The Development of Oral Solid Dosage Forms Using the Direct-Compression Tableting of Spray-Dried Bacteriophages Suitable for Targeted Delivery and Controlled Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Active Ingredient, Bacteriophage Felix O1
2.2. Phage Titration Using Double-Layer Plaque Assay
2.3. Spray-Drying of Bacteriophage Felix O1 in pH-Responsive Formulations
2.4. Assessing the Water Content and Morphology of Spray-Dried Powder Using Thermogravimetric Analysis (TGA)
2.5. Tablet Production Using Direct Compression
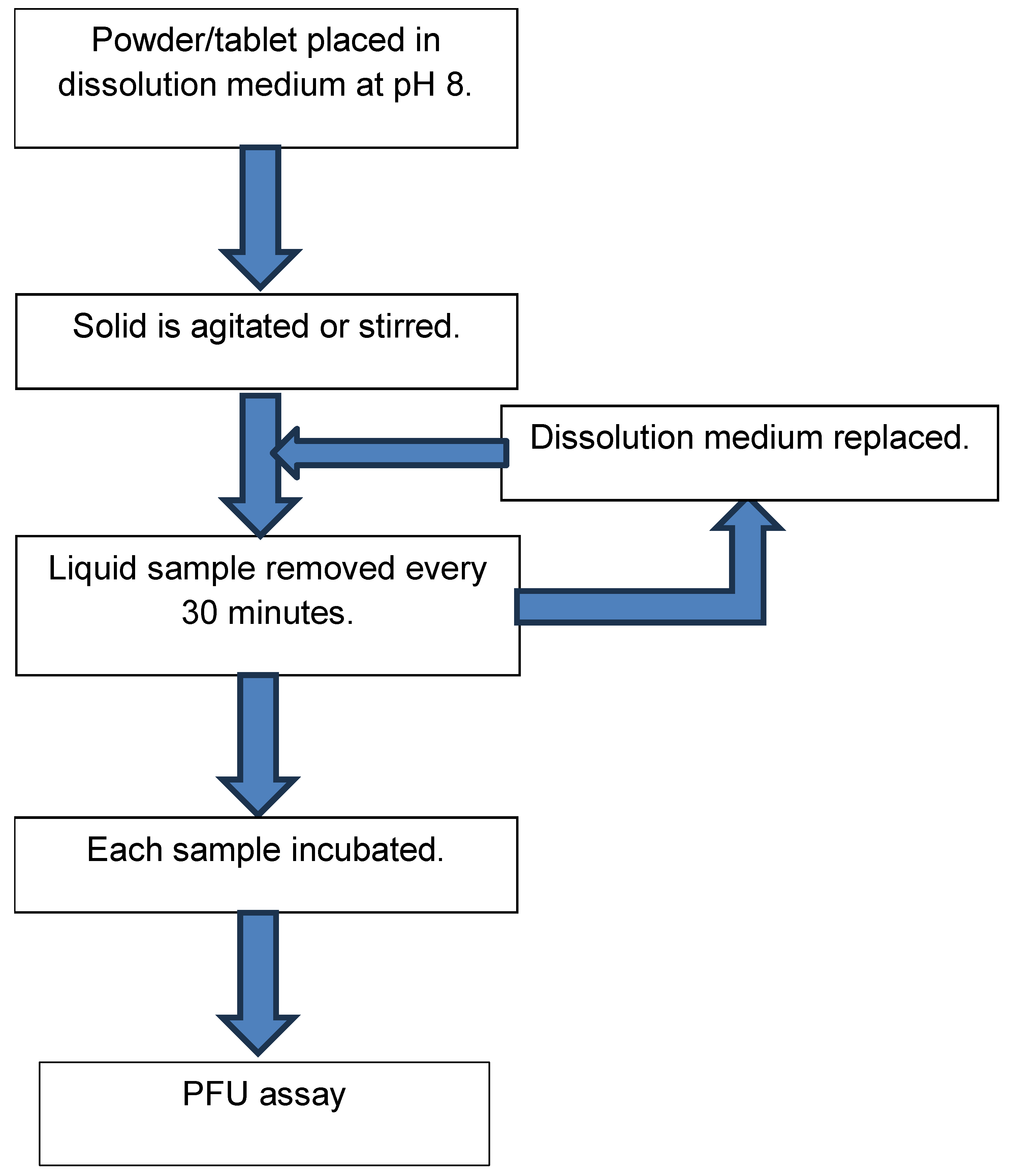
2.6. Dissolution Testing of Formulated Powders and Tablets
2.7. Tablet Friability Tests
2.8. Tablet Disintegration Tests
2.9. Statistical Analysis of Results
3. Results
3.1. Assessing the Effects of Processing on Phage Viability
3.2. Initial Dissolution Analysis of Powders and Tablets Containing PS21-S100 and PS21-L100 in Simulated Intestinal Fluid (SIF)
3.3. Analysis of the Effect of Simulated Gastric Fluid (SGF) on Phage Viability
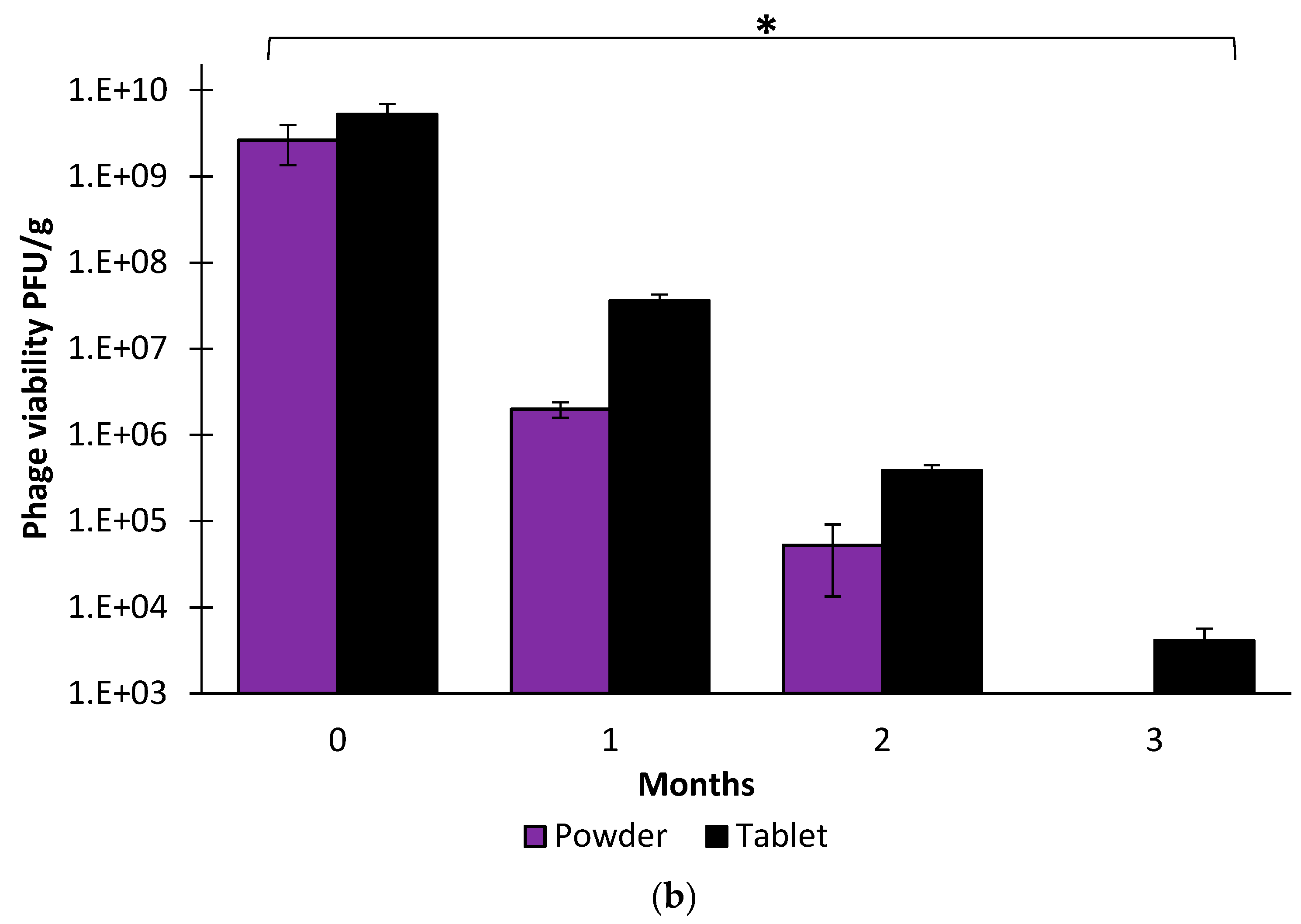
3.4. Stability Studies of Powders and Tablets Containing PS21-S100 and PS21-L100
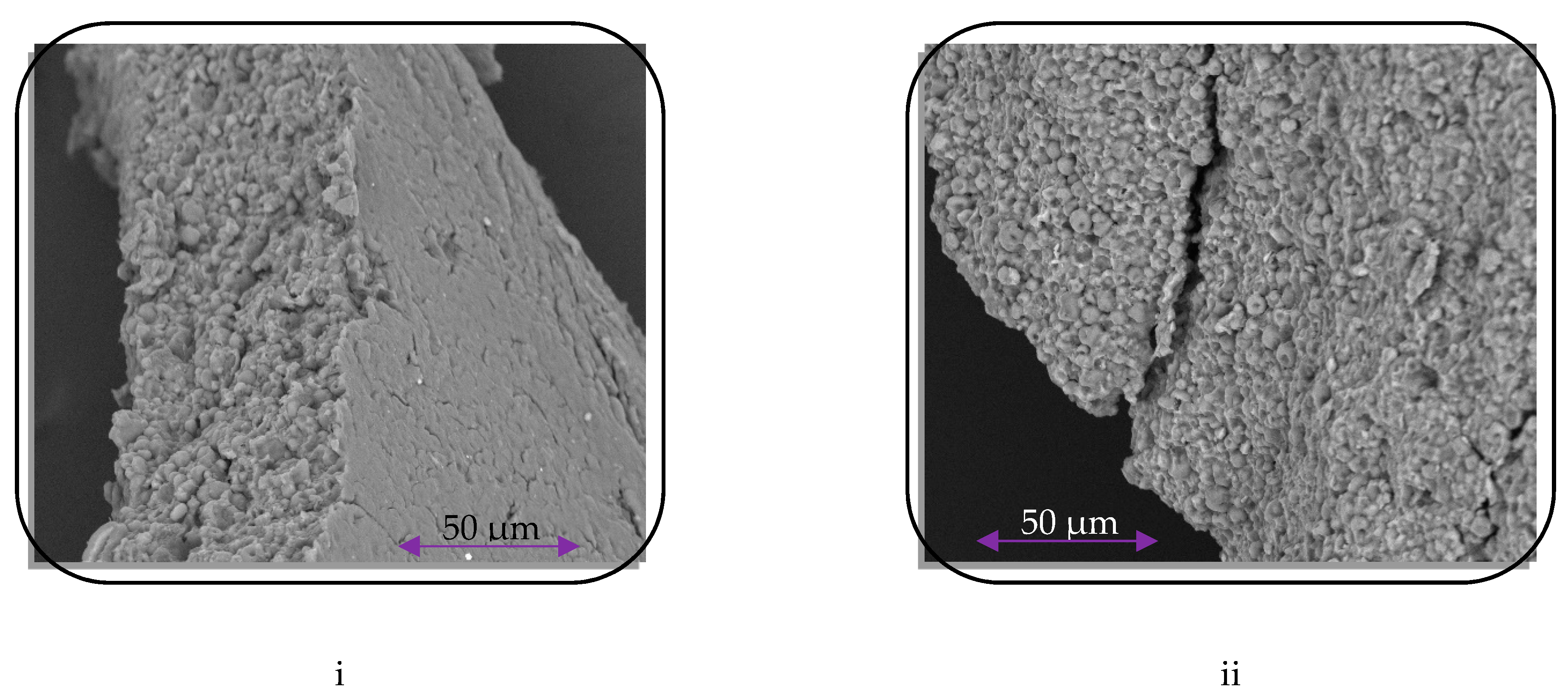
3.5. Tablet Friability
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yen, C.; Shih, S.-M.; Tate, J.E.; Wu, F.-T.; Huang, Y.-C.; Parashar, U.D.; Hsiung, C.A. Intussusception-related Hospitalizations Among Infants Before and After Private Market Licensure of Rotavirus Vaccines in Taiwan, 2001–2013. Pediatr. Infect. Dis. J. 2017, 36, e252. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Aladvidze, Z.; Morris, J.G. Bacteriophage Therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st Century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Mokomane, M.; Kasvosve, I.; de Melo, E.; Pernica, J.M.; Goldfarb, D.M. The global problem of childhood diarrhoeal diseases: Emerging strategies in prevention and management. Ther. Adv. Infect. Dis. 2018, 5, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance—A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Wallin, M.; Kutter, E.; Morales, S.; Britton, W.; Li, J.; Chan, H.-K. Storage stability of inhalable phage powders containing lactose at ambient conditions. Int. J. Pharm. 2019, 560, 11–18. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Kwok, P.C.L.; Khanal, D.; Morales, S.; Kutter, E.; Li, J.; Chan, H.-K. Inhalable bacteriophage powders: Glass transition temperature and bioactivity stabilization. Bioeng. Transl. Med. 2020, 5, e10159. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Okamoto, Y.; Morales, S.; Kutter, E.; Chan, H.-K. Hydrogel formulations containing non-ionic polymers for topical delivery of bacteriophages. Int. J. Pharm. 2021, 605, 120850. [Google Scholar] [CrossRef]
- Khanal, D.; Chang, R.Y.K.; Hick, C.; Morales, S.; Chan, H.-K. Enteric-coated bacteriophage tablets for oral administration against gastrointestinal infections. Int. J. Pharm. 2021, 609, 121206. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Liang, L.; Wanga, H.; Kariukic, S.; Nageld, T.E.; Connerton, I.F.; Vehring, R. Spray-dried anti-Campylobacter bacteriophage CP30A powder suitable for global distribution without cold chain infrastructure. Int. J. Pharm. 2019, 569, 118601. [Google Scholar] [CrossRef]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, Stabilisation and Encapsulation of Bacteriophage for Phage Therapy. Adv. Colloid Interface Sci. 2017, 249, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Resch, G. Editorial: Manufacturing, Formulation and Delivery Issues for Phage Therapy to Become A Reality. Front. Microbiol. 2020, 11, 584137. [Google Scholar] [CrossRef]
- Vinner, G.K.; Rezaie-Yazdi, Z.; Leppanen, M.; Stapley, A.G.F.; Leaper, M.C.; Malik, D.J. Microencapsulation of Salmonella-specific bacteriophage Felix O1 using spray-drying in a pH-responsive formulation and direct compression tableting of powders into a solid oral dosage form. Pharmaceuticals 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Tabare, E.; Dauchot, T.; Cochez, C.; Glonti, T.; Antoine, C.L.; Pirnay, J.-P.; Delcenserie, T.D.; Goole, J.; Eudragit, F.S. Microparticles Containing Bacteriophages, Prepared by Spray-Drying for Oral Administration. Pharmaceuticals 2019, 12, 1602. [Google Scholar] [CrossRef]
- Vinner, G.K.; Malik, D.J. High precision microfluidic microencapsulation of bacteriophages for enteric delivery. Res. Microbiol. 2018, 169, 522–530. [Google Scholar] [CrossRef]
- WHO. Annex 2 Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products. WHO Techn. Rep. Ser. 2009, 953, 87–130. [Google Scholar]
- Rathore, A.S. Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009, 27, 546–553. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, 110–128. [Google Scholar] [CrossRef]
- Abedon, S. Chapter 1—Phage therapy pharmacology: Calculating phage dosing. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 1–40. [Google Scholar]
- Yuen, K.-H. The transit of dosage forms through the small intestine. Int. J. Pharm. 2010, 395, 9–16. [Google Scholar] [CrossRef]
- Karthikeyan, J.S.; Salvi, D.; Karwe, M.V. Modelling of fluid flow, carbohydrate digestion, and glucose absorption in human small intestine. J. Food Eng. 2021, 292, 110339. [Google Scholar] [CrossRef]
- Syamov, R.M. Treatment and prophylaxis of cholera with bacteriophage. Bull. WHO 1963, 28, 361–367. [Google Scholar]
- Grasmeijer, N.; Frijlink, H.W.; Hinrichs, W.L.J. Model to predict inhomogeneous protein–sugar distribution in powders prepared by spray drying. J. Aerosol Sci. 2016, 101, 22–33. [Google Scholar] [CrossRef]
- Leung, S.S.; Parumasivam, T.; Gao, F.G.; Carrigy, N.B.; Vehring, R.; Finlay, W.H.; Morales, S.; Britton, W.J.; Kutter, E.; Chan, H.K. Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections. Pharm. Res. 2016, 33, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Roe, K.D.; Labuza, T.P. Glass transition and crystallization of amorphous trehalose-sucrose mixtures. Int. J. Food Prop. 2005, 8, 559–574. [Google Scholar] [CrossRef]
- Vandenheuvel, D.; Meeus, J.; Lavigne, R.; Van Den Mooter, G. Instability of bacteriophages in spray-dried trehalose powders is caused by crystallization of the matrix. Int. J. Pharm. 2014, 472, 202–205. [Google Scholar] [CrossRef]
- Bogdanova, E.; Fureby, A.M.; Kocherbitov, V. Hydration Enthalpies of Amorphous Sucrose, Trehalose and Maltodextrins and Their Relationship with Heat Capacities. Phys. Chem. Chem. Phys. 2021, 23, 14433–14448. [Google Scholar] [CrossRef]
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdi, Z.R.; Leaper, M.C.; Malik, D.J. The Development of Oral Solid Dosage Forms Using the Direct-Compression Tableting of Spray-Dried Bacteriophages Suitable for Targeted Delivery and Controlled Release. Processes 2023, 11, 3146. https://doi.org/10.3390/pr11113146
Yazdi ZR, Leaper MC, Malik DJ. The Development of Oral Solid Dosage Forms Using the Direct-Compression Tableting of Spray-Dried Bacteriophages Suitable for Targeted Delivery and Controlled Release. Processes. 2023; 11(11):3146. https://doi.org/10.3390/pr11113146
Chicago/Turabian StyleYazdi, Zahra Rezaie, Mark C. Leaper, and Danish J. Malik. 2023. "The Development of Oral Solid Dosage Forms Using the Direct-Compression Tableting of Spray-Dried Bacteriophages Suitable for Targeted Delivery and Controlled Release" Processes 11, no. 11: 3146. https://doi.org/10.3390/pr11113146
APA StyleYazdi, Z. R., Leaper, M. C., & Malik, D. J. (2023). The Development of Oral Solid Dosage Forms Using the Direct-Compression Tableting of Spray-Dried Bacteriophages Suitable for Targeted Delivery and Controlled Release. Processes, 11(11), 3146. https://doi.org/10.3390/pr11113146






