Study on Phase Change Flow and Heat Transfer Characteristics of Microalgae Slurry in the Absorber Tube of a Parabolic trough Solar Collector
Abstract
:1. Introduction
2. Physical and Computational Models
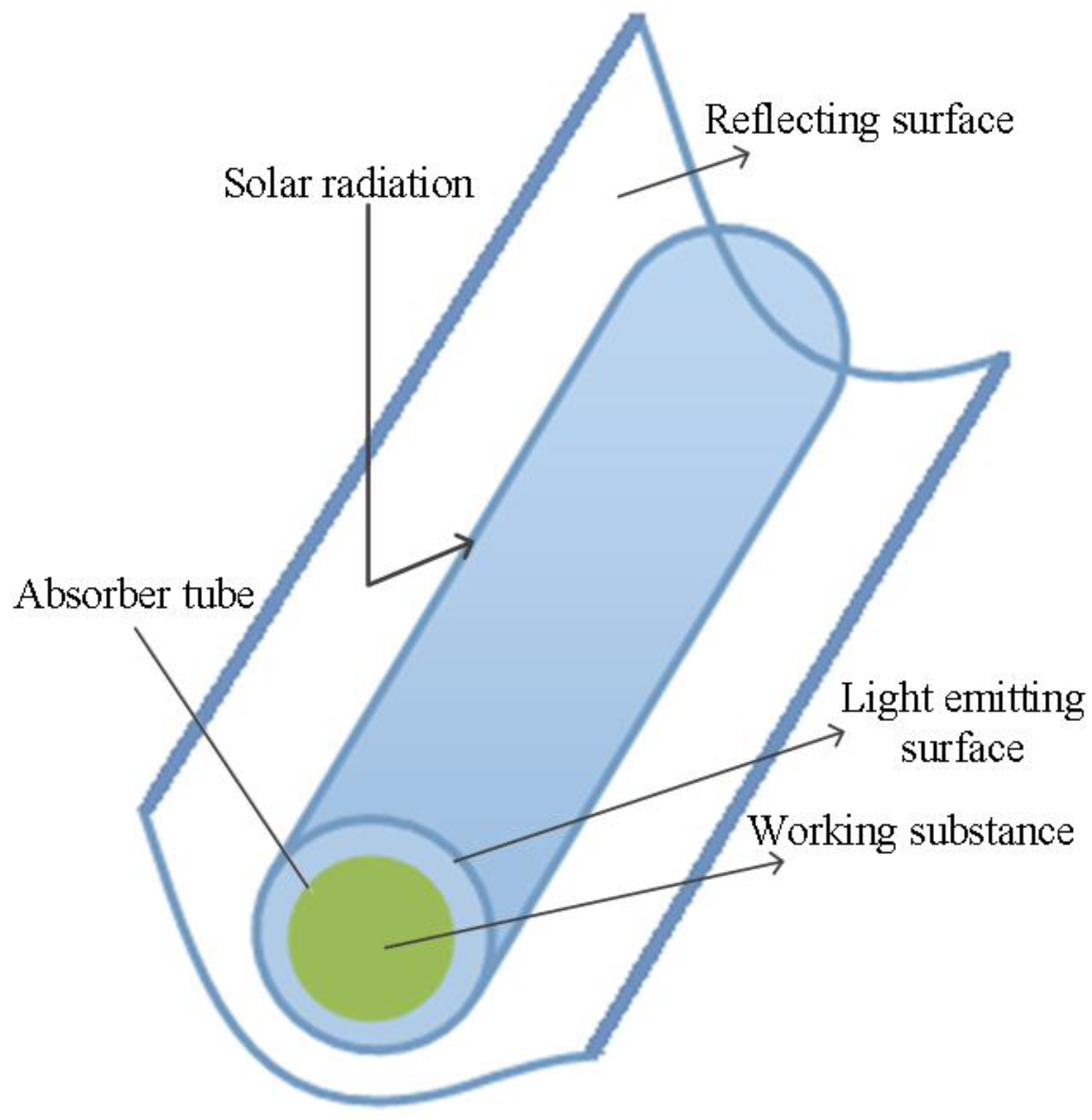
2.1. The Physical Model
2.2. Governing Equations
2.3. Boundary Conditions and Numerical Method
2.4. Grid Independence Checking and Model Validation
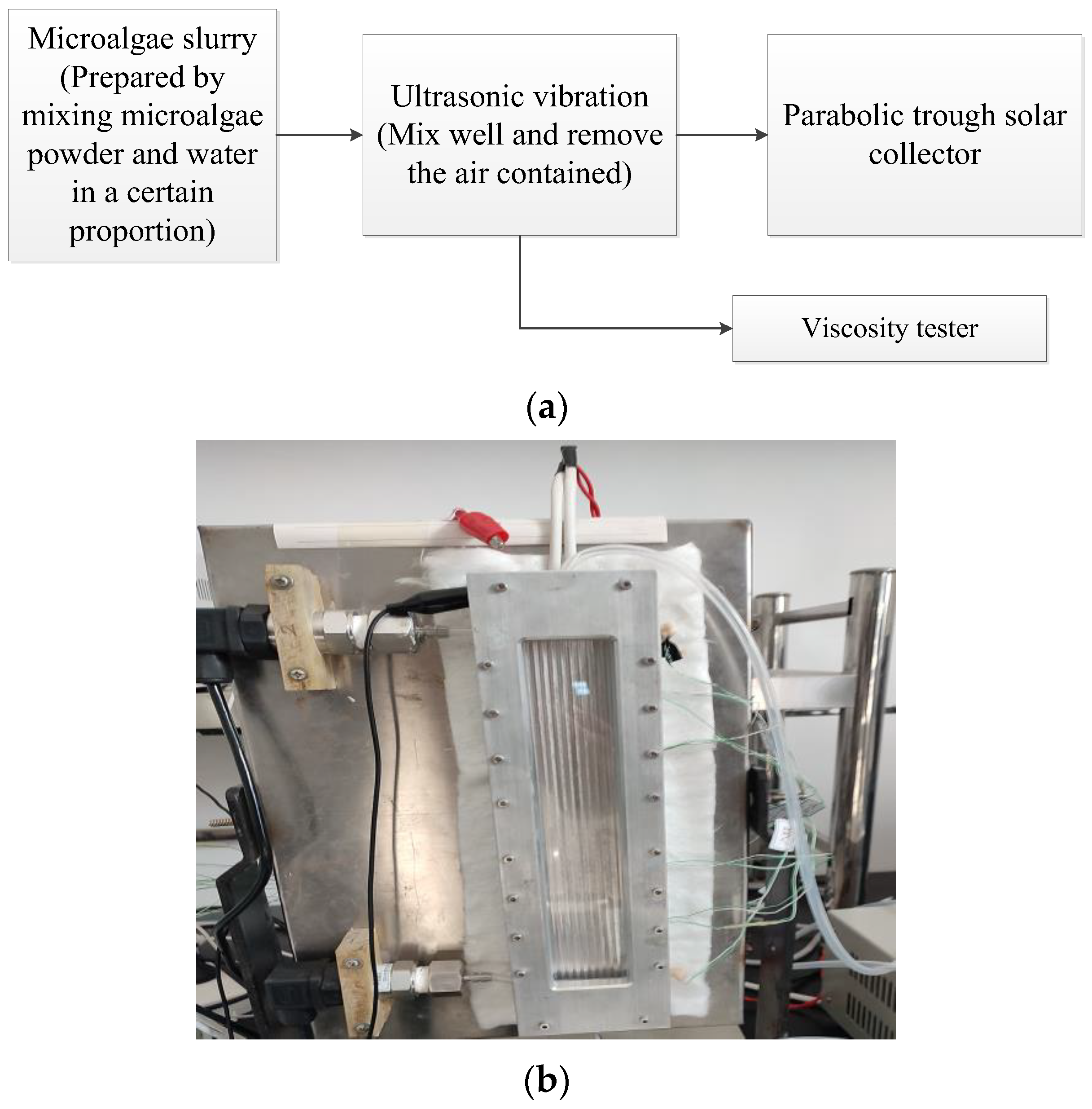
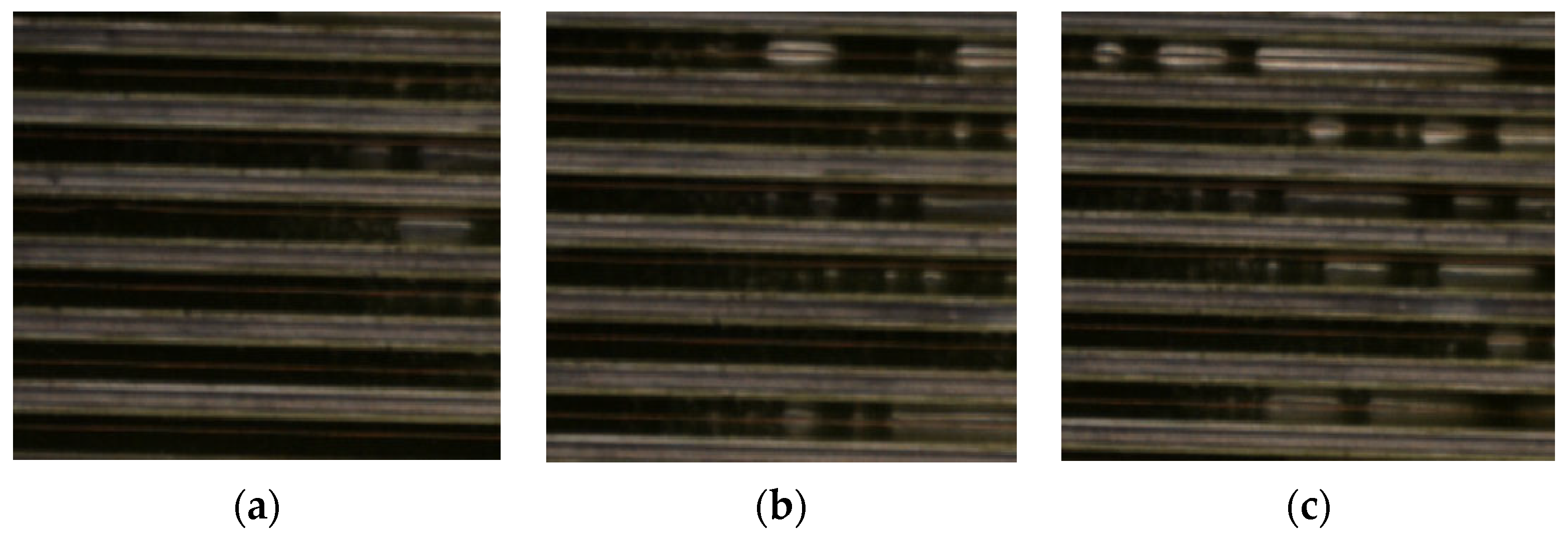
2.5. Experimental Flow Chart and Phase Change Process Observation Device
3. Results and Discussion
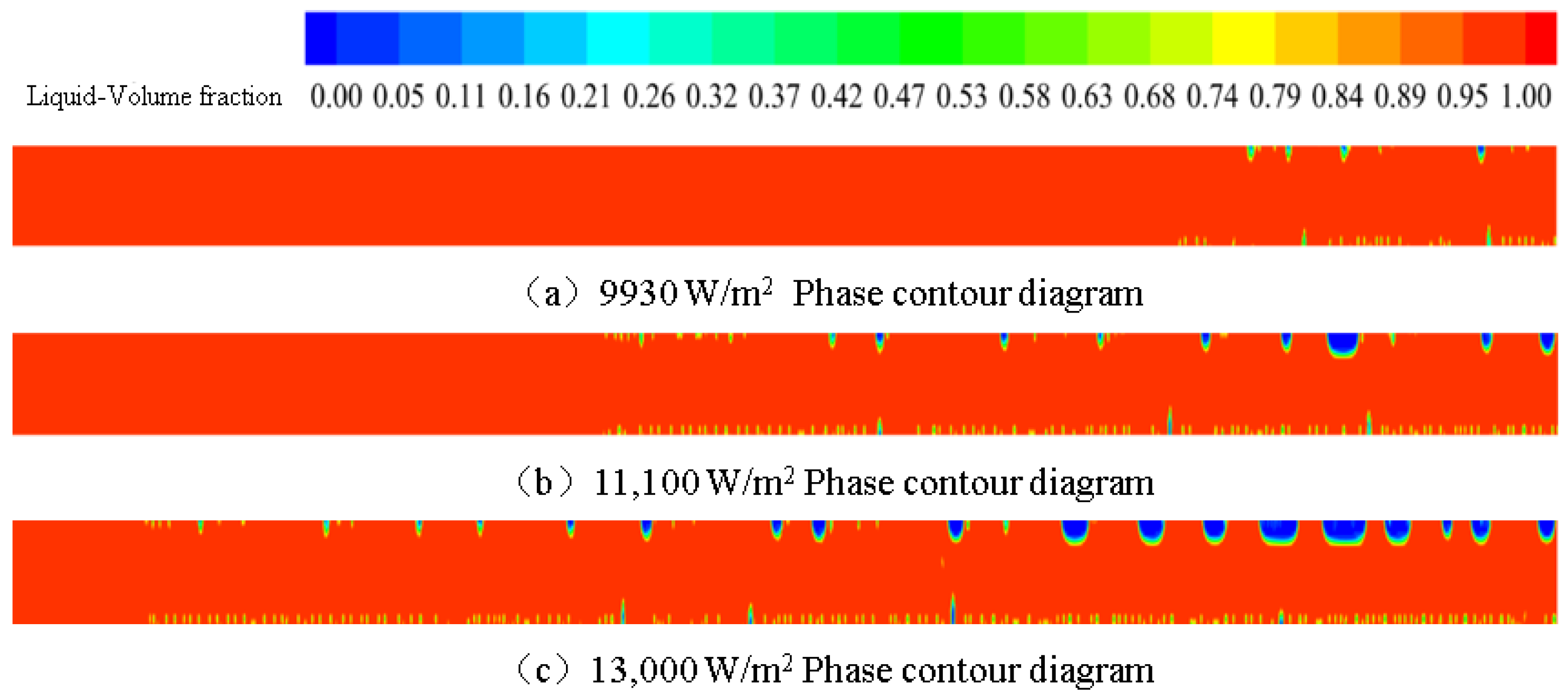
3.1. Effect of Wall Heat Flux on Phase Change Flow and Heat Transfer
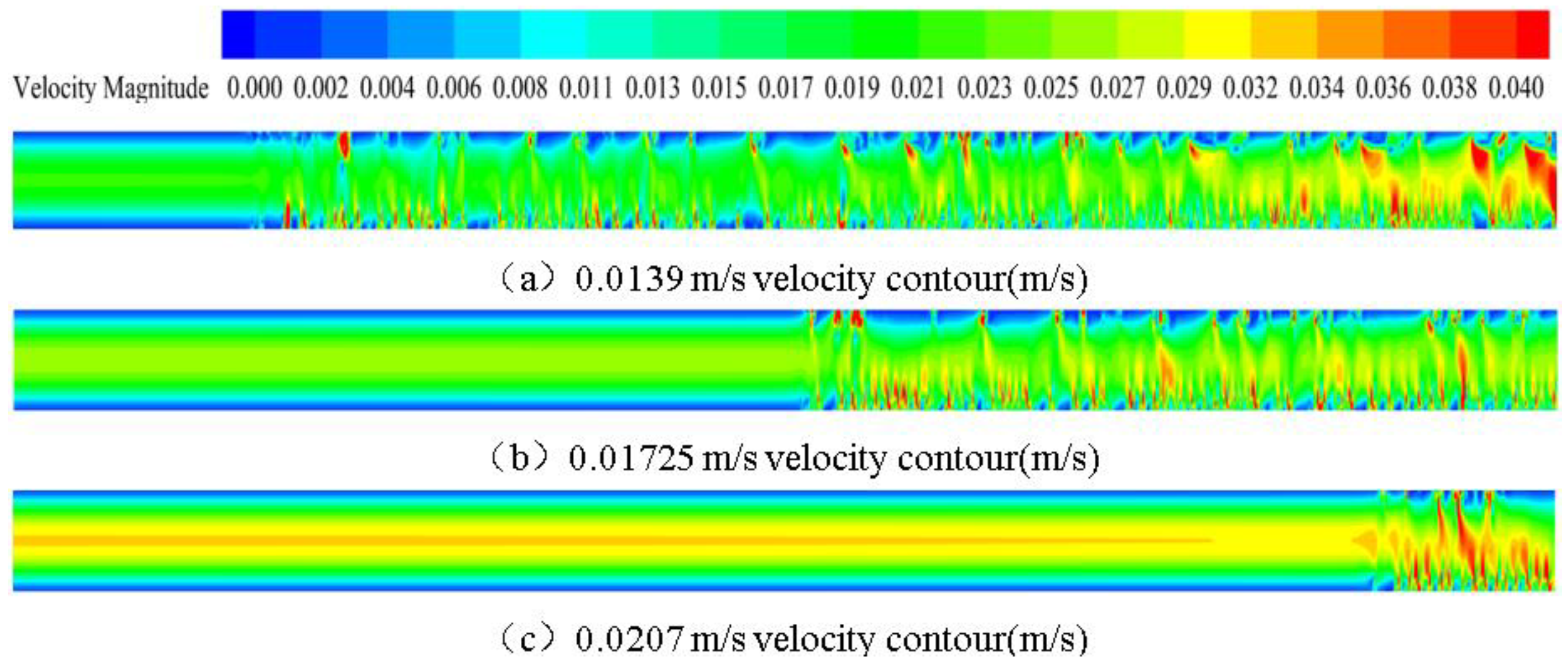
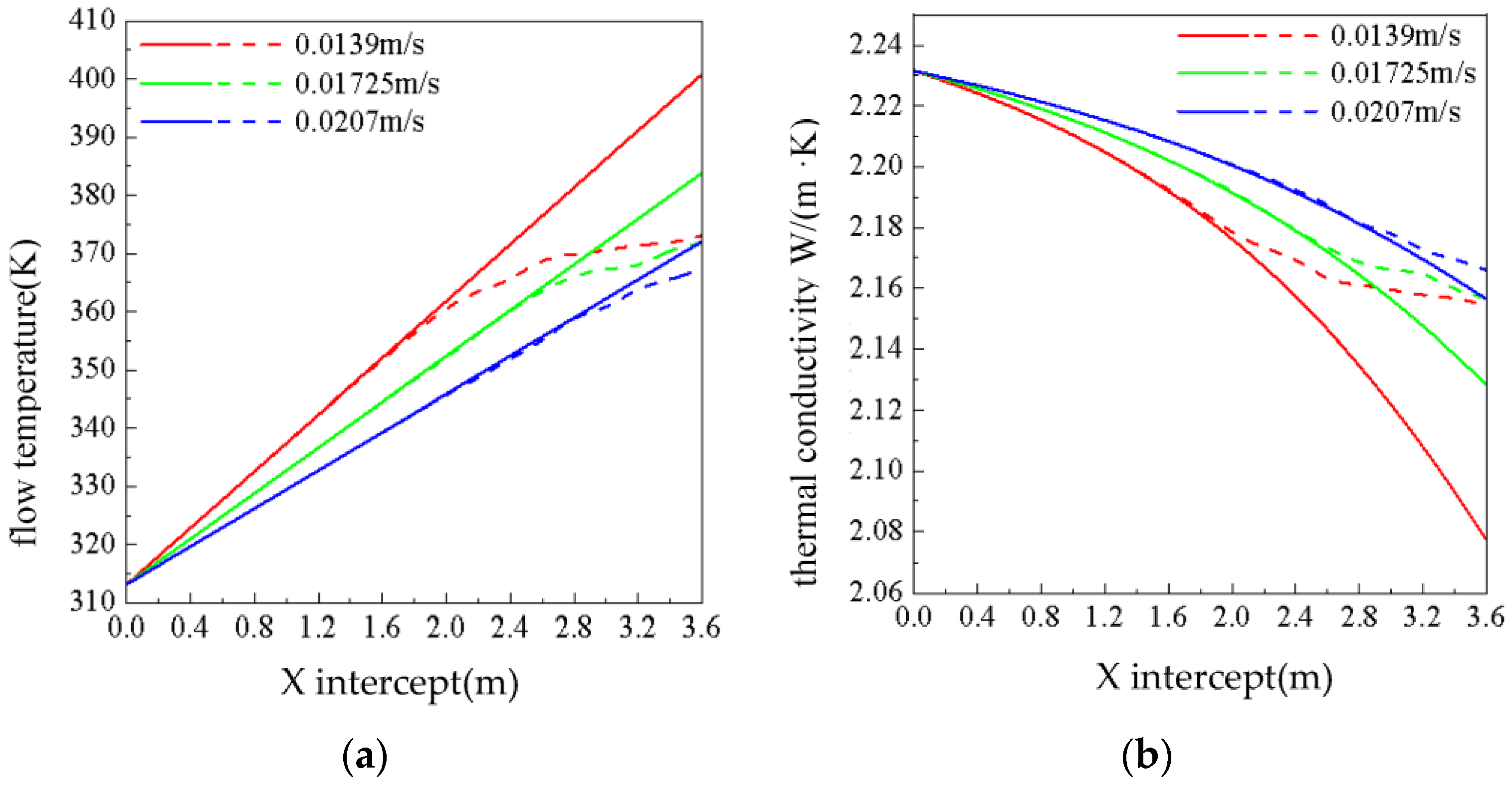
3.2. Effect of the Flow Rate on Phase Change Flow and Heat Transfer
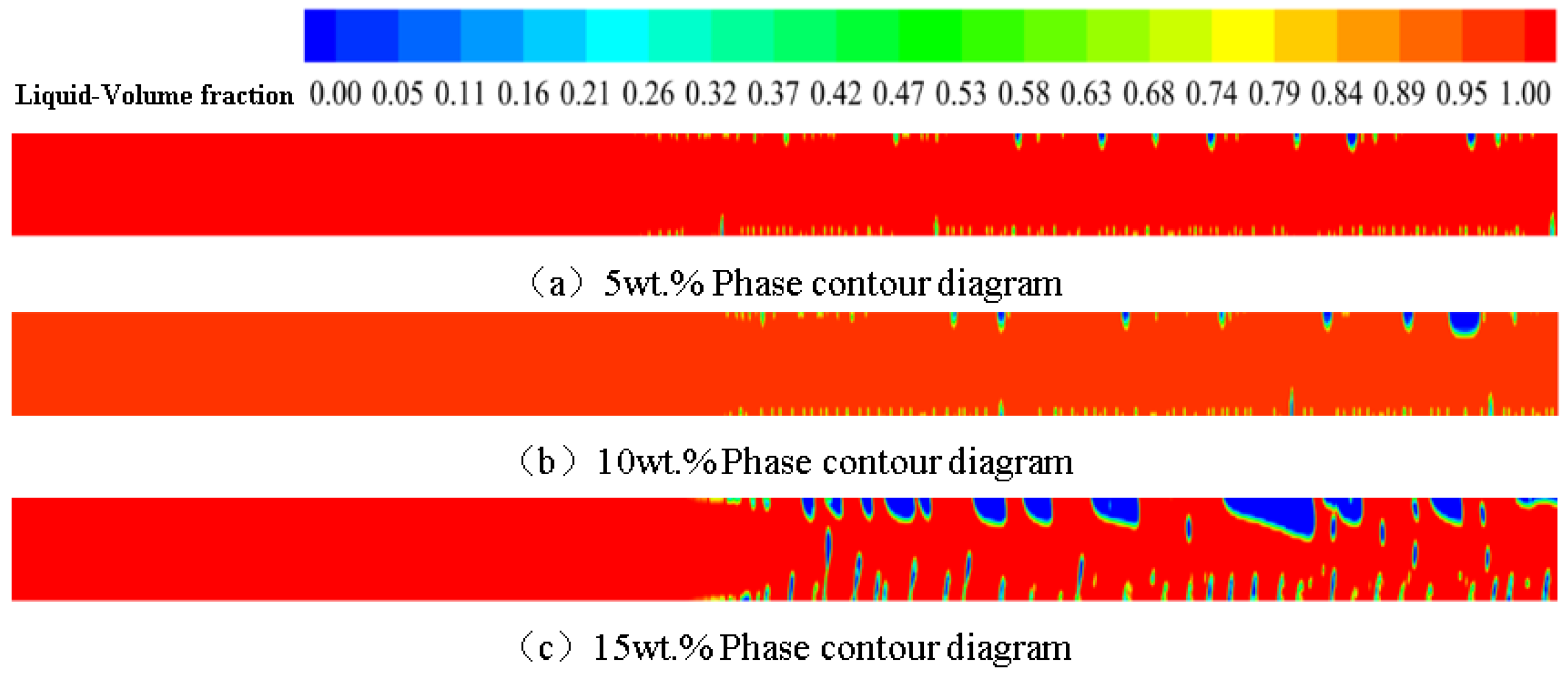
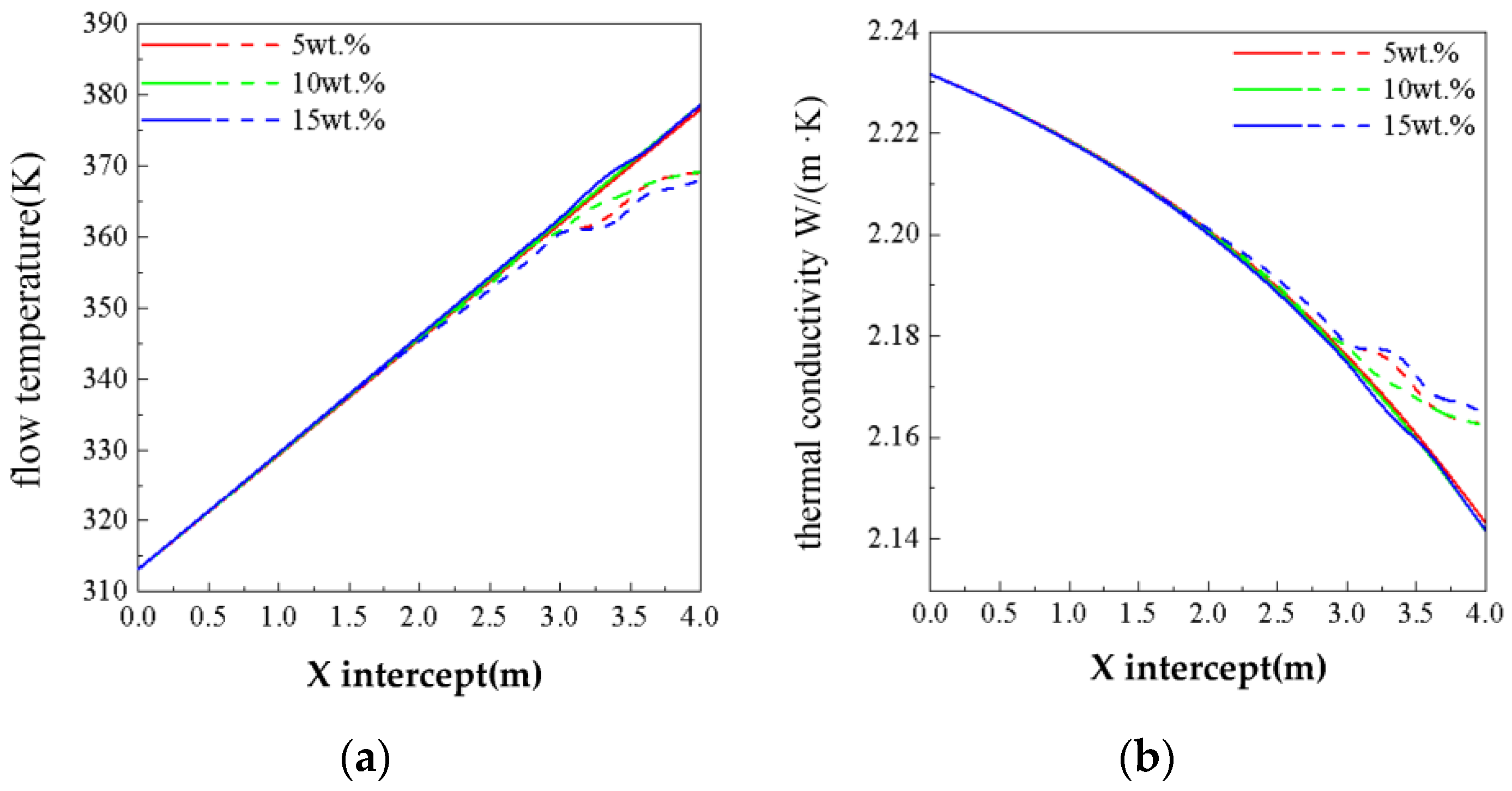
3.3. Effect of Concentration on Phase Change Flow and Heat Transfer
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Berenguer, C.V.; Perestrelo, R.; Pereira, J.A.; Câmara, J.S. Management of Agri-Food Waste Based on Thermochemical Processes towards a Circular Bioeconomy Concept: The Case Study of the Portuguese Industry. Processes 2023, 11, 2870. [Google Scholar] [CrossRef]
- Verdelho Vieira, V.; Cadoret, J.-P.; Acien, F.G.; Benemann, J. Clarification of Most Relevant Concepts Related to the Microalgae Production Sector. Processes 2022, 10, 175. [Google Scholar] [CrossRef]
- Velmozhina, K.; Shinkevich, P.; Zhazhkov, V.; Politaeva, N.; Korablev, V.; Vladimirov, I.; Morales, T.C. Production of Biohydrogen from Microalgae Biomass after Wastewater Treatment and Air Purification from CO2. Processes 2023, 11, 2978. [Google Scholar] [CrossRef]
- Liu, P.; Zhuang, H.; Qian, Y.; Yang, J.; Pan, Y.; Zhou, Z.; Jia, L.; Qi, F. Recent advances in mass spectrometric studies on the reaction process of biomass pyrolysis. Fuel Process. Technol. 2022, 237, 107473. [Google Scholar] [CrossRef]
- Romero-Borbón, E.; Oropeza-González, A.E.; González-García, Y.; Córdova, J. Thermochemical and Enzymatic Saccharification of Water Hyacinth Biomass into Fermentable Sugars. Processes 2022, 10, 210. [Google Scholar] [CrossRef]
- Encinas-Vazquez, I.A.; Carrillo-Pérez, E.; Mártin-García, A.R.; Del-Toro-Sánchez, C.L.; Márquez-Ríos, E.; Bastarrachea, L.J.; Rodríguez-Figueroa, J.C. Effects of High-Intensity Ultrasound Pretreatment on the Exopolysaccharide Concentration and Biomass Increase in Cheese Whey Kefir. Processes 2023, 11, 1905. [Google Scholar] [CrossRef]
- Lee, D.; Nam, H.; Seo, M.W.; Lee, S.H.; Tokmurzin, D.; Wang, S.; Park, Y.K. Recent progress in the catalytic thermochemical conversion process of biomass for biofuels. Chem. Eng. J. 2022, 447, 137501. [Google Scholar] [CrossRef]
- Xiao, C.; Liao, Q.; Fu, Q.; Huang, Y.; Chen, H.; Zhang, H.; Xia, A.; Zhu, X.; Reungsang, A.; Liu, Z. A solar-driven continuous hydrothermal pretreatment system for biomethane production from microalgae biomass. Appl. Energy 2019, 236, 1011–1018. [Google Scholar] [CrossRef]
- Chen, H.; Fu, Q.; Liao, Q.; Zhang, H.; Huang, Y.; Xia, A.; Zhu, X. Rheological properties of microalgae slurry for application in hydrothermal pretreatment systems. Bioresour. Technol. 2017, 249, 599–604. [Google Scholar] [CrossRef]
- Eshgarf, H.; Afrand, M. An experimental study on rheological behavior of non-Newtonian hybrid nano-coolant for application in cooling and heating systems. Exp. Therm. Fluid Sci. 2016, 76, 221–227. [Google Scholar] [CrossRef]
- Yoo, G.; Park, M.S.; Yang, J.W. Lipid content in microalgae determines the quality of biocrude and Energy Return On Investment of hydrothermal liquefaction. Appl. Energy 2015, 156, 354–361. [Google Scholar] [CrossRef]
- Luo, W.; Han, H.Z.; Yu, R.T.; Cai, L.; Gao, R.C. Numerical Study on Flow and Heat Transfer Characteristics of the Annular Side for Eccentric Helically Coiled Tube in Tube Heat Exchanger. J. Northeast. Electr. Power Univ. 2021, 6, 53–60. [Google Scholar]
- Zhang, X.; Ma, D.; Xia, G. Numerical Study of Enhanced Heat Transfer with locally Denser Sinusoidal Wavy Microchannels. J. Northeast. Electr. Power Univ. 2021, 4, 43–51. [Google Scholar]
- Zhang, L.; Li, J.; Xu, X.; Liu, F.; Guo, Y.; Yang, Z.; Hu, T. High spatial granularity residential heating load forecast based on Dendrite net model. Energy 2023, 269, 126787. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, T.; Zhang, L.; Yang, Z.; McLoone, S.; Menhas, M.I.; Guo, Y. A novel dynamic opposite learning enhanced Jaya optimization method for high efficiency plate–fin heat exchanger design optimization. Eng. Appl. Artif. Intell. 2023, 119, 105778. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, Q.; Fu, Q.; Chen, H.; Huang, Y.; Xia, A.; Zhu, X.; Reungsang, A.; Liu, Z.; Li, J. Rheological properties of microalgae slurry under subcritical conditions for hydrothermal hydrolysis systems. Algal Res. 2018, 33, 78–83. [Google Scholar] [CrossRef]
- Fu, Q.; Zhang, H.; Chen, H.; Liao, Q.; Xia, A.; Huang, Y.; Zhu, X.; Reungsang, A.; Liu, Z. Hydrothermal hydrolysis pretreatment of microalgae slurries in a continuous reactor under subcritical conditions for large–scale application. Bioresour. Technol. 2018, 266, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, H.; Ling, X.; Liu, L.; Huhe, T. Investigation of heat and mass transfer and gas–liquid thermodynamic process paths in a humidifier. Energy 2022, 261, 125156. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, K.; Yin, B. Numerical method for gas-liquid two-phase flow with phase change heat transfer considering compressibility using OpenFOAM. Int. J. Therm. Sci. 2023, 188, 108195. [Google Scholar] [CrossRef]
- Odumosu, O.A.; Xu, H.; Wang, T.; Che, Z. Growth of elongated vapor bubbles during flow boiling heat transfer in wavy microchannels. Appl. Therm. Eng. 2023, 223, 119987. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Hemmatian, F.; Dahkaee, K.P.; Hlawitschka, M.W.; Kantzas, A. Detailed study of single bubble behavior and drag correlations in Newtonian and non-Newtonian liquids for the design of bubble columns. Chem. Eng. Res. Des. 2022, 179, 119–129. [Google Scholar] [CrossRef]
- Gkotsis, P.K.; Evgenidis, S.P.; Karapantsios, T.D. Influence of Newtonian and non-Newtonian fluid behaviour on void fraction and bubble size for a gas-liquid flow of sub-millimeter bubbles at low void fractions. Exp. Therm. Fluid Sci. 2019, 109, 109912. [Google Scholar] [CrossRef]
- Hao, C. Investigation on Flow, Heat Transfer and Hydrothermal Hydrolysis of Microalgae Slurry in a Tubular Reactor; Chongqing University: Chongqing, China, 2020; pp. 45–50. [Google Scholar]
- Hong, Z. The Heat Transfer and Hydrothermal Hydrolysis Properties of Microalgae Slurry in a Circular Tube; Chongqing University: Chongqing, China, 2018. [Google Scholar]




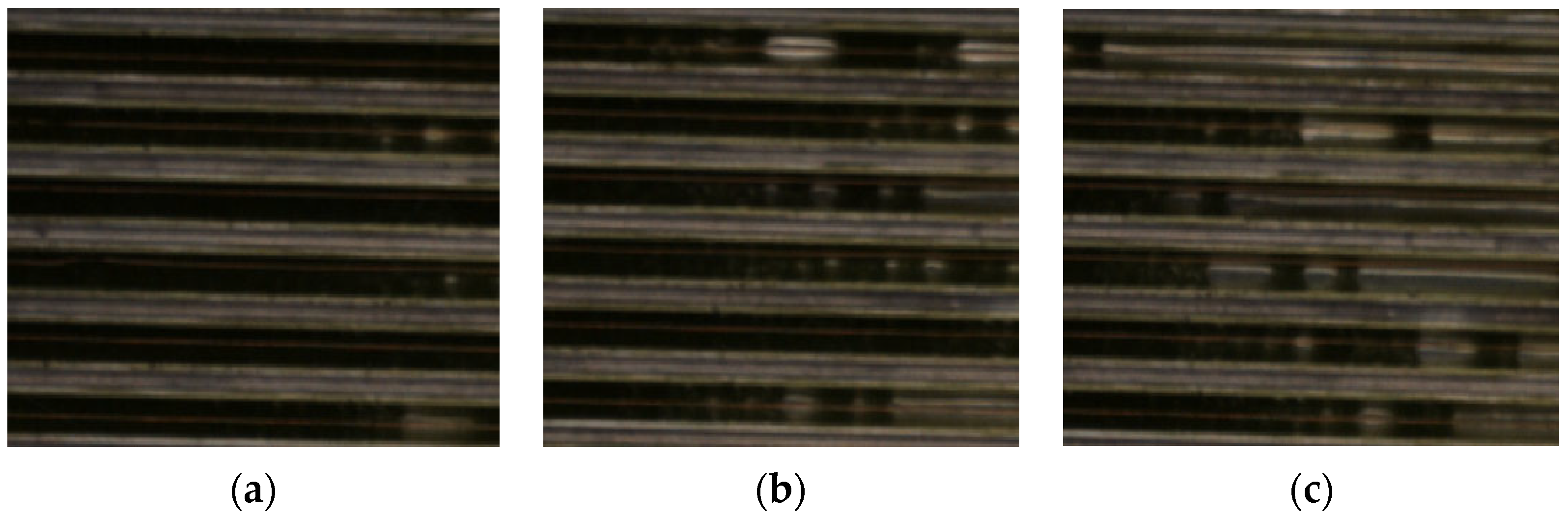
| Case | Volumetric Flowrate L/h | Heat Flux W/m2 | Inlet Temperature K | Simulated Outlet Temperature K | Error % |
|---|---|---|---|---|---|
| 1 | 60 | 11,100 | 313.15 | 412.11 | 0.097 |
| 2 | 60 | 11,100 | 313.15 | 411.71 | Baseline |
| 3 | 60 | 11,100 | 313.15 | 411.58 | 0.032 |
| Case | Volumetric Flowrate L/h | Experimental Inlet Temperature K | Thermometer Error (Inlet Temperature) K | Experimental Outlet Temperature K | Experimental Standard Deviation (Outlet Temperature) K |
|---|---|---|---|---|---|
| 1 | 50 | 353.15 | ±1 | 377.52 | 0.87 |
| 2 | 60 | 353.15 | ±1 | 365.45 | 0.72 |
| Case | Volumetric Flowrate L/h | Heat Flux W/m2 | Inlet Temperature K | Experimental Outlet Temperature K | Simulated Outlet Temperature K | Error % |
|---|---|---|---|---|---|---|
| 1 | 50 | 11,100 | 353.15 | 377.52 | 379.98 | 0.7 |
| 2 | 60 | 11,100 | 353.15 | 365.45 | 366.67 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Dong, N.; Chen, Q. Study on Phase Change Flow and Heat Transfer Characteristics of Microalgae Slurry in the Absorber Tube of a Parabolic trough Solar Collector. Processes 2023, 11, 3107. https://doi.org/10.3390/pr11113107
Han W, Dong N, Chen Q. Study on Phase Change Flow and Heat Transfer Characteristics of Microalgae Slurry in the Absorber Tube of a Parabolic trough Solar Collector. Processes. 2023; 11(11):3107. https://doi.org/10.3390/pr11113107
Chicago/Turabian StyleHan, Wei, Nanhang Dong, and Qicheng Chen. 2023. "Study on Phase Change Flow and Heat Transfer Characteristics of Microalgae Slurry in the Absorber Tube of a Parabolic trough Solar Collector" Processes 11, no. 11: 3107. https://doi.org/10.3390/pr11113107
APA StyleHan, W., Dong, N., & Chen, Q. (2023). Study on Phase Change Flow and Heat Transfer Characteristics of Microalgae Slurry in the Absorber Tube of a Parabolic trough Solar Collector. Processes, 11(11), 3107. https://doi.org/10.3390/pr11113107







