Effects of MgO and Rare-Earth Oxides (Y2O3, Yb2O3, Dy2O3) on the Structural Characteristics and Electrical Properties of BaTiO3
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
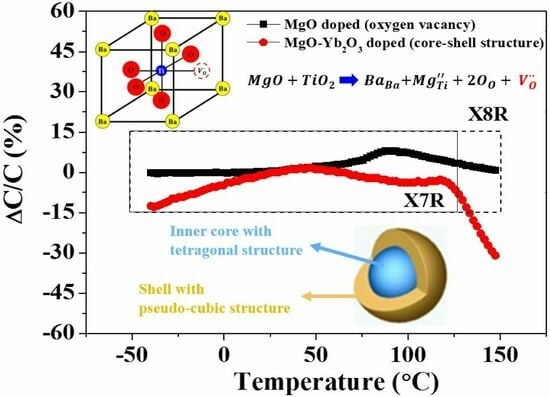
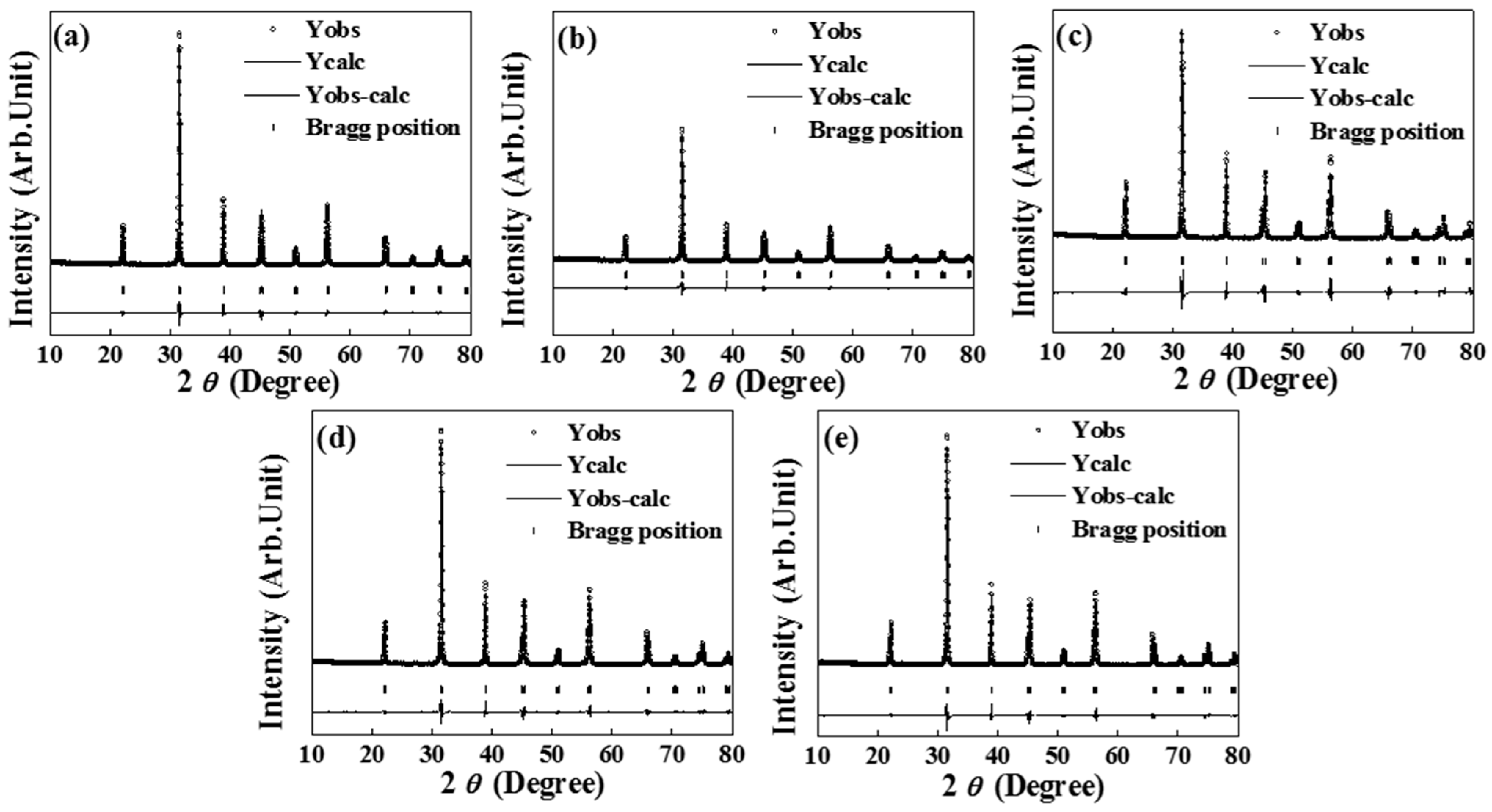
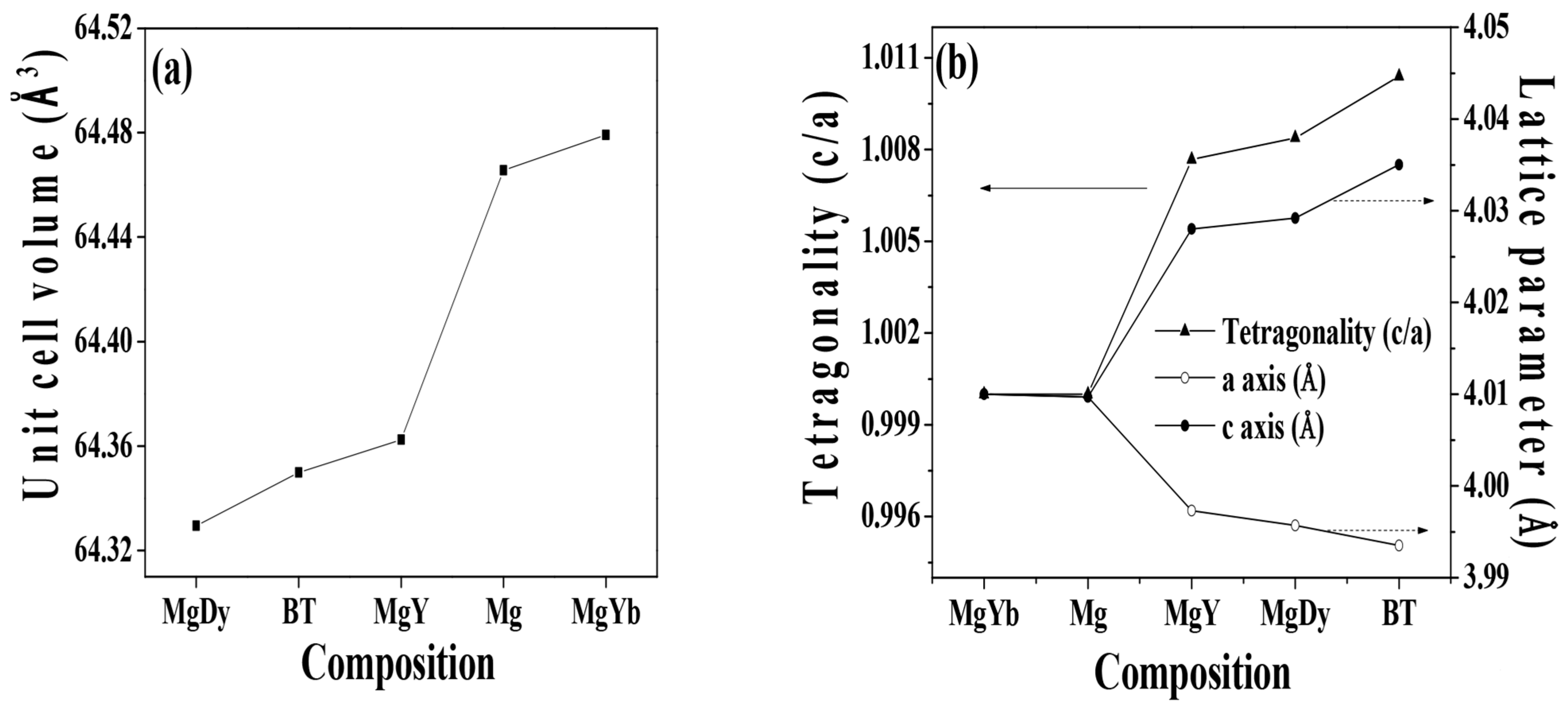
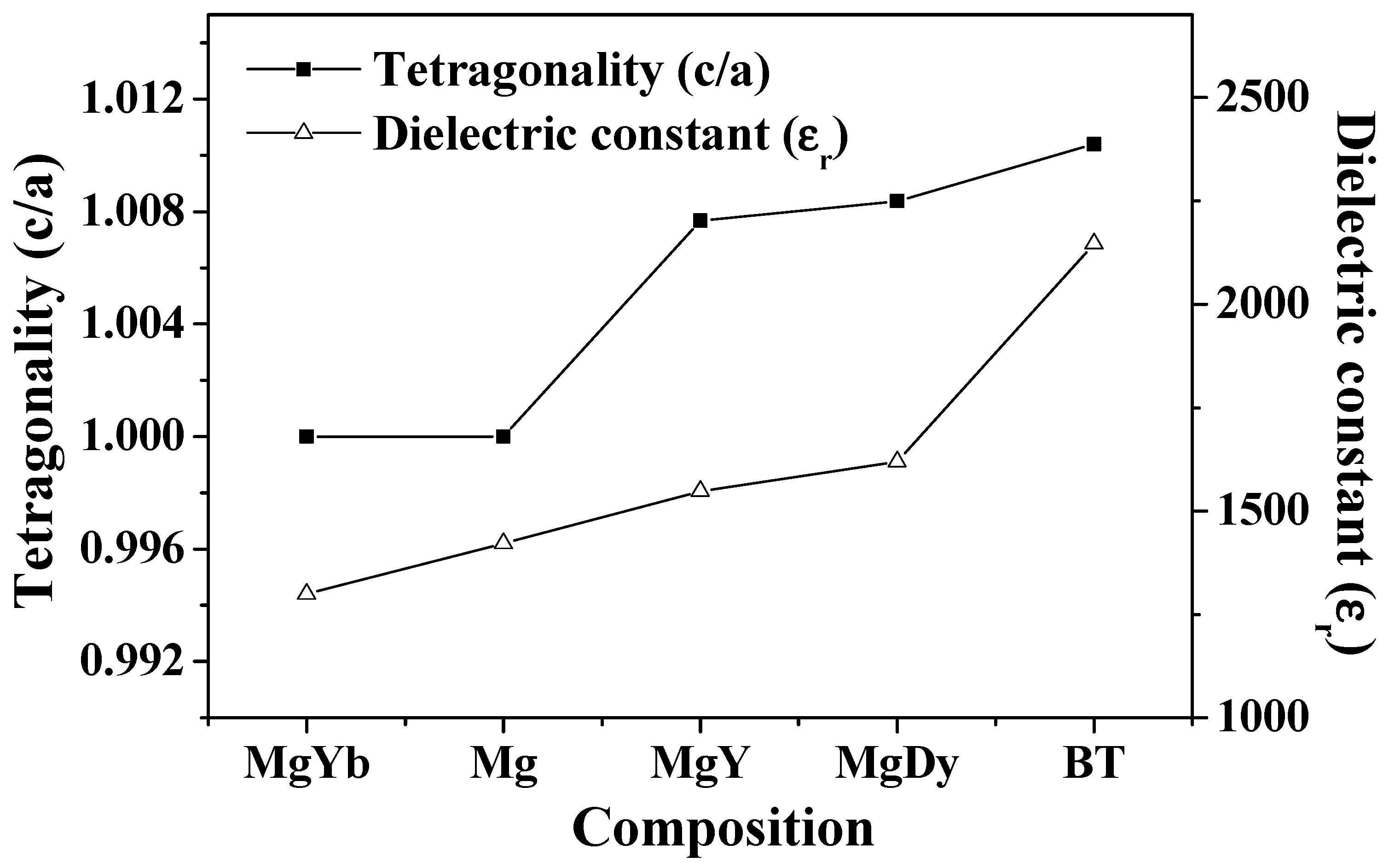
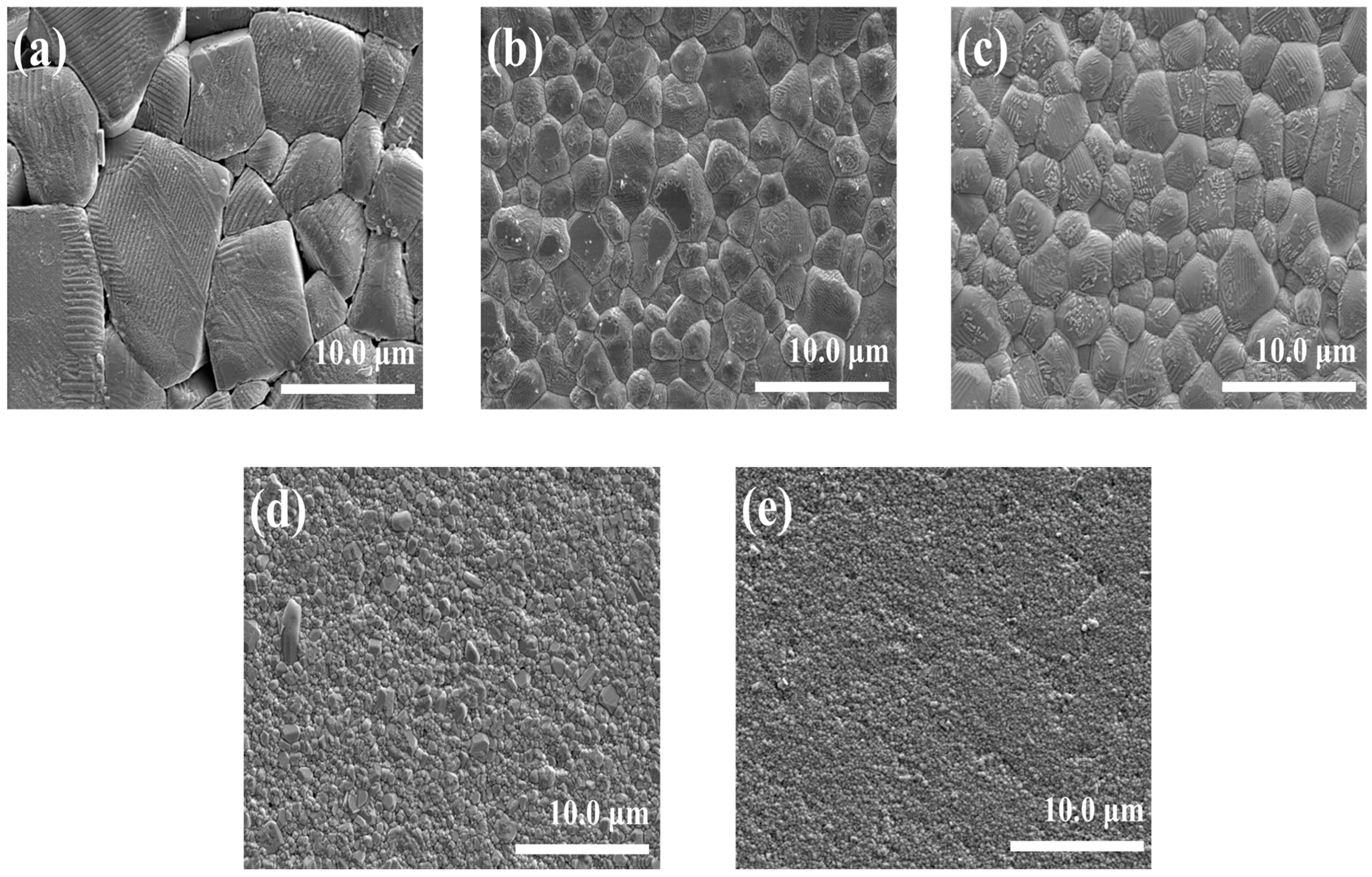
3.1. Physical Properties
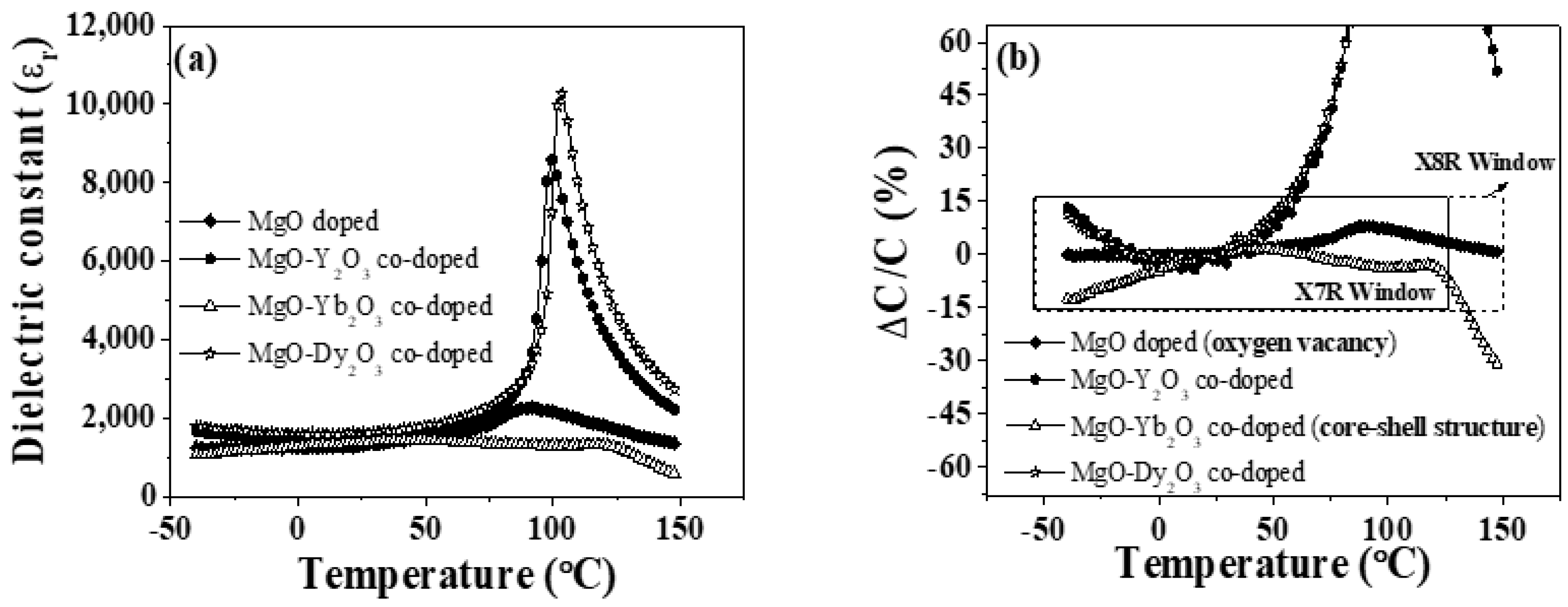
3.2. Dielectric and Electrical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, Y.; Chen, W.; Chen, S.; Langhammer, H.T. Microstructure and dielectric properties of dysprosium-doped barium titanate ceramics. Ceramica 2005, 51, 214–218. [Google Scholar] [CrossRef]
- Hao, H.; Liu, M.; Liu, H.; Zhang, S.; Shu, X.; Wang, T.; Yao, Z.; Cao, M. Design, Fabrication and dielectric properties on core-double shell BaTiO3-based ceramics for MLCC application. RSC Adv. 2015, 5, 8868–8876. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Kim, J.Y.; Tian, Z.; Fang, J.; Hur, K.H.; Li, L. High performance of BaTiO3-Based BME MLCC Nanopowder Prepared by Aqueous Chemical Coating method. J. Am. Ceram. Soc. 2012, 95, 1628–1633. [Google Scholar] [CrossRef]
- Hernandez, V.I.; García-Guti, D.I.; Aguilar-Garib, J.A.; Nava-Quintero, R.J. Characterization of precipitates formed in X7R 0603 BME-MLCC during sintering. Ceram. Int. 2021, 47, 310–319. [Google Scholar] [CrossRef]
- MYoon, M.S.; Ur, S.C. Effects of A-site Ca and B-site Zr substitution on dielectric properties and microstructure in tin-doped BaTiO3-CaTiO3 composites. Ceram. Int. 2008, 34, 1941–1948. [Google Scholar]
- Zhu, X.; Zhu, J.; Zhou, S.; Liu, Z.; Ming, N.; Hesse, D. BaTiO3 nanocrystals: Hydrothermal synthesis and structural characterization. J. Cryst. Growth 2005, 283, 553–562. [Google Scholar] [CrossRef]
- Beck, H.P.; Eiser, W.; Haberkorn, R. Pitfalls in the synthesis of nanoscaled perovskite type compounds. Part I: Influence of different sol-gel preparation methods and characterization of nanoscaled BaTiO3. J. Eur. Ceram. Soc. 2001, 21, 687–693. [Google Scholar] [CrossRef]
- Shimooka, H.; Kuwabara, M. Crystallinity and Stoichiometry of Nano-Structured Sol-Gel Derived BaTiO3 Monolithic Gels. J. Am. Ceram. Soc. 1996, 79, 2983–2985. [Google Scholar] [CrossRef]
- Wei, X.; Xu, G.; Ren, Z.; Shen, G.; Han, G. Room-Temperature Synthesis of BaTiO3 Nanoparticles in Large Batches. J. Am. Ceram. Soc. 2008, 91, 3774–3780. [Google Scholar] [CrossRef]
- Kozerozhets, I.; Semenov, E.; Kozlova, L.; Ioni, Y.; Avdeeva, V.; Ivakin, Y. Mechanism to form nanosized oxides when burning aqueous carbohydrate salt solutions. Mater. Chem. Phys. 2023, 309, 128387. [Google Scholar] [CrossRef]
- Song, Y.H.; Han, Y.H. Effects of Rare-Earth Oxides on Temperature Stability of Acceptor-Doped BaTiO3. Jpn. J. Appl. Phys. 2005, 44, 6143. [Google Scholar] [CrossRef]
- Jeong, J.; Han, Y.H. Effects of MgO-Doping on Electrical Properties and Microstructure of BaTiO3. Jpn. J. Appl. Phys. 2004, 43, 5373. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, K.J.; Yoon, Y.J.; Hong, M.H.; Hong, J.O.; Hur, K.H. Role of Yttrium and magnesium in the formation of core-shell structure of BaTiO3 grains in MLCC. J. Eur. Ceram. Soc. 2008, 28, 1213–1219. [Google Scholar] [CrossRef]
- Sakabe, Y.; Hamaji, Y.; Sano, H.; Wada, N. Effects of Rare-Earth Oxides on the Reliability of X7R Dielectrics. Jpn. J. Appl. Phys. 2002, 41, 5668. [Google Scholar] [CrossRef]
- Saito, H.; Chazono, H.; Kishi, H.; Yamaoka, N. X7R Multilayer Ceramic Capacitors with Nickel Electrodes. Jpn. J. Appl. Phys. 1991, 30, 2307. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, C.H.; Yoon, Y.J.; Song, S.M.; Kim, Y.T.; Hur, K.H. Doping behaviors of dysprosium, yttrium and holmium in BaTiO3 ceramics. J. Eur. Ceram. Soc. 2009, 29, 1735–1741. [Google Scholar] [CrossRef]
- Li, Y.X.; Yao, X.; Wang, X.S.; Hao, Y.B. Studies of dielectric properties of rare earth (Dy, Tb, Eu) doped barium titanate sintered in pure nitrogen. Ceram. Int. 2012, 38, S29–S32. [Google Scholar] [CrossRef]
- Wang, M.; Xue, K.; Zhang, K.; Li, L. Dielectric properties of BaTiO3-based ceramics are tuned by defect dipoles and oxygen vacancies under a reducing atmosphere. Ceram. Int. 2022, 48, 22212–22220. [Google Scholar] [CrossRef]
- Kishi, H.; Okino, Y.; Honda, M.; Iguchi, Y.; Imaeda, M.; Takahashi, Y.; Ohsato, H.; Okuda, T. The effect of MgO and rare-earth oxide on formation behavior of core-shell structure in BaTiO3. Jpn. J. Appl. Phys. 1997, 36, 5954. [Google Scholar] [CrossRef]
- Yang, W.C.; Hu, C.T.; Lin, I.N. Effect of Y2O3/MgO Co-doping on the electrical properties of base-metal-electroded BaTiO3 materials. J. Eur. Ceram. Soc. 2004, 24, 1479–1483. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, S.; Zhou, X.; Li, B.; Chen, Z. Effect of sintering atmospheres on the microstructure and dielectric properties of Yb/Mg co-doped BaTiO3 ceramics. Mater. Lett. 2005, 59, 2457–2460. [Google Scholar] [CrossRef]
- Gong, H.; Wang, X.; Zhang, S.; Yang, X.; Li, L. Influence of sintering temperature on core-shell structure evolution and reliability in Dy modified BaTiO3 dielectric ceramics. Phys. Status Solidi 2014, 211, 1213–1218. [Google Scholar] [CrossRef]
- Hahn, D.W.; Han, Y.H. Electrical properties of Yb-Doped BaTiO3. Jpn. J. Appl. Phys. 2009, 48, 111406. [Google Scholar] [CrossRef]
- Nfissi, A.; Ababou, Y.; Belhajji, M.; Sayouri, S.; Hajji, L.; Bennani, M.N. Investigation of Ba and Ti site occupation effects on structural, optical and dielectric properties of sol gel processed Y-doped BaTiO3 ceramics. Opt. Mater. 2021, 122, 111708. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Z.; Lu, Z.; Wang, X. Effects of sintering temperature and Bi2O3, Y2O3 and MgO co-doping on the dielectric properties of X8R BaTiO3-based ceramics. Ceram. Int. 2022, 48, 2377–2384. [Google Scholar] [CrossRef]
- Kishi, H.; Kohzu, N.; Sugino, J.; Ohsato, H.; Iguchi, Y.; Okuda, T. The effect of Rare-earth (La, Sm, Dy, Ho and Er) and Mg on the microstructure in BaTiO3. J. Eur. Ceram. Soc. 1999, 19, 1043–1046. [Google Scholar] [CrossRef]
- Lin, Y.T.; Ou, S.F.; Lin, M.H.; Song, Y.R. Effect of MgO addition on the microstructure and dielectric properties of BaTiO3 ceramics. Ceram. Int. 2018, 44, 3531–3535. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–766. [Google Scholar] [CrossRef]
- Badapanda, T.; Senthil, V.; Panigrahi, S.; Anwar, S. Diffuse of phase transition behavior of dysprosium doped barium titanate ceramic. J. Electroceram. 2013, 31, 55–60. [Google Scholar] [CrossRef]
- Tihtih, M.; Ibrahim, J.E.F.M.; Basyooni, M.A.; En-nadir, R.; Belaid, W.; Hussainova, I.; Kocserha, I. Development of yttrium-doped BaTiO3 for next-generation multilayer ceramic capacitors. ACS Omega 2023, 8, 8448–8460. [Google Scholar] [CrossRef] [PubMed]
- Arlt, G.; Hennings, D.; de With, G. Dielectric properties of fine-grained barium titanate ceramics. J. Appl. Phys. 1985, 58, 1619–1625. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, S.J.; Kim, D.Y. Influence of excess Ba concentration on the dielectric nonlinearity in Mn and V-doped BaTiO3 multilayer ceramic capacitors. J. Appl. Phys. 2013, 114, 224103. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, M.Y.; Nam, C.H.; Seo, J.W.; Wi, S.K.; Hur, K.H. Correlation between tetragonality (c/a) and direct current (dc) bias characteristics of BaTiO3-based multilayer ceramic capacitors. Appl. Phys. Lett. 2015, 107, 072906. [Google Scholar] [CrossRef]
- Tutuncu, G.; Li, B.; Bowman, K.; Jones, J.L. Domain wall motion and electromechanical strain in lead free piezoelectrics: Insight from the model system (1-x)Ba(Zr0.2Ti0.8)O3-x(Ba0.7Ca0.3)TiO3 using in situ high-energy X-ray diffraction during application of electric fields. J. Appl. Phys. 2014, 115, 144104. [Google Scholar] [CrossRef]
- Qi, T.; Grinberg, I.; Rappe, A.M. Correlations between tetragonality, A.M.R.; polarization, and ionic displacement in PbTiO3-derived ferroelectric perovskite solid solutions. Phys. Rev. B 2010, 82, 134113. [Google Scholar] [CrossRef]
- Gong, H.; Wang, X.; Zhang, S.; Wen, H.; Li, L. Grain size effect on electrical and reliability characteristics of modified fine-grained BaTiO3 ceramics for MLCCS. J. Eur. Ceram. Soc. 2014, 34, 1733–1739. [Google Scholar] [CrossRef]
- Yoon, S.H.; Randall, C.A.; Hur, K.H. Influence of Grain Size on Impedance Spectra and Resistance Degradation Behavior in Acceptor (Mg)-Doped BaTiO3 Ceramics. J. Am. Ceram. Soc. 2009, 92, 2944–2952. [Google Scholar] [CrossRef]
- Shlyakhtina, A.; Fedtke, P.; Busch, A.; Kolbanev, I.; Barfels, T.; Wienecke, M.; Sokolov, A.; Ulianov, V.; Trounov, V.; Shcherbakova, L. Effect of Ca-doping on the electrical conductivity of oxide ion conductor Yb2Ti2O7. Solid State Ion. 2008, 179, 1004–1008. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, Y.S.; Hong, J.O.; Sinn, D.S. Effect of pyrochlore (Y2Ti2O7) phase on the resistance degradation on yttrium-doped BaTiO3 ceramic capacitors. J. Mater. Res. 2007, 22, 2539–2543. [Google Scholar] [CrossRef]
- Hennings, D.; Schnell, A.; Simon, G. Diffuse Ferroelectric Phase Transitions in Ba(Ti1-yZry)O3 ceramics. J. Am. Ceram. Soc. 1982, 65, 539–544. [Google Scholar] [CrossRef]
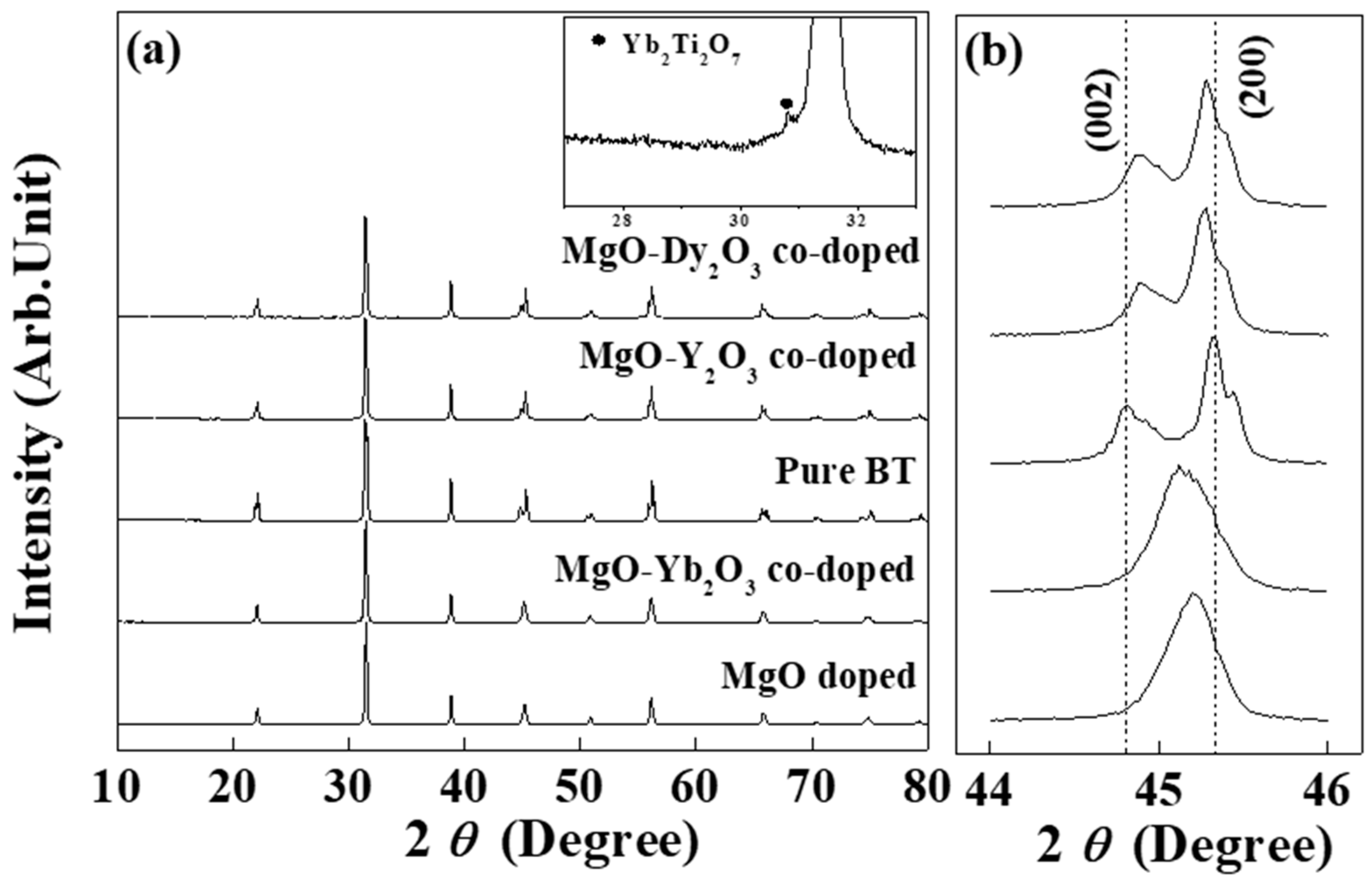

| Additives | Abbreviation | BaTiO3 | MgO | Y2O3 | Yb2O3 | Dy2O3 |
|---|---|---|---|---|---|---|
| Pure BaTiO3 | BT | 100 | 0 | 0 | 0 | 0 |
| MgO | Mg | 100 | 1 | 0 | 0 | 0 |
| MgO-Y2O3 | Mg/Y | 100 | 1 | 1 | 0 | 0 |
| MgO-Yb2O3 | Mg/Yb | 100 | 1 | 0 | 1 | 0 |
| MgO-Dy2O3 | Mg/Dy | 100 | 1 | 0 | 0 | 1 |
| Specimen | Sintering Condition (°C/h) | Apparent Density (g/cm3) | Theoretical Density (g/cm3) | Relative Density (%) |
|---|---|---|---|---|
| MgO-Dy2O3 | 1350 °C/1 h | 5.8140 | 6.0200 | 96.58 |
| Pure BT | 5.8030 | 6.0018 | 96.42 | |
| MgO-Y2O3 | 5.7845 | 6.0170 | 96.14 | |
| MgO | 5.6686 | 6.0080 | 94.35 | |
| MgO-Yb2O3 | 1350 °C/5 h | 5.7683 | 6.0170 | 95.87 |
| Specimen | Lattice Parameter (Å) | Tetragonality (c/a) | Unit-Cell Volume (Å3) | Rbragg | GoF | |
|---|---|---|---|---|---|---|
| a-Axis | c-Axis | |||||
| Pure BT | 3.9935 | 4.0350 | 1.01039 | 64.3499 | 2.87 | 2.6 |
| MgO-Dy2O3 | 3.9957 | 4.0292 | 1.00838 | 64.3294 | 2.30 | 2.3 |
| MgO-Y2O3 | 3.9973 | 4.0280 | 1.00768 | 64.3625 | 2.35 | 2.4 |
| MgO | 4.0097 | 4.0097 | 1 | 64.4656 | 2.21 | 2.1 |
| MgO-Yb2O3 | 4.0100 | 4.0100 | 1 | 64.4792 | 1.79 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Kim, E.S. Effects of MgO and Rare-Earth Oxides (Y2O3, Yb2O3, Dy2O3) on the Structural Characteristics and Electrical Properties of BaTiO3. Processes 2023, 11, 3235. https://doi.org/10.3390/pr11113235
Park JH, Kim ES. Effects of MgO and Rare-Earth Oxides (Y2O3, Yb2O3, Dy2O3) on the Structural Characteristics and Electrical Properties of BaTiO3. Processes. 2023; 11(11):3235. https://doi.org/10.3390/pr11113235
Chicago/Turabian StylePark, Jae Hoon, and Eung Soo Kim. 2023. "Effects of MgO and Rare-Earth Oxides (Y2O3, Yb2O3, Dy2O3) on the Structural Characteristics and Electrical Properties of BaTiO3" Processes 11, no. 11: 3235. https://doi.org/10.3390/pr11113235
APA StylePark, J. H., & Kim, E. S. (2023). Effects of MgO and Rare-Earth Oxides (Y2O3, Yb2O3, Dy2O3) on the Structural Characteristics and Electrical Properties of BaTiO3. Processes, 11(11), 3235. https://doi.org/10.3390/pr11113235