Investigation of a Jet-Based Direct Mixing Process for Improved Structuring of Conductive Battery Hetero-Agglomerates
Abstract
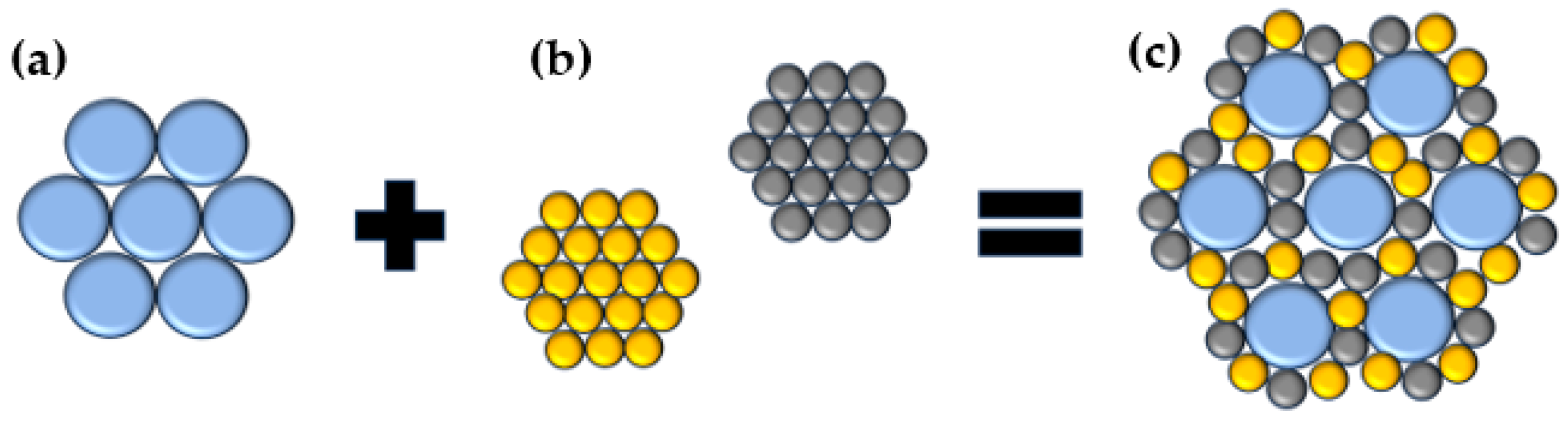
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Operation of the Feeding Process
2.2.2. Jet-Based Direct Mixing Process
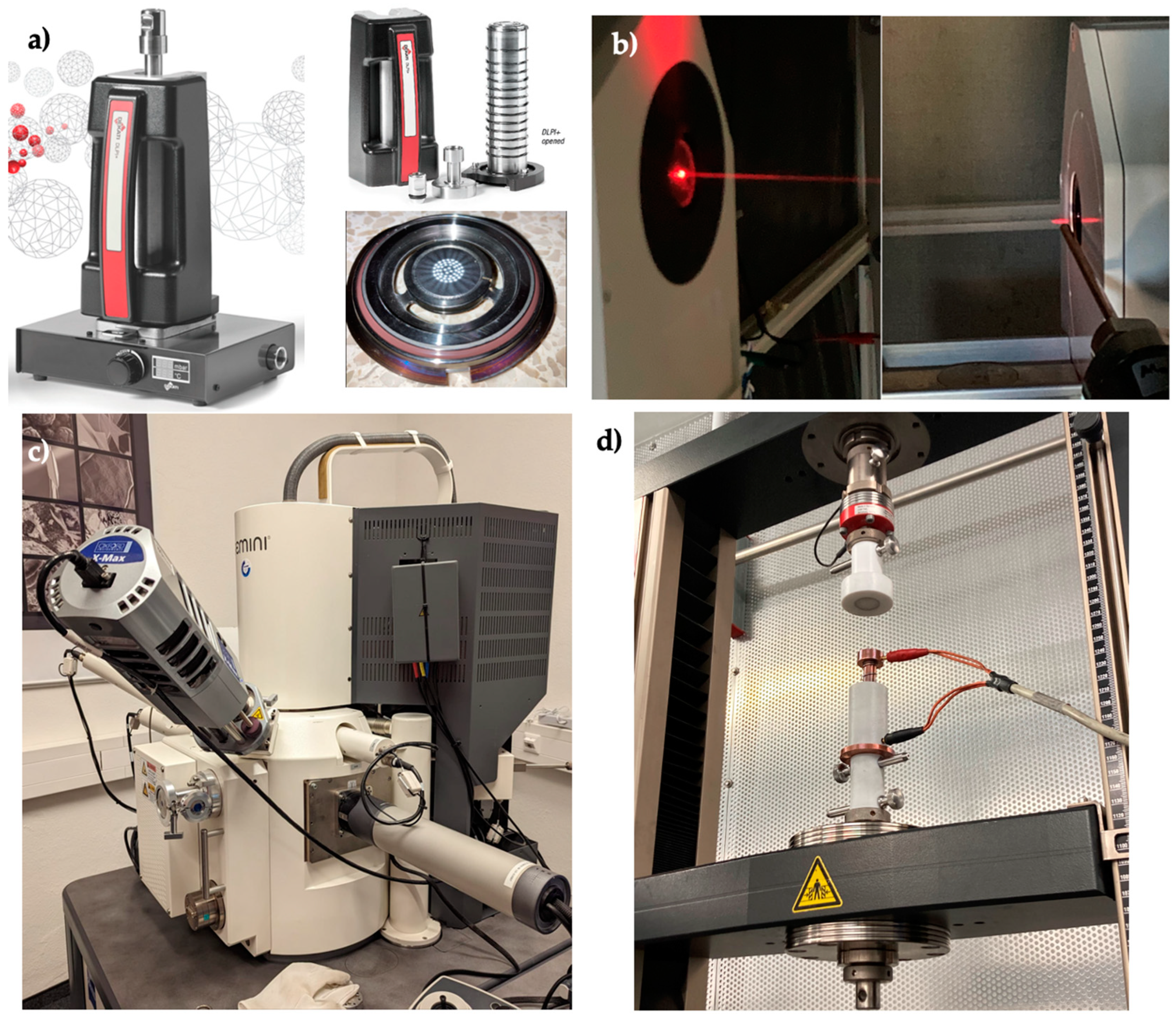
2.2.3. Measurement Technology
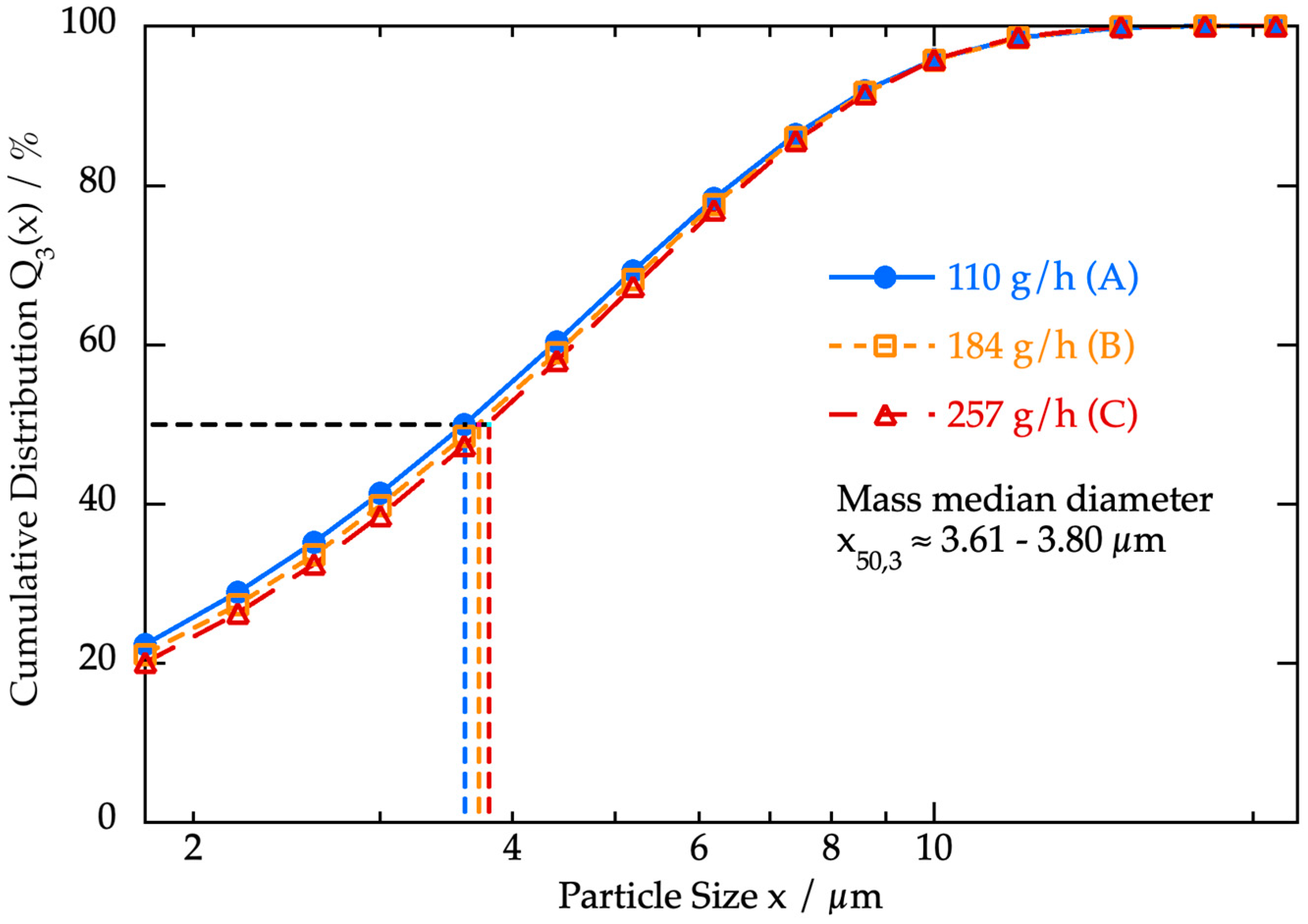
3. Results and Discussion
3.1. Characterisation of the Brush Disperser
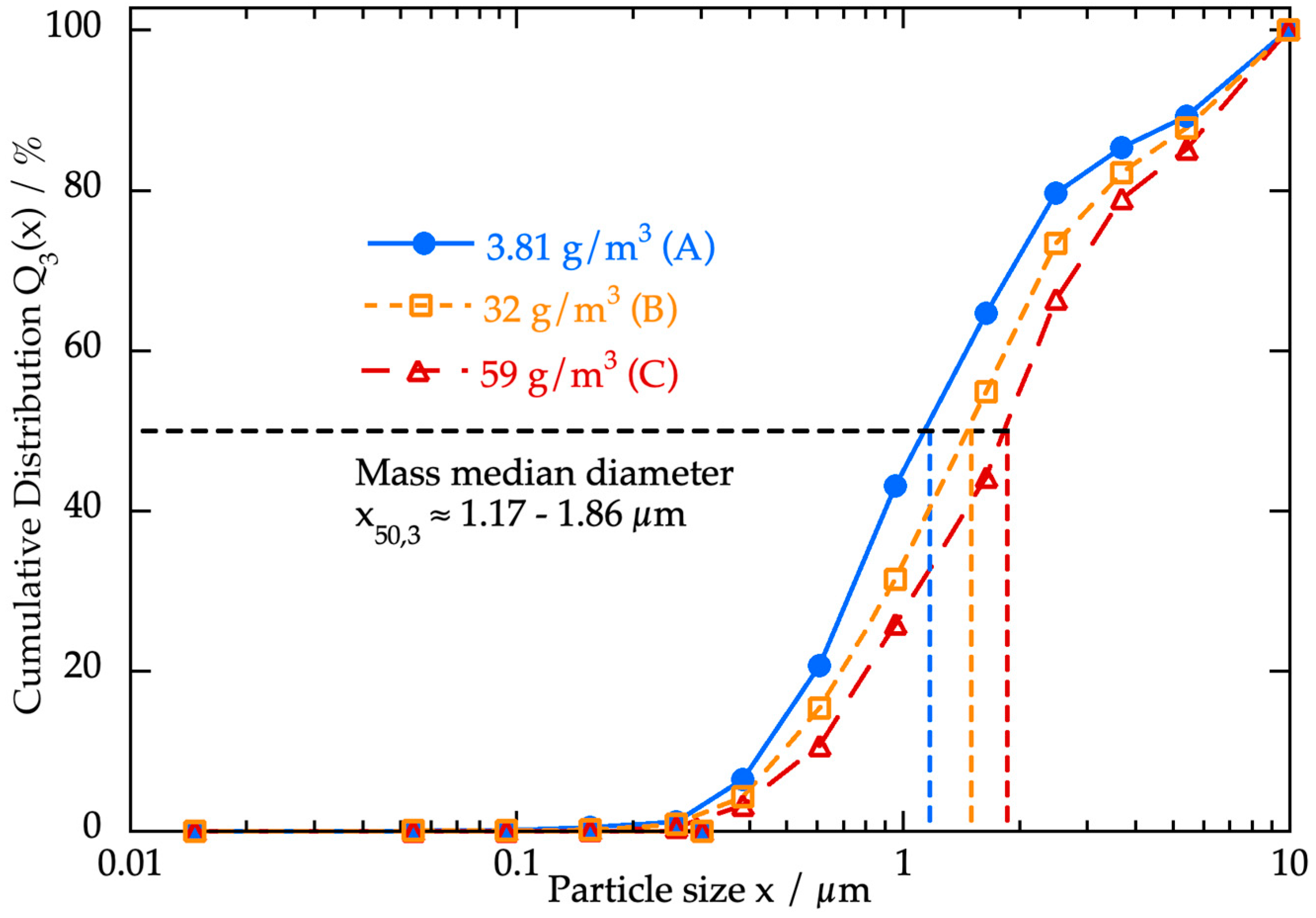
3.2. Investigation of the Feed Material
3.3. Examination of the Cathode Composite
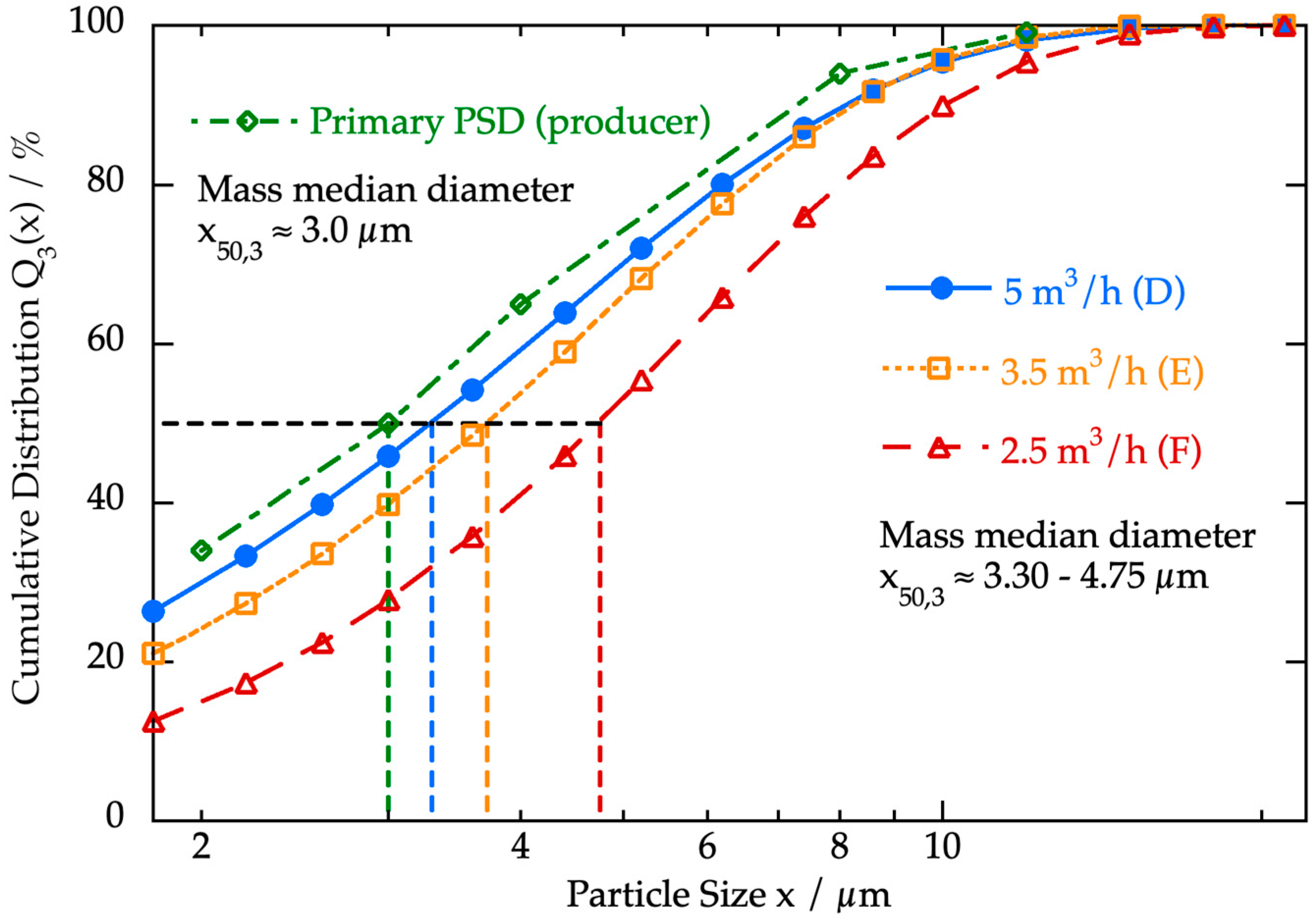
3.3.1. Cascade Impactor
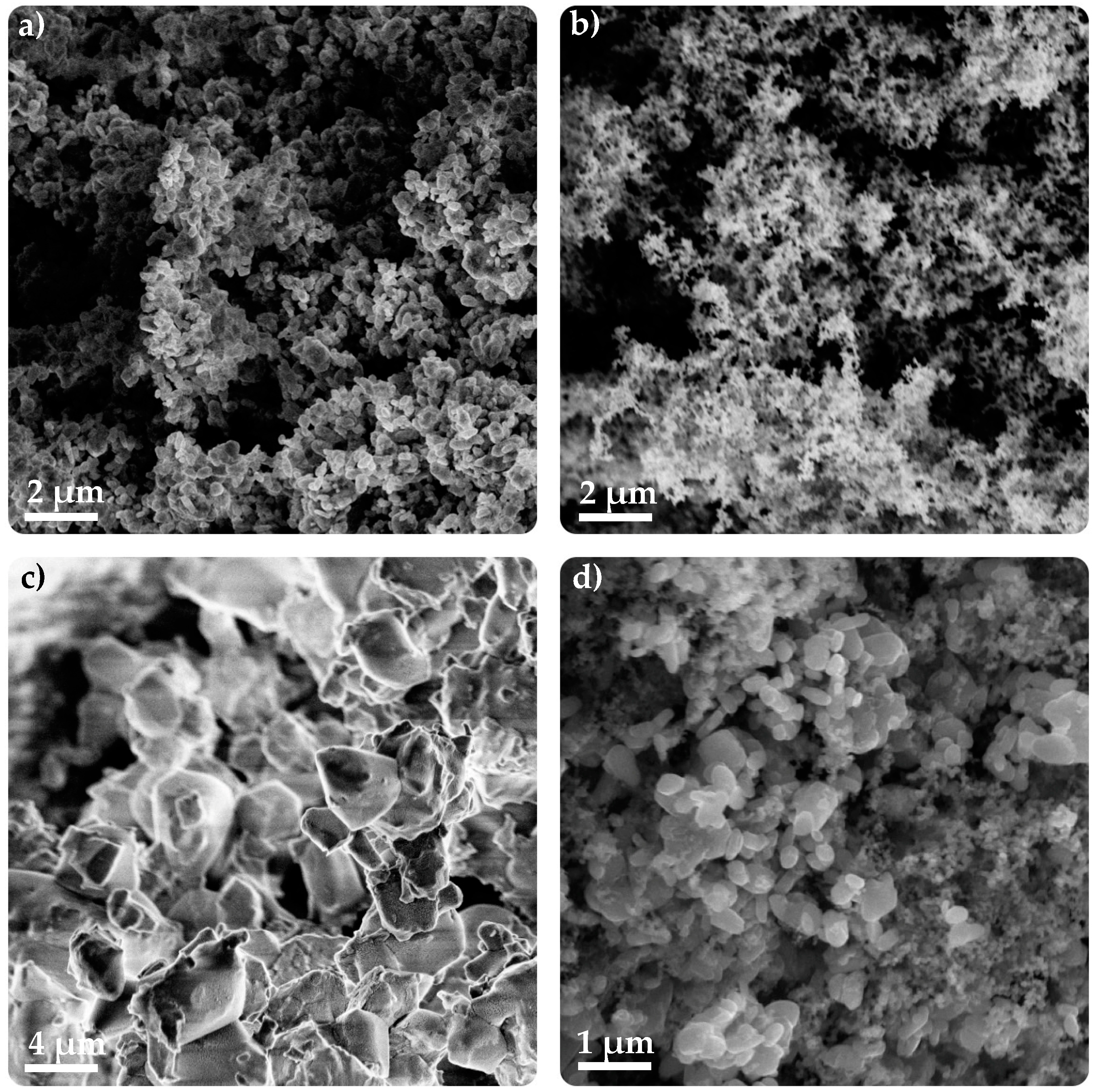
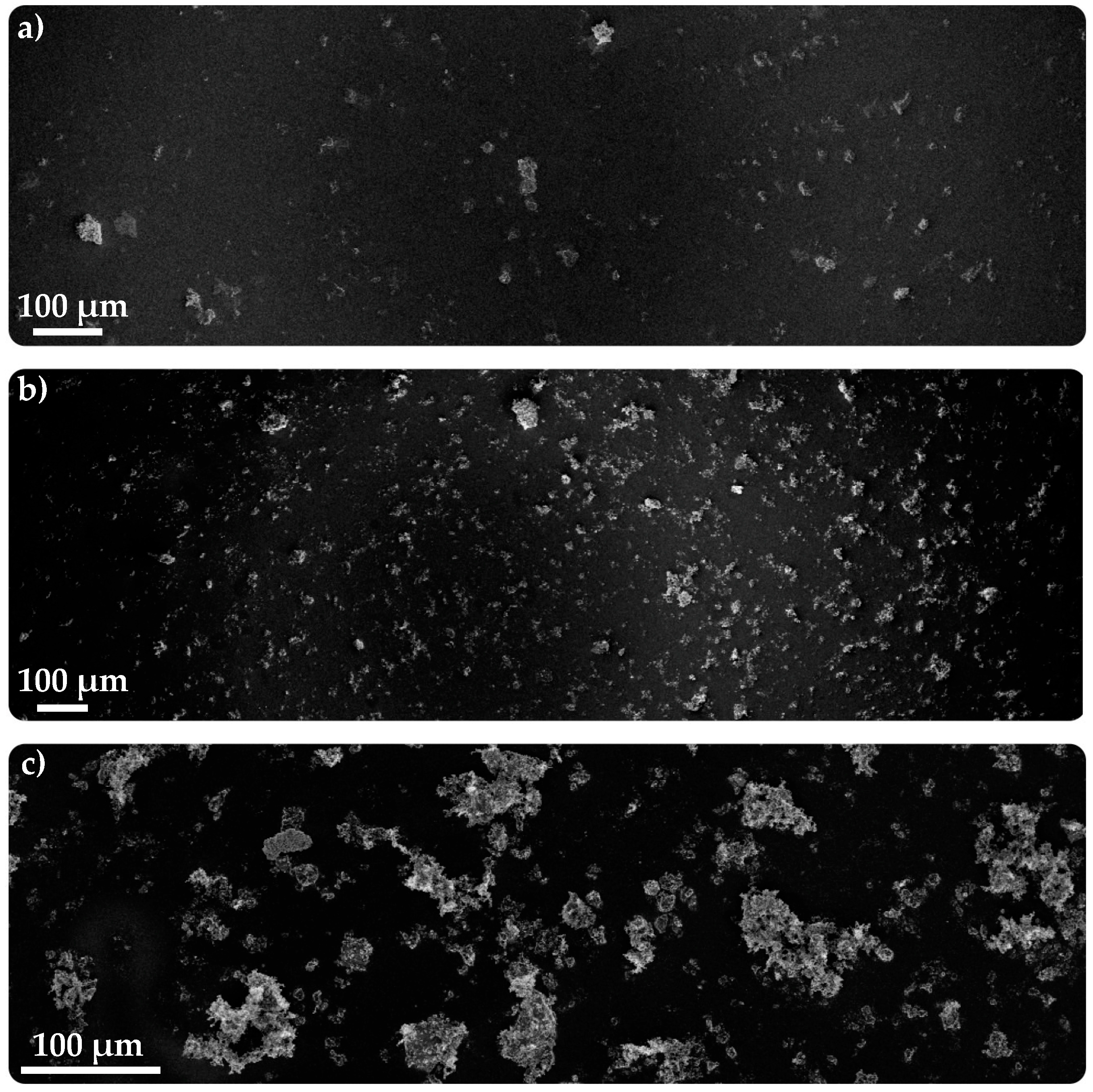
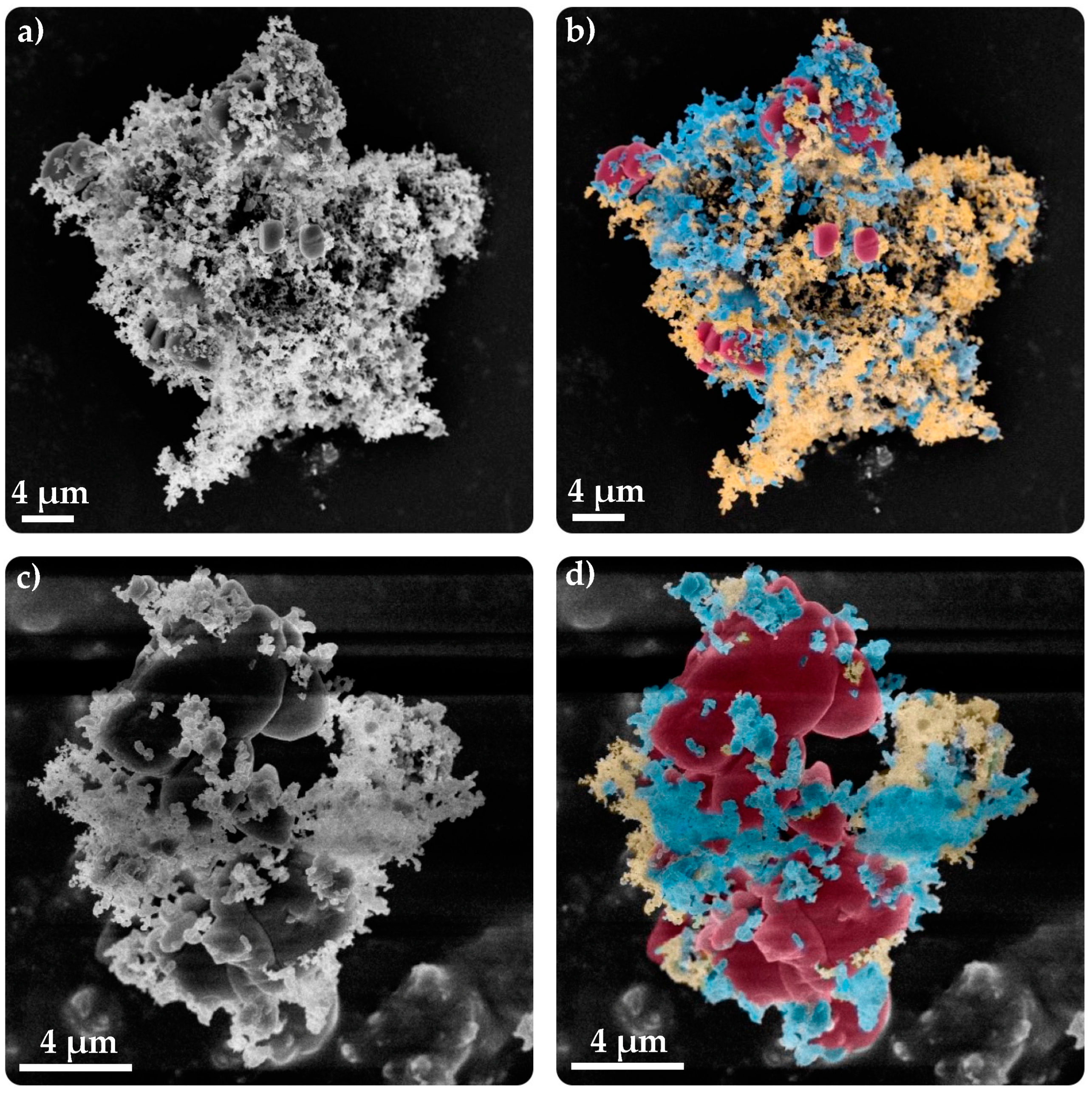
3.3.2. SEM–FIB
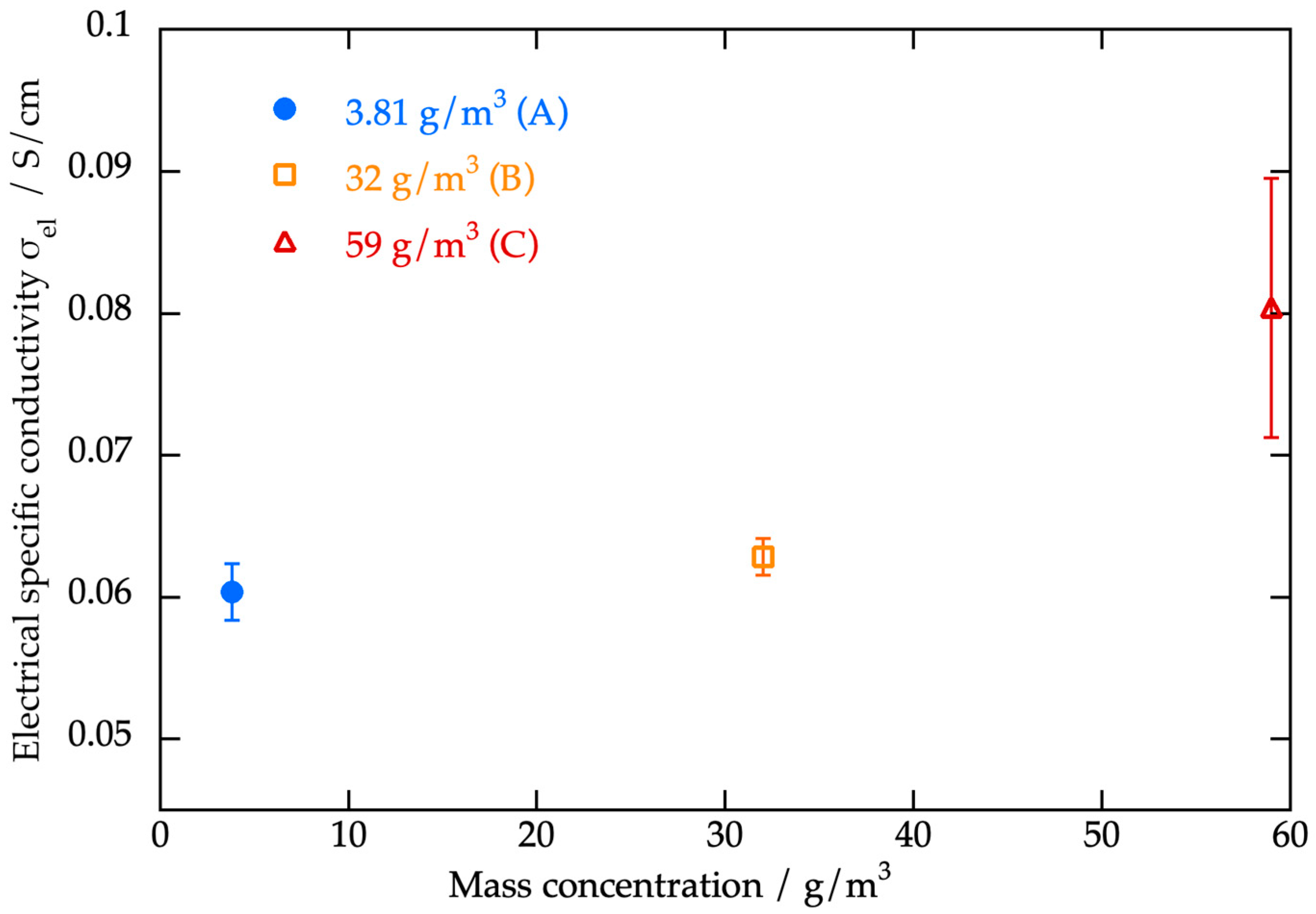
3.3.3. Electrical Conductivity Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liua, H.; Cheng, X.; Chong, Y.; Yuana, H.; Huanga, J.-Q.; Zhang, Q. Advanced electrode processing of lithium ion batteries: A review of powder technology in battery fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]
- Chen, Q.; Gong, S.; Moll, J.; Zhao, D.; Kumar, S.K.; Colby, R.H. Mechanical Reinforcement of Polymer Nanocomposites from Percolation of a Nanoparticle Network. ACS Macro Lett. 2015, 4, 398–402. [Google Scholar] [CrossRef]
- Tahara, K. Pharmaceutical formulation and manufacturing using particle/powder technology for personalized medicines. Adv. Powder Technol. 2020, 31, 387–392. [Google Scholar] [CrossRef]
- Rhein, F.; Nirschl, H.; Kaegi, R. Separation of Microplastic Particles from Sewage Sludge Extracts Using Magnetic Seeded Filtration. Water Res. X 2022, 17, 100155. [Google Scholar] [CrossRef] [PubMed]
- Rhein, F.; Kaiser, S.; Rhein, M.; Nirschl, H. Agglomerate processing and recycling options in magnetic seeded filtration. Chem. Eng. Sci. 2021, 238, 116577. [Google Scholar] [CrossRef]
- Adin, H.; Adin, M.S. Effect of particles on tensile and bending properties of jute epoxy composites. Mater. Test. 2022, 64, 401–411. [Google Scholar] [CrossRef]
- Schmaltz, T.; Wicke, T.; Weymann, L.; Voß, P.; Neef, C.; Thielmann, A. Solid-State Battery Roadmap 2035+; Fraunhofer ISI: Karlsruhe, Germany, 2022. [Google Scholar] [CrossRef]
- Sveinbjörnsson, D.Þ.; Vegge, T.; Norby, P.; Mogensen, M.B. Design and Characterisation of Solid Electrolytes for All-Solid-State Lithium Batteries; Department of Energy Conversion and Storage, Technical University of Denmark: Kongens Lyngby, Denmark, 2014. [Google Scholar]
- Janek, J.; Zeier, W.G.A. Solid future for battery development. Nat. Energy 2016, 1, 16141. [Google Scholar] [CrossRef]
- Helmers, L.; Froböse, L.; Friedrich, K.; Steffens, M.; Kern, D.; Michalowski, P.; Kwade, A. Sustainable Solvent-Free Production and Resulting Performance of Polymer Electrolyte-Based All-Solid-State Battery Electrodes. Energy Technol. 2021, 9, 2000923. [Google Scholar] [CrossRef]
- Dixit, M.B.; Muralidharan, N.; Parejiya, A.; Amin, R.; Essehli, R.; Belharouak, I. Current Status and Prospects of Solid-State Batteries as the Future of Energy Storage. In Management and Applications of Energy Storage Devices; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Kartini, E.; Genardy, C.T. The Future of All Solid State Battery. IOP Conf. Ser. Mater. Sci. Eng. 2020, 924, 012038. [Google Scholar] [CrossRef]
- Hu, Y.-S. Batteries, Getting solid. Nat. Energy 2016, 1, 16042. [Google Scholar] [CrossRef]
- Bhalode, P.; Ierapetritou, M. A review of existing mixing indices in solid-based continuous blending operations. Powder Technol. 2020, 373, 195–209. [Google Scholar] [CrossRef]
- Lacey, P.M.C. Developments in the theory of particle mixing. J. Appl. Chem. 2007, 4, 257–268. [Google Scholar] [CrossRef]
- Poux, M.; Fayolle, P.; Bridoux, D.; Bousquet, J. Powder mixing: Some practical rules applied to agitated systems. Powder Technol. 1991, 68, 213–234. [Google Scholar] [CrossRef]
- Salameh, S.; Gómez-Hernández, J.; Goulas, A.; van Bui, H.; van Ommen, J.R. Advances in scalable gas-phase manufacturing and processing of nanostructured solids: A review. Particuology 2017, 30, 15–39. [Google Scholar] [CrossRef]
- Yao, W.; Guangsheng, G.; Fei, W.; Jun, W. Fluidization and agglomerate structure of SiO2 nanoparticles. Powder Technol. 2002, 124, 152–159. [Google Scholar] [CrossRef]
- Cerbelaud, M.; Videcoq, A.; Abélard, P.; Ferrando, R. Simulation of the heteroagglomeration between highly size-asymmetric ceramic particles. J. Colloid Interface Sci. 2009, 332, 360–365. [Google Scholar] [CrossRef]
- Gockeln, M.; Pokhrel, S.; Meierhofer, F.; Glenneberg, J.; Schowalter, M.; Rosenauer, A.; Fritsching, U.; Busse, M.; Mädler, L.; Kun, R. Fabrication and performance of Li4Ti5O12/C Li-ion battery electrodes using combined double flame spray pyrolysis and pressure-based lamination technique. J. Power Sources 2018, 374, 97–106. [Google Scholar] [CrossRef]
- Perez-Vaquero, J.; Valverde, J.M.; Quintanilla, M.A.S. Flow properties of CO2 sorbent powders modified with nanosilica. Powder Technol. 2013, 249, 443–455. [Google Scholar] [CrossRef]
- Grossmann, H.K.; Grieb, T.; Meierhofer, F.; Hodapp, M.-J.; Noriler, D.; Gröhn, A.; Meier, H.F.; Fritsching, U.; Wegner, K.; Mädler, L. Nanoscale mixing during double-flame spray synthesis of heterostructured nanoparticles. J. Nanopart. Res. 2015, 17, 174. [Google Scholar] [CrossRef]
- Eggersdorfer, M.L.; Kadau, D.; Herrmann, H.J.; Pratsinis, S.E. Fragmentation and restructuring of soft-agglomerates under shear. J. Colloid Interface Sci. 2010, 342, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Hasebe, S.; Kousaka, Y. Dispersion of aggregates of fine powder by acceleration in an air stream and its application to the evaluation of adhesion between particles. Powder Technol. 1997, 91, 25–30. [Google Scholar] [CrossRef]
- Ammar, Y.; Dehbi, A.; Reeks, M.W. Break-Up of Aerosol Agglomerates in Highly Turbulent Gas Flow. Flow Turbul. Combust. 2012, 89, 465–489. [Google Scholar] [CrossRef]
- Froeschke, S.; Kohler, S.; Weber, A.P.; Kasper, G. Impact fragmentation of nanoparticle agglomerates. J. Aerosol Sci. 2003, 34, 275–288. [Google Scholar] [CrossRef]
- Seipenbusch, M.; Toneva, P.; Peukert, W.; Weber, A.P. Impact fragmentation of metal nanoparticle agglomerates. Part. Part. Syst. Charact. 2007, 24, 193–200. [Google Scholar] [CrossRef]
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21–33. [Google Scholar] [CrossRef]
- Gudziol, H.; Blau, B.; Stadeler, M. Investigations of nasal deposition efficiency of wheaten flour and corn starch. Laryngorhinootologie 2009, 88, 398–404. [Google Scholar] [CrossRef]
- Myojo, T.; Oyabu, T.; Nishi, K.; Kadoya, C.; Tanaka, I.; Ono-Ogasawara, M.; Sakae, H.; Shirai, T. Aerosol generation and measurement of multi-wall carbon nanotubes. J. Nanopart. Res. 2009, 11, 91–99. [Google Scholar] [CrossRef]
- Holunga, D.M.; Flagan, R.C.; Atwater, H.A. A Scalable Turbulent Mixing Aerosol Reactor for Oxide-Coated Silicon Nanoparticles. Ind. Eng. Chem. Res. 2005, 44, 6332–6341. [Google Scholar] [CrossRef]
- Teleki, A.; Buesser, B.; Heine, M.C.; Krumeich, F.; Akhtar, M.K.; Pratsinls, S.E. Role of Gas-Aerosol Mixing during in Situ Coating of Flame-Made Titania Particles. Ind. Eng. Chem. Res. 2009, 48, 85–92. [Google Scholar] [CrossRef]
- Minnermann, M.; Grossmann, H.K.; Pokhrel, S.; Thiel, K.; Hagelin-Weaver, H.; Bäumer, M.; Mädler, L. Double flame spray pyrolysis as a novel technique to synthesize alumina-supported cobalt Fischer-Tropsch catalysts. Catal. Today 2013, 214, 90–99. [Google Scholar] [CrossRef]
- Kolck, V.; Witte, J.; Schmidt, E.; Kruggel-Emden, H. Analysis of process parameter sensitivities of jet-based direct mixing gas phase hetero-agglomeration by DEM/CFD-modelling. Powder Technol. 2023, 429, 118963. [Google Scholar] [CrossRef]
- Molaiyan, P.; Mailhiot, S.E.; Voges, K.; Kantola, A.M.; Hu, T.; Michalowski, P.; Kwade, A.; Telkki, V.-V.; Lassi, U. Investigation of the structure and ionic conductivity of a Li3InCl6 modified by dry room annealing for solid-state Li-ion battery applications. Mater. Des. 2023, 227, 111690. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Mathur, G.N.; Varadan, V.K. Lithium Iron Phosphates as Cathode Materials in Lithium Ion Batteries for Electric Vehicles. Proc. SPIE Nanosen. Biosens. Info-Tech Sens. Syst. 2012, 8344, 122–129. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials für lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Oh, S.W.; Myung, S.-T.; Oh, S.-M.; Oh, K.H.; Amine, K.; Scrosati, B.; Sun, Y.-K. Double Carbon Coating of LiFePO4 as High Rate Electrode for Rechargeable Lithium Batteries. Adv. Mater. 2010, 22, 4842–4845. [Google Scholar] [CrossRef] [PubMed]
- Verein Deutscher Ingenieure, e.V. Richtlinie VDI 2066 Blatt 5: Particulate Matter Measurement. Dust Measurement in Flowing Gases. In Particle Size Selective Measurement by Impaction Method–Cascade Impactor; Beuth: Berlin, Germany, 1994. [Google Scholar]
- Appelhans, C. Zur Frage der drucklosen Messung der elektrischen Leitfähigkeit von pulverförmigen Werkstoffen. Mater. Werkst. 1980, 11, 205–208. [Google Scholar] [CrossRef]
- Haselrieder, W.; Ivanov, S.; Tran, H.Y.; Theil, S.; Froböse, L.; Westphal, B.; Wohlfahrt-Mehrens, M.; Kwade, A. Influence of formulation method and related processes on structural, electrical and electrochemical properties of LMS/NCA-blend electrodes. Prog. Solid State Chem. 2014, 42, 157–174. [Google Scholar] [CrossRef]






| Material | Limestone (CaCO3) | Cathode Material | ||
|---|---|---|---|---|
| Lithium Iron Phosphate (LFP) | Carbon Black (CB) | Sodium Chloride (NaCl) | ||
| Mean particle diameter [µm] | 3.0 | 0.8 | 0.05 | 9.22 |
| Density [kg/m3] | 2700 | 3890 | 2100 | 2200 |
| Weight per cent [%] | / | 58 | 4 | 38 |
| Variation of | Mass Flow | Volume Flow | ||||
|---|---|---|---|---|---|---|
| Case | A | B | C | D | E | F |
| Set pre-pressure [kPa] | 150 | 150 | 150 | 250 | 150 | 85 |
| Result. volume flow [m3/h] | 3.5 | 3.5 | 3.5 | 5 | 3.5 | 2.5 |
| Set feed speed [mm/h] | 300 | 500 | 700 | 500 | 500 | 500 |
| Result. mass flow [g/h] | 110 | 184 | 257 | 184 | 184 | 184 |
| Result. mass concentration [g/m3] | 31.4 | 52.6 | 73.4 | 36.8 | 52.6 | 73.6 |
| Material | NaCl | LFP + CB | ||||
|---|---|---|---|---|---|---|
| Case | A | B | C | D | E | F |
| Set pre-pressure [kPa] | 250 | 150 | 85 | 250 | 150 | 85 |
| Result. Volume flow [m3/h] | 5 | 3.5 | 2.5 | 5 | 3.5 | 2.5 |
| Set feed speed [mm/h] | 51 | 300 | 395 | 91 | 535 | 703 |
| Result. Mass flow [g/h] | 14.1 | 82.7 | 109 | 24.04 | 141 | 186 |
| Result. Mass concentration [g/m3] | 2.81 | 23.6 | 43.6 | 4.81 | 40.4 | 74.3 |
| Tamping density [g/cm3] | 0.878 (constant) | 0.841 (constant) | ||||
| Material | NaCl and LFP–CB Mixture | ||
|---|---|---|---|
| Case | A | B | C |
| Volume flow [m3/h] | 10 | 7 | 5 |
| Mass flow [g/h] | 38.1 | 224 | 295 |
| Mass concentration [g/m3] | 3.81 | 32 | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witte, J.; Hua, Z.C.; Kolck, V.; Kruggel-Emden, H.; Heinrich, S.; Schmidt, E. Investigation of a Jet-Based Direct Mixing Process for Improved Structuring of Conductive Battery Hetero-Agglomerates. Processes 2023, 11, 3243. https://doi.org/10.3390/pr11113243
Witte J, Hua ZC, Kolck V, Kruggel-Emden H, Heinrich S, Schmidt E. Investigation of a Jet-Based Direct Mixing Process for Improved Structuring of Conductive Battery Hetero-Agglomerates. Processes. 2023; 11(11):3243. https://doi.org/10.3390/pr11113243
Chicago/Turabian StyleWitte, Joscha, Zhi Cheng Hua, Victor Kolck, Harald Kruggel-Emden, Stefan Heinrich, and Eberhard Schmidt. 2023. "Investigation of a Jet-Based Direct Mixing Process for Improved Structuring of Conductive Battery Hetero-Agglomerates" Processes 11, no. 11: 3243. https://doi.org/10.3390/pr11113243
APA StyleWitte, J., Hua, Z. C., Kolck, V., Kruggel-Emden, H., Heinrich, S., & Schmidt, E. (2023). Investigation of a Jet-Based Direct Mixing Process for Improved Structuring of Conductive Battery Hetero-Agglomerates. Processes, 11(11), 3243. https://doi.org/10.3390/pr11113243








