Coal gangue is the waste produced during coal mining and processing, accounting for about 10~15% of the total coal production [
1]. The accumulation of coal gangue leads to environmental pollution, including soil and water pollution caused by the weathering and leaching of coal gangue, harmful gas and dust caused by the spontaneous combustion of coal gangue, and a waste of land resources caused by coal gangue stacking [
1,
2,
3]. However, the processing and utilization of coal gangue not only reduces the impact of coal gangue on the environment, but it also turns coal gangue into a resource to increase economic benefits. It has been proven that kaolin can be prepared from coal gangue with more than 80% kaolin mineral content [
4,
5]. The technology of kaolin preparation from coal gangue has been applied industrially in numerous countries, which alleviates the shortage of high-quality, non-coal measured kaolin. However, it must be noted that the impurities in coal gangue must be removed to prepare qualified kaolin. The impurities in coal gangue mainly include carbon, iron, titanium, quartz, and so on [
6], among which, carbon is the most abundant. At present, some research has been conducted on the removal of carbon from coal gangue. Zhang [
7] found that the optimum calcination temperature was 700 °C by calcining coal gangue for decarburization. Cheng [
8] found that decarbonization via calcination was a two-step reaction, with the temperature ranges of the first and second steps being 350–550 °C and 580–830 °C, respectively. Yuan [
9,
10,
11] conducted a comparative study on the convective calcination and static calcination of coal measured kaolin, finding that under the same calcination temperature, kaolin products with higher whiteness and lower COD (Chemical Oxygen Demand, such as pyrite and organic matter) values can be obtained via fluidized calcination. Yuan [
9] also discovered that the COD value of coal gangue decreased with an increase in calcination temperature. At 500 °C, the COD value of the kaolin product decreased to 2217 μg/g, and at 900 °C, the COD value was reduced to 460 μg/g. He [
12] separated carbon from kaolin in coal measures using the electric separation method. When the voltage was 32 kv, the feed frequency was 5 Hz, and the flow rate was 20 cm
3/s; the decarbonization efficiency was higher, reaching 27.78%. Peng [
13] used the gravity separation method to remove carbon from coal gangue. The ignition loss (LOI) of the kaolin product obtained via a float and sink experiment was 44.22% lower than that obtained from raw materials. The kaolin decarburized by the gravity separation method was whiter than that carburized by raw materials. Huang [
14] confirmed that carbon had a great influence on the COD value of coal gangue, removing carbon from raw materials via the flotation method. The COD value of carbon products obtained after flotation was 216,202 μg/g, much higher than that of raw materials and kaolin products. Huang [
15] also found that the COD value of coal gangue existed in different grain sizes in raw materials. Regarding the employment of the calcination method for decarburization, the optimum grain size for reducing the COD value of raw materials was in the range of −74~+60 μm when the calcination temperature was 450 °C, indicating the COD value of raw materials could be reduced from 27,517 μg/g to 585 μg/g. Huang [
16] also studied the combined process of flotation and calcination, finding that the COD value of the kaolin obtained via flotation was 3533 μg/g. When the kaolin was calcined under the condition of 300 °C, the COD value of the calcined kaolin was close to 2700 μg/g, and the temperature was much lower than that of direct calcination without flotation. If the kaolin obtained from flotation was calcined at 450 °C, the COD value of the calcined kaolin could be as low as 290 μg/g. Therefore, compared with direct calcination, the process of flotation followed by calcination reduced the calcination temperature, calcination cost, and COD value of kaolin products.
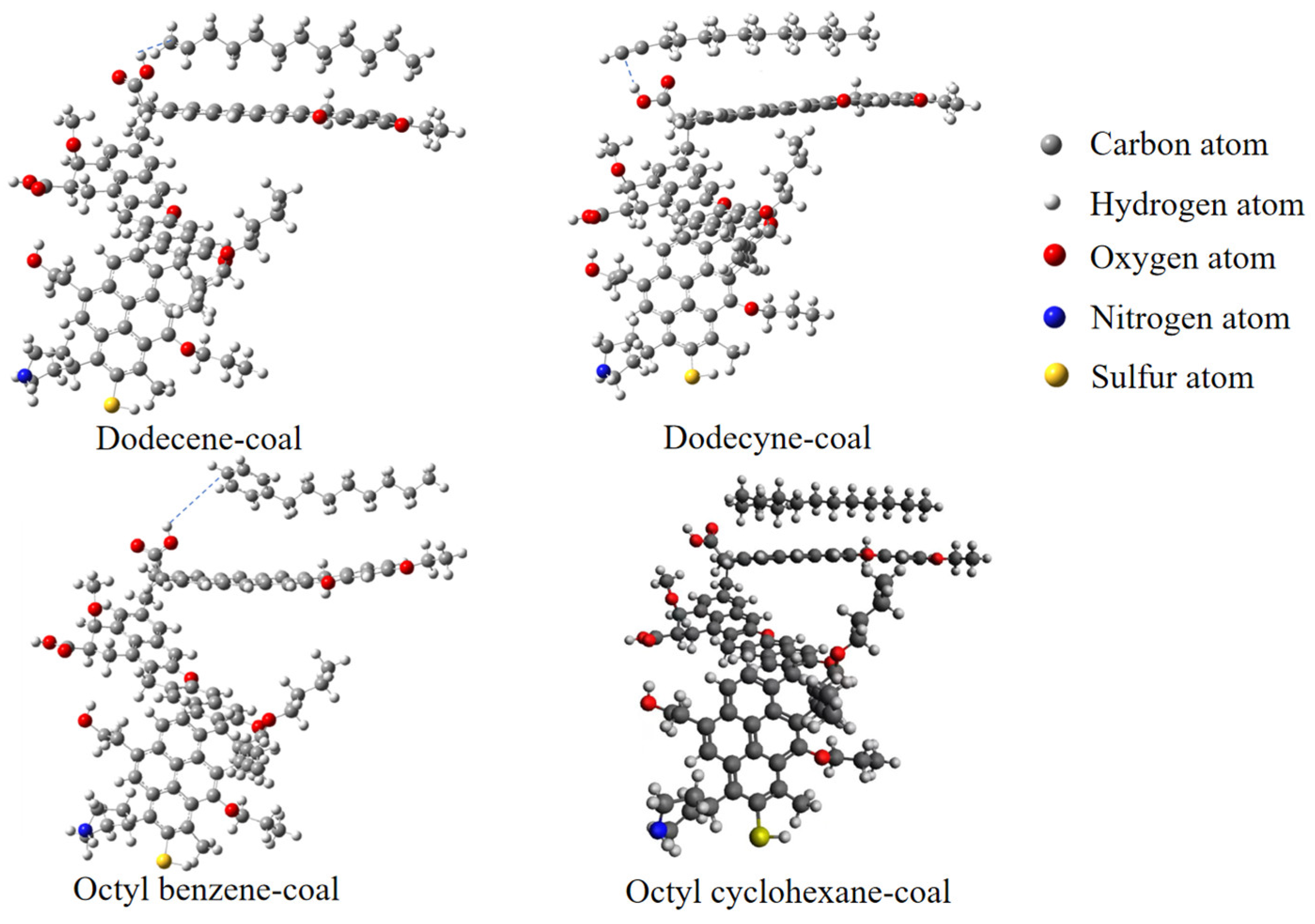
Regarding the above, it is obvious that the main methods of decarbonization include calcination, electric separation, gravity separation, flotation, etc. In fact, although carbon in coal gangue is an impurity during the preparation of kaolin, it becomes a valuable energy after being enriched, especially for low ash coal gangue with higher carbon contents. However, at present, the most commonly used method of decarbonization is calcination, which often results in the loss of carbon. In addition to consuming heat energy, calcination also brings certain atmospheric pollution, and it sometimes leads to the destruction of the layered structure of the kaolinite’s crystal phase. Flotation is an effective method to recover carbon resources, but there are very few studies on the removal of carbon from coal gangue via flotation, and the published research results on flotation all focus upon fixed and commonly used flotation agents, such as kerosene or diesel. At present, there is no comparative study of different flotation agents in the flotation process of coal gangue. Therefore, this paper mainly compares the interaction between coal molecules in coal gangue and various flotation agents, and it reveals the mechanism of action. Since collectors play a very important role in flotation reagents, it is necessary to study collectors in depth. During industrial production processes, kerosene and diesel are often used as collectors, and they have different carbon chain lengths. Therefore, it is essential to examine the interaction between agents with different carbon chain lengths and coal molecules. In addition, considering that kerosene and diesel oil interact with coal molecules in gangue through their alkyl groups, it is necessary to study the mechanism of action between other groups and coal molecules, except alkyl groups. In short, this paper focused on researching the interaction mechanism between collectors with different chain lengths and different groups and coal molecules; it also attempted to explore the mechanism concerning the flotation decarbonization of coal gangue.