Biodegradation and Utilization of the Pesticides Glyphosate and Carbofuran by Two Yeast Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Strains
2.3. Growth Media and Culture Conditions
2.4. Molecular Identification
2.5. Biodegradation of Carbofuran and Glyphosate
2.6. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
2.7. Statistical Analysis
3. Results
3.1. Molecular Identification
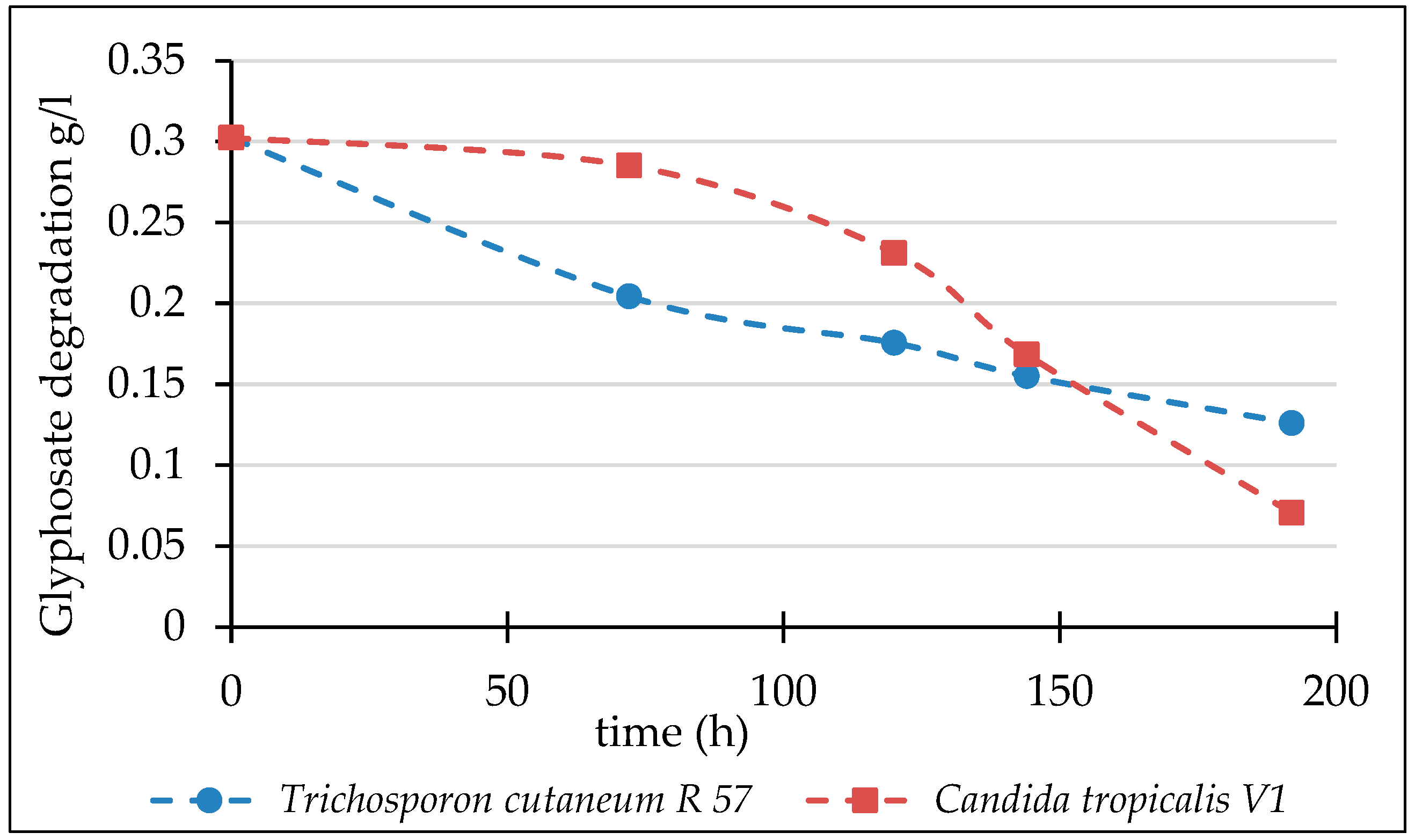
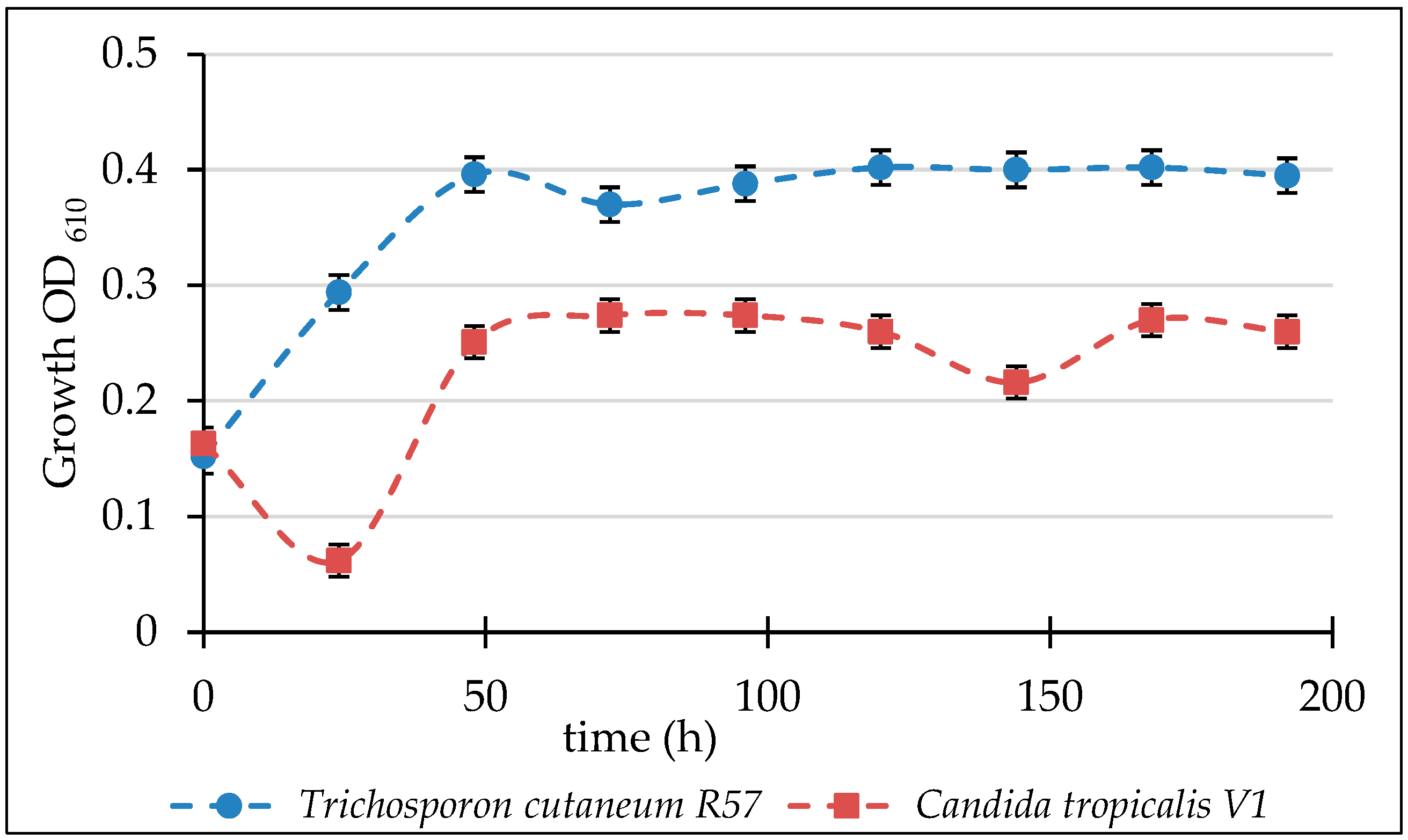
3.2. Biodegradation of Glyphosate
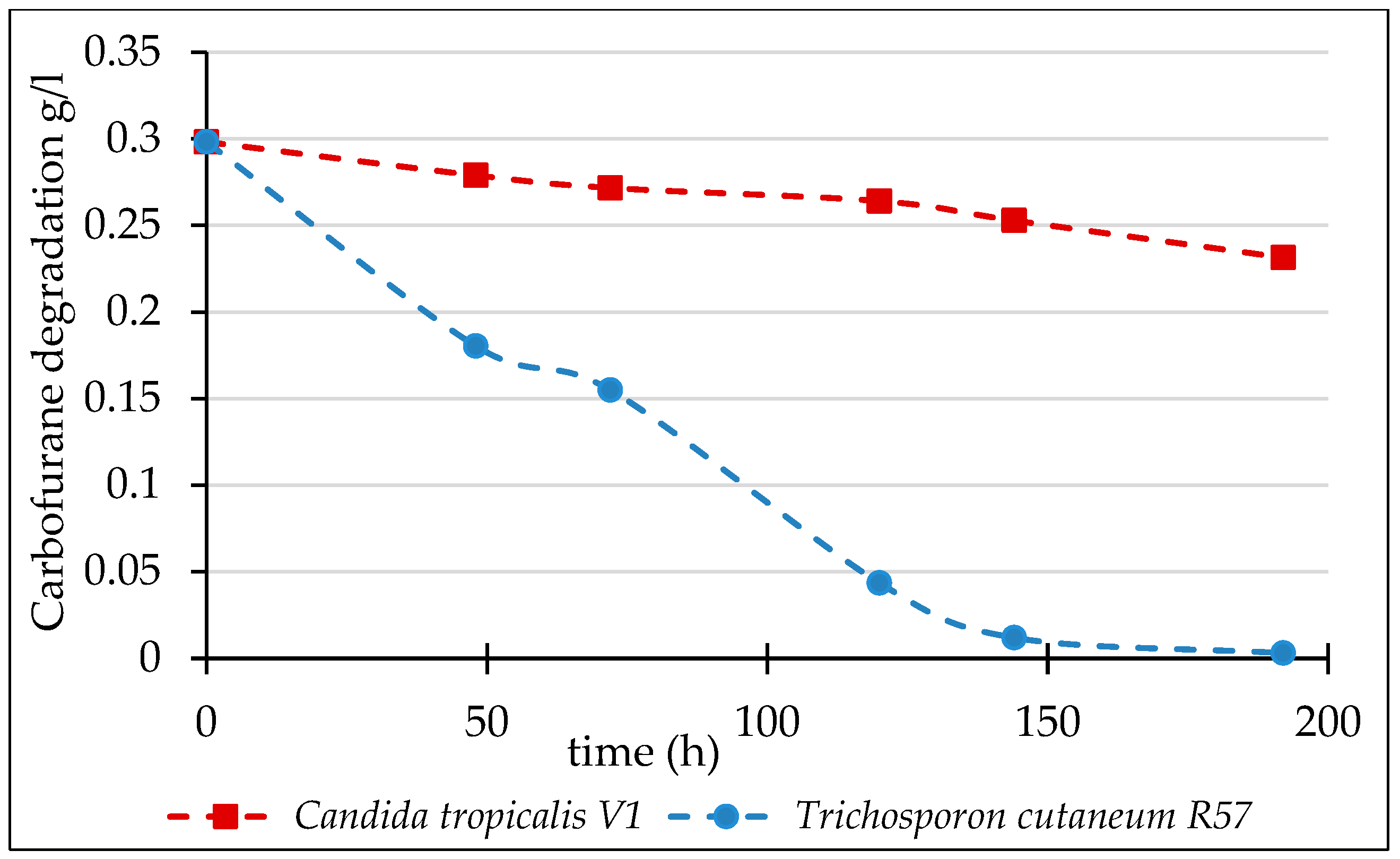
3.3. Biodegradation of Carbofuran
3.4. Intermediate Metabolic Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal. 1989. Available online: https://www.basel.int/TheConvention/Overview/tabid/1271/Default.aspx (accessed on 1 October 2023).
- Bruce-Vanderpuije, P.; Megson, D.; Reiner, E.; Bradley, L.; Adu-Kumi, S.; Gardella, J., Jr. The state of POPs in Ghana—A review on persistent organic pollutants: Environmental and human exposure. Environ. Pollut. 2018, 245, 331–342. [Google Scholar] [CrossRef] [PubMed]
- United State Environmental Protection Agency (USEPA). 2018. Available online: https://www.epa.gov/pm-pollution/health-and-environmental-effects-particulate-matter-pm (accessed on 6 September 2023).
- Štefanac, T.; Grgas, D.; Landeka Dragičević, T. Xenobiotics—Division and Methods of Detection: A Review. J. Xenobiot. 2021, 11, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Lallas, P. The Stockholm Convention on Persistent Organic Pollutants. Am. J. Int. Law 2001, 95, 692–708. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef]
- Kumari, S.; Kumar, D.; Khurana, S. Microbial degradation of pesticides: Microbial potential for degradation of pesticides. In Development in Wastewater Treatment Research and Processes; Shah, M., Rodriguez-Couto, S., Kapoor, R., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; pp. 41–67. [Google Scholar] [CrossRef]
- Wang, X.; Sial, M.U.; Bashir, M.A.; Bilal, M.; Raza, Q.-U.-A.; Ali Raza, H.M.; Rehim, A.; Geng, Y. Pesticides Xenobiotics in Soil Ecosystem and Their Remediation Approaches. Sustainability 2022, 14, 3353. [Google Scholar] [CrossRef]
- Castrejón-Godínez, M.; Tovar-Sánchez, E.; Valencia-Cuevas, L.; Rosas-Ramírez, M.; Rodríguez, A.; Mussali-Galante, P. Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review. Microorganisms 2021, 9, 2322. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Montero, P.J.; Vega-Verduga, C.; Alulema-Pullupaxi, P.; Fernández, L.; Paz, J.L. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules 2020, 25, 5550. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Carles, L.; Donnadieu, F.; Batisson, I.; Artigas, J. Glyphosate-degrading behavior of five bacterial strains isolated from stream biofilms. J. Hazard. Mater. 2021, 420, 126651. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Seralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Annett, R.; Habibi, H.R.; Hontela, A. Impacts of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Saquib, Q.; Siddiqui, M.; Ansari, S.; Alwathnani, H.; Al-Khedhairy, A. Carbofuran cytotoxicity, DNA damage, oxidative stress, and cell death in human umbilical vein endothelial cells: Evidence of vascular toxicity. J. Appl. Toxicol. 2021, 41, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Environmental Protection Agency. Pt. 355, App. A. 2008. Available online: https://www.govinfo.gov/content/pkg/CFR-2011-title40-vol28/pdf/CFR-2011-title40-vol28-part355-appA.pdf (accessed on 6 September 2023).
- Miglani, R.; Parveen, N.; Kumar, A.; Ansari, M.A.; Khanna, S.; Rawat, G.; Panda, A.K.; Bisht, S.S.; Upadhyay, J.; Ansari, M.N. Degradation of Xenobiotic Pollutants: An Environmentally Sustainable Approach. Metabolites 2022, 12, 818. [Google Scholar] [CrossRef]
- Bobate, S.; Bokade, P.; Bajaj, A. Engineered yeasts as biocatalysts for pesticide degradation. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Daverey, A., Dutta, K., Joshi, S., Gea, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 449–474. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmed, I.; Shahzad, A.; Khalid, N.; Mehboob, F.; Ahad, K.; Ali, G. Molecular identification and characterization of Pseudomonas sp. NCCP-407 for phenol degradation isolated from industrial waste. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 341–346. [Google Scholar] [CrossRef]
- Singh, B.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed]
- Kujur, R.; Deb, S.; Das, S. Genome analysis of Pseudomonas species reveals that Pseudomonas panacis Park et al. 2005 is a later heterotypic synonym of Pseudomonas marginalis (Brown 1918) Stevens 1925 (Approved Lists 1980). Int. J. Syst. Evol. Microbiol. 2022, 72, 005354. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhu, S.; Zhu, S.; Xie, H.; Su, K.; Yan, T.; Wang, J.; Wang, J.; Wang, F.; Sun, F. Biodegradation of organochlorine pesticide endosulfan by bacterial strain Alcaligenes faecalis JBW4. J. Environ. Sci. 2013, 25, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Nag, M.; Lahiri, D.; Dutta, B.; Jadav, G.; Ray, R. Biodegradation of used polyethylene bags by a new marine strain of Alcaligenes faecalis LNDR-1. Environ. Sci. Pollut. Res. 2021, 28, 41365–41379. [Google Scholar] [CrossRef]
- Martínková, L.; Uhnáková, B.; Pátek, M.; Nesvera, J.; Kren, V. Biodegradation potential of the genus Rhodococcus. Environ. Int. 2009, 35, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Tengku-Mazuki, T.; Subramaniam, K.; Zakaria, N.; Convey, P.; Abdul Khalil, K.; Lee, G.; Zulkharnain, A.; Shaharuddin, N.; Ahmad, A. Optimization of phenol degradation by Antarctic bacterium Rhodococcus sp. Antarct. Sci. 2020, 32, 486–495. [Google Scholar] [CrossRef]
- Asim, N.; Hassan, M.; Shafique, F.; Ali, M.; Nayab, H.; Shafi, N.; Khawaja, S.; Manzoor, S. Characterizations of novel pesticide-degrading bacterial strains from industrial wastes found in the industrial cities of Pakistan and their biodegradation potential. PeerJ 2021, 9, e12211. [Google Scholar] [CrossRef]
- Yan, X.; Jin, W.; Wu, G.; Jiang, W.; Yang, Z.; Ji, J.; Qiu, J.; He, J.; Jiang, J. Hydrolase CehA and monooxygenase CfdC are responsible for carbofuran degradation in Sphingomonas strain CDS-1. Appl. Environ. Microbiol. 2018, 84, e00805-18. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Cecchi, G.; Landinez-Torres, A.; Pecoraro, L.; Russo, F.; Wu, B.; Cai, L.; Liu, X.Z.; Tosi, S.; Vareses, G.C.; et al. Fungi as a toolbox for sustainable bioremediation of pesticides in soil and water. Plant Biosyst. 2018, 152, 474–488. [Google Scholar] [CrossRef]
- Mishra, S.; Zhang, W.; Lin, Z.; Pang, S.; Huang, Y.; Bhatt, P.; Chen, S. Carbofuran toxicity and its microbial degradation in contaminated environments. Chemosphere 2020, 259, 127419. [Google Scholar] [CrossRef] [PubMed]
- El-Gendi, H.; Saleh, A.K.; Badierah, R.; Redwan, E.M.; El-Maradny, Y.A.; El-Fakharany, E.M. A comprehensive insight into fungal enzymes: Structure, classification, and their role in mankind’s challenges. J. Fungi 2021, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Mori, T.; Ohno, H.; Ichinose, H.; Kawagishi, H.; Hirai, H. White-rot fungus Phanerochaete chrysosporium metabolizes chloropyridinyl-type neonicotinoid insecticides by an n-dealkylation reaction catalyzed by two cytochrome P450s. J. Hazard. Mater. 2021, 402, 123831. [Google Scholar] [CrossRef]
- Hu, K.; Peris, A.; Torán, J.; Eljarrat, E.; Sarrà, M.; Blánquez, P.; Caminal, G. Exploring the degradation capability of trametes versicolor on selected hydrophobic pesticides through setting sights simultaneously on culture broth and biological matrix. Chemosphere 2020, 250, 126293. [Google Scholar] [CrossRef] [PubMed]
- Beltrán-Flores, E.; Sarrà, M.; Blánquez, P. Pesticide bioremediation by trametes versicolor: Application in a fixed-bed reactor, sorption contribution and bioregeneration. Sci. Total Environ. 2021, 794, 148386. [Google Scholar] [CrossRef]
- Soares, P.; Birolli, W.; Ferreira, I.; Porto, A. Biodegradation pathway of the organophosphate pesticides chlorpyrifos, methyl parathion and profenofos by the marine-derived fungus Aspergillus sydowii CBMAI 935 and its potential for methylation reactions of phenolic compounds. Mar. Pollut. Bull. 2021, 166, 112185. [Google Scholar] [CrossRef]
- Carranza, C.; Barberis, C.; Chiacchiera, S.; Magnoli, C. Assessment of growth of Aspergillus spp. from agricultural soils in the presence of glyphosate. Rev. Argent. Microbiol. 2017, 49, 384–393. [Google Scholar] [CrossRef]
- Njoku, K.; Eludini, P.; Adesuyi, A.; Ude, E.; Oyelami, A. Physiological and molecular characterization of active fungi in pesticides contaminated soils for degradation of glyphosate. Res. Sq. 2020, 1, 14. [Google Scholar] [CrossRef]
- Correa, L.; Bezerra, A.; Honorato, L.; Cortêz, A.; Souza, J.; Souza, E. Amazonian soil fungi are efficient degraders of glyphosate herbicide; novel isolates of Penicillium, Aspergillus, and Trichoderma. Braz. J. Biol. 2021, 83, e242830. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Song, X.; Zheng, C.; Sun, S.; Zhuang, B.; Tao, B. Transcriptome analysis and identification of candidate genes involved in glyphosate resistance in the fungus Fusarium verticillioides. J. Environ. Sci. Health. Part B 2021, 56, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Seo, S.; Lim, J.; Song, J.; Seo, J.; Kim, P. Screening of Carbofuran-Degrading Bacteria Chryseobacterium sp. BSC2-3 and Unveiling the Change in Metabolome during Carbofuran Degradation. Metabolites 2022, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Metabolism of carbofuran by Aspergillus niger and Fusarium graminareum. J. Environ. Sci. Health B 1998, 33, 253–266. [Google Scholar] [CrossRef]
- Seo, J.; Jeon, J.; Kim, S.; Kang, S.; Han, J.; Hur, H. Fungal biodegradation of carbofuran and carbofuran phenol by fungus Mucor ramannianus: Identification of metabolites. Water Sci. Technol. 2007, 55, 163–167. [Google Scholar] [CrossRef]
- Ruiz-Hidalgo, K.; Mass-Mora, M.; Barbieri, E.; Caraz-Rojas, E.; Rodrguez, C. Degradation of carbofuran by Tremetes versicolor in rice husk as a potential lignocellulosic substrate for biomixture: From mineralization to toxicity reduction. Process Biochem. 2014, 49, 2266–2271. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S.; Al-Zomyh, S.; Alrumman, S. Biodegradation and detoxification of aliphatic and aromatic hydrocarbons by new yeast strains. Ecotoxicol. Environ. Saf. 2018, 151, 28–34. [Google Scholar] [CrossRef]
- Iheanacho, C.; Okerentugba, P.; Orji, F.; Ataikiru, T. Hydrocarbon degradation potentials of indigeneous fungal isolates from a petroleum hydrocarbon contaminated soil in Sakpenwa community, Niger Delta. Glob. Adv. Res. J. Environ. Sci. Toxicol. 2014, 3, 6–11. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.; Ali, S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Haz. Mat. 2023, 448, 130944. [Google Scholar] [CrossRef]
- Rana, S.; Handa, S.; Aggarwal, Y.; Puri, S.; Chatterjee, M. Role of Candida in the bioremediation of pollutants: A review. Lett. App. Microbiol. 2023, 76, ovad103. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Tang, Z.; Bao, H.; Wu, D.; Deng, X.; Guo, G.; Ye, B.-C.; Dai, B. Degradation of pendimethalin by the yeast YC2 and determination of its two main metabolites. RSC Adv. 2018, 9, 491–497. [Google Scholar] [CrossRef]
- Ebadi, T.; Najafpour, G.; Younesi, H.; Mohammadi, M. Rapid biodegradation of diazinon using a novel strain of Candida pseudolambica. Environ. Technol. Innov. 2022, 25, 102218. [Google Scholar] [CrossRef]
- Becerra, K.; Ghosh, S.; Godoy, L. Pesticide and Yeast Interaction in Alcoholic Fermentation: A Mini-Review. Fermentation 2023, 9, 266. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, R.; Singh, D. Identification of enzyme(s) capable of degrading endosulfan and endosulfan sulfate using in silico techniques. Enzym. Microb. Technol. 2019, 124, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, S.; Hu, M.; Hao, W.; Geng, P.; Zhang, Y. Biodegradation of carbofuran by Pichia anomala strain HQ-C-01 and its application for bioremediation of contaminated soils. Biol. Fertil. Soils 2011, 47, 917–923. [Google Scholar] [CrossRef]
- Liu, D.; Coloe, S.; Baird, R.; Pederson, J. Rapid mini-preparation of fungal DNA for PCR. J. Clin. Microbiol. 2000, 38, 471. [Google Scholar] [CrossRef]
- Jaeger, E.; Carroll, N.; Choudhury, S.; Dunlop, A.; Towler, H.; Matheson, M.; Adamson, P.; Okhravi, N. Rapid detection and identification of Candida, Aspergillus, and Fusarium species in ocular samples using nested PCR. J. Clin. Microbiol. 2000, 38, 2902–2908. [Google Scholar] [CrossRef]
- Gupta, J.; Rathour, R.; Singh, R.; Thakur, I. Production and characterization of extracellular polymeric substances (EPS) generated by a carbofuran degrading strain Cupriavidus sp. ISTL7. Bioresour. Technol. 2019, 282, 417–424. [Google Scholar] [CrossRef]
- Ariffin, F.; Rahman, S. Biodegradation of Carbofuran: A Review. J. Environ. Microbiol. Toxicol. 2020, 8, 50–57. [Google Scholar] [CrossRef]
- Umar Mustapha, M.; Halimoon, N.; Wan Johari, W.L.; Abd Shukor, M.Y. Enhanced Carbofuran Degradation Using Immobilized and Free Cells of Enterobacter sp. Isolated from Soil. Molecules 2020, 25, 2771. [Google Scholar] [CrossRef] [PubMed]
- Costas-Ferreira, C.; Durán, R.; Faro, L. Toxic Effects of Glyphosate on the Nervous System: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 4605. [Google Scholar] [CrossRef]
- Romero, M.; Reinoso, E.; Kiernan, A.; Córdoba, S. Biodegradation of glyphosate by wild yeasts. Rev. Mex. Mic. 2004, 19, 45–50. [Google Scholar]
- Liu, Z.; Zeng, G.; Wang, J.; Zhong, H.; Ding, Y.; Yuan, X. Effects of monorhamnolipid and Tween 80 on the degradation of phenol by Candida tropicalis. Proc. Biochem. 2010, 45, 805–809. [Google Scholar] [CrossRef]
- Chandran, P.; Das, N. Degradation of diesel oil by immobilized Candida tropicalis and biofilm formed on gravels. Biodegradation 2011, 22, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.; El-Banna, A.; Saad Allah, M. Efficiency of Candida tropicalis for Potential Degradation of Metalaxyl in the Aqueous Media. Curr. Microbiol. 2020, 77, 2991–2999. [Google Scholar] [CrossRef]
- Aleksieva, Z.; Ivanova, D.; Godjevargova, T.; Atanasov, B. Degradation of some phenol derivatives by Trichosporon cutaneum R57. Proc. Biochem. 2002, 37, 1215–1219. [Google Scholar] [CrossRef]
- Yan, Q.; Hong, Q.; Han, P.; Dong, X.; Shen, Y.; Li, S. Isolation and characterization of a carbofuran-degrading strain Novosphingobium sp. FND-3. FEMS Microbiol. Lett. 2007, 271, 207–213. [Google Scholar] [CrossRef]
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial degradation of phenol and phenolic derivatives. Eng. Life Sci. 2013, 13, 76–87. [Google Scholar] [CrossRef]
- Gerginova, M.; Zlateva, P.; Peneva, N.; Alexieva, Z. Influence of phenolic substrates utilised by yeast Trichosporon cutaneum on the degradation kinetics. Biotechnol. Biotechnol. Equip. 2014, 28, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, N.; Valladares, A.; Georgieva, N.; Müller, R. Phenol degradation by Trichosporon cutaneum R57 in the presence of copper ions. J. Chem. Technol. Metall. 2015, 50, 613–618. [Google Scholar]
- Park, M.; Lee, S.; Han, T.; Oh, B.; Shim, J.; Kim, I. A New Intermediate in the Degradation of Carbofuran by Sphingomonas sp. Strain SB5. J. Microbiol. Biotechnol. 2006, 16, 1306–1310. [Google Scholar]
- Malhotra, H.; Kaur, S.; Phale, P. Conserved Metabolic and Evolutionary Themes in Microbial degradation of Carbamate Pesticides. Front. Microbiol. 2021, 12, 648868. [Google Scholar] [CrossRef] [PubMed]
- Duc, H. Enhancement of carbofuran degradation by immobilized Bacillus sp. strain DT1. Environ. Eng. Res. 2022, 27, 210158. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide Glyphosate: Toxicity and Microbial Degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef] [PubMed]



| Pesticide | Degradation Products | m/z of Fragment Ions (Relative Abundanse, %) |
|---|---|---|
| Glyphosate | Methylglycine | 89(6), 30(100), 29(3), 28(9), 15(3) |
| Glycine | 75(4), 44(6),30(100), 29(5), 28(8) | |
| Carbofuran | Carbofuran-7-phenol | 164(100), 149(83), 131(32), 123(29), 122(28), 121(21), 103(17),77(14) |
| Pyruvic acid | 88(5), 45(13), 44(18), 43(100), 42(9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoyanova, K.; Gerginova, M.; Peneva, N.; Dincheva, I.; Alexieva, Z. Biodegradation and Utilization of the Pesticides Glyphosate and Carbofuran by Two Yeast Strains. Processes 2023, 11, 3343. https://doi.org/10.3390/pr11123343
Stoyanova K, Gerginova M, Peneva N, Dincheva I, Alexieva Z. Biodegradation and Utilization of the Pesticides Glyphosate and Carbofuran by Two Yeast Strains. Processes. 2023; 11(12):3343. https://doi.org/10.3390/pr11123343
Chicago/Turabian StyleStoyanova, Katya, Maria Gerginova, Nadejda Peneva, Ivayla Dincheva, and Zlatka Alexieva. 2023. "Biodegradation and Utilization of the Pesticides Glyphosate and Carbofuran by Two Yeast Strains" Processes 11, no. 12: 3343. https://doi.org/10.3390/pr11123343
APA StyleStoyanova, K., Gerginova, M., Peneva, N., Dincheva, I., & Alexieva, Z. (2023). Biodegradation and Utilization of the Pesticides Glyphosate and Carbofuran by Two Yeast Strains. Processes, 11(12), 3343. https://doi.org/10.3390/pr11123343






