3D Printed, Single-Use Bioreactor with Integrated Inline Sensors for Microbial and Mammalian Cell Cultivation—A Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Additive Manufacturing and Characterization of Printed Material
2.2. Biochemical Engineering Investigations
2.2.1. Computational Fluid Dynamics
2.2.2. Experimental Biochemical Engineering Investigations
2.3. Cultivations
2.3.1. Controller Setup
2.3.2. E. coli Fed-Batch
2.3.3. CHO Fed-Batch
3. Results and Discussion
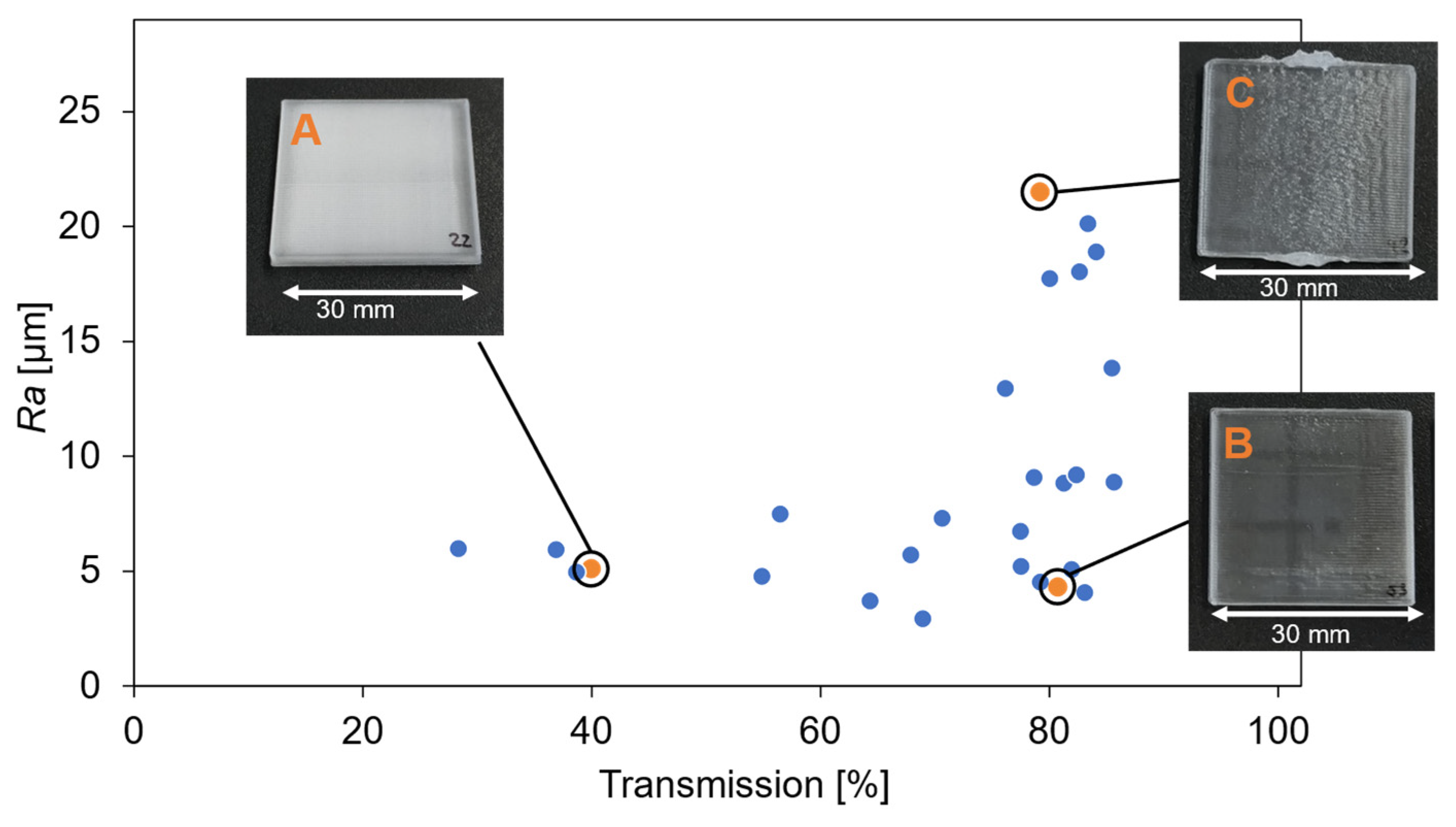
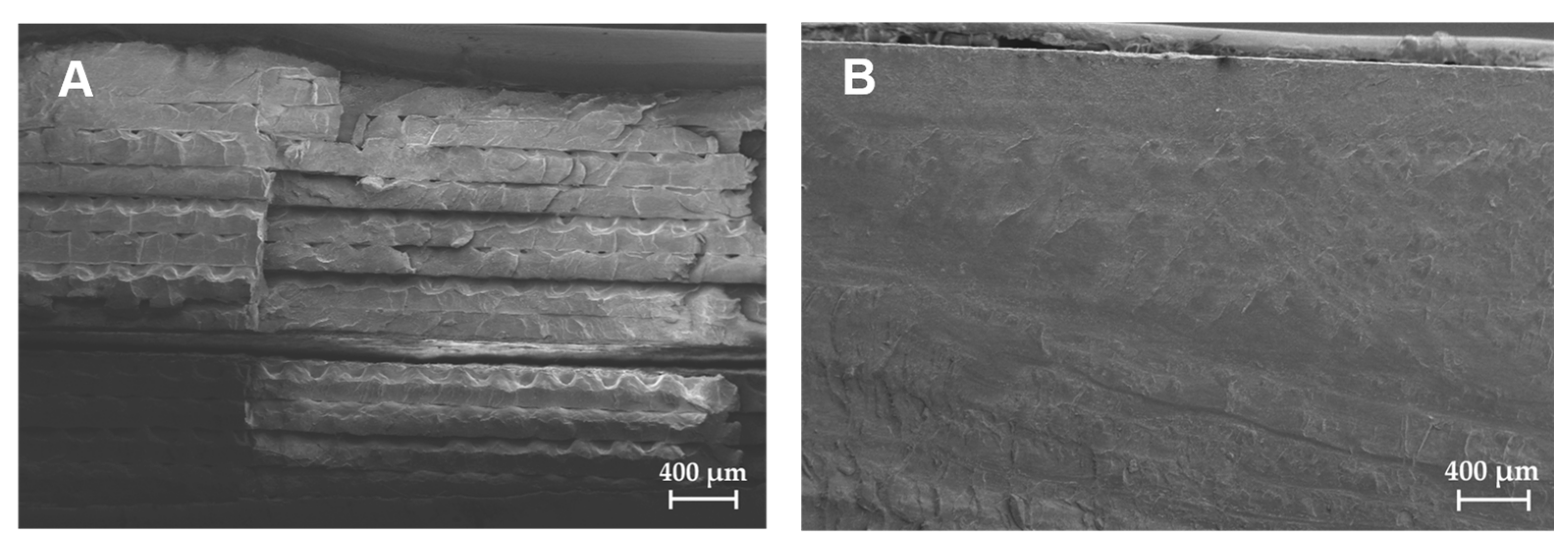
3.1. Printing Parameter and Material Evaluation
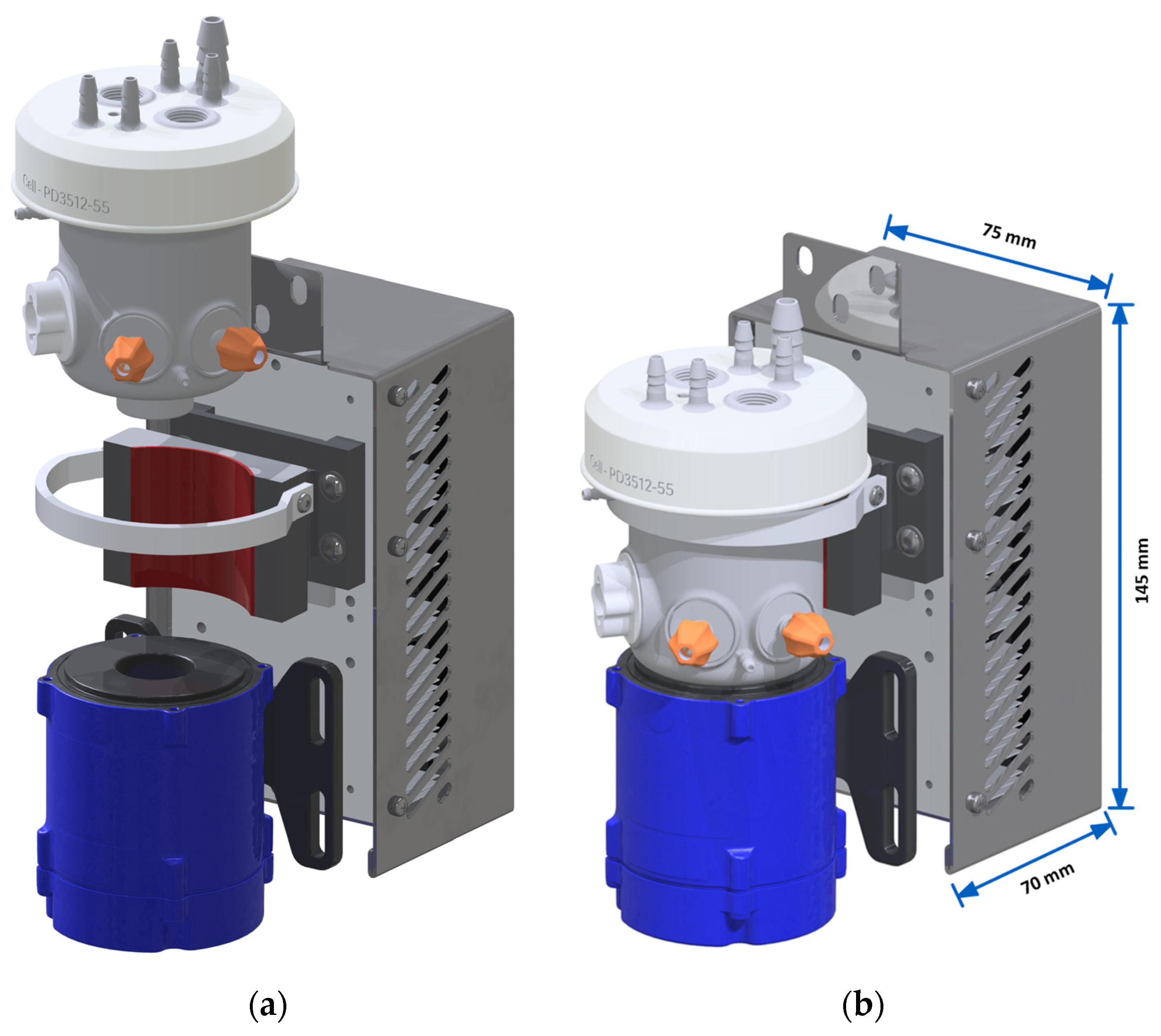
3.2. Bioreactor Design and Assembly
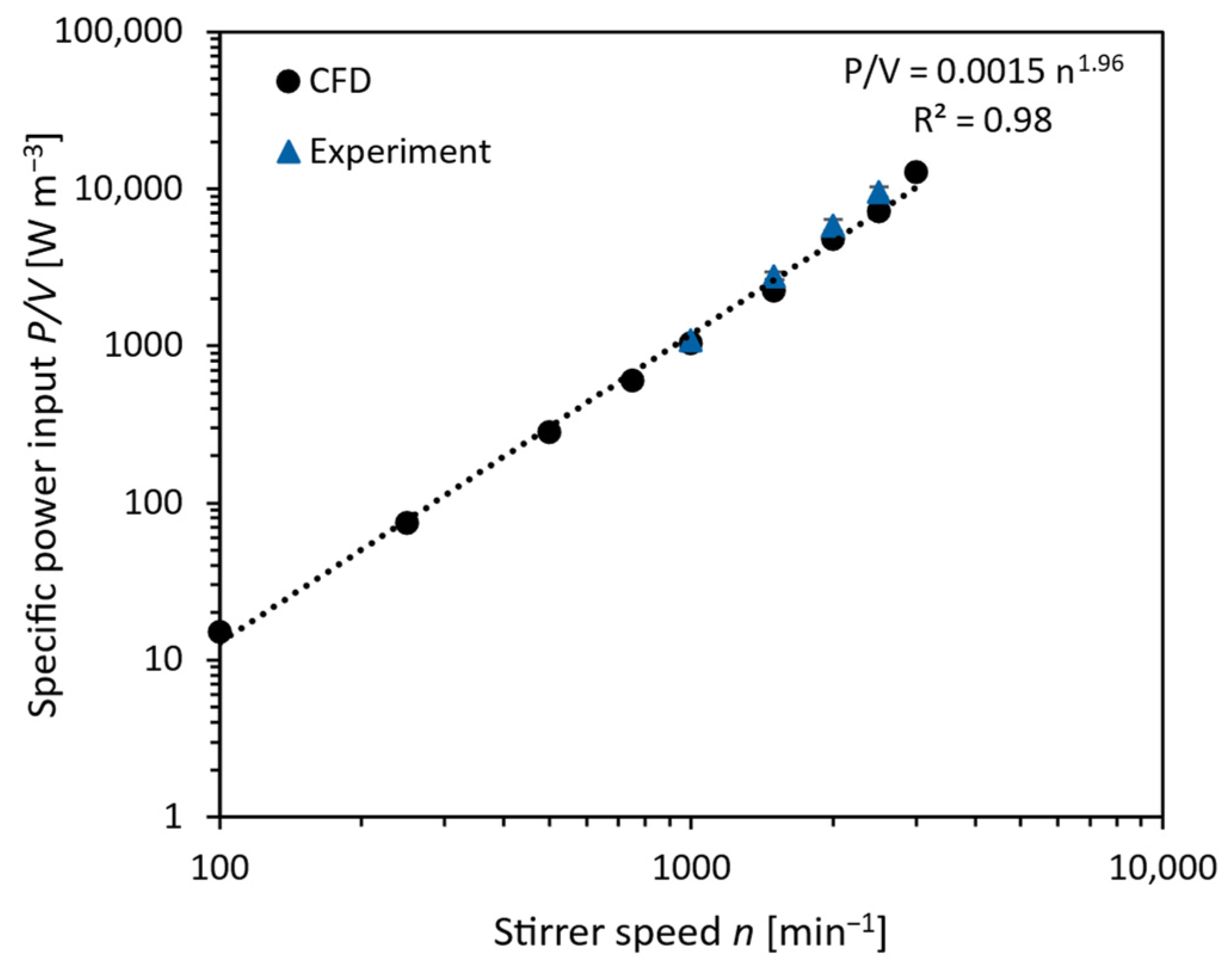
3.3. Process Engineering Parameters
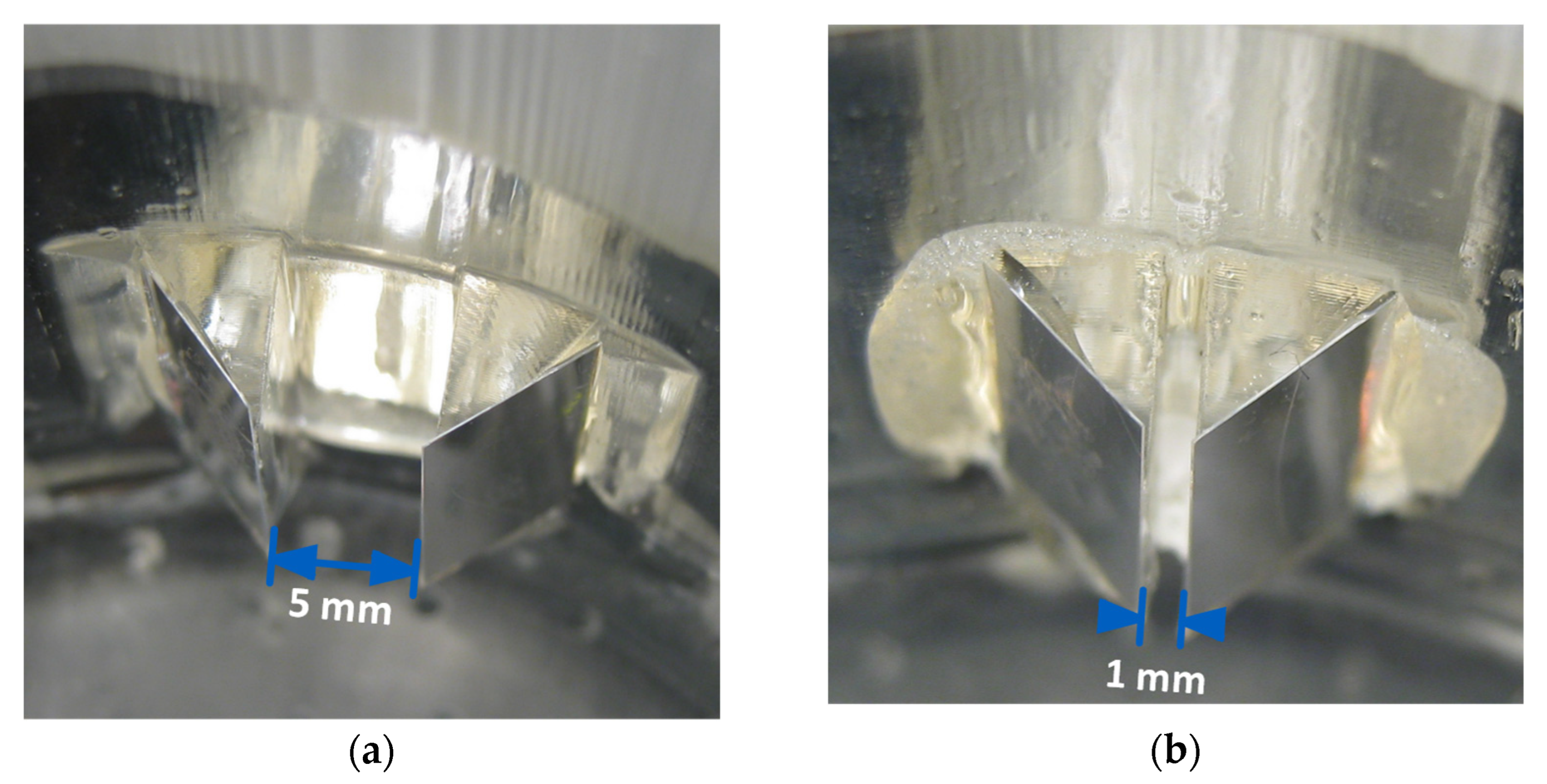
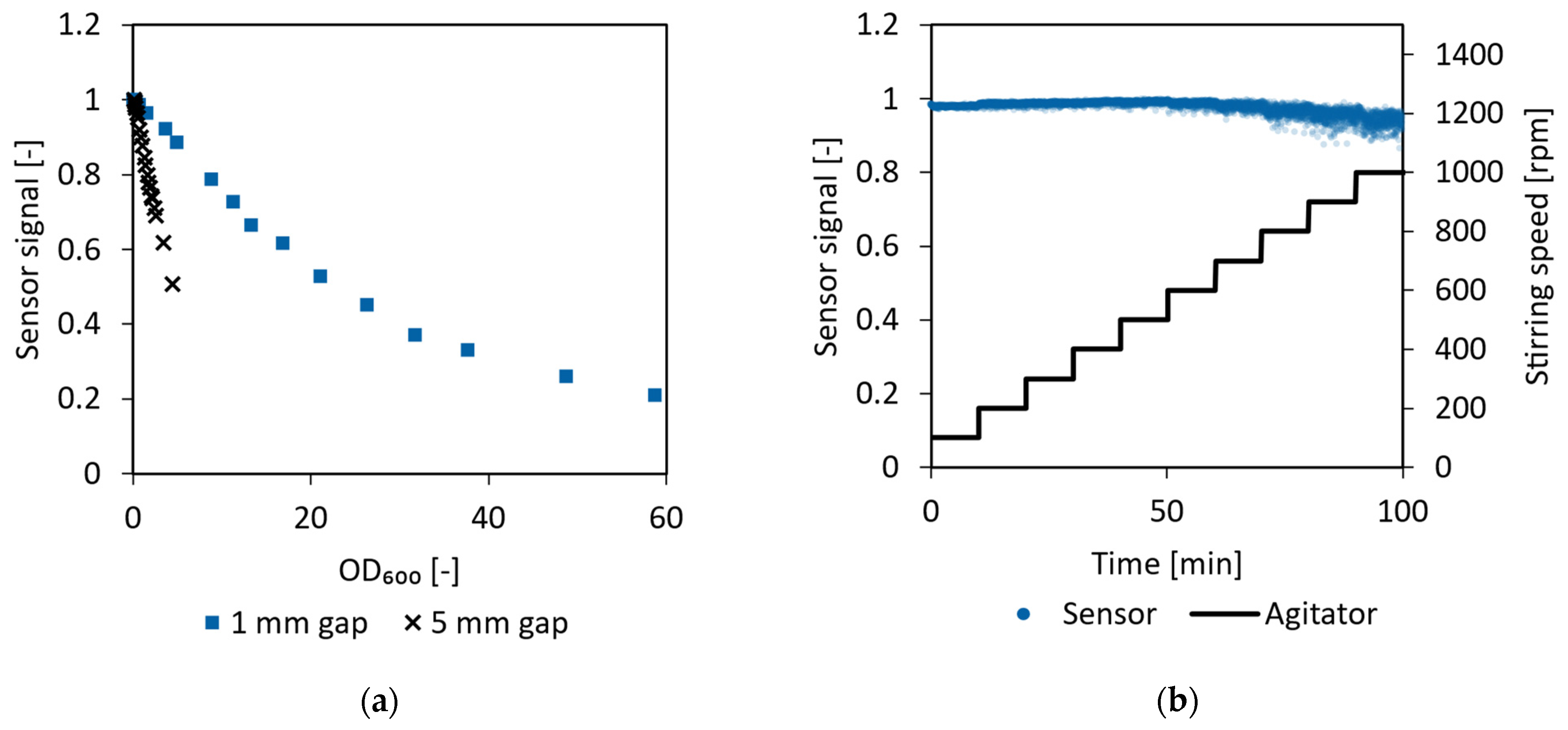
3.4. Sensor Design
3.5. Cultivations
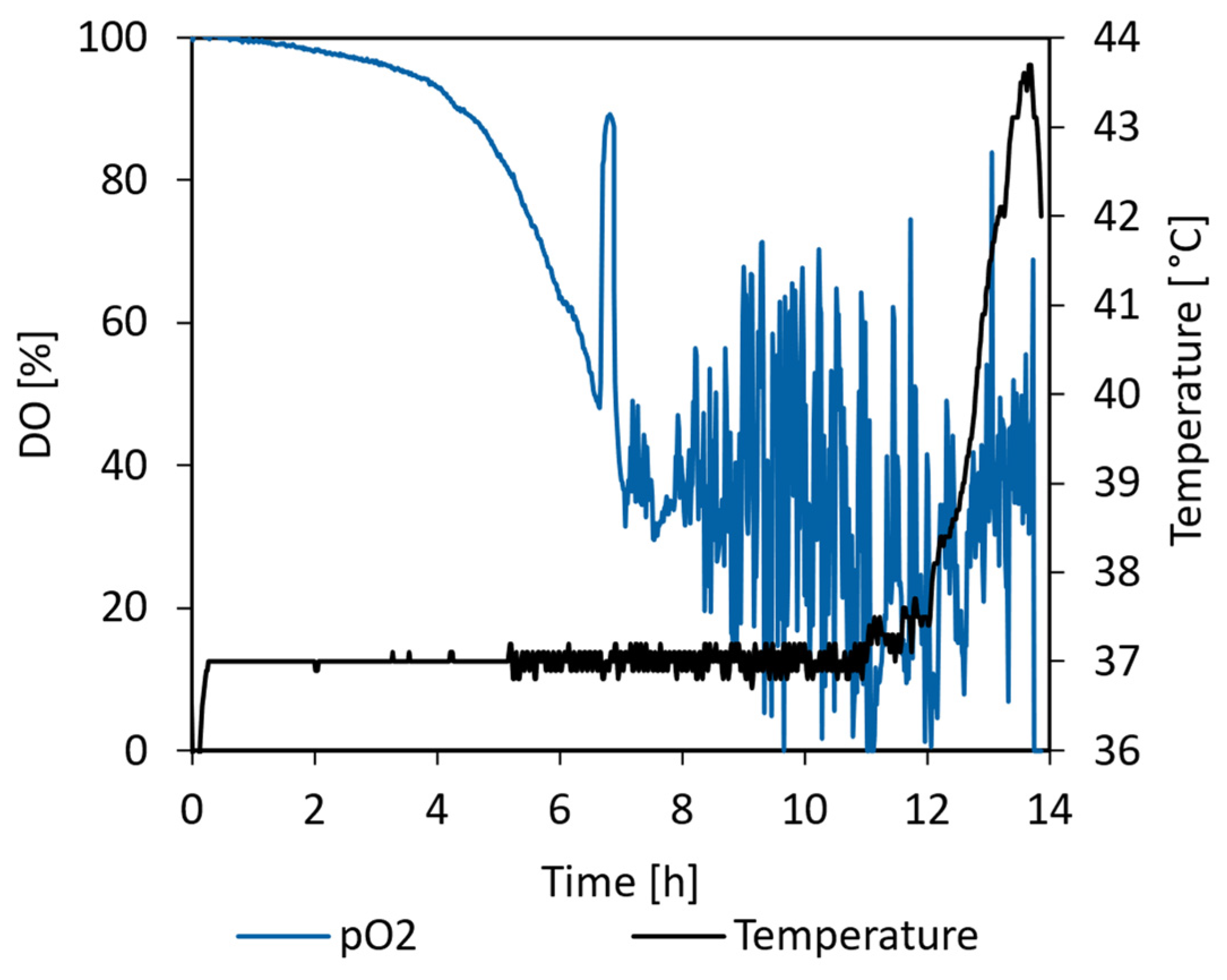
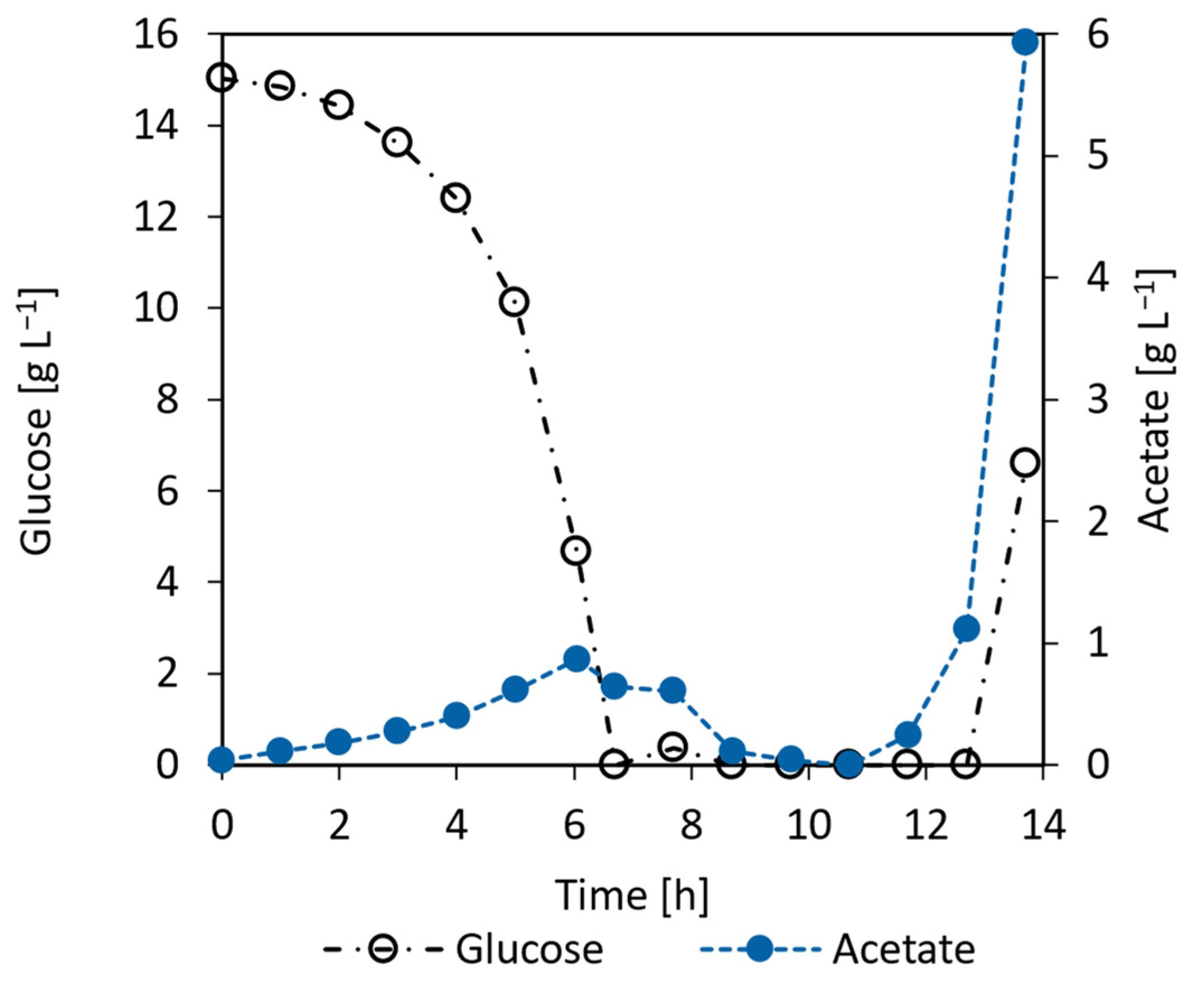
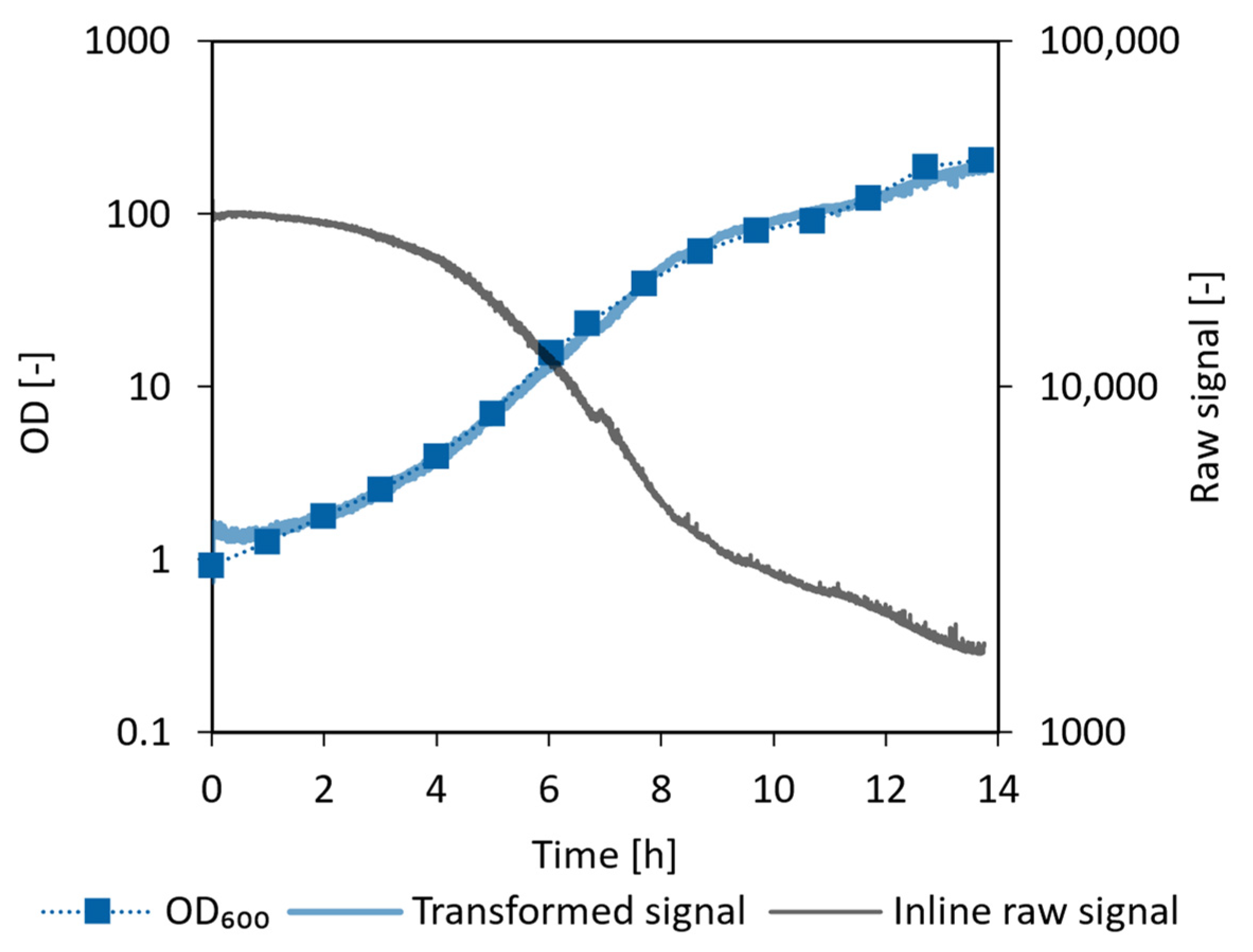
3.5.1. E. coli Proof of Concept Cultivation
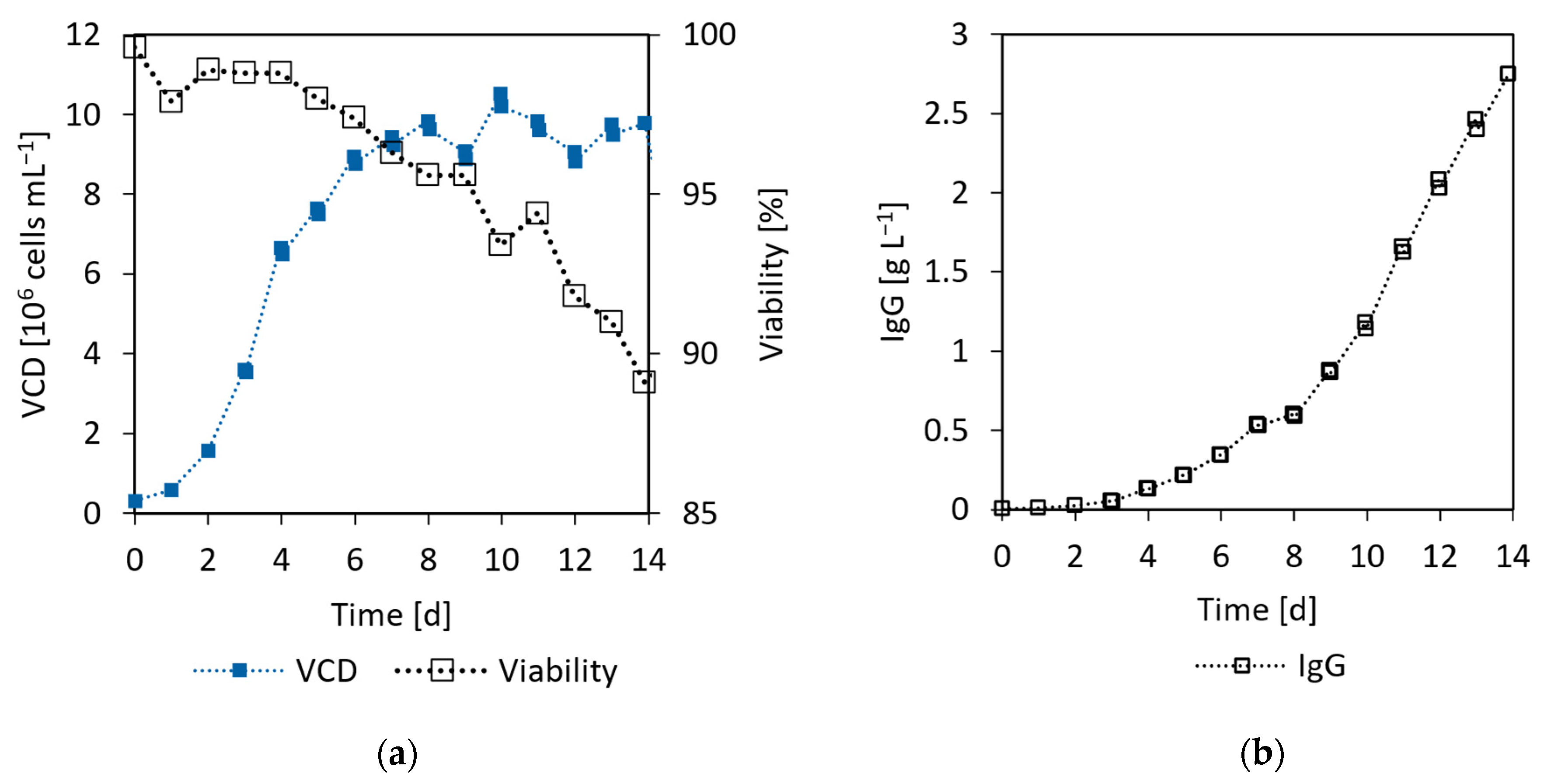
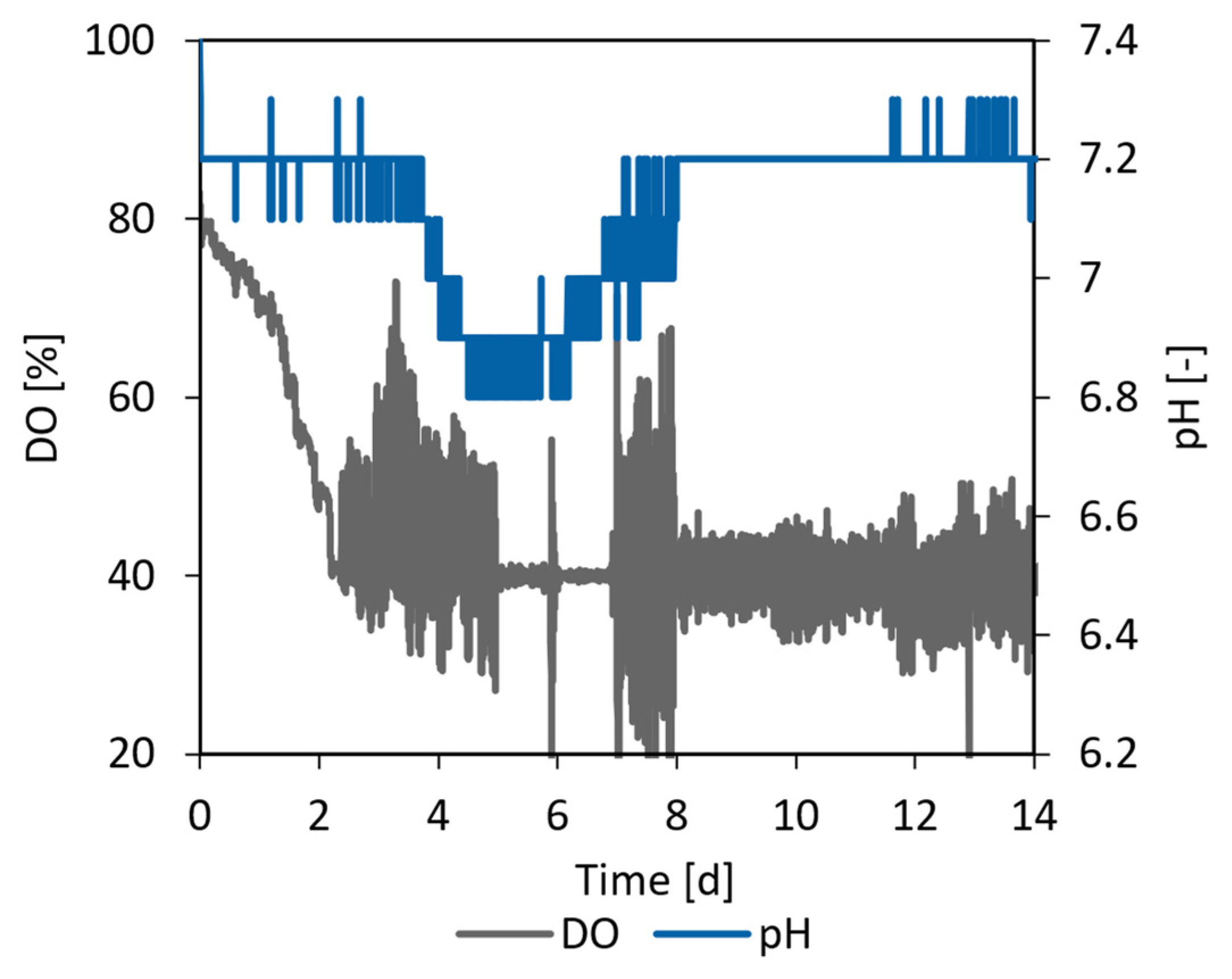
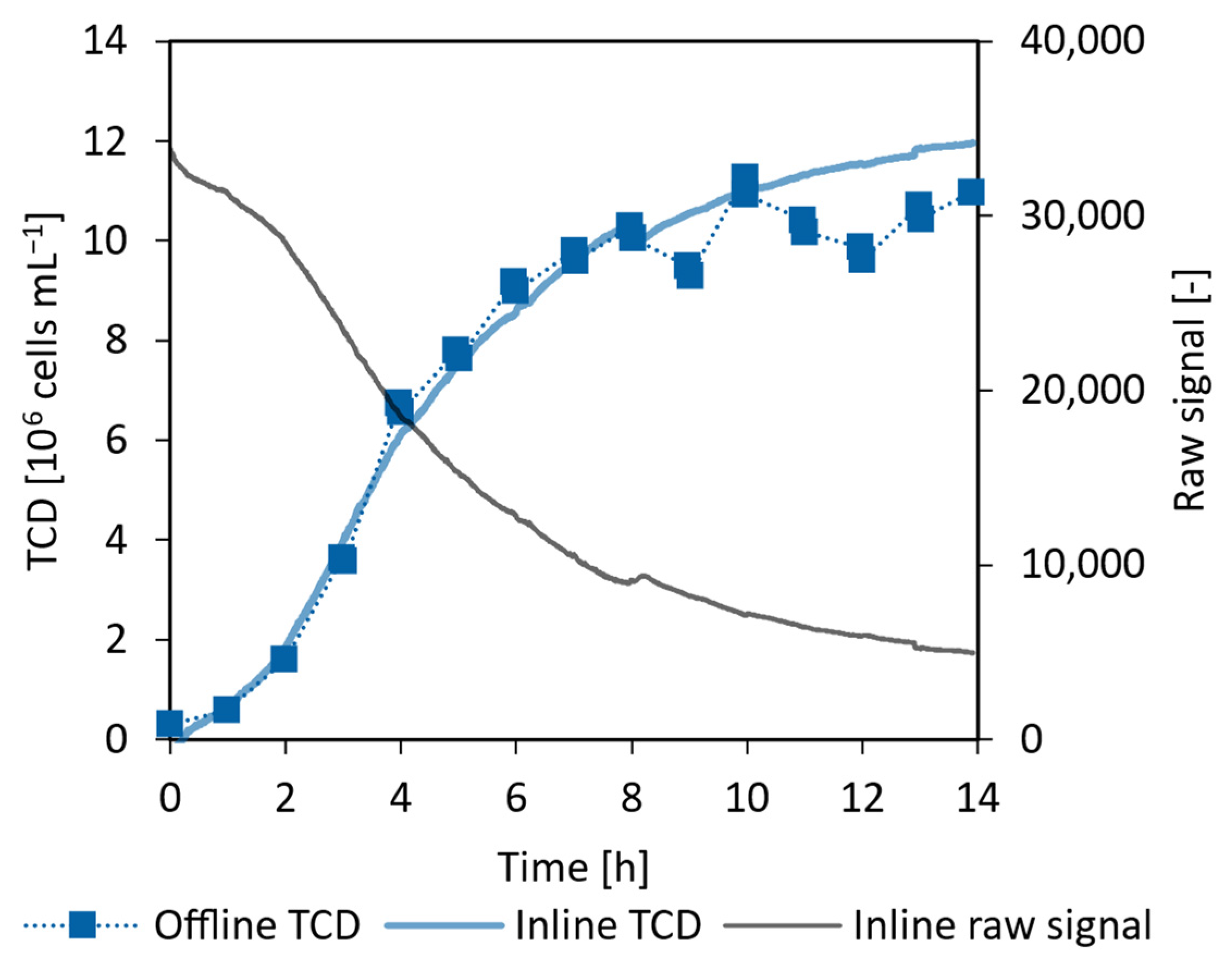
3.5.2. CHO Proof of Concept Cultivation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| APF | Arburg Plastics Freeforming |
| CDW | Cell dry weight |
| CFD | Computational fluid dynamics |
| CHO | Chinese hamster ovary |
| DO | Dissolved oxygen |
| EOM | Electro-optical module |
| IgG | Immunoglobulin G |
| SMMA | Styrene methyl methacrylate |
| SLS | Selective laser sintering |
| TCD | Total cell density |
| VCD | Viable cell density |
| WFI | Water for injection |
Nomenclature
| Latin symbols | ||
| a | Model parameter in Equations (9) and (10) | [-] |
| BMraw | Biomass sensor raw signal | [-] |
| b | Model parameter in Equations (9) and (10) | [-] |
| c | Model parameter in Equations (9) and (10) | [-] |
| c | Concentration | [g L−1] |
| Conversion factor | [g L−1] | |
| F | Feed rate | [g h−1] |
| Surface tension force | [N] | |
| Gravitational acceleration | [m s−2] | |
| I | Electric current | [A] |
| kLa | Volumetric oxygen mass transfer coefficient | [h−1] |
| Kt | Motor constant | [N cm A−1] |
| M | Moment | [N m] |
| n | Stirring speed | [rpm] |
| P/V | Specific power input | [W m−3] |
| Ra | Average surface roughness | [µm] |
| t | Time | [h] |
| Velocity | [m s−1] | |
| Tip speed | [m s−1] | |
| V | Volume | [m3] |
| w | Width | [mm] |
| Yx/s | Yield | [g g−1] |
| Greek symbols | ||
| Volume fraction | [-] | |
| θ | Mixing time | [s] |
| Local interface curvature | [m−1] | |
| µ | Specific growth rate | [h−1] |
| Kinematic viscosity | [m2 s−1] | |
| Density | [kg m−3] | |
| Surface tension | [N m−1] | |
| Χ | Physical properties | [-] |
References
- Sandner, V.; Pybus, L.P.; McCreath, G.; Glassey, J. Scale-Down Model Development in Ambr Systems: An Industrial Perspective. Biotechnol. J. 2019, 14, 1700766. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Clark, C.; Ryder, T.; Sparks, C.; Zhou, J.; Wang, M.; Russell, R.; Scott, C. Characterization of TAP Ambr 250 Disposable Bioreactors, as a Reliable Scale-down Model for Biologics Process Development. Biotechnol. Prog. 2017, 33, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-T.; Aulakh, R.P.S.; Traul, D.L.; Yuk, I.H. Advanced Microscale Bioreactor System: A Representative Scale-down Model for Bench-Top Bioreactors. Cytotechnology 2012, 64, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Moroney, J.; Hoshan, L.; Jiang, R.; Xu, S. Systematic Evaluation of High-Throughput Scale-down Models for Single-Use Bioreactors (SUB) Using Volumetric Gas Flow Rate as the Criterion. Biochem. Eng. J. 2019, 151, 107307. [Google Scholar] [CrossRef]
- Junne, S.; Neubauer, P. How Scalable and Suitable Are Single-Use Bioreactors? Curr. Opin. Biotechnol. 2018, 53, 240–247. [Google Scholar] [CrossRef]
- Oosterhuis, N.M.G.; Junne, S. Design, Applications, and Development of Single-Use Bioreactors. In Bioreactors; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 261–294. ISBN 978-3-527-68336-9. [Google Scholar]
- Nogueira, D.E.S.; Cabral, J.M.S.; Rodrigues, C.A.V. Single-Use Bioreactors for Human Pluripotent and Adult Stem Cells: Towards Regenerative Medicine Applications. Bioengineering 2021, 8, 68. [Google Scholar] [CrossRef]
- Kaiser, S.C. An Approach for Rapid Manufacture and Qualification of a Single-Use Bioreactor Prototype. In Single-Use Technology in Biopharmaceutical Manufacture; Eibl, R., Eibl, D., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 235–246. ISBN 978-1-119-47783-9. [Google Scholar]
- Priyadarshini, B.M.; Dikshit, V.; Zhang, Y. 3D-Printed Bioreactors for In Vitro Modeling and Analysis. IJB 2020, 6, 267. [Google Scholar] [CrossRef]
- Sharma, R.; Harrison, S.T.L.; Tai, S.L. Advances in Bioreactor Systems for the Production of Biologicals in Mammalian Cells. ChemBioEng Rev. 2022, 9, 42–62. [Google Scholar] [CrossRef]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2008–2012). Anal. Chem. 2013, 85, 487–508. [Google Scholar] [CrossRef]
- Beal, J.; Farny, N.G.; Haddock-Angelli, T.; Selvarajah, V.; Baldwin, G.S.; Buckley-Taylor, R.; Gershater, M.; Kiga, D.; Marken, J.; Sanchania, V.; et al. Robust Estimation of Bacterial Cell Count from Optical Density. Commun. Biol. 2020, 3, 512. [Google Scholar] [CrossRef]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General Calibration of Microbial Growth in Microplate Readers. Sci. Rep. 2016, 6, 38828. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.; Lucy, C.A. Quantitative Chemical Analysis, 9th ed.; W.H. Freeman & Company: New York, NY, USA, 2016; ISBN 978-1-4641-3538-5. [Google Scholar]
- AARBURG Gmbh Whitepaper: Droplets to the Beat of Milliseconds, Arburg Plastic Freeforming: Current Investigations on Part Optimization. Kunststoffe Int. 2018, 11, 15–21.
- Volpato, N.; Kretschek, D.; Foggiatto, J.A.; Gomez Da Silva Cruz, C.M. Experimental Analysis of an Extrusion System for Additive Manufacturing Based on Polymer Pellets. Int. J. Adv. Manuf. Technol. 2015, 81, 1519–1531. [Google Scholar] [CrossRef]
- Rehfeld, J.S.; Kuhnke, L.M.; Ude, C.; John, G.T.; Beutel, S. Investigation and Evaluation of a 3D-printed Optical Modified Cultivation Vessel for Improved Scattered Light Measurement of Biotechnologically Relevant Organisms. Eng. Life Sci. 2023, 23, e2300204. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.; Nussbaumer, T.; Kolar, J.W. Bearingless 300-W PMSM for Bioreactor Mixing. IEEE Trans. Ind. Electron. 2011, 59, 1376–1388. [Google Scholar] [CrossRef]
- Zhong, J.-J. Bioreactor Engineering. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 165–177. ISBN 978-0-08-088504-9. [Google Scholar]
- Hammond, M.; Nunn, H.; Rogers, G.; Lee, H.; Marghitoiu, A.-L.; Perez, L.; Nashed-Samuel, Y.; Anderson, C.; Vandiver, M.; Kline, S. Identification of a Leachable Compound Detrimental to Cell Growth in Single-Use Bioprocess Containers. PDA J. Pharm. Sci. Technol. 2013, 67, 123–134. [Google Scholar] [CrossRef]
- INEOS Styrolution NAS 30 Styrene Methyl Methacrylate Technical Datasheet. 2016. Available online: https://www.ineos-styrolution.com/Product/NAS_NAS-30_SKU401000260276.html (accessed on 28 April 2023).
- Eibl, R.; Steiger, N.; Fritz, C.; Eisenkrätzer, D.; Bär, J.; Müller, D.; Eibl, D. Recommendation for Leachables Studies-Standardized Cell Culture Test for the Early Identification of Critical Films, 1st ed.; DECHEMA: Frankfurt am Main, Germany, 2014; ISBN 978-3-89746-149-9. [Google Scholar]
- Hirt, C.W.; Nichols, B.D. Volume of Fluid (VOF) Method for the Dynamics of Free Boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Menter, F. Zonal Two Equation K-w Turbulence Models for Aerodynamic Flows. In Proceedings of the 23rd Fluid Dynamics, Plasmadynamics, and Lasers Conference, Orlando, FL, USA, 6 July 1993; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1993. [Google Scholar]
- Roache, P.J. Perspective: A Method for Uniform Reporting of Grid Refinement Studies. J. Fluids Eng. 1994, 116, 405–413. [Google Scholar] [CrossRef]
- Bauer, I.; Dreher, T.; Eibl, D.; Glöckler, R.; Husemann, U.; John, G.T.; Kaiser, S.C.; Kampeis, P.; Kauling, J. Recommendations for Process Engineering Characterisation of Single-Use Bioreactors and Mixing Systems by Using Experimental Methods; DECHEMA: Frankfurt am Main, Germany, 2020; ISBN 978-3-89746-227-4. [Google Scholar]
- Schirmer, C.; Nussbaumer, T.; Schöb, R.; Pörtner, R.; Eibl, R.; Eibl, D. Development, Engineering and Biological Characterization of Stirred Tank Bioreactors. In Biopharmaceuticals; Yeh, M.-K., Chen, Y.-C., Eds.; InTech: London, UK, 2018; ISBN 978-1-78923-718-4. [Google Scholar]
- Schirmer, C.; Dreher, T.; Leupold, M.; Glaser, R.; Castan, A.; Brown, J.; Eibl, D.; Glöckler, R. Recommendation for Biological Evaluation of Bioreactor Performance for Microbial Processes; DECHEMA Biotechnologie: Frankfurt am Main, Germany, 2019; ISBN 978-3-89746-217-5. [Google Scholar]
- Biener, R.; Steinkämper, A.; Hofmann, J. Calorimetric Control for High Cell Density Cultivation of a Recombinant Escherichia Coli Strain. J. Biotechnol. 2010, 146, 45–53. [Google Scholar] [CrossRef]
- Xu, S.; Hoshan, L.; Jiang, R.; Gupta, B.; Brodean, E.; O’Neill, K.; Seamans, T.C.; Bowers, J.; Chen, H. A Practical Approach in Bioreactor Scale-up and Process Transfer Using a Combination of Constant P / V and Vvm as the Criterion. Biotechnol. Prog. 2017, 33, 1146–1159. [Google Scholar] [CrossRef]
- Ali, S.; Perez-Pardo, M.A.; Aucamp, J.P.; Craig, A.; Bracewell, D.G.; Baganz, F. Characterization and Feasibility of a Miniaturized Stirred Tank Bioreactor to Perform E. Coli High Cell Density Fed-Batch Fermentations. Biotechnol. Prog. 2012, 28, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Knabben, I.; Regestein, L.; Marquering, F.; Steinbusch, S.; Lara, A.R.; Büchs, J. High Cell-Density Processes in Batch Mode of a Genetically Engineered Escherichia Coli Strain with Minimized Overflow Metabolism Using a Pressurized Bioreactor. J. Biotechnol. 2010, 150, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, S.; Zahnow, C.; Dreher, T.; Bargh, N.; Husemann, U.; Greller, G. Superior Scalability of a Single-Use Bioreactor Family from 0.25 to 2000 L. Sartorius 2017. Available online: https://www.sartorius.com/download/921544/ambr-biostat-superior-scalability-poster-en-sartorius-data.pdf (accessed on 28 April 2023).
- Nienow, A.W. Reactor Engineering in Large Scale Animal Cell Culture. Cytotechnology 2006, 50, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Ott, V.; Weiss, N.; Neubauer, P.; Eibl, D.; Eibl, R. Process Intensification Using a One-Step Inoculum Production and High-Seeded Fed-Batch Processes. Chem. Ing. Tech. 2022, 94, 1977–1984. [Google Scholar] [CrossRef]







| Symbol | Value | Meaning |
|---|---|---|
| Time dependent feed rate in g h−1 | ||
| 0.4 h−1 | Target growth rate | |
| 595 g L−1 | Feed glucose concentration | |
| 1.22 g mL−1 | Feed density | |
| 0.42 g g−1 | Yield CDW/glucose | |
| 23–29 | OD600 at start of feed phase | |
| 0.33 g L−1 | Conversion factor | |
| ≈55 mL | Bioreactor volume at feed start in mL | |
| 5.2–6.8 h | Process duration at feed start |
| Symbol | Value | Meaning |
|---|---|---|
| Inline OD measurement | ||
| Raw signal of biomass sensor | ||
| −5.22 | Fit parameter a | |
| 2.01 × 105 | Fit parameter b | |
| 2.20 × 108 | Fit parameter c |
| Symbol | Value | Meaning |
|---|---|---|
| Inline TCD measurement | ||
| Raw signal of biomass sensor | ||
| 7.09 × 10−2 | Fit parameter a | |
| −4.31 × 10−4 | Fit parameter b | |
| 1.25 × 10−19 | Fit parameter c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, S.L.; Seidel, S.; Seefeldt, A.; Romang, M.; Kastl, S.; Gensel, J.; Neumeyer, T.; John, G.T.; Eibl, D. 3D Printed, Single-Use Bioreactor with Integrated Inline Sensors for Microbial and Mammalian Cell Cultivation—A Case Study. Processes 2023, 11, 3231. https://doi.org/10.3390/pr11113231
Schneider SL, Seidel S, Seefeldt A, Romang M, Kastl S, Gensel J, Neumeyer T, John GT, Eibl D. 3D Printed, Single-Use Bioreactor with Integrated Inline Sensors for Microbial and Mammalian Cell Cultivation—A Case Study. Processes. 2023; 11(11):3231. https://doi.org/10.3390/pr11113231
Chicago/Turabian StyleSchneider, Samuel Lukas, Stefan Seidel, Andressa Seefeldt, Michael Romang, Simon Kastl, Julia Gensel, Thomas Neumeyer, Gernot Thomas John, and Dieter Eibl. 2023. "3D Printed, Single-Use Bioreactor with Integrated Inline Sensors for Microbial and Mammalian Cell Cultivation—A Case Study" Processes 11, no. 11: 3231. https://doi.org/10.3390/pr11113231
APA StyleSchneider, S. L., Seidel, S., Seefeldt, A., Romang, M., Kastl, S., Gensel, J., Neumeyer, T., John, G. T., & Eibl, D. (2023). 3D Printed, Single-Use Bioreactor with Integrated Inline Sensors for Microbial and Mammalian Cell Cultivation—A Case Study. Processes, 11(11), 3231. https://doi.org/10.3390/pr11113231








