Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources
Abstract
:1. Introduction
2. Ascorbic Acid Applications
2.1. AA as Dietary Supplement
2.2. AA in Cosmetics
2.3. AA Industrial Applications
3. Ascorbic Acid Extraction
3.1. Pretreatments
3.2. Conventional Solvent Extraction
3.3. Ultrasound-Assisted Extraction
3.4. Microwave-Assisted Extraction
3.5. Pressurized Liquid Extraction
3.6. Supercritical Fluid Extraction
3.7. Other Extraction Methods
3.8. Purification of AA from Organic Solvents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182. [Google Scholar]
- Iqbal, K.; Khan, A.; Khattak, M. Biological significance of ascorbic acid (vitamin C) in human health–A review. Pak. J. Nutr. 2004, 3, 5–13. [Google Scholar]
- Mazurek, A.; Włodarczyk-Stasiak, M. A New Method for the Determination of Total Content of Vitamin C, Ascorbic and Dehydroascorbic Acid, in Food Products with the Voltammetric Technique with the Use of Tris(2-carboxyethyl)phosphine as a Reducing Reagent. Molecules 2023, 28, 812. [Google Scholar]
- Tucaliuc, A.; Cîșlaru, A.; Kloetzer, L.; Blaga, A.C. Strain Development, Substrate Utilization, and Downstream Purification of Vitamin C. Processes 2022, 10, 1595. [Google Scholar]
- Lim, S.M.; Lau, M.S.L.; Tiong, E.I.J.; Goon, M.M.; Lau, R.J.C.; Yeo, W.S.; Lau, S.Y.; Mubarak, N.M. Process design and economic studies of two-step fermentation for production of ascorbic acid. SN Appl. Sci. 2020, 2, 816. [Google Scholar] [CrossRef]
- Yang, W.; Xu, H. Industrial Fermentation of Vitamin C. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Wiley: Hoboken, NJ, USA, 2016; pp. 161–192. [Google Scholar] [CrossRef]
- Zhou, M.; Bi, Y.; Ding, M.; Yuan, Y. One-Step Biosynthesis of Vitamin C in Saccharomyces cerevisiae. Front. Microbiol. 2021, 12, 643472. [Google Scholar] [CrossRef]
- Wang, P.; Zeng, W.; Xu, S.; Du, G.; Zhou, J.; Chen, J. Current challenges facing one-step production of l-ascorbic acid. Biotechnol. Adv. 2018, 36, 1882–1899. [Google Scholar] [CrossRef] [PubMed]
- Pappenberger, G.; Hohmann, H.-P. Industrial Production of l-Ascorbic Acid (Vitamin C) and d-Isoascorbic Acid. In Biotechnology of Food and Feed Additives; Zorn, H., Czermak, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–188. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Cotruţ, R.; Bădulescu, L. UPLC Rapid Quantification of Ascorbic Acid in Several Fruits and Vegetables Extracted Using Different Solvents. Agric. Agric. Sci. Procedia 2016, 10, 160–166. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. Factors Affecting Vitamin C Status and Prevalence of Deficiency: A Global Health Perspective. Nutrients 2020, 12, 1963. [Google Scholar] [PubMed]
- FAO Joint; World Health Organization. Human Vitamin and Mineral Requirements; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Rivas, M.Á.; Casquete, R.; Martín, A.; Córdoba, M.d.G.; Aranda, E.; Benito, M.J. Strategies to Increase the Biological and Biotechnological Value of Polysaccharides from Agricultural Waste for Application in Healthy Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 5937. [Google Scholar] [CrossRef] [PubMed]
- Selahvarzi, A.; Ramezan, Y.; Sanjabi, M.R.; Namdar, B.; Akbarmivehie, M.; Mirsaeedghazi, H.; Azarikia, F. Optimization of ultrasonic-assisted extraction of phenolic compounds from pomegranate and orange peels and their antioxidant activity in a functional drink. Food Biosci. 2022, 49, 101918. [Google Scholar] [CrossRef]
- Abdurrahman Isa, A.; Samsuri, S.; Aini Amran, N. Integration of Maceration and Freeze Concentration for Recovery of Vitamin C from Orange Peel Waste. IOP Conf. Ser. Earth Environ. Sci. 2019, 268, 012101. [Google Scholar] [CrossRef]
- Arora, M.; Kaur, P. Phytochemical screening of orange peel and pulp. Int. J. Res. Eng. Technol. 2013, 2, 517–522. [Google Scholar]
- Roussos, P.A. Phytochemicals and antioxidant capacity of orange (Citrus sinensis (L.) Osbeck cv. Salustiana) juice produced under organic and integrated farming system in Greece. Sci. Hortic. 2011, 129, 253–258. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Sharma, K.; Akansha, C.E. Comparative studies of proximate, mineral and phytochemical compositions of pomegranate (Punica granatum) in peel, seed and whole fruit powder. Methods 2018, 17, 18. [Google Scholar]
- Marra, F.; Petrovicova, B.; Canino, F.; Maffia, A.; Mallamaci, C.; Muscolo, A. Pomegranate Wastes Are Rich in Bioactive Compounds with Potential Benefit on Human Health. Molecules 2022, 27, 5555. [Google Scholar]
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martínez, J.; Godoy, H.T. Vitamin C in camu-camu [Myrciaria dubia (H.B.K.) McVaugh]: Evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Mittu, B.; Bhat, Z.R.; Chauhan, A.; Kour, J.; Behera, A.; Kaur, M. Chapter 16—Ascorbic acid. In Nutraceuticals and Health Car; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 289–302. [Google Scholar] [CrossRef]
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12, 2008. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef]
- Cerullo, G.; Negro, M.; Parimbelli, M.; Pecoraro, M.; Perna, S.; Liguori, G.; Rondanelli, M.; Cena, H.; D’Antona, G. The Long History of Vitamin C: From Prevention of the Common Cold to Potential Aid in the Treatment of COVID-19. Front. Immunol. 2020, 11, 574029. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Dresen, E.; Lee, Z.-Y.; Hill, A.; Notz, Q.; Patel, J.J.; Stoppe, C. History of scurvy and use of vitamin C in critical illness: A narrative review. Nutr. Clin. Pract. 2023, 38, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Granger, M.; Eck, P. Chapter Seven—Dietary Vitamin C in Human Health. In Advances in Food and Nutrition Research; Eskin, N.A.M., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 83, pp. 281–310. [Google Scholar]
- Corpe, C.P.; Eck, P.; Wang, J.; Al-Hasani, H.; Levine, M. Intestinal Dehydroascorbic Acid (DHA) Transport Mediated by the Facilitative Sugar Transporters, GLUT2 and GLUT8. J. Biol. Chem. 2013, 288, 9092–9101. [Google Scholar] [CrossRef]
- Frikke-Schmidt, H.; Tveden-Nyborg, P.; Lykkesfeldt, J. l-dehydroascorbic acid can substitute l-ascorbic acid as dietary vitamin C source in guinea pigs. Redox Biol. 2016, 7, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bürzle, M.; Suzuki, Y.; Ackermann, D.; Miyazaki, H.; Maeda, N.; Clémençon, B.; Burrier, R.; Hediger, M.A. The sodium-dependent ascorbic acid transporter family SLC23. Mol. Asp. Med. 2013, 34, 436–454. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two Faces of Vitamin C—Antioxidative and Pro-Oxidative Agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.-J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Baron, J.H. Sailors’ scurvy before and after James Lind–A reassessment. Nutr. Rev. 2009, 67, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Ceglie, G.; Macchiarulo, G.; Marchili, M.R.; Marchesi, A.; Rotondi Aufiero, L.; Di Camillo, C.; Villani, A. Scurvy: Still a threat in the well-fed first world? Arch. Dis. Child. 2019, 104, 381. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral. Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar]
- Ivanova, I.P.; Trofimova, S.V.; Piskarev, I.M. Evaluation of prooxidant properties of ascorbic acid. Biophysics 2013, 58, 453–456. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Abobaker, A.; Alzwi, A.; Alraied, A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 2020, 72, 1517–1528. [Google Scholar] [CrossRef]
- Carr, A.C.; Rowe, S. The Emerging Role of Vitamin C in the Prevention and Treatment of COVID-19. Nutrients 2020, 12, 3286. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in correspondence to Its Dietary and Pharmacological Concentrations. Oxidative Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef] [PubMed]
- Van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef]
- Villagran, M.; Ferreira, J.; Martorell, M.; Mardones, L. The Role of Vitamin C in Cancer Prevention and Therapy: A Literature Review. Antioxidants 2021, 10, 1894. [Google Scholar] [CrossRef]
- Shenoy, N.; Creagan, E.; Witzig, T.; Levine, M. Ascorbic Acid in Cancer Treatment: Let the Phoenix Fly. Cancer Cell 2018, 34, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination Therapy with Vitamin C Could Eradicate Cancer Stem Cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Bedhiafi, T.; Inchakalody, V.P.; Fernandes, Q.; Mestiri, S.; Billa, N.; Uddin, S.; Merhi, M.; Dermime, S. The potential role of vitamin C in empowering cancer immunotherapy. Biomed. Pharmacother. 2022, 146, 112553. [Google Scholar] [CrossRef]
- Kuhn, S.-O.; Meissner, K.; Mayes, L.M.; Bartels, K. Vitamin C in sepsis. Curr. Opin. Anesthesiol. 2018, 31, 55. [Google Scholar] [CrossRef]
- Kashiouris, M.G.; L’Heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A., III; Yager, D.R.; Natarajan, R. Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Moores, J. Vitamin C: A wound healing perspective. Br. J. Community Nurs. 2013, 18, S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of vitamin C in skin diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Shibuya, S.; Sakaguchi, I.; Ito, S.; Kato, E.; Watanabe, K.; Izuo, N.; Shimizu, T. Topical Application of Trisodium Ascorbyl 6-Palmitate 2-Phosphate Actively Supplies Ascorbate to Skin Cells in an Ascorbate Transporter-Independent Manner. Nutrients 2017, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Jacques, C.; Genies, C.; Bacqueville, D.; Tourette, A.; Borotra, N.; Chaves, F.; Sanches, F.; Gaudry, A.L.; Bessou-Touya, S.; Duplan, H. Ascorbic acid 2-glucoside: An ascorbic acid pro-drug with longer-term antioxidant efficacy in skin. Int. J. Cosmet. Sci. 2021, 43, 691–702. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The Use of Ascorbic Acid as a Food Additive: Technical-Legal Issues. Ital. J. Food Saf. 2016, 5, 4313. [Google Scholar] [CrossRef]
- Final Report of the Safety Assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as Used in Cosmetics1. Int. J. Toxicol. 2005, 24, 51–111. [CrossRef]
- Hieu, T.V.; Guntoro, B.; Qui, N.H.; Quyen, N.T.K.; Hafiz, F.A.A. The application of ascorbic acid as a therapeutic feed additive to boost immunity and antioxidant activity of poultry in heat stress environment. Vet. World 2022, 15, 685–693. [Google Scholar] [CrossRef]
- Hassan, F.A.; Shalaby, A.G.; Elkassas, N.E.M.; El-Medany, S.A.; Hamdi Rabie, A.; Mahrose, K.; Abd El-Aziz, A.; Bassiony, S. Efficacy of ascorbic acid and different sources of orange peel on growth performance, gene expression, anti-oxidant status and microbial activity of growing rabbits under hot conditions. Anim. Biotechnol. 2023, 34, 2480–2491. [Google Scholar] [CrossRef]
- Ostrenko, K.; Nekrasov, R.; Ovcharova, A.; Lemiasheuski, V.; Kutin, I. The Effect of Lithium Salt with Ascorbic Acid on the Antioxidant Status and Productivity of Gestating Sows. Animals 2022, 12, 915. [Google Scholar] [CrossRef]
- Cao, F.; Tian, W.; Wang, M.; Wang, M.; Li, L. Stability enhancement of lead-free CsSnI3 perovskite photodetector with reductive ascorbic acid additive. InfoMat 2020, 2, 577–584. [Google Scholar] [CrossRef]
- Cai, Z.; Li, H.; Wu, J.; Zhu, L.; Ma, X.; Zhang, C. Ascorbic acid stabilised copper nanoclusters as fluorescent sensors for detection of quercetin. RSC Adv. 2020, 10, 8989–8993. [Google Scholar] [CrossRef]
- Sun, K.; Qiu, J.; Liu, J.; Miao, Y. Preparation and characterization of gold nanoparticles using ascorbic acid as reducing agent in reverse micelles. J. Mater. Sci. 2009, 44, 754–758. [Google Scholar] [CrossRef]
- Palomba, M.; Carotenuto, G.; Longo, A. A Brief Review: The Use of L-Ascorbic Acid as a Green Reducing Agent of Graphene Oxide. Materials 2022, 15, 6456. [Google Scholar] [CrossRef] [PubMed]
- Lie, J.; Liu, J.-C. Closed-vessel microwave leaching of valuable metals from spent lithium-ion batteries (LIBs) using dual-function leaching agent: Ascorbic acid. Sep. Purif. Technol. 2021, 266, 118458. [Google Scholar] [CrossRef]
- Chen, D.; Rao, S.; Wang, D.; Cao, H.; Xie, W.; Liu, Z. Synergistic leaching of valuable metals from spent Li-ion batteries using sulfuric acid- l-ascorbic acid system. Chem. Eng. J. 2020, 388, 124321. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant Material for the Extraction of Bioactive Compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Irina, I.; Cédric, P.; Ghoul, M.; Boudhrioua, N. Antioxidants of Maltease orange peel: Comparative investigation of the efficiency of four extraction methods. J. Appl. Pharm. Sci. 2017, 7, 126–135. [Google Scholar]
- Karatas, F.; Kamışlı, F. Variations of vitamins (A, C and E) and MDA in apricots dried in IR and microwave. J. Food Eng. 2007, 78, 662–668. [Google Scholar] [CrossRef]
- Marques, L.G.; Silveira, A.M.; Freire, J.T. Freeze-drying characteristics of tropical fruits. Dry. Technol. 2006, 24, 457–463. [Google Scholar] [CrossRef]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef]
- Pongmalai, P.; Devahastin, S.; Chiewchan, N.; Soponronnarit, S. Enhancement of microwave-assisted extraction of bioactive compounds from cabbage outer leaves via the application of ultrasonic pretreatment. Sep. Purif. Technol. 2015, 144, 37–45. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Colnar, D.; Voća, S.; Lorenzo, J.M.; Munekata, P.E.S.; Barba, F.J.; Dobričević, N.; Galić, A.; Dujmić, F.; Pliestić, S.; et al. Effect of ultrasound pre-treatment and drying method on specialized metabolites of honeyberry fruits (Lonicera caerulea var. kamtschatica). Ultrason. Sonochem. 2019, 56, 372–377. [Google Scholar] [CrossRef]
- Fonteles, T.V.; Leite, A.K.F.; Silva, A.R.A.; Carneiro, A.P.G.; Miguel, E.d.C.; Cavada, B.S.; Fernandes, F.A.N.; Rodrigues, S. Ultrasound processing to enhance drying of cashew apple bagasse puree: Influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016, 31, 237–249. [Google Scholar] [CrossRef]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The effect of Pulsed Electric Field as a pre-treatment step in Ultrasound Assisted Extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for Extraction and Production of Bioactive Compounds to be Used as Nutraceuticals and Food Ingredients: An Overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Hassan, F.A.; Elkassas, N.; Salim, I.; El-Medany, S.; Aboelenin, S.M.; Shukry, M.; Taha, A.E.; Peris, S.; Soliman, M.; Mahrose, K. Impacts of Dietary Supplementations of Orange Peel and Tomato Pomace Extracts as Natural Sources for Ascorbic Acid on Growth Performance, Carcass Characteristics, Plasma Biochemicals and Antioxidant Status of Growing Rabbits. Animals 2021, 11, 1688. [Google Scholar] [CrossRef] [PubMed]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Suryanti, V.; Marliyana, S.; Putri, H. Effect of germination on antioxidant activity, total phenolics, β-carotene, ascorbic acid and α-tocopherol contents of lead tree sprouts (Leucaena leucocephala (lmk.) de Wit). Int. Food Res. J. 2016, 23, 167. [Google Scholar]
- Fracassetti, D.; Costa, C.; Moulay, L.; Tomás-Barberán, F.A. Ellagic acid derivatives, ellagitannins, proanthocyanidins and other phenolics, vitamin C and antioxidant capacity of two powder products from camu-camu fruit (Myrciaria dubia). Food Chem. 2013, 139, 578–588. [Google Scholar] [CrossRef]
- Czech, A.; Malik, A.; Sosnowska, B.; Domaradzki, P. Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. Int. J. Food Sci. 2021, 2021, 6662259. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Krishan, G.; Buecker, R.D. Process for the Manufacure of an Amla Composition. EPO Patent EP2044849A1, 27 September 2007. [Google Scholar]
- Fochesato, A.; Paiaro, E. Extraction Method of Mixtures Comprising Ascorbic Acid from a Dry Ground Vegetable Product. EP1889616A2, 20 February 2008. [Google Scholar]
- Yongjun, L. Extraction Method of Vitamin C in Kiwi Fruits, Vitamin C Obtained by Extraction Method and Application of Vitamin C. CN Patent CN112898249A, 4 June 2021. [Google Scholar]
- Weisi, Z.; Luteng, W.; Xinhong, L.; Xiujuan, X.; Huifan, L. Extraction Method of Vitamin C. CN Patent CN114149390A, 8 March 2022. [Google Scholar]
- Chuanyun, C.; Shengming, L.; Haibo, W. Extraction Method of Lemon Vitamin C. CN Patent CN107298663A, 27 October 2017. [Google Scholar]
- Lei, W.; Xiaole, X.; Tingting, J. Extraction Method of Acerola Cherry Vitamin C. CN Patent CN111349062A, 30 June 2020. [Google Scholar]
- Weiyun, M.; Zhihong, Z.; Lu, L.; Jikun, C.; Jinxiang, H.; Wangrong, G. Preparing Method of Sweet Persimmon Vitamin C. CN Patent CN106588839A, 26 April 2017. [Google Scholar]
- Yang, Y.; Qin, Y.; Fenfen, Z.; Wanliang, M.; Zhenxing, H. Method for Extracting Vitamin C from SWEET TEA. CN Patent CN104292196A, 21 January 2015. [Google Scholar]
- Heping, Z. Method for Extracting Vitamin C from Ginseng Fruits. CN Patent CN104544131A, 29 April 2015. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefining 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and Ultrasound-Assisted Extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef] [PubMed]
- Yuanyong, J.; Jicheng, W. Method for Extracting Vitamin C from Ephedra Fruits. CN Patent CN106432155A, 22 February 2017. [Google Scholar]
- Yuanyong, J.; Jicheng, W. Extraction Method of Pouteria Caimito. CN Patent CN106432154A, 22 February 2017. [Google Scholar]
- Baolei, F.; Zengyi, Q.; Guoliang, W.; Xiaofeng, G.; Sheng, X.; Tingting, L.; Mingsheng, L. Preparation Method of Vitamin C Granules in Rosa roxburghii. CN Patent CN110623951A, 31 December 2019. [Google Scholar]
- Ahmad, J.; Langrish, T.A.G. Optimisation of total phenolic acids extraction from mandarin peels using microwave energy: The importance of the Maillard reaction. J. Food Eng. 2012, 109, 162–174. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Plazzotta, S.; Ibarz, R.; Manzocco, L.; Martín-Belloso, O. Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Ultrason. Sonochem. 2020, 63, 104954. [Google Scholar] [CrossRef]
- Duan, G.; Li, Y.; Yu, Y.; Chen, B.; Duan, H. Method for Extracting Ascorbic Acid from Vegetables and Fruits. CN Patent CN102023191A, 20 April 2011. [Google Scholar]
- Yanying, S. Method for Extracting Vitamin C from Folium Rubi Suavissimi. CN Patent CN107056734A, 18 August 2017. [Google Scholar]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA-J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Elgndi, M.A.; Filip, S.; Pavlić, B.; Vladić, J.; Stanojković, T.; Žižak, Ž.; Zeković, Z. Antioxidative and cytotoxic activity of essential oils and extracts of Satureja montana L., Coriandrum sativum L. and Ocimum basilicum L. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2017, 128, 128–137. [Google Scholar] [CrossRef]
- Aresta, A.; Cotugno, P.; De Vietro, N.; Massari, F.; Zambonin, C. Determination of Polyphenols and Vitamins in Wine-Making by-Products by Supercritical Fluid Extraction (SFE). Anal. Lett. 2020, 53, 2585–2595. [Google Scholar] [CrossRef]
- King, J.W. Modern supercritical fluid technology for food applications. Annu. Rev. Food Sci. Technol. 2014, 5, 215–238. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef]
- Samsuri, S.; Amran, N.A.; Yahya, N.; Jusoh, M. Review on Progressive Freeze Concentration Designs. Chem. Eng. Commun. 2016, 203, 345–363. [Google Scholar] [CrossRef]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Microwave-assisted extraction of bioactive compounds from cashew apple (Anacardium occidenatale L.) bagasse: Modeling and optimization of the process using response surface methodology. J. Food Meas. Charact. 2021, 15, 4781–4793. [Google Scholar] [CrossRef]
- Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.J.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels. Molecules 2011, 16, 2218–2232. [Google Scholar] [CrossRef]
- Rajakaruna, A.; Manful, C.F.; Abu-Reidah, I.M.; Critch, A.L.; Vidal, N.P.; Pham, T.H.; Cheema, M.; Thomas, R. Application of solvent pH under pressurized conditions using accelerated solvent extraction and green solvents to extract phytonutrients from wild berries. Food Biosci. 2022, 47, 101471. [Google Scholar] [CrossRef]
- Stan, M.; Soran, M.L.; Marutoiu, C. Extraction and HPLC determination of the ascorbic acid content of three indigenous spice plants. J. Anal. Chem. 2014, 69, 998–1002. [Google Scholar] [CrossRef]
- Dakuyo, R.; Konaté, K.; Kaboré, K.; Sanou, A.; Konkobo, F.A.; Bazié, D.; Sama, H.; Dicko, M.H. Ascorbic acid, pigments, anti-nutritional factors, and nutraceutical potential of Anacardium occidentale fruits as affected by temperature. Int. J. Food Prop. 2023, 26, 471–488. [Google Scholar] [CrossRef]
- Anticona, M.; Blesa, J.; Lopez-Malo, D.; Frigola, A.; Esteve, M.J. Effects of ultrasound-assisted extraction on physicochemical properties, bioactive compounds, and antioxidant capacity for the valorization of hybrid Mandarin peels. Food Biosci. 2021, 42, 101185. [Google Scholar] [CrossRef]
- Um, M.; Han, T.-H.; Lee, J.-W. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci. Biotechnol. 2018, 27, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; da Costa, S.C.; Madrona, G.S. Camu-camu bioactive compounds extraction by ecofriendly sequential processes (ultrasound assisted extraction and reverse osmosis). Ultrason. Sonochem. 2020, 64, 105017. [Google Scholar] [CrossRef]
- Šic Žlabur, J.; Brajer, M.; Voća, S.; Galić, A.; Radman, S.; Rimac-Brnčić, S.; Xia, Q.; Zhu, Z.; Grimi, N.; Barba, F.J.; et al. Ultrasound as a Promising Tool for the Green Extraction of Specialized Metabolites from Some Culinary Spices. Molecules 2021, 26, 1866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hamauzu, Y. Phenolic compounds, ascorbic acid, carotenoids and antioxidant properties of green, red and yellow bell peppers. J. Food Agric. Env. 2003, 1, 22–27. [Google Scholar]
- Andarwulan, N.; Kurniasih, D.; Apriady, R.A.; Rahmat, H.; Roto, A.V.; Bolling, B.W. Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. J. Funct. Foods 2012, 4, 339–347. [Google Scholar] [CrossRef]
- Hassan, S.; Adam, F.; Abu Bakar, M.R.; Abdul Mudalip, S.K. Evaluation of solvents’ effect on solubility, intermolecular interaction energies and habit of ascorbic acid crystals. J. Saudi Chem. Soc. 2019, 23, 239–248. [Google Scholar] [CrossRef]
- Frenich, A.G.; Torres, M.E.H.; Vega, A.B.; Vidal, J.L.M.; Bolaños, P.P. Determination of Ascorbic Acid and Carotenoids in Food Commodities by Liquid Chromatography with Mass Spectrometry Detection. J. Agric. Food Chem. 2005, 53, 7371–7376. [Google Scholar] [CrossRef] [PubMed]
- Chebrolu, K.K.; Jayaprakasha, G.K.; Yoo, K.S.; Jifon, J.L.; Patil, B.S. An improved sample preparation method for quantification of ascorbic acid and dehydroascorbic acid by HPLC. LWT 2012, 47, 443–449. [Google Scholar] [CrossRef]
- Jankowski, C.; Poulin, G.; Dean, P.R. Extraction of L-Ascorbic acid from Plant Tissues. CN Patent CA1132586A, 28 September 1982. [Google Scholar]
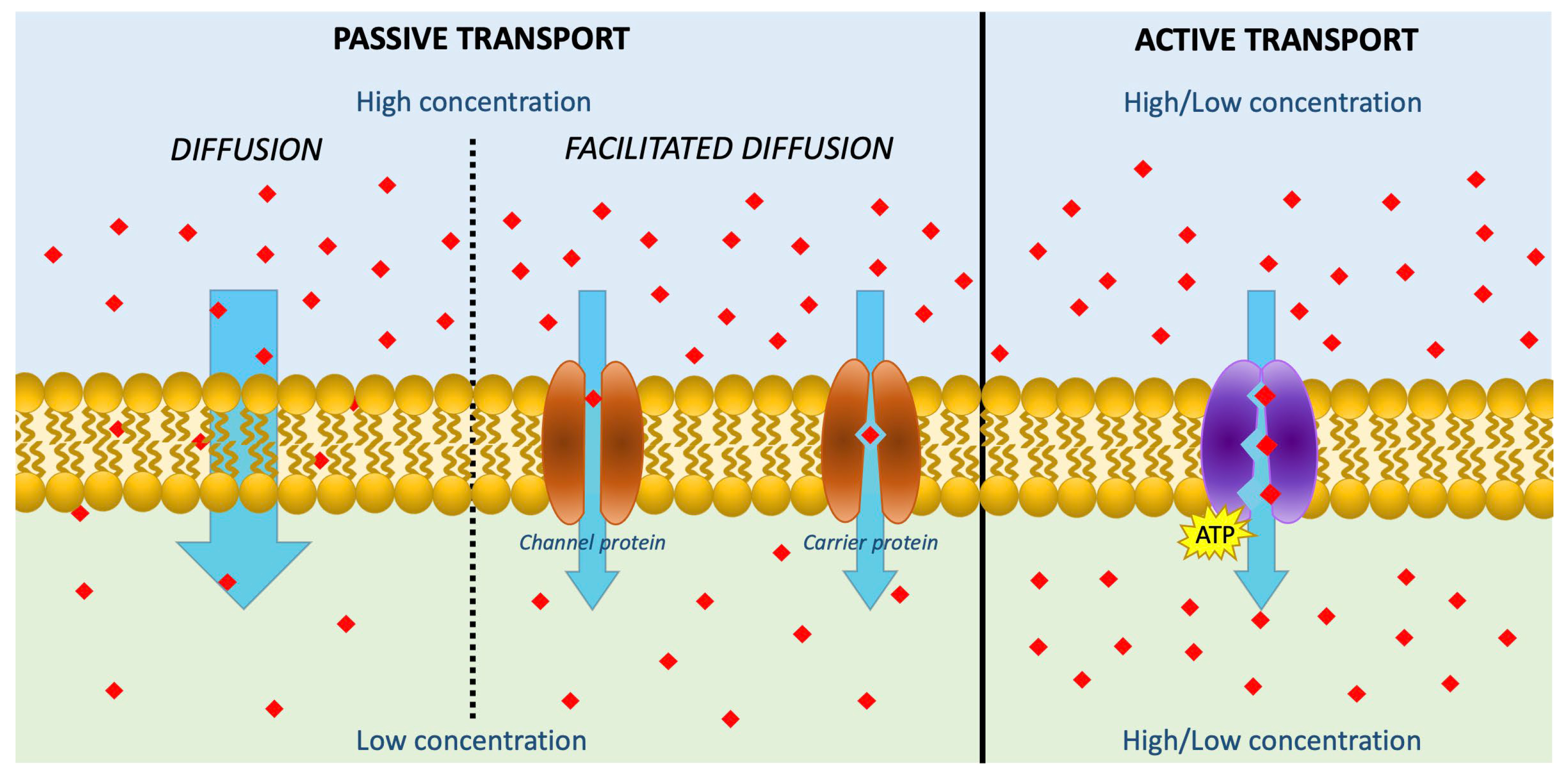
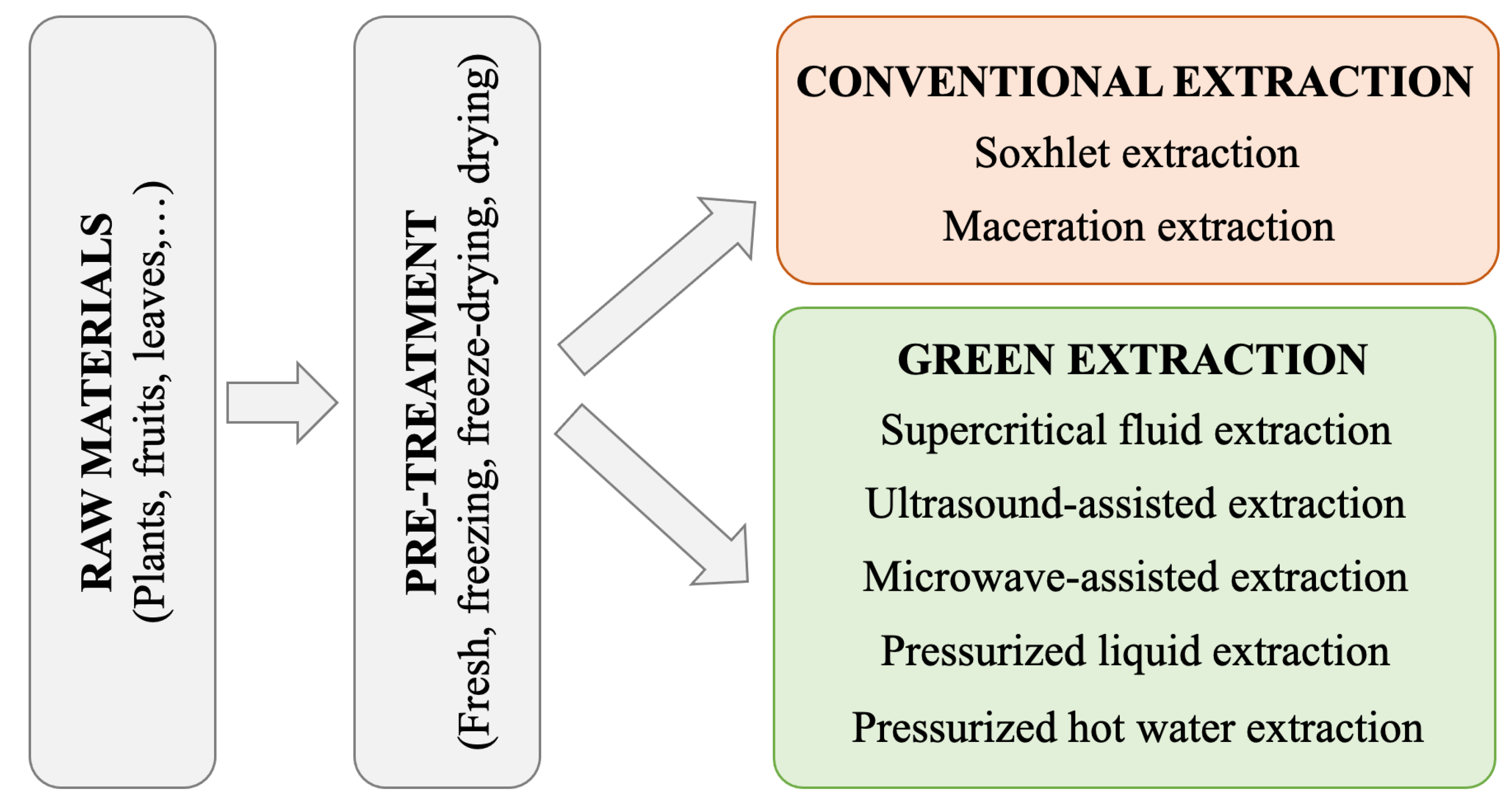
| Parameters | Source | Pretreatment | Yield (mg/100 g) | Ref. |
|---|---|---|---|---|
| 1:2 w/v in 95% ethanol for 24 h | Orange | Fresh | 59 | [88] |
| Tomato pomace | 14 | |||
| 1:25 in ethanol for 24 h | Grapefruit peels | Freeze-dried | 113 | [89] |
| Lemon peels | 59 | |||
| Orange peels | 110 | |||
| Acetic acid 1% | Oenanthe linearis | Shed-dried | 1539 | [86] |
| Sonchus arvensis | 1211 | |||
| Citric acid 3% | Orange | Frozen | 38 | [11] |
| Ethanol, 24 h, and 160 rpm | Lead tree sprouts | Freeze-dried after 3 days of germination | 180 | [90] |
| Methanol 5%, citric acid (21 g/L) and EDTA (0.5 g/L) | Camu-camu | Spray-dried | 3510 | [91] |
| Camu-camu seeds and peels | Fluid-bed dried | 9040 | ||
| Metaphosphoric acid-acetic acid | Orange | Freeze-dried | 50 | [92] |
| Pomelo | 72 | |||
| Mandarin | 42 | |||
| Lemon | 59 | |||
| Lime | 41 | |||
| Red grapefruit peel | 34 | |||
| Green grapefruit peel | 67 | |||
| White grapefruit peel | 44 | |||
| A mixture of methanol, ethanol, acetone, and water | Pomegranate peels | Fresh | 99 | [93] |
| Pomegranate pulp | 85 |
| Patent Number | Method Description | Ref. |
|---|---|---|
| EP2044849A1 | Amla compounds were extracted using ethanol, and the extract was enriched by different additions of amla juice followed by vacuum concentration | [94] |
| EP1889616A2 | AA was extracted from dog rose, acerola, and camu-camu using an aqueous solution with ethanol and a dissolved gas (CO2), also applying a magnetic field | [95] |
| CN112898249A | AA was extracted from lyophilized and milled peels and pulp of kiwi fruits using an oxalic acid solution | [96] |
| CN114149390A | AA was extracted from golden pomelo using a debitterizing solution | [97] |
| CN107298663A | AA was extracted from lemon | [98] |
| CN111349062A | Acerola cherry juice was pre-treated with cellulase and pectinase before the CSE | [99] |
| CN106588839A | AA extracted from sweet persimmon with cold water | [100] |
| CN104292196A | AA was extracted from sweet tea powder with water in a ratio of 1:10–1:40, adding pH buffer to pH 5.0–7.0 | [101] |
| CN104544131A | AA was extracted from ginseng fruits, ginkgo biloba extract, honeysuckle extract and sodium sulfite, using an aqueous solution of oxalic acid, hydrochloric acid and metaphosphoric acid as an extraction solution at 40–60 °C for 1–30 min | [102] |
| Process | Parameters | Source | Pretreatment | Yield (mg/100 g) | Ref. |
|---|---|---|---|---|---|
| MAE | 1% citric acid in water, 560 W, 110 s | Cashew apples | Dried at 40 °C | 239 | [121] |
| Ethanol–water (70:30), 540 W, 50 s | Peach waste | Frozen | 108 | [112] | |
| Methanol, 10% power, 15 min | Potato peels | Freeze-dried | 144 | [122] | |
| MAE+ UAE | Ethanol and 3% metaphosphoric acid, US 37 kHz for 30 min, MW 100 W for 2 min | Cabbage leaves | Fresh | 287 | [81] |
| PEF | Ethanol 100%, 10 μs, 1000 Hz and 1.0 kV cm−1 | Orange peel | Freeze-dried | 1029 | [19] |
| PEF+ UAE | Ethanol 100% PEF: 10 μs, 1000 Hz and 1.0 kV cm−1 US: 37 kHz | Orange peel | Freeze-dried | 870 | [19] |
| PFC | −12 °C, 400 rpm, 20 min circulation time | Orange peel | Drying in oven (35 °C, 12 h) and maceration | 72 | [16] |
| PLE | Distilled water, 70 °C, 10 MPa, 3 mL/min, 2 h | Camu-camu | Freeze-drying | 30,040 | [22] |
| Ethanol and water (70:30) pH 2.5, 2 cycles, 1500 psi | Blueberry | Fresh | 17 | [123] | |
| Ethanol and water (70:30) pH 11.5, 2 cycles, 1500 psi | Rosehip berry | 109 | |||
| Ethanol and water (70:30) pH 11.5, 2 cycles, 1500 psi | Partridgeberry | 83 | |||
| SFE | Ethanol, 70 °C, 250 bar, 2–0.4 mL/min | Grapes seeds | Freeze-drying | 272 | [117] |
| UAE | Acetic acid 8%, 95 W, 35 kHz, 10 min | Parsley | / | 264 | [124] |
| Acetone (80:20) in water for 24 h under stirring, then US for 2 h | Cashew apples | Dried at 50 °C for 48 h | 388 | [125] | |
| Ethanol 50%, 400 W for 30 min | Orange peel | / | 54 | [106] | |
| Mandarin peel | 136 | [126] | |||
| Ethanol 50%, 200 W, 40 kHz for 30 min at 30 °C | Rugosa rose fruit | Freeze-dried | 638 | [127] | |
| Ethanol 100% 2 h | Grapefruit peel | ||||
| Ethanol 100%, 37 kHz | Orange peel | Freeze-dried | 1229 | [19] | |
| Hydrochloric acid 0.1% in methanol | Genova citrus lemon | / | 6013 | [128] | |
| Water, 5 min, 50 °C, 30% amplitude, then reverse osmosis | Camu-camu | / | 619 | [129] | |
| Water, 30 min, 35 kHz, 40 °C | Turmeric | Dried at 55 °C | 17 | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susa, F.; Pisano, R. Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources. Processes 2023, 11, 3167. https://doi.org/10.3390/pr11113167
Susa F, Pisano R. Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources. Processes. 2023; 11(11):3167. https://doi.org/10.3390/pr11113167
Chicago/Turabian StyleSusa, Francesca, and Roberto Pisano. 2023. "Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources" Processes 11, no. 11: 3167. https://doi.org/10.3390/pr11113167
APA StyleSusa, F., & Pisano, R. (2023). Advances in Ascorbic Acid (Vitamin C) Manufacturing: Green Extraction Techniques from Natural Sources. Processes, 11(11), 3167. https://doi.org/10.3390/pr11113167











