Exploration of Eco-Friendly Hydrochar’s Potential in Advanced Oxidative Processes for Dicamba Degradation within a Circular Bio-Economy Framework
Abstract
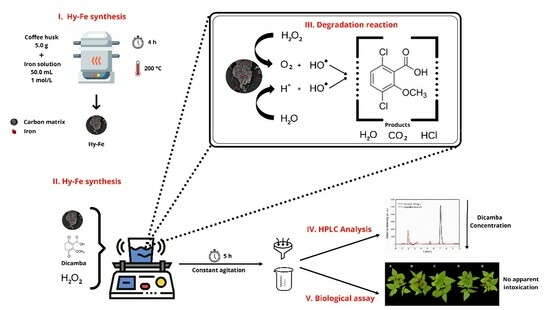
:1. Introduction
2. Materials and Methods
2.1. The Standards and Reagents
2.2. Precursor Biomass
2.3. Composite Production
2.4. Hy-Fe Characterization
2.5. Dicamba Degradation Assay
2.6. Optimization of the Degradation Process
2.7. Evaluation with Sensitive Plant Species
2.8. Decontamination of Personal Protective Equipment (PPE)
3. Results and Discussion
3.1. This Hy-Fe Characterization
3.2. Dicamba Degradation Assay
3.3. Biological Assay with the Fenton-like Degraded Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPEA. Agrotóxicos No Brasil: Padrões de Uso, Política Da Regulação e Prevenção Da Captura Regulatória; IPEA: Brasília, Brazil, 2019; Volume 76.
- Procópio, S.O.; Pires, F.R.; Menezes, C.C.E.; Barroso, A.L.L.; Moraes, R.V.; Silva, M.V.V.; Queiroz, R.G.; Carmo, M.L. Efeitos de Dessecantes No Controle de Plantas Daninhas Na Cultura Da Soja. Planta Daninha 2006, 24, 193–197. [Google Scholar] [CrossRef]
- Krzyszowska, A.J.; Allen, R.D.; Vance, G.F. Assessment of the Fate of Two Herbicides in a Wyoming Rangeland Soil: Column Studies. J. Environ. Qual. 1994, 23, 1051–1058. [Google Scholar] [CrossRef]
- Andrade, S.R.B.; Silva, A.A.; Lima, C.F.; D’Antonino, L.; Queiroz, M.E.L.R.; França, A.C.; Felipe, R.S.; Victoria Filho, R. Ametryn Leaching on Red-Yellow Latosol and Red-Yellow Ultisol with Different PH Values. Planta Daninha 2010, 28, 655–663. [Google Scholar] [CrossRef]
- Grover, R. Mobility of Dicamba, Picloram and 2,4-D in Soil Columns. Weed Sci. 1977, 25, 159–162. Available online: https://www.cambridge.org/core/journals/weed-science/article/abs/mobility-of-dicamba-picloram-and-24d-in-soil-columns/A4314DE4E62F91F57F45C0D1CE0DE2DC (accessed on 14 November 2023). [CrossRef]
- Underwood, M.G.; Soltani, N.; Hooker, D.C.; Robinson, D.E.; Vink, J.P.; Swanton, C.J.; Sikkema, P.H. The Addition of Dicamba to POST Applications of Quizalofop-p-Ethyl or Clethodim Antagonizes Volunteer Glyphosate-Resistant Corn Control in Dicamba-Resistant Soybean. Weed Technol. 2016, 30, 639–647. [Google Scholar] [CrossRef]
- Beyki, T.; Asadollahzadeh, M.J. Selective Removal of Dicamba from Aqueous Samples Using Molecularly Imprinted Polymer Nanospheres. J. Water Environ. Nanotechnol. 2016, 1, 19–25. [Google Scholar] [CrossRef]
- Furtado, R.D.; Hoff, R.B. Removal of Imazethapyr and Imazapic from the Effluent of Aero-Agricultural Operations: Efficiency of a Treatment System Using Ozone. Water. Air. Soil Pollut. 2017, 228, 438. [Google Scholar] [CrossRef]
- Bahieldin, A.; Dyer, W.E.; Qu, R. Concentration Effects of Dicamba on Shoot Regeneration in Wheat. Plant Breed. 2000, 119, 437–439. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Dosemeci, M.; Samanic, C.; Lubin, J.; Lynch, C.F.; Knott, C.; Barker, J.; Hoppin, J.A.; Sandler, D.P.; Coble, J.; et al. Pesticides and Lung Cancer Risk in the Agricultural Health Study Cohort. Am. J. Epidemiol. 2004, 160, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.A.; Fleck, N.C. Análise Do Risco Da Ocorrência de Biotipos de Plantas Daninhas Resistentes Aos Herbicidas. Planta Daninha 1997, 15, 152–161. [Google Scholar] [CrossRef]
- Campos, S.X.d.; Vieira, E.M. Estudo Da Degradação Do Herbicida Ácido 2,4-Diclorofenoxiacético (2,4-D) Por Meio Da Radiação Gama Do Cobalto-60 Em Solução Aquosa Contendo Ácido Húmico. Quim. Nova 2002, 25, 529–532. [Google Scholar] [CrossRef]
- Qiang, Z.; Liu, C.; Dong, B.; Zhang, Y. Degradation Mechanism of Alachlor during Direct Ozonation and O3/H2O2 Advanced Oxidation Process. Chemosphere 2010, 78, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Wantala, K.; Khemthong, P.; Wittayakun, J.; Grisdanurak, N. Visible Light-Irradiated Degradation of Alachlor on Fe-TiO2 with Assistance of H2O2. Korean J. Chem. Eng. 2011, 28, 2178–2183. [Google Scholar] [CrossRef]
- Yang, Y.; Ghatge, S.; Ko, Y.; Yoon, Y.; Ahn, J.-H.; Kim, J.J.; Hur, H.-G. Non-Specific Degradation of Chloroacetanilide Herbicides by Glucose Oxidase Supported Bio-Fenton Reaction. Chemosphere 2021, 292, 133417. [Google Scholar] [CrossRef]
- Xin, Y.; Liu, H.; Han, L.; Zhou, Y. Comparative Study of Photocatalytic and Photoelectrocatalytic Properties of Alachlor Using Different Morphology TiO 2/Ti Photoelectrodes. J. Hazard. Mater. 2011, 192, 1812–1818. [Google Scholar] [CrossRef]
- Deng, X.; Yang, Y.; Mei, Y.; Li, J.; Guo, C.; Yao, T.; Guo, Y.; Xin, B.; Wu, J. Construction of Fe3O4@FeS2@C@MoS2 Z-Scheme Heterojunction with Sandwich-like Structure: Enhanced Catalytic Performance in Photo-Fenton Reaction and Mechanism Insight. J. Alloys Compd. 2021, 901, 163437. [Google Scholar] [CrossRef]
- Rossi, A.F.; Martins, R.C.; Quinta-Ferreira, R.M. Reuse of Homogeneous Fenton’s Sludge from Detergent Industry as Fenton’s Catalyst. J. Adv. Oxid. Technol. 2013, 16, 298–305. [Google Scholar] [CrossRef]
- Dulova, N.; Trapido, M.; Dulov, A. Catalytic Degradation of Picric Acid by Heterogeneous Fenton-Based Processes. Environ. Technol. 2011, 32, 439–446. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent Advances in Utilization of Biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Camps, B.M.; Tomlinson, T.; Initiative, I.B. The Use of Biochar in Composting Compost and Biochar: In Competition for Feedstocks? Biochar Benefits to the Composting Processes; MDPI: Basel, Switzerland, 2015. [Google Scholar]
- Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Bioresource Technology Role of Biochar as an Additive in Organic Waste Composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Lee, D.; Cheng, Y.; Wong, R.; Wang, X. Bioresource Technology Adsorption Removal of Natural Organic Matters in Waters Using Biochar. Bioresour. Technol. 2018, 260, 413–416. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and Utilization of Biochar: A Review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Ajmal, Z.; Muhmood, A.; Usman, M.; Kizito, S.; Lu, J.; Dong, R.; Wu, S. Phosphate Removal from Aqueous Solution Using Iron Oxides: Adsorption, Desorption and Regeneration Characteristics. J. Colloid Interface Sci. 2018, 528, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mofradnia, S.R.; Ashouri, R.; Tavakoli, Z.; Shahmoradi, F.; Rashedi, H.; Yazdian, F. Effect of Zero-Valent Iron/Starch Nanoparticle on Nitrate Removal Using MD Simulation. Int. J. Biol. Macromol. 2019, 121, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Dionysiou, D.D.; Liu, H. The Use of Zero-Valent Iron for Groundwater Remediation and Wastewater Treatment: A Review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, I.K.M.; Tsang, D.C.W.; Cao, X.; Lin, D.; Wang, L.; Graham, N.J.D.; Alessi, D.S.; Komárek, M.; Sik, Y.; et al. Multifunctional Iron-Biochar Composites for the Removal of Potentially Toxic Elements, Inherent Cations, and Hetero-Chloride from Hydraulic Fracturing Wastewater. Environ. Int. 2019, 124, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Wang, A. Removal of Methylene Blue from Aqueous Solution Using Chitosan-g-Poly(Acrylic Acid)/Montmorillonite Superadsorbent Nanocomposite. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 47–53. [Google Scholar] [CrossRef]
- Guimarães, T.; Andrade Luciano, V.; Stefani Ventura Silva, M.; Paula de Carvalho Teixeira, A.; Moreira da Costa, M.; Pereira Lopes, R. Biochar-Iron Composites: An Efficient Material for Dyes Removal. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100645. [Google Scholar] [CrossRef]
- Guimarães, T.; De Oliveira, A.F.; Lopes, R.P.; De Carvalho Teixeira, A.P. Biochars Obtained from Arabica Coffee Husks by a Pyrolysis Process: Characterization and Application in Fe(Ii) Removal in Aqueous Systems. New J. Chem. 2020, 44, 3310–3322. [Google Scholar] [CrossRef]
- Guimarães, T.; de Aguiar, A.; da Silva, E.; Mielke, K.; da Costa, M.; da Silva, A.; Teixeira, A.; Lopes, R. Dicamba Degradation by Fenton-Like Process Using Iron/Biochar Composites. J. Braz. Chem. Soc. 2023, 00, 1–9. [Google Scholar] [CrossRef]
- Guimarães, T.; Paquini, L.D.; Lyrio Ferraz, B.R.; Roberto Profeti, L.P.; Profeti, D. Efficient Removal of Cu(II) and Cr(III) Contaminants from Aqueous Solutions Using Marble Waste Powder. J. Environ. Chem. Eng. 2020, 8, 103972. [Google Scholar] [CrossRef]
- Veiga, P.A.d.S.; Schultz, J.; Matos, T.T.d.S.; Fornari, M.R.; Costa, T.G.; Meurer, L.; Mangrich, A.S. Production of High-Performance Biochar Using a Simple and Low-Cost Method: Optimization of Pyrolysis Parameters and Evaluation for Water Treatment. J. Anal. Appl. Pyrolysis 2020, 148, 104823. [Google Scholar] [CrossRef]
- Ibrahim, A.F.M.; Dandamudi, K.P.R.; Deng, S.; Lin, Y.S. Pyrolysis of Hydrothermal Liquefaction Algal Biochar for Hydrogen Production in a Membrane Reactor. Fuel 2020, 265, 116935. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Chen, T.; Dong, C.; Sun, Y. Synthesis of Magnetic Biochar Composites for Enhanced Uranium(VI) Adsorption. Sci. Total Environ. 2019, 651, 1020–1028. [Google Scholar] [CrossRef]
- Wei, Y.; Shen, C.; Xie, J.; Bu, Q. Study on Reaction Mechanism of Superior Bamboo Biochar Catalyst Production by Molten Alkali Carbonates Pyrolysis and Its Application for Cellulose Hydrolysis. Sci. Total Environ. 2020, 712, 136435. [Google Scholar] [CrossRef]
- Ghani, W.A.W.A.K.; Mohd, A.; da Silva, G.; Bachmann, R.T.; Taufiq-Yap, Y.H.; Rashid, U.; Al-Muhtaseb, A.H. Biochar Production from Waste Rubber-Wood-Sawdust and Its Potential Use in C Sequestration: Chemical and Physical Characterization. Ind. Crops Prod. 2013, 44, 18–24. [Google Scholar] [CrossRef]
- Tang, Y.; Alam, M.S.; Konhauser, K.O.; Alessi, D.S.; Xu, S.; Tian, W.J.; Liu, Y. Influence of Pyrolysis Temperature on Production of Digested Sludge Biochar and Its Application for Ammonium Removal from Municipal Wastewater. J. Clean. Prod. 2019, 209, 927–936. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Astruc, D. Biochar as a Support for Nanocatalysts and Other Reagents: Recent Advances and Applications; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 426, ISBN 4843550159. [Google Scholar]
- Álvarez, M.L.; Gascó, G.; Palacios, T.; Paz-Ferreiro, J.; Méndez, A. Fe Oxides-Biochar Composites Produced by Hydrothermal Carbonization and Pyrolysis of Biomass Waste. J. Anal. Appl. Pyrolysis 2020, 151, 104893. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Liu, J.; Xiao, Y.; Wang, T.; Xue, Y. Magnetic Biochar Prepared by Electromagnetic Induction Pyrolysis of Cellulose: Biochar Characterization, Mechanism of Magnetization and Adsorption Removal of Chromium (VI) from Aqueous Solution. Bioresour. Technol. 2021, 337, 125429. [Google Scholar] [CrossRef]
- Chu, J.H.; Kang, J.K.; Park, S.J.; Lee, C.G. Application of Magnetic Biochar Derived from Food Waste in Heterogeneous Sono-Fenton-like Process for Removal of Organic Dyes from Aqueous Solution. J. Water Process Eng. 2020, 37, 101455. [Google Scholar] [CrossRef]
- Debalina, B.; Reddy, R.B.; Vinu, R. Production of Carbon Nanostructures in Biochar, Bio-Oil and Gases from Bagasse via Microwave Assisted Pyrolysis Using Fe and Co as Susceptors. J. Anal. Appl. Pyrolysis 2017, 124, 310–318. [Google Scholar] [CrossRef]
- Gasparov, L.V.; Tanner, D.B.; Romero, D.B.; Berger, H.; Margaritondo, G.; Forró, L. Infrared and Raman Studies of the Verwey Transition in Magnetite. Phys. Rev. B-Condens. Matter Mater. Phys. 2000, 62, 7939–7944. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, H.; Hu, X.; Wang, R.; Dong, W. Productions of Phenolic Rich Bio-Oil Using Waste Chilli Stem Biomass by Catalytic Pyrolysis: Evaluation of Reaction Parameters on Products Distributions. J. Energy Inst. 2021, 97, 233–239. [Google Scholar] [CrossRef]
- Jiang, S.F.; Xi, K.F.; Yang, J.; Jiang, H. Biochar-Supported Magnetic Noble Metallic Nanoparticles for the Fast Recovery of Excessive Reductant during Pollutant Reduction. Chemosphere 2019, 227, 63–71. [Google Scholar] [CrossRef]
- Renata, P. Magnetized Biochar as a Gold Nanocatalyst Support for p-Nitrophenol Reduction. A. Short Report. J. Braz. Chem. Soc. 2021, 32, 1680–1686. [Google Scholar]
- Curi, N.; Da Motta, P.E.F.; Fabris, J.D.; De Oliveira, L.C.A. Espectroscopia Mössbauer Na Caracterização de Compostos Ferrosos Em Solos e Sua Relação Com Retenção de Fósforo. Quim. Nova 2008, 31, 1467–1471. [Google Scholar] [CrossRef]
- Shan, A.; Idrees, A.; Zaman, W.Q.; Abbas, Z.; Ali, M.; Rehman, M.S.U.; Hussain, S.; Danish, M.; Gu, X.; Lyu, S. Synthesis of NZVI-Ni@BC Composite as a Stable Catalyst to Activate Persulfate: Trichloroethylene Degradation and Insight Mechanism. J. Environ. Chem. Eng. 2021, 9, 104808. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-Pot Synthesis and Characterization of Engineered Hydrochar by Hydrothermal Carbonization of Biomass with ZnCl2. Chemosphere 2020, 254, 126866. [Google Scholar] [CrossRef]
- Sangami, S.; Manu, B. Synthesis of Green Iron Nanoparticles Using Laterite and Their Application as a Fenton-like Catalyst for the Degradation of Herbicide Ametryn in Water. Environ. Technol. Innov. 2017, 8, 150–163. [Google Scholar] [CrossRef]
- Mechakra, H.; Sehili, T.; Kribeche, M.A.; Ayachi, A.A.; Rossignol, S.; George, C. Use of Natural Iron Oxide as Heterogeneous Catalyst in Photo-Fenton-like Oxidation of Chlorophenylurea Herbicide in Aqueous Solution: Reaction Monitoring and Degradation Pathways. J. Photochem. Photobiol. A Chem. 2016, 317, 140–150. [Google Scholar] [CrossRef]
- Ruipérez, F.; Mujika, J.I.; Ugalde, J.M.; Exley, C.; Lopez, X. Pro-Oxidant Activity of Aluminum: Promoting the Fenton Reaction by Reducing Fe(III) to Fe(II). J. Inorg. Biochem. 2012, 117, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Orrico, A.C.A.; Orrico Junior, M.A.P.; De Lucas Junior, J.; Fernandes, R.A.M.; Sunada, S.; Rodrigues, J.P. Revista Agrarian. Rev. Agrar. 2011, 4, 222–227. [Google Scholar]












| Treatments | Application |
|---|---|
| T1 | H2O |
| T2 | [dicamba]0 1.00 mg L−1 |
| T3 | [dicamba]0 5.00 mg L−1 |
| T4 | [dicamba]0 10.00 mg L−1 |
| T5 | [dicamba]0 50.00 mg L−1 |
| T6 | Solution of [dicamba]0 1.00 mg L−1 degraded* using 0.20 g Hy-Fe |
| T7 | Solution of [dicamba]0 5.00 mg L−1 degraded* using 0.20 g Hy-Fe |
| T8 | Solution of [dicamba]0 10.00 mg L−1 degraded* using 0.20 g Hy-Fe |
| T9 | Solution of [dicamba]0 50.00 mg L−1 degraded* using 0.20 g Hy-Fe |
| T10 | Solution of [dicamba]0 50.00 mg L−1 degraded* using 0.20 g Hy-Fe, subjected to periodic stirring |
| T11 | Solution of [dicamba]0 50.00 mg L−1 degraded* using 0.20 g Hy-Fe, for system at rest |
| T12 | Solution of [dicamba]0 50.00 mg L−1 degraded* using 0.60 g Hy-Fe |
| T13 | Solution of [dicamba]0 50.00 mg L−1 degraded* using 1.00 g Hy-Fe |
| Sample | Elementary Analysis, % | |||
|---|---|---|---|---|
| C | H | N | S | |
| Hy-Fe | 52.30 ± 0.30 | 5.20 ± 0.09 | 1.49 ± 0.05 | 0.24 ± 0.01 |
| Treatment | Description | DMAS * | *** |
|---|---|---|---|
| T1 | H2O | 2.62 | a |
| T2 | [dicamba]0 1.00 mg L−1 | 2.47 | a |
| T3 | [dicamba]0 5.00 mg L−1 | 2.23 | b |
| T4 | [dicamba]0 10.00 mg L−1 | 2.08 | c |
| T5 | [dicamba]0 50.00 mg L−1 | 1.79 | d |
| T6 | Solution of [dicamba]0 1.00 mg L−1 degraded** using 0.20 g Hy-Fe | 2.53 | a |
| T7 | Solution of [dicamba]0 5.00 mg L−1 degraded** using 0.20 g Hy-Fe | 2.53 | a |
| T8 | Solution of [dicamba]0 10.00 mg L−1 degraded** using 0.20 g Hy-Fe | 2.59 | a |
| T9 | Solution of [dicamba]0 50.00 mg L−1 degraded** using 0.20 g Hy-Fe | 2.59 | a |
| T10 | Solution of [dicamba]0 50.00 mg L−1 degraded** using 0.20 g Hy-Fe, periodic stirring | 2.52 | a |
| T11 | Solution of [dicamba]0 50.00 mg L−1 degraded** using 0.20 g Hy-Fe, for system at rest | 2.61 | a |
| T12 | Solution of [dicamba]0 50.00 mg L−1 degraded** using 0.60 g Hy-Fe | 2.56 | a |
| T13 | Solution of [dicamba]0 50.00 mg L−1 degraded** using 1.00 g Hy-Fe | 2.50 | a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, T.; Silva, E.M.G.d.; Aguiar, A.C.M.d.; Costa, M.M.d.; Mielke, K.C.; Mendes, K.F.; Silva, A.A.d.; Teixeira, A.P.d.C.; Moreira, R.P.L. Exploration of Eco-Friendly Hydrochar’s Potential in Advanced Oxidative Processes for Dicamba Degradation within a Circular Bio-Economy Framework. Processes 2023, 11, 3244. https://doi.org/10.3390/pr11113244
Guimarães T, Silva EMGd, Aguiar ACMd, Costa MMd, Mielke KC, Mendes KF, Silva AAd, Teixeira APdC, Moreira RPL. Exploration of Eco-Friendly Hydrochar’s Potential in Advanced Oxidative Processes for Dicamba Degradation within a Circular Bio-Economy Framework. Processes. 2023; 11(11):3244. https://doi.org/10.3390/pr11113244
Chicago/Turabian StyleGuimarães, Tiago, Elisa Maria Gomes da Silva, Adalin Cezar Moraes de Aguiar, Marcelo Moreira da Costa, Kamila Cabral Mielke, Kassio Ferreira Mendes, Antonio Alberto da Silva, Ana Paula de Carvalho Teixeira, and Renata Pereira Lopes Moreira. 2023. "Exploration of Eco-Friendly Hydrochar’s Potential in Advanced Oxidative Processes for Dicamba Degradation within a Circular Bio-Economy Framework" Processes 11, no. 11: 3244. https://doi.org/10.3390/pr11113244
APA StyleGuimarães, T., Silva, E. M. G. d., Aguiar, A. C. M. d., Costa, M. M. d., Mielke, K. C., Mendes, K. F., Silva, A. A. d., Teixeira, A. P. d. C., & Moreira, R. P. L. (2023). Exploration of Eco-Friendly Hydrochar’s Potential in Advanced Oxidative Processes for Dicamba Degradation within a Circular Bio-Economy Framework. Processes, 11(11), 3244. https://doi.org/10.3390/pr11113244