Sludge Reduction and Surface Investigation in Electrochemical Machining by Complexing and Reducing Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Tests Procedure
2.2. Characterization
3. Results and Discussion
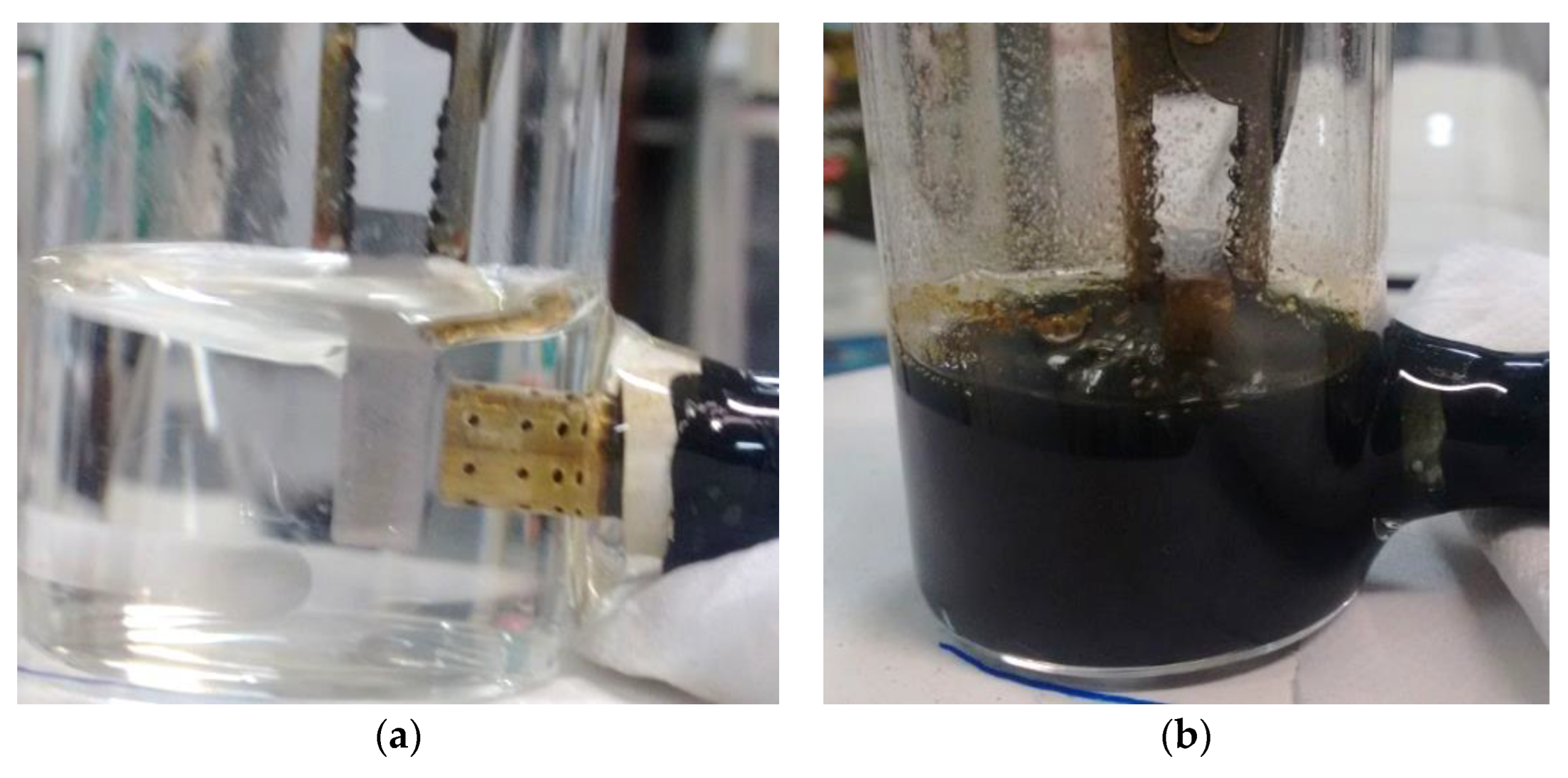
3.1. Complexing Agent Presence on Electrolyte Composition
3.2. Reducing Agent Presence on Electrolyte Composition
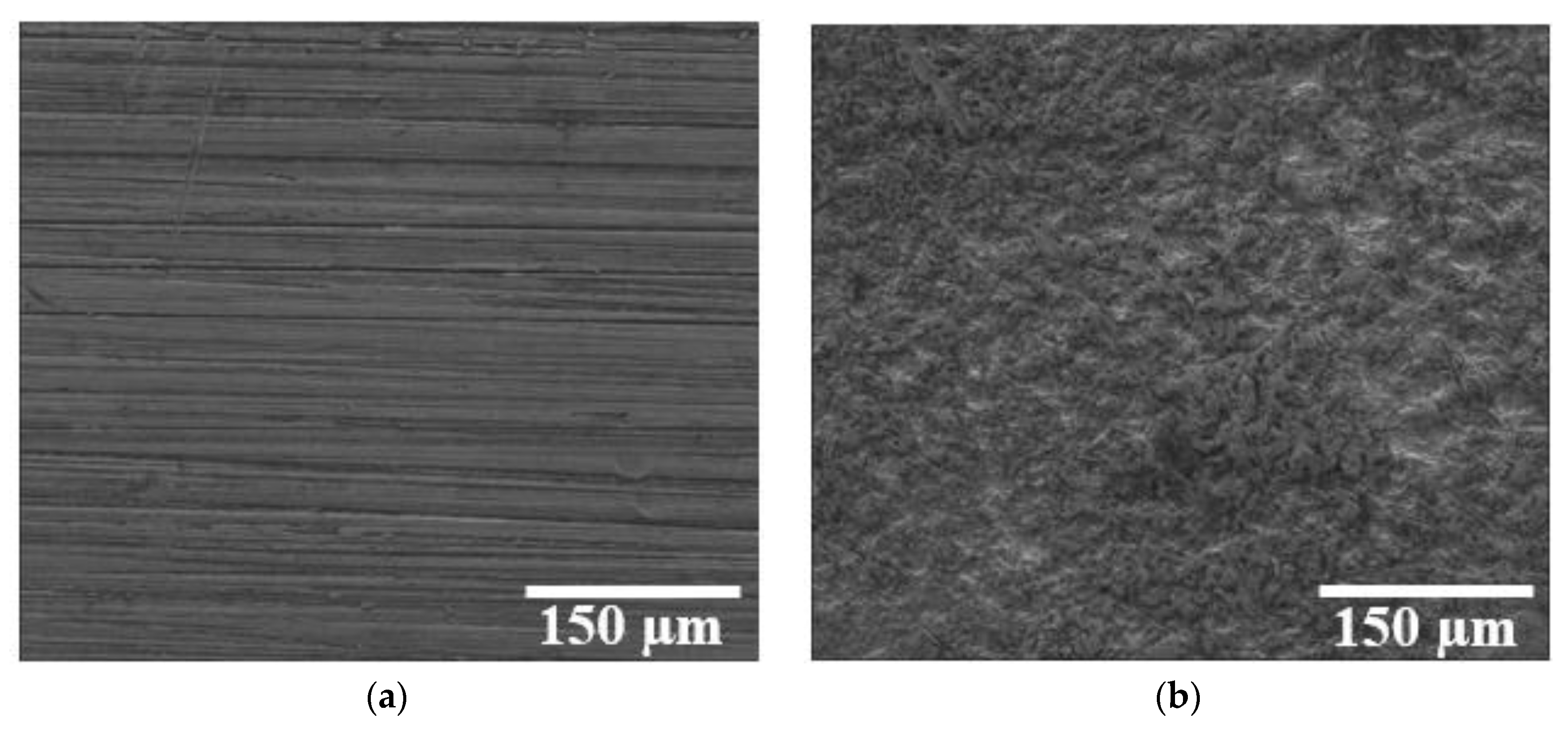
3.3. Workpiece Surface Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
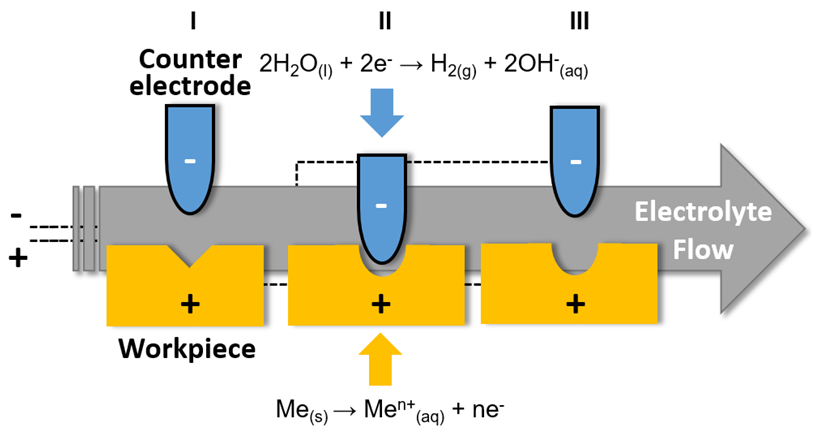
Appendix A. Overall ECM Process and the Redox Reactions Involved: I—Initial Condition; II—During ECM Process; III—Final Condition
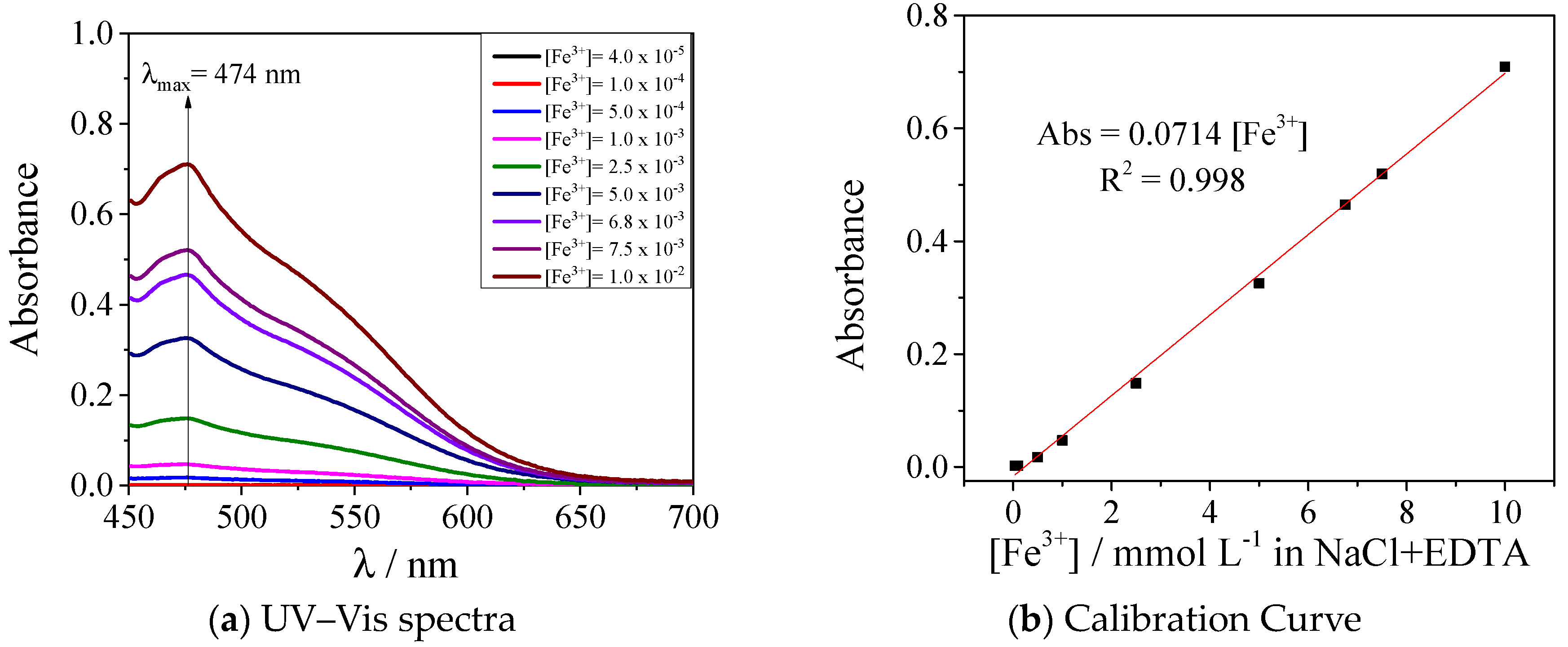
Appendix B. UV–VIS Calibration Curves

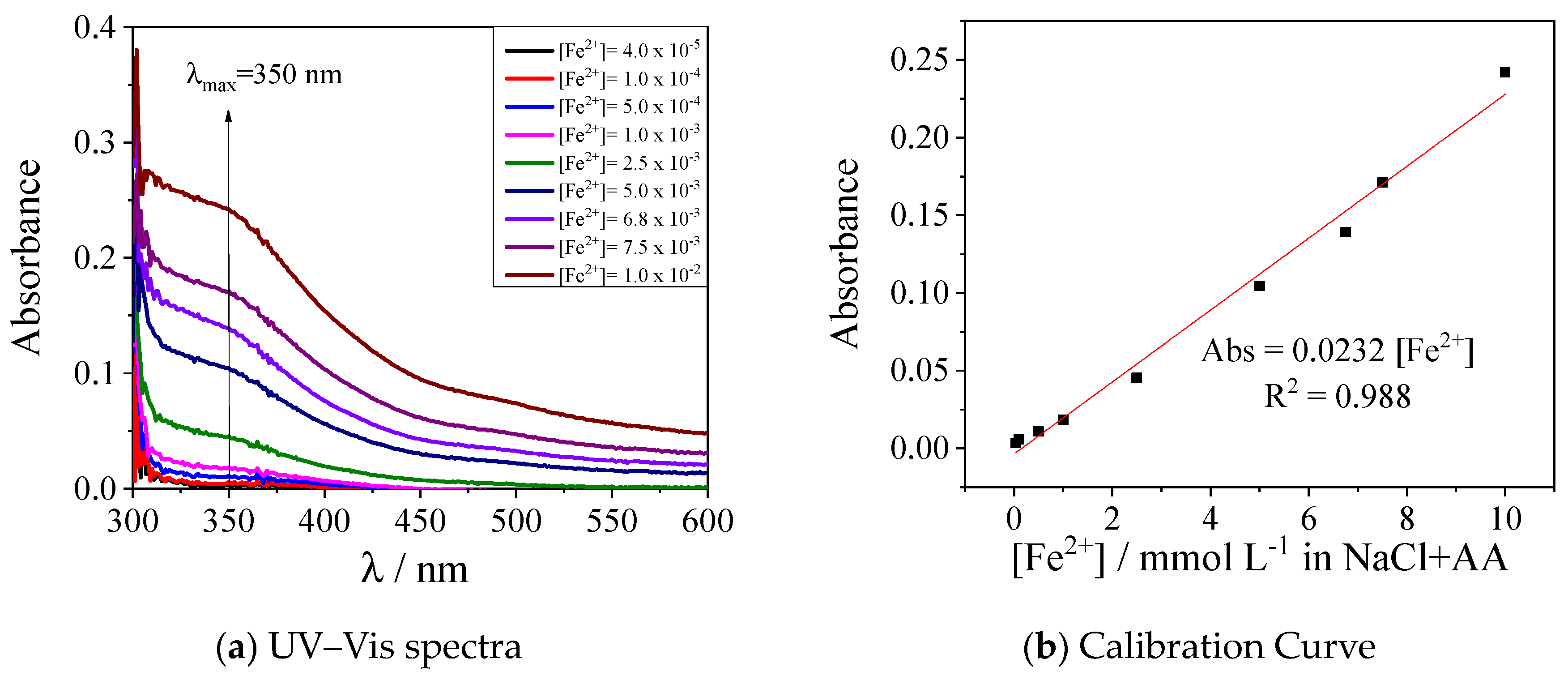

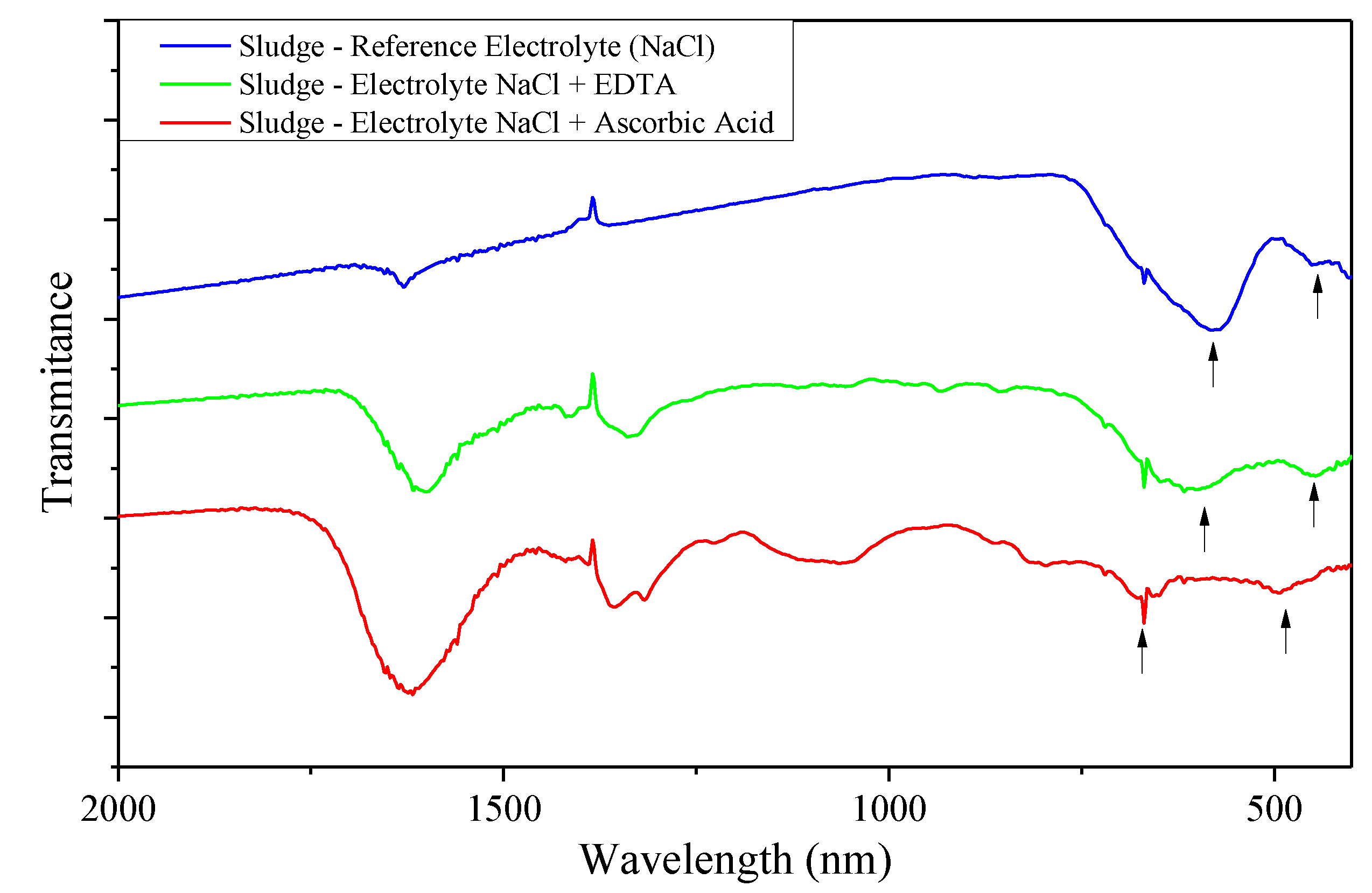
Appendix C. FTIR Spectra
References
- Mi, D.; Natsu, W. Design of ECM tool electrode with controlled conductive area ratio for holes with complex internal features. Precis. Eng. 2017, 47, 54–61. [Google Scholar] [CrossRef]
- Patel, D.S.; Sharma, V.; Jain, V.K.; Ramkumar, J. Sustainable Electrochemical Micromachining Using Atomized Electrolyte Flushing. J. Electrochem. Soc. 2021, 168, 043504. [Google Scholar] [CrossRef]
- Hsue, A.W.-J.; Huang, Z.-Y. Deionized Water Electrochemical Machining Hybridized with Alumina Powder Polishing for Microcavity of M-333 Mold Steel. Processes 2022, 10, 152. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, N. Electrochemical Milling of TB6 Titanium Alloy in NaNO3 Solution. J. Electrochem. Soc. 2019, 166, E35–E49. [Google Scholar] [CrossRef]
- Wang, K.; Yan, Y.; Zhou, P.; Zhang, C.; Kang, R.; Guo, D. Preparation of Flat and Smooth Copper Surface by Jet Electrochemical Machining and Electrochemical Polishing. J. Electrochem. Soc. 2020, 167, 163501. [Google Scholar] [CrossRef]
- Manikandan, N.; Kumanan, S.; Sathiyanarayanan, C. Multiple performance optimization of electrochemical drilling of Inconel 625 using Taguchi based Grey Relational Analysis. Eng. Sci. Technol. Int. J. 2017, 20, 662–671. [Google Scholar] [CrossRef]
- Wang, D.; He, B.; Zhu, Z.; Ge, Y.; Zhu, D. Localization of the Anodic Dissolution of Inconel 718 by Using an Electrodeposited Nickel Film. J. Electrochem. Soc. 2018, 165, E282–E288. [Google Scholar] [CrossRef]
- Núñez, P.J.; García-plaza, E.; Hernando, M.; Trujillo, R. Characterization of Surface Finish of Electropolished Stainless Steel AISI 316L with Varying Electrolyte Concentrations. Procedia Eng. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Rotty, C.; Mandroyan, A.; Doche, M.-L.; Monney, S.; Hihn, J.-Y.; Rouge, N. Electrochemical Superfinishing of Cast and ALM 316L Stainless Steels in Deep Eutectic Solvents: Surface Microroughness Evolution and Corrosion Resistance. J. Electrochem. Soc. 2019, 166, C468–C478. [Google Scholar] [CrossRef]
- Schubert, N.; Schneider, M.; Michaelis, A. Electrochemical Machining of cemented carbides. Int. J. Refract. Met. Hard Mater. 2014, 47, 54–60. [Google Scholar] [CrossRef]
- Deconinck, D.; Van Damme, S.; Albu, C.; Hotoiu, L.; Deconinck, J. Study of the effects of heat removal on the copying accuracy of the electrochemical machining process. Electrochim. Acta 2011, 56, 5642–5649. [Google Scholar] [CrossRef]
- Lohrengel, M.M.; Rataj, K.P.; Münninghoff, T. Electrochemical Machining—Mechanisms of anodic dissolution. Electrochim. Acta 2016, 201, 348–353. [Google Scholar] [CrossRef]
- Walther, B.; Schilm, J.; Michaelis, A.; Lohrengel, M.M. Electrochemical dissolution of hard metal alloys. Electrochim. Acta 2007, 52, 7732–7737. [Google Scholar] [CrossRef]
- Datta, M. Anodic dissolution of metals at high rates. IBM J. Res. Dev. 1993, 37, 207–226. [Google Scholar] [CrossRef]
- Shen, Z.-Y.; Tsui, H.-P. An Investigation of Ultrasonic-Assisted Electrochemical Machining of Micro-Hole Array. Processes 2021, 9, 1615. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Physico–chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Mohammad, A.E.K.; Wang, D. Electrochemical mechanical polishing technology: Recent developments and future research and industrial needs. Int. J. Adv. Manuf. Technol. 2016, 86, 1909–1924. [Google Scholar] [CrossRef]
- Ringbom, A.; Sittonen, S.; Saxén, B. The Fe(III)-EDTA-H2O2 complex and its analytical use. Anal. Chim. Acta 1957, 16, 541–545. [Google Scholar] [CrossRef]
- Zaitoun, M.A.; Lin, C.T. Chelating Behavior between Metal Ions and EDTA in Sol-Gel Matrix. J. Phys. Chem. B 1997, 5647, 1857–1860. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Karmakar, S.; Bhowal, A.; Das, P.; Sharma, M. Reduction of hexavalent chromium using L-ascorbic acid in rotating reactors. Int. J. Environ. Sci. Technol. 2022, 19, 5757–6780. [Google Scholar] [CrossRef]
- Kocot, P.; Karocki, A.; Stasicka, Z. Photochemistry of the Fe (III)–EDTA complexes A mechanistic study. J. Photochem. Photobiol. A Chem. 2006, 179, 176–183. [Google Scholar] [CrossRef]
- Klocke, F.; Zeis, M.; Harst, S.; Klink, A.; Veselovac, D.; Baumgärtner, M. Modeling and Simulation of the Electrochemical Machining (ECM) Material Removal Process for the Manufacture of Aero Engine Components. Procedia CIRP 2013, 8, 265–270. [Google Scholar] [CrossRef]
- Gotic, M.; Music, S. Mössbauer, FT-IR and FE SEM investigation of iron oxides precipitated from FeSO4 solutions. J. Mol. Struct. 2007, 836, 445–453. [Google Scholar] [CrossRef]
- Mishra, D.; Arora, R.; Lahiri, S.; Amritphale, S.S.; Chandra, N. Synthesis and Characterization of Iron Oxide Nanoparticles by Solvothermal Method 1. Prot. Met. Phys. Chem. Surf. 2014, 50, 628–631. [Google Scholar] [CrossRef]
- Davies, M.B. Reactions of L-Ascorbic Acid with Transition Metal Complexes. Polyhedron 1992, 11, 285–321. [Google Scholar] [CrossRef]
- Lohrengel, M.M.; Klu, I.; Rosenkranz, C.; Bettermann, H.; Schultze, J.W. Microscopic investigations of electrochemical machining of Fe in NaNO3. Electrochim. Acta 2003, 48, 3203–3211. [Google Scholar] [CrossRef]
- Skinn, B.; Lucatero, S.; Hall, T.; Snyder, S.; Taylor, E.J.; Inman, M. Electrochemical Machining Recycling for Metal Recovery and Waste Elimination. In Proceedings of the International Manufacturing Science and Engineering Conference, Detroit, MI, USA, 9–13 June 2014; ASME: New York, NY, USA, 2014; Volume 1, pp. 1–7. [Google Scholar]
- Skinn, B.; Lucatero, S.; Hall, T.; Snyder, S.; Taylor, E.J.; Inman, M. Apparatus and Method for Recovery of Material Generated during Electrochemical Material Removal in Acidic Electrolytes. U.S. Patent Application n. 20160230303 A1, 10 April 2018. [Google Scholar]
- Zander, D.; Schupp, A.; Beyss, O.; Rommes, B.; Klink, A. Oxide Formation during Transpassive Material Removal of Martensitic 42CrMo4 Steel by Electrochemical Machining. Materials 2021, 14, 402. [Google Scholar] [CrossRef]


| Experimental Parameter | Value |
|---|---|
| Anodic dissolution time | 180 s |
| Voltage applied | 5.0 V |
| Current density | 1.5 ± 0.2 A |
| Electrolyte temperature (before anodic dissolution) | 22 ± 3 °C |
| Mixing level | 800~1000 RPM |
| Electrolyte volume | 30 mL |
| Workpiece surface area | 1.0 cm2 |
| Counter-electrode surface area | 2.5 cm2 |
| Chemical Element | Specification (%) SAE 4144M Steel | Workpiece before ECM Process (%) | Workpiece after ECM Process | ||
|---|---|---|---|---|---|
| 100 g L−1 NaCl | +15 g L−1 EDTA (pH > 10.0) | +20 g L−1 Ascorbic Acid (pH < 5.0) | |||
| C | 0.42~0.46 | 0.453 | 0.456 | 0.46 | 0.46 |
| Si | 0.20~0.30 | 0.268 | 0.267 | 0.266 | 0.269 |
| Mn | 0.90~1.10 | 1.07 | 1.06 | 1.06 | 1.06 |
| P | max 0.025 | 0.0251 | 0.0253 | 0.0241 | 0.0237 |
| S | 0.010~0.020 | 0.0195 | 0.017 | 0.0178 | 0.017 |
| Cr | 1.15~1.30 | 1.26 | 1.25 | 1.27 | 1.26 |
| Ni | max 0.25 | 0.196 | 0.198 | 0.197 | 0.201 |
| Mo | 0.25~0.35 | 0.309 | 0.31 | 0.309 | 0.314 |
| Cu | max 0.35 | 0.157 | 0.159 | 0.159 | 0.16 |
| Fe | Remaining | 96 | 96 | 96 | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercal, G.; de Alvarenga, G.; Vidotti, M. Sludge Reduction and Surface Investigation in Electrochemical Machining by Complexing and Reducing Agents. Processes 2023, 11, 2186. https://doi.org/10.3390/pr11072186
Cercal G, de Alvarenga G, Vidotti M. Sludge Reduction and Surface Investigation in Electrochemical Machining by Complexing and Reducing Agents. Processes. 2023; 11(7):2186. https://doi.org/10.3390/pr11072186
Chicago/Turabian StyleCercal, Gustavo, Gabriela de Alvarenga, and Marcio Vidotti. 2023. "Sludge Reduction and Surface Investigation in Electrochemical Machining by Complexing and Reducing Agents" Processes 11, no. 7: 2186. https://doi.org/10.3390/pr11072186
APA StyleCercal, G., de Alvarenga, G., & Vidotti, M. (2023). Sludge Reduction and Surface Investigation in Electrochemical Machining by Complexing and Reducing Agents. Processes, 11(7), 2186. https://doi.org/10.3390/pr11072186









