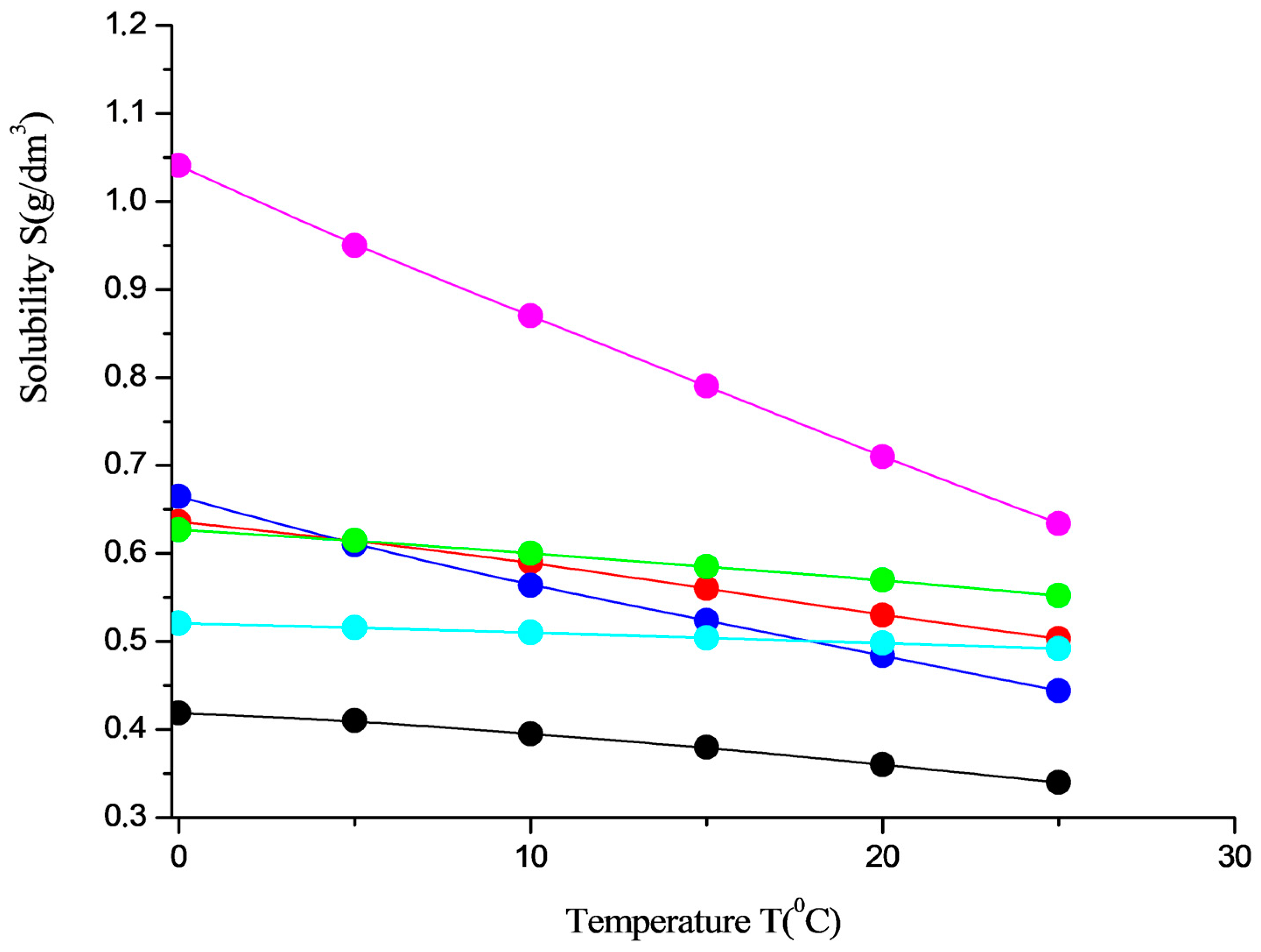
4. Solubility in Water Solutions in the Natural Range of Temperatures
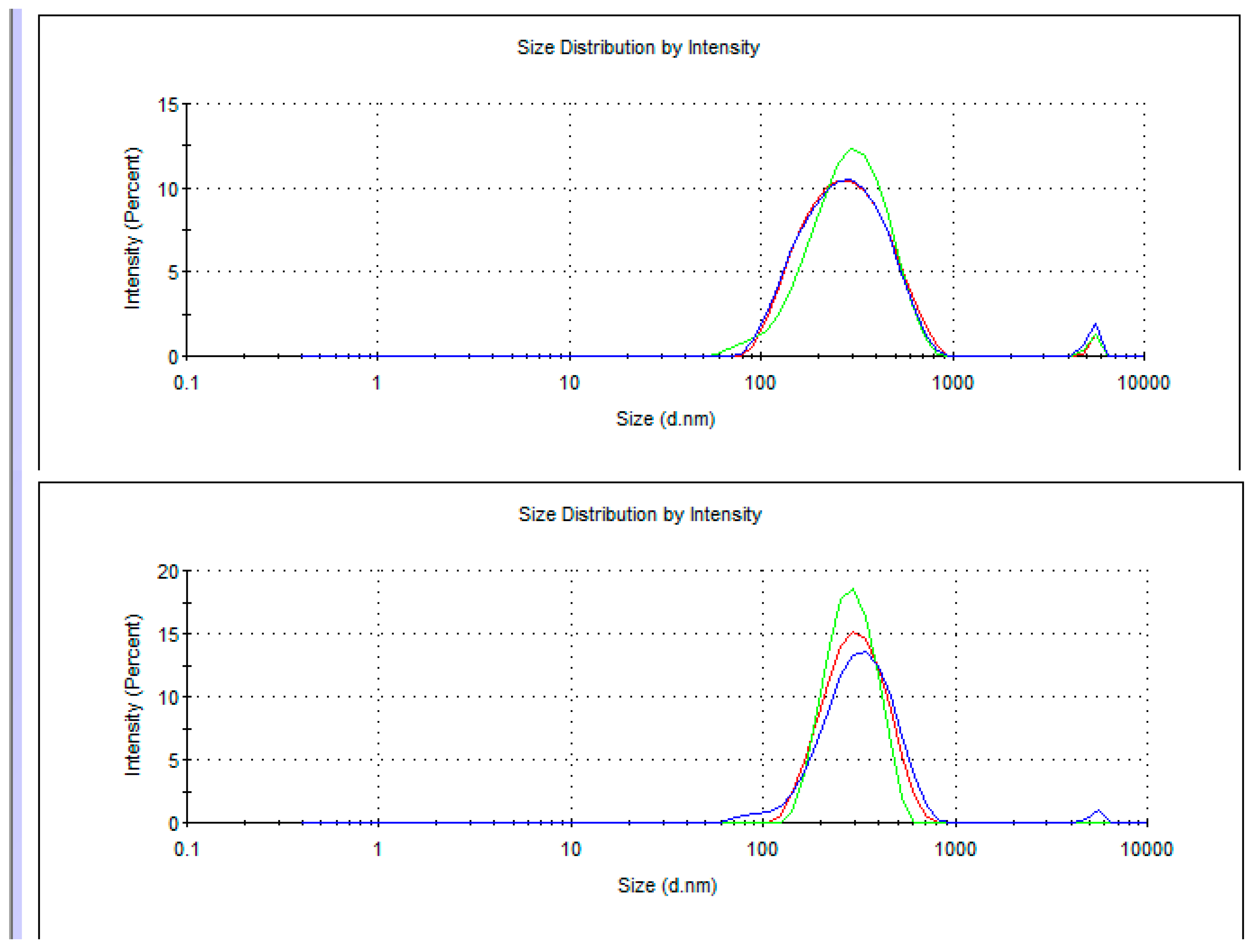
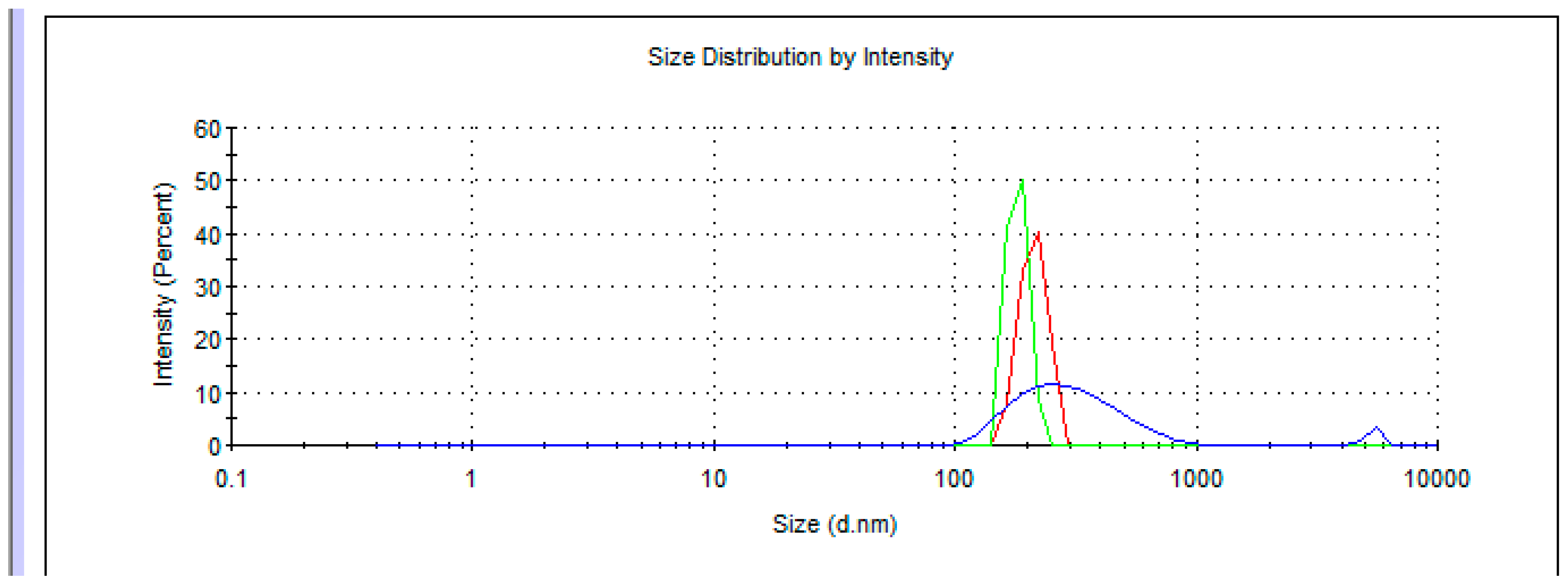
5. Dynamic Light Scattering in Water Solutions
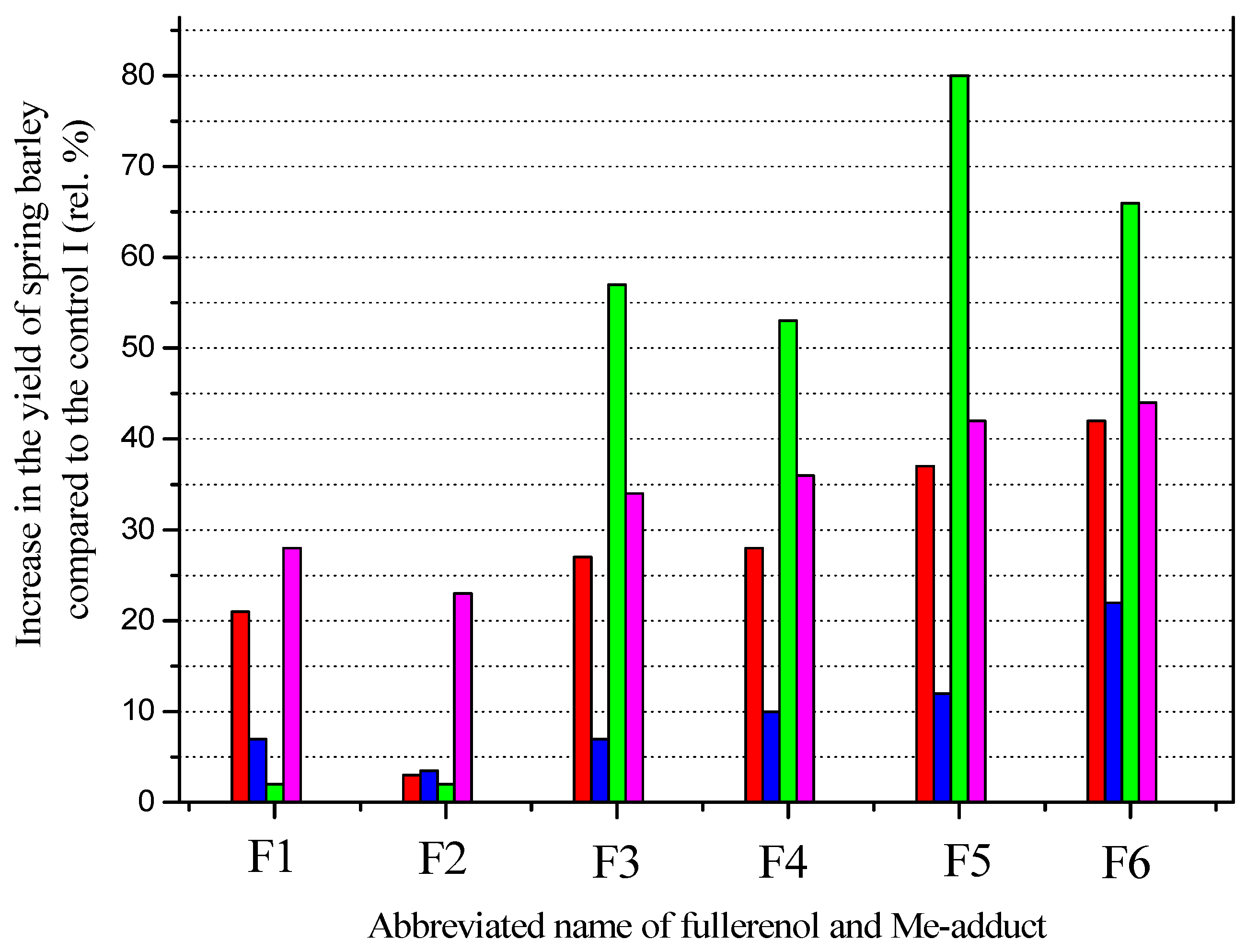
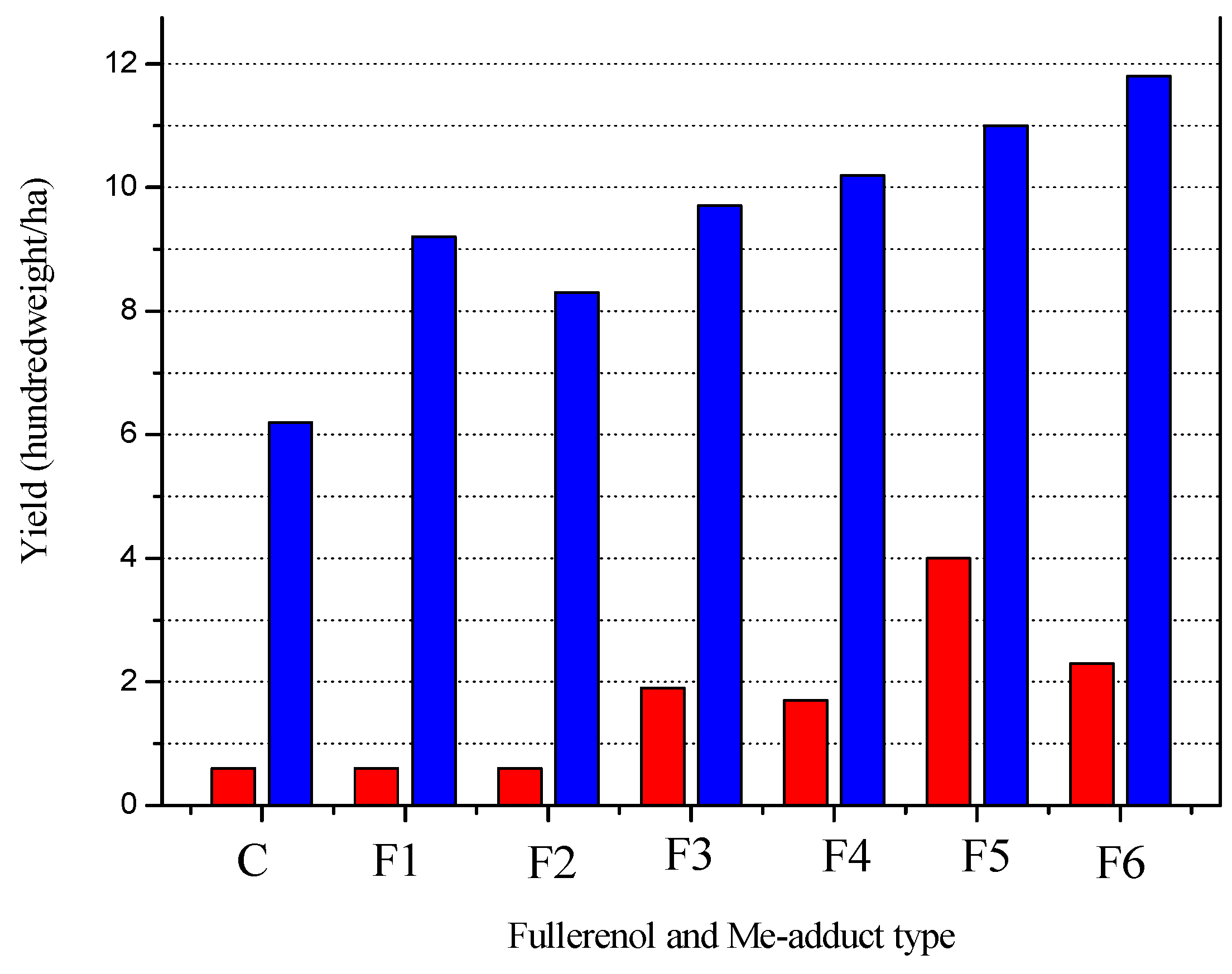
6. The Effect of Micro-Fertilizers Based on Me-Adducts on the Yield of Spring Barley
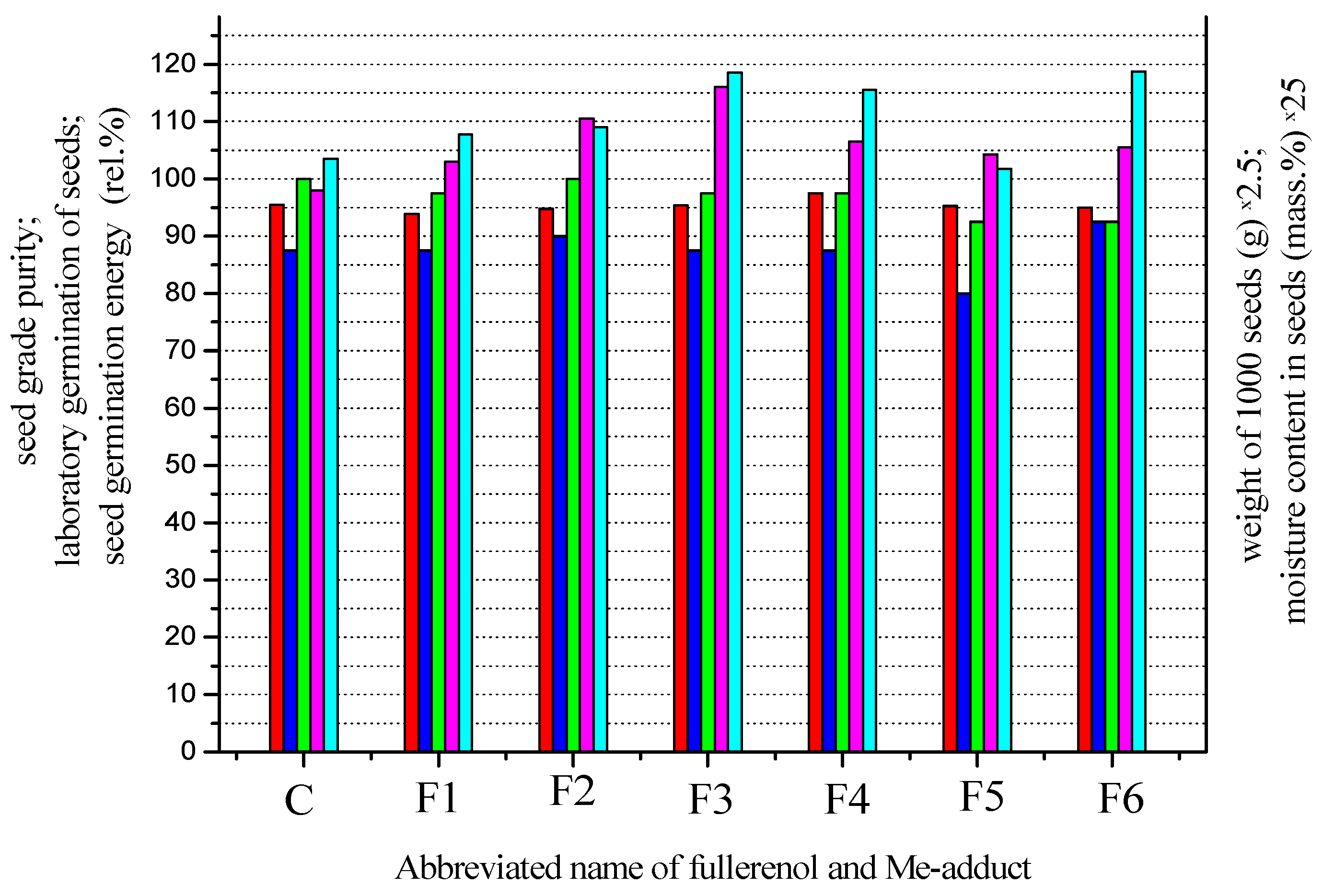
7. Quality Control of Seeds, Processed by Fullerenols and Me-Adducts
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharoyko, V.V.; Ageev, S.V.; Meshcheriakov, A.A.; Akentiev, A.V.; Noskov, B.A.; Rakipov, I.T.; Charykov, N.A.; Kulenova, N.A.; Shaimardanova, B.K.; Podolsky, N.E.; et al. Physicochemical study of water-soluble C60(OH)24 fullerenol. J. Mol. Liquids 2020, 311, 113360–113411. [Google Scholar] [CrossRef]
- Charykov, N.A.; Keskinov, V.A.; Tsvetkov, K.A.; Kanbar, A.; Semenov, K.N.; Gerasimova, L.V.; Shaimardanov, Z.K.; Shaimardanova, B.K.; Kulenova, N.A. Solubility of Rare Earth Chlorides in Ternary Water-Salt Systems in the Presence of a Fullerenol—C60(OH)24 Nanoclusters at 25 °C. Models of Nonelectrolyte Solubility in Electrolyte Solutions. Processes 2021, 9, 349. [Google Scholar] [CrossRef]
- Semenov, K.N.; Charykov, N.A.; Postnov, V.N.; Sharoyko, V.V.; Vorotyntsev, I.V.; Galagudza, M.M.; Murin, I.V. Fullerenols: Physicochemical properties and applications. Prog. Solid State Chem. 2016, 44, 59–74. [Google Scholar] [CrossRef]
- Podolsky, N.E.; Marcos, M.A.; Cabaleiro, D.; Semenov, K.N.; Lugo, L.; Petrov, A.V.; Charykov, N.A.; Sharoyko, V.V.; Vlasov, T.D.; Murin, I.V. Physico-chemical properties of C60(OH)22–24 water solutions: Density, viscosity, refraction index, isobaric heat capacity and antioxidant activity. J. Mol. Liquids 2019, 278, 342–355. [Google Scholar] [CrossRef]
- Panova, G.G.; Semenov, K.N.; Shilova, O.A.; Kornyukhin, D.L.; Shpanev, A.M.; Anikina, L.M.; Khamova, T.V.; Artemyeva, A.M.; Kanash, E.V.; Charykov, N.A.; et al. Ifluence of carbon and silica ash nanonaterials on the resistance of spring barley to root rot disease. Agrophysics 2018, N3, 48–58. [Google Scholar] [CrossRef]
- Panova, G.G.; Kanash, E.V.; Semenov, K.N.; Charykov, N.A.; Khomyakov, Y.V.; Anikina, L.M.; Artem’eva, A.M.; Kornyukhin, D.L.; Vertebnyi, V.E.; Sinyavina, N.G.; et al. Fullerene derivatives stimulate the production process, growth and the resistance to oxidative stress in wheat and barley plants. Agric. Biol. 2018, 53, 38–49. [Google Scholar] [CrossRef]
- Panova, G.G.; Semenov, K.N.; Shilova, O.A.; Khomyakov, Y.u.V.; Anikina, L.M.; Charykov, N.A.; Artemjeva, A.M.; Kanash, E.V.; Khamova, T.B.; Udalova, O.R.; et al. Water soluble derivatives of fullerenes and silica-ash nanocomposites as promising nanomaterials for use in plant production. Agrophysics 2015, N4, 37–48. [Google Scholar]
- Bityutskii, N.P.; Yakkonen, K.L.; Lukina, K.A.; Semenov, K.N. Fullerenol increases effectiveness of foliar iron fertilization in iron-deficient cucumber. PLoS ONE 2020, 15, e0232765. [Google Scholar] [CrossRef]
- Yin, J.J.; Lao, F.; Meng, J.; Fu, P.P.; Zhao, Y.; Xing, G.; Gao, X.; Sun, B.; Wang, P.C.; Chen, C.; et al. Inhibition of Tumor Growth by Endohedral Metallofullerenol Nanoparticles Optimized as Reactive Oxygen Species Scavenger. Mol. Pharmacol. 2008, 74, 1132–1140. [Google Scholar] [CrossRef]
- Shafiq, F.; Iqbal, M.; Ali, M.; Ashraf, M.A. Fullerenol regulates oxidative stress and tissue ionic homeostasis in spring wheat to improve net-primary productivity under salt-stress. Ecotoxicol. Environ. Saf. 2021, 211, 111901. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon nanomaterials: Production, impact on plant development, agricultural and environmental applications. Chem. Biol. Technol. Agric. 2016, 3, 17–26. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front. Plant Sci. 2016, 7, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- State Standard of Russian Federation №12039-82; Seeds of Agricultural Crops. Methods for Determining Viability. Standardinform: Moscow, Russia, 1982.
- State Standard of Russian Federation №12037-81; Seeds of Agricultural Crops. Methods for Determining the Purity and Waste of Seeds. Standardinform: Moscow, Russia, 1981.
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of Synthesis and Antioxidant Potential of Fullerenol Nanoparticles. J. Nanomater. 2015, 2015, 567073. [Google Scholar] [CrossRef]
- Aoshima, H.; Kokubo, K.E.N.; Shirakawa, S.; Ito, M.; Yamana, S.; Oshima, T. Antimicrobial Activity of Fullerenes and Their Hydroxylated Derivatives. Biocontrol Sci. 2009, 14, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sachkova, A.S.; Kovel, E.S.; Kudryasheva, N.S.; Guseynov, O.A.; Churilov, G.N.; Dubinina, I.A.; Bondar, A.A. On mechanism of antioxidant properties of fullerenols. Biochem. Biophys. Rep. 2017, 9, 1–8. [Google Scholar] [PubMed]
- Gondal, A.H.; Tayyiba, L. Prospects of Using Nanotechnology in Agricultural Growth, Environment and Industrial Food Products. Rev. Agric. Sci. 2022, 10, 68–81. [Google Scholar] [CrossRef]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef]
- Chen, Y.W.; Hwang, K.C.; Yen, C.C.; Lai, Y.L. Regulatory, integrative and comparative physiology. Am. J. Physiol. 2004, 287, 21–26. [Google Scholar]
- State Standard of Russian Federation №12042-80; Seeds of Agricultural Crops. Methods for Determining the Mass of 1000 Seeds. Standardinform: Moscow, Russia, 1980.
- State Standard of Russian Federation №12041-82; Seeds of Agricultural Crops. Methods for Determining Humidity. Standardinform: Moscow, Russia, 1982.
- State Standard of Russian Federation №12038-84; Seeds of Agricultural Crops. Methods for Determining Germination. Standardinform: Moscow, Russia, 1984.
- State Standard of Russian Federation №10469-76; Barley Seeds. Varietal and Sowing Qualities. Standardinform: Moscow, Russia, 1976.
- Yurkevich, V.M.; Kabanova, N.V.; Sokolik AIYurin, V.M. Reaction of agricultural plants to the presence of heavy metals in the environment. Belorus. State Univ. 2011, 6, 39–46. [Google Scholar]
- Sadenova, M.; Kulenova, N.; Gert, S.; Beisekenov, N.; Levin, E. Innovative Approaches for Improving the Quality and Resilience of Spring Barley Seeds: The Role of Nanotechnology and Phytopathological Analysis. Plants 2023, 12, 3892. [Google Scholar] [CrossRef] [PubMed]
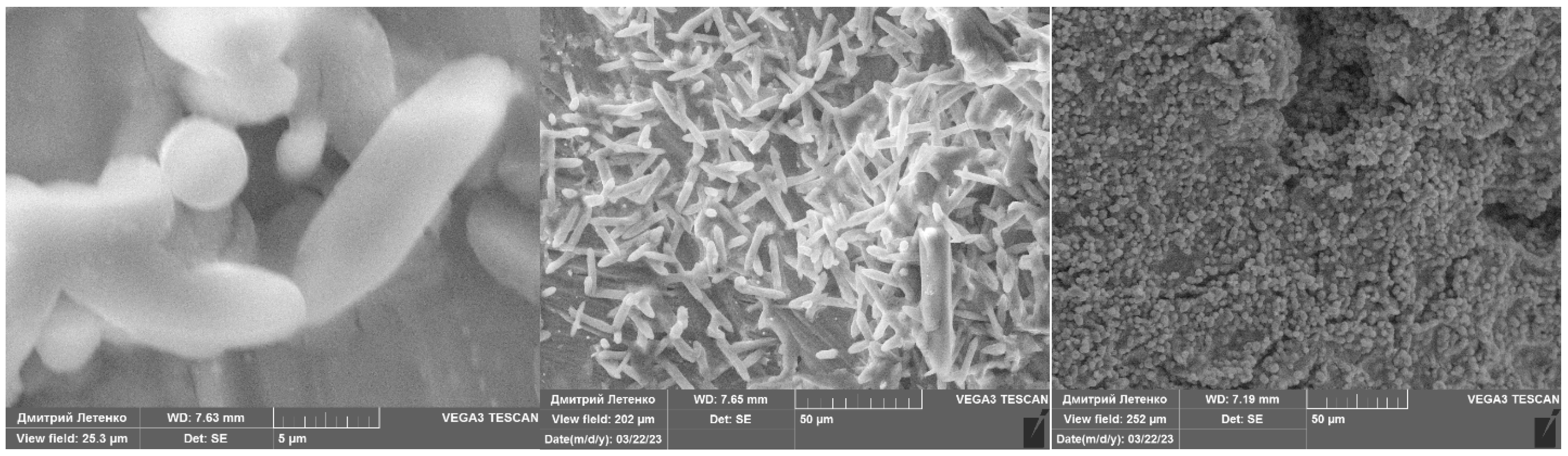
| No | Me in Adduct | ) (60 C Atoms). | Me-Adducts Formula | ||||
|---|---|---|---|---|---|---|---|
| C | O | H | Na | Me | |||
| 1 | Na | 60 | 44 ± 2 | 40 ± 2 | 24 ± 1 | 0 | |
| 2 | Co | 60 | 48 ± 6 | 48 ± 5 | 12 ± 3 | 6 ± 2 | |
| 3 | Cu | 60 | 42 ± 8 | 36 ± 5 | 4 ± 2 | 10 ± 4 | |
| 4 | Mn | 60 | 42 ± 9 | 36 ± 4 | 4 ± 2 | 10 ± 5 | |
| 5 | Zn | 60 | 44 ± 7 | 40 ± 5 | 8 ± 3 | 8 ± 3 | |
| 6 | Gd | 60 | 46 ± 8 | 44 ± 6 | 6 ± 3 | 6 ± 3 | |
| 7 | Tb | 60 | 44 ± 8 | 40 ± 7 | 6 ± 3 | 6 ± 3 | |
| No | (103 cm2/g) | (g/dm3) | |
|---|---|---|---|
| 1 | Co | 0.915 | 1.093 |
| 2 | Cu | 0.572 | 1.748 |
| 3 | Mn | 0.850 | 1.176 |
| 4 | Zn | 0.391 | 2.557 |
| 5 | Gd | 0.568 | 1.760 |
| 6 | Tb | 0.733 | 1.364 |
| No | Process | |
|---|---|---|
| 1. | —MED | |
| 2. | —T-RE-OMD | |
| 3. | —SOD | |
| 4. | —FCO | |
| 5. | —MED | |
| 6. | —T-RE-OMD | |
| 7. | —SOD | |
| 8. | —FCO | |
| 9. | —MED | |
| 10. | —T-RE-OMD | |
| 11. | —SOD | |
| 12. | —FCO | |
| 13. | —MED | |
| 14. | —T-RE-OMD | |
| 15. | —SOD | |
| 16. | —FCO | |
| 17. | —MED | |
| 18. | —T-RE-OMD | |
| 19. | —SOD | |
| 20. | —FCO | |
| 21. | —MED | |
| 22. | —T-RE-OMD | |
| 23. | —SOD | |
| 24. | —FCO |
| No | System | Crystal Hydrate | Temperature (°C) | Solubility S (g/dm3) |
|---|---|---|---|---|
| 1. | 25 | 0.340 | ||
| 2. | 0 | 0.419 | ||
| 3. | 25 | 0.503 | ||
| 4. | 0 | 0.636 | ||
| 5. | 25 | 0.444 | ||
| 6. | 0 | 0.665 | ||
| 7. | 25 | 0.634 | ||
| 8. | 0 | 1.041 | ||
| 9. | 25 | 0.552 | ||
| 10. | 0 | 0.627 | ||
| 11. | 25 | 0.492 | ||
| 12. | 0 | 0.521 |
| Me-Adduct | Concentration C (g/dm3) | |||||
|---|---|---|---|---|---|---|
| 0.34 | - | 65 | 105 | 7 | ||
| 0.55 | - | - | 105 | 5 | ||
| 0.49 | - | - | 108 | 5 | ||
| 0.88 | - | - | 120 | 5 | ||
| 0.72 | - | - | 120 | 6 | ||
| 0.50 | - | 65 | 103 | 7 | ||
| Me-adduct | ||||||
| 2 | ||||||
| - | 2 | |||||
| - | 2 | |||||
| - | 3 | |||||
| - | 3 | |||||
| 2 | ||||||
| Me-adduct | μm∙cm/V∙s | μm∙cm/V∙s | μm∙cm/V∙s | |||
| −20 | −35 | −60 | −1.4 | −1.8 | −0.5 | |
| - | −35 | −50 | - | −2.1 | −0.7 | |
| - | −35 | −50 | - | −2.0 | −0.7 | |
| - | −40 | −50 | - | −2.3 | −0.7 | |
| - | −40 | −55 | - | −2.4 | −0.6 | |
| −20 | −30 | −60 | −1.5 | −2.1 | −0.5 | |
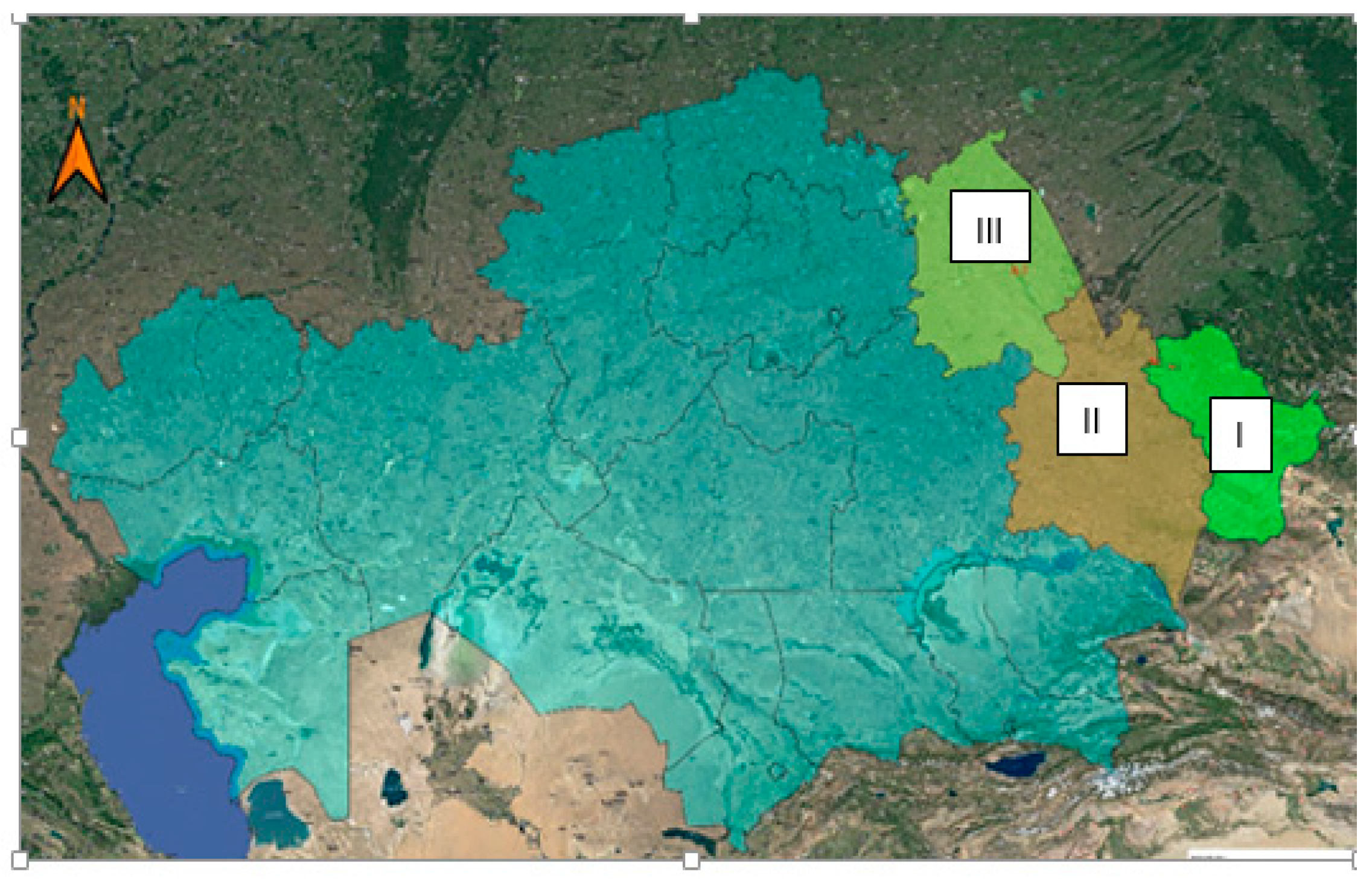
| The Name of the Indicator | Polygon I | Polygon II | Polygon III |
|---|---|---|---|
| Soil type | Ordinary chernozem, leached, of medium mechanical composition | Dark brown, light mechanical composition, subject to water and wind erosion | Chestnut, light mechanical composition, highly susceptible to water and wind erosion |
| Humus content range, % | 4. 1 ÷ 5.3 | 1.0 ÷ 1.6 | 0.6 ÷ 1.3 |
| The amount of precipitation for the entire growing season, mm | 182 | 123 | 136 |
| Average temperature for the entire growing season, °C | 23.5 | 18.1 | 21.2 |
| The Abbreviated Name of Fullerenol and Me-Adducts in the Field Experiment | Full Name of Fullerenol and Me-Adducts |
|---|---|
| C | Control (without processing) |
| F1 | Fullerenol-24 |
| F2 | Fullerenol-d [13,14] |
| F3 | |
| F4 | |
| F5 | |
| F6 |
| Date of Sowing of Spring Barley | Data of the First Treatment by Fullerenols and Me-Adducts | Data of the Second Treatment by Fullerenols and Me-Adducts | |
|---|---|---|---|
| Polygon I | 5 May 2022 | 8 June 2022 | 15 June 2022 |
| Polygon II | 12 May 2022 | 16 June 2022 | 23 June 2022 |
| Polygon III | 26 May 2022 | 29 June 2022 | 6 July 2022 |
| Polygon III-a | 26 May 2022 | 29 June 2022 | 6 July 2022 |
| Fullerenol and Me-Adduct | Seed Grade Purity (Rel.%) | Weight of 1000 Seeds (g) | Moisture Content in Seeds (Rel. Mass. %) | Laboratory Germination of Seeds (Rel.%) | Seed Germination Energy (Rel.%) |
|---|---|---|---|---|---|
| C | 95.5 | 39.2 | 4.14 | 87.5 | 100.0 |
| F1 | 93.9 | 41.2 | 4.31 | 87.5 | 97.5 |
| F2 | 94.8 | 44.2 | 4.36 | 90.0 | 100.0 |
| F3 | 95.4 | 46.4 | 4.74 | 87.5 | 97.5 |
| F4 | 97.5 | 42.6. | 4.62 | 87.5 | 97.5 |
| F5 | 95.3 | 41.7 | 4.07 | 80.0 | 92.5 |
| F6 | 95.0 | 42.2 | 4.75 | 92.5 | 92.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulenova, N.A.; Charykov, N.A.; Keskinov, V.A.; Gur’eva, A.A.; German, V.P.; Letenko, D.G. Research and Possible Agronomic Applications of C60(OH)24 Adducts with Heavy Metals for Crop Treatment. Processes 2023, 11, 3354. https://doi.org/10.3390/pr11123354
Kulenova NA, Charykov NA, Keskinov VA, Gur’eva AA, German VP, Letenko DG. Research and Possible Agronomic Applications of C60(OH)24 Adducts with Heavy Metals for Crop Treatment. Processes. 2023; 11(12):3354. https://doi.org/10.3390/pr11123354
Chicago/Turabian StyleKulenova, Natalia A., Nikolay A. Charykov, Viktor A. Keskinov, Anastasiia A. Gur’eva, Valeriia P. German, and Dmitry G. Letenko. 2023. "Research and Possible Agronomic Applications of C60(OH)24 Adducts with Heavy Metals for Crop Treatment" Processes 11, no. 12: 3354. https://doi.org/10.3390/pr11123354
APA StyleKulenova, N. A., Charykov, N. A., Keskinov, V. A., Gur’eva, A. A., German, V. P., & Letenko, D. G. (2023). Research and Possible Agronomic Applications of C60(OH)24 Adducts with Heavy Metals for Crop Treatment. Processes, 11(12), 3354. https://doi.org/10.3390/pr11123354









