1. Introduction
The globe is facing a number of environmental issues because of increasing manufacturing activities. Due to the recent increase in the manufacturing of electronic devices and their ever-shortening spans of life, today, electronic waste (e-waste) has emerged as one of the major environmental concerns [
1]. Currently, rapidly emerging countries, such as India and China, are the primary contributors of e-waste [
2]. According to Wang et al. [
3], 20–50 million tons of e-waste are generated worldwide each year, which is growing at a rate of 3–5% annually [
4]. India has been ranked as the first among the top five e-waste generators with an estimated yearly generation of 2 million tons [
5]. Among these e-wastes, waste printed circuit boards (WPCBs) make up 3–6% of total e-waste and they are heterogeneous mixture of organic materials, glass fibers, and metals [
6]. They normally contain 30% plastics (polyethylene, polypropylene, polyesters, PVC, epoxy resins, PTFE, and nylon), 30% ceramic (primarily silica or alumina with some amount of mica) [
7], alkaline earth oxides, and barium titanate [
8]. They also contain 40% metals, with copper accounting for 10–27% of the total weight of WPCBs. Other metals found are gold, silver, iron, and palladium. Some toxic metals such as antimony, cadmium, lead, nickel, arsenic, mercury, and chromium can have major environmental consequences if not recycled carefully [
7].
PCBs are mainly manufactured with resins containing fire flame retardants such as tetrabromobisphenol A (TBBPA) and polybrominated diphenyl ethers (PBDEs) [
9]. To facilitate their recycling, many sophisticated methods have been developed and applied in China and India [
10], which include mechanical dismantling, to separate plastics and metals, hydrometallurgical and pyrometallurgical facilities, and amalgamation to recover valuable metals [
11]. However, the separation of plastics with brominated flame retardants is not applied. Hence, the incineration of WPCBs and resulting waste produces a high quantity of dioxins, furans, and HBr with brominated phenols as by-products [
12,
13]. The brominated chemicals, such as their chlorinated congeners, can be hazardous to human skin, liver, and digestive tract. A number of studies have been carried out about the toxicity and health risks concerning human exposure to TBBPA on recycling sites [
10,
14,
15]. Therefore, environmentally acceptable disposal or recycling of WPCBs is a big challenge [
12]. The dibenzodioxins generated were mostly contained in the char matrix; nevertheless, research has revealed that polybrominated dibenzodioxins/furans (PBDD/Fs) were released at ppb and ppm levels during the breakdown process [
13,
16]. By adopting more appropriate processing conditions during recycling, it is possible to minimize the amount to which they are generated.
Pyrolysis appears to be one of the most promising technologies, allowing large polymer chains to be broken down into lighter polymer compounds with smaller molecular weight. Thermal decomposition of WPCBs initiates at 250 °C and continues to 900 °C [
17]. Abdou et al. [
17] observed the degradation of WPCBs below 550 °C and the polymeric matrix was not completely removed. Although the reaction process is influenced by the chemical composition and residence time, it is critical to developing methods and procedures for obtaining pyrolysis products with low halogen yields and reducing harmful emissions as much as possible. If the pyrolysis products have low yields of halogens, they can be utilized appropriately [
16]. Furthermore, simplicity and effectiveness are especially important in developing a technique to produce the ideal synthetic liquid fuel. Moreover, WPCBs include a relatively high content of ash, so its energy density (e.g., heating value) and thermolysis efficiency are low. Co-pyrolysis of biomass with WPBCs could be an alternative technology that improves these two criteria [
18]. A high amount of hydrogen present in biomass may lead to dehalogenation and prevention of PBDD/Fs which promotes HBr/HCl fixation in char. Furthermore, the biomass has a higher H/C molar ratio which can act as a hydrogen donor to WPCBs [
19]. Water, one of the most abundant components in biomass, is expected to act as a reactive agent, promoting further cracking of the WPCBs tar and the production of more volatile chemicals that improves pyrolysis oil yields [
18,
20]. Co-pyrolysis of biomass has been shown in several studies to boost oil yield and quality while maintaining the overall process. However, because of the various reactivities of biomass and e-waste, this process is relatively complex. Pyrolysis product yields and characteristics are influenced by the temperature and blend ratio of feedstock [
18,
21].
Generally, the knowledge of the pyrolysis kinetics of the main thermal decomposition process is critical to predicting the pyrolysis behavior of a material and helps to design a suitable reactor, as well as the mathematical modeling of the reactor for process optimization. Process parameters, heat and mass transport limits, physical and chemical properties of the sample, and systematic errors are all factors that might affect the kinetic parameters. The key procedural challenges are dehalogenation and avoidance of highly toxic emissions (especially PBDD/Fs). The aim of this study is to probe the pyrolysis behavior of WPCB, cotton stalk (CS), and its blend. Three model-free methods, such as Kissinger–Akahira–Sunose (KAS), Flynn–Wall–Ozawa (FWO), and Starink, were used to calculate apparent activation energy (Eα) and pre-exponential factor (A). Furthermore, with calculated Eα values, the possible decomposition mechanism was probed using the Criado method. There is no single work in the published literature that focused on the co-pyrolysis of these residues. The comparison of the thermal decomposition of PCB, CS, and its blend can be the first expanding research effort to understand the synergistic behavior of the mixture.
2. Kinetic Modeling
The concept of the single-step, solid-state reactions can be represented by the Arrhenius equation. It means that the reactant molecules are in an endothermic equilibrium with the active molecules that are involved in the reaction.
where
is the activation energy (kJ mol
−1) and
A is the pre-exponential factor (>1).
R refers to the gas constant (kJ mol
−1 K
−1). T represents the temperature in
K and
κ(
T) is a rate constant. The value of
E was supposed to be a constant for single-step reactions and it is the energy needed to turn inactive molecules into active molecules. To describe the kinetic expression from the mass balance of a reacting solid and the conversion degrees for a single-phase reaction, the thermogravimetric results can be expressed as [
22]:
where
n is reaction order.
A general kinetic Equation (2) is based on assumption that the rate of process
dα/
dt is a function of two variables only: degree of conversion (
α) and temperature (
T). Assuming that the function of two variables can be substituted by a product of two functions variables,
κ(
T) and
f(
α). Therefore, the rate of heterogeneous solid reactions can be described as [
23]:
where
κ(
T) and
t are rate constant and time, respectively. α is the degree of conversion, ranging from 0 to 1.
f(
α) is the reaction model that represents a certain solid-state mechanism [
24]. The degree of conversion can be defined as:
where
m0,
mt, and
mf are the initial, instantaneous, and final mass of the sample. Integrating Equation (1) into Equation (3), the rate of a solid-state reaction can be expressed as:
where
E, A, and reaction model
f(α) are called kinetic triplets [
23]. If the reaction is studied under non-isothermal conditions at a linear heating rate,
β =
dT/
dt. In which Equation (5) becomes:
Actual solid-state reaction is complex, which includes multiple solid–gaseous reactions with different rates, such as adsorption, desorption, sublimation of gaseous products or reactants on the surface of a reacting solid, and diffusion of gaseous products or reactants through a solid [
24,
25].
Therefore, the effective activation energy derived from the overall kinetic data is a composite number determined by the activation energies of the individual stages and their relative contributions to the overall reaction rate. As a result, the effective activation energy is usually a function of either temperature and conversion, or merely temperature. That is why the concept of variable activation energy
Eα is more suitable to the non-elementary nature of heterogeneous solid-state reactions. It should be used to explain how overall reaction rates are affected by temperature [
26]. According to the previous studies, this obtained activation energy is the apparent one and does not have any mechanistic significance [
27].
2.1. Model-Free Methods
There are two basic methods to calculate the kinetic triplets of complex solid-state reactions, namely model-fitting and model-free (or iso-conversion) methods. The model-fitting approaches use a single unpredictable heating rate [
26], whereas the model-free method uses several heating rates, which eliminates mass transfer constraints. Dhaundiyal et al. [
15] employed a model-fitting approach (Coats Redfern method [
28]) to test the validity of the model-free method and produce the best appropriate reaction model for the decomposition of weed and lignocellulosic materials, respectively. They observed that kinetic parameters derived at various heating rates for models, differed significantly from those computed using the model-free method.
The force-fitting of non-isothermal data to a hypothetical reaction model is the initial cause of disagreement between model-free and model-based runs. Arrhenius parameters are calculated in the form of g(α) in the model fitting of Coats Redfern method, which is previously assumed and not able to discriminate separately temperature dependence of rate constant and conversion resulting in erroneous estimates of Arrhenius parameters.
The possibility of multi-reaction pathways is another key cause of disagreement, as the activation energy determined using the model-based method is a function of
T and
α. However, the calculated value of activation energy represents the average value for the overall process. It is derived in such a way that it is invariant with the reaction mechanism and kinetics that depend on the change in temperature and degree of conversion. With model-free methods, there is an opportunity for estimating the relative reactivity of solids by comparing the respective consumed time to accomplish the same extent of conversion at the same temperature. Therefore, these are called the iso-conversion method, and the parameters are calculated as a function of the conversion rate [
25,
27]. In other words, these methods used the rate data associated with a constant conversion to eliminate the reaction rate dependency on the conversion [
27]. Therefore, iso-conversional approaches, as recommended by [
27,
29], are more realistic and exact when modelling non-isothermal pyrolytic kinetics.
By integrating Equation (6), replacing
E/
RT by
x, and making rearranging Equation (7) can be written.
where
p(
x) is a function known as the Arrhenius integral, which can be calculated numerically or by using various approximations. When we utilize Doyle’s estimate [
29,
30] for
we get the popular equation proposed by Ozawa [
31] for determining the activation energy by iso-conversion methods from Equation (3). Doyle [
29,
30] reported that for 20 <
E/
RT < 60,
logp (
E/
RT) may be closely approximated by Equation (8) [
32]:
That can be reduced to Flynn–Wall–Ozawa equation [
33]:
where
Eα is the apparent activation energy or can also be called variable activation energy. The plot of
ln β against
1/T at different heating rates gives a straight line with the slope −1.052
Eα/R. Finding the slope for different (
α) reveals the dependency of
Eα on
α [
33].
Murray and White [
34,
35] provided a more precise estimate for 20 <
E/
RT < 50 in the form of Kissinger, sometimes known as the Kissinger–Akahira–Sunose (KAS) equation [
15,
36].
For a constant value of α, plotting
ln (β/T2) vs. 1/
T will give a straight line with a slope of
Eα/R. The profile of apparent activation energy can be generated for the different conversions [
37]. Starink approximated the expression of FWO and KAS method, which can be transformed into the same general formula as [
38]:
where
s = 0 and
B = 0.4567 for FWO method, and
s = 2 and
B = 1 for KAS method. According to Starink, both parameters can be adjusted to
s = 1.8 and
B = 1.0033. As a result, the Starink equation can be rewritten as [
30,
31]:
where
C = , for a given conversion fraction
α, the points of
ln (β/T1.8) versus 1/
T at different heating rates can be fitted to a straight line and the slope of the line corresponds to 1.0037
. As a result, the slope of the straight line can be used to estimate the value of
.
2.2. Criado Method
Many studies that have examined experimental data for the solid-state mechanism have used reference theoretical curves, referred to as “master plots” in their analyses [
34]. It uses the concept of reduced time plots, introduced by Ozawa [
24,
34] for
α against
t/
tα, where
tα is the time to reach a certain value of
α (usually 0.5 to 0.9). In this sense, the master plot is a characteristic curve independent of the measurement condition, which is readily obtained from experimental results [
39].
To use this method for the determination of the reaction mechanism, the known value of
Eα is required [
40]. For that, experimental data can be compared against a collection of theoretical model plots and the model that exactly matches the data can be chosen. This makes it possible to view the data in terms of the mechanism described by the chosen reaction model. The master theoretical curves can be generated through the
Z(
α) function [
39,
40]:
Z(
α) versus
α for different reaction mechanisms can be plotted as presented in
Table 1 [
29,
31,
36,
40]. The equation is given by:
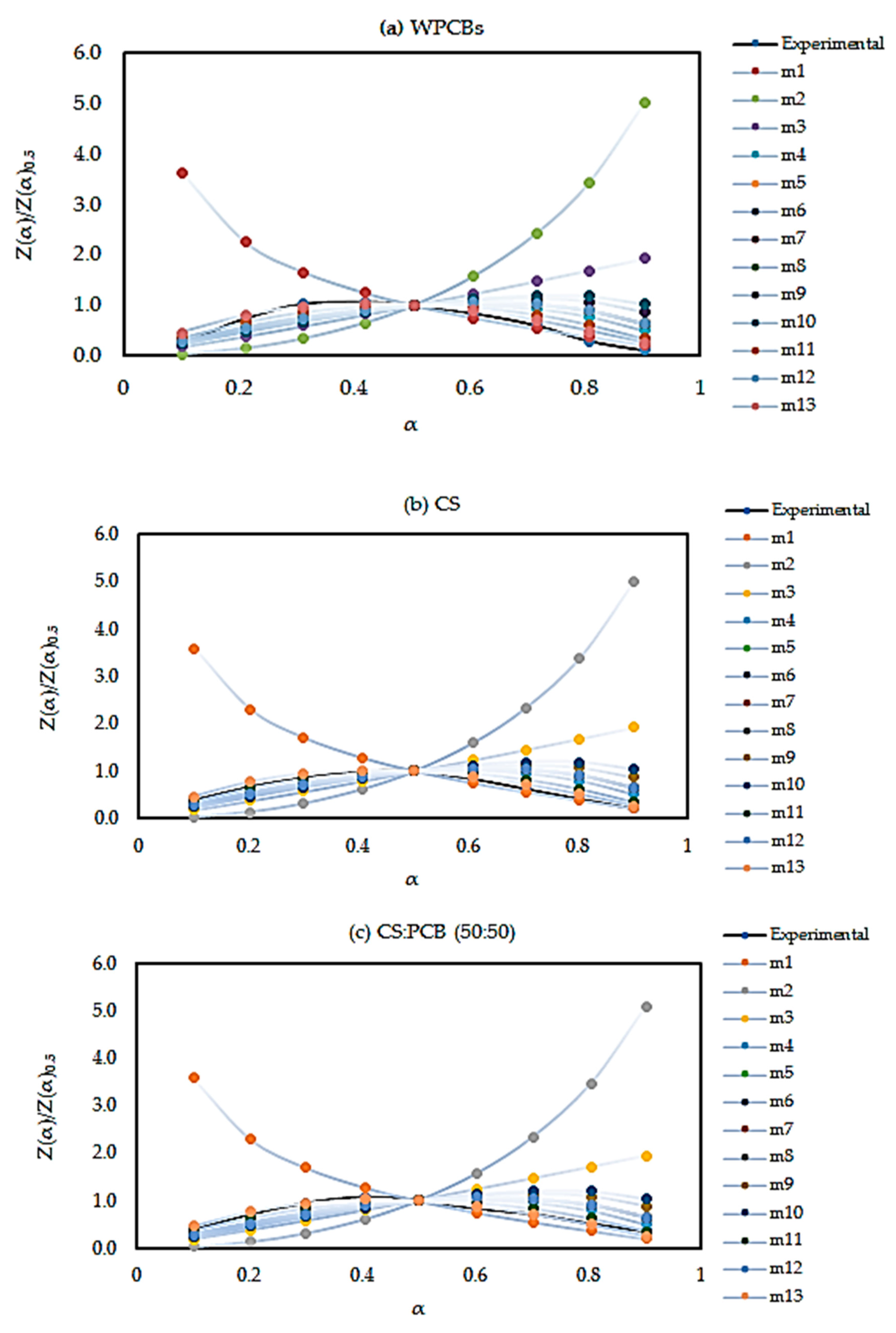
By plotting vs α for different mechanisms, a theoretical curve will be obtained. By the derived apparent activation energy plot, the experimental curve can be made. By comparing the two curves, the most reliable reaction mechanism can be found.
Table 1.
Different degradation mechanisms with f(α) and g(α).
Table 1.
Different degradation mechanisms with f(α) and g(α).
| No. | Function Name | Mechanisms | f(α) | g(α) |
|---|
| m1 | Jander equation | Diffusion, 3D (spherical symmetry) | 3/2(1 − α)2/3[1 − (1 − α)1/3]−1 | [1 − (1 − α)1/3]1/2 |
| m2 | G–B equation | Diffusion, 3D (column symmetry) | 3/2[(1 − α)−1/3 − 1]−1 | 1 − 2α/3 − (1 − α)2/3 |
| m3 | Anti–Jander equation | Diffusion, 3D | 3/2(1 + α)2/3[(1 + α)1/3 −1]−1 | [(1 + α)1/3 − 1]2 |
| m4 | Z–L–T equation | Diffusion, 3D | 3/2(1 − α)4/3[(1 − α)−1/3 − 1]−1 | [(1 − α)−1/3 − 1]2 |
| m5 | Avrami–Erofeev Equation | Random nucleation and nuclei growth, n = 3 | 3(1 − α) [−ln (1 − α)]2/3 | [−ln (1 − α)]1/3 |
| m6 | Avrami–Erofeev Equation | Random nucleation and nuclei growth, n = 2 | 2(1 − α) [−ln (1 − α)]1/2 | [−ln (1 − α)]1/2 |
| m7 | Avrami–Erofeev Equation | Random nucleation and nuclei growth, n = 3/2 | 3/2(1 − α) [−ln (1 − α)]1/3 | [−ln (1 − α)]2/3 |
| m8 | Avrami–Erofeev Equation | Random nucleation and nuclei growth, n = 4/3 | 4/3(1 − α) [−ln (1 − α)]1/4 | [−ln (1 − α)]3/4 |
| m9 | Geometrical contraction | Shrinkage geometric shape (column symmetry) | 3(1 − α)2/3 | 1 − (1 − α)1/3 |
| m10 | Geometrical contraction | Shrinkage geometric shape, (spherical symmetry) | 2(1 − α)1/2 | 1 − (1 − α)1/2 |
| m11 | Reaction order n = 2 | Chemical reaction | (1 − α)2 | (1 − α)−1 −1 |
| m12 | Reaction order n = 1 | Chemical reaction | (1 − α) | −ln(1-α) |
| m13 | Reaction order n = 3 | Chemical reaction | (1 − α)3 | ((1 − α)−2 −1)/2 |
6. Conclusions
The thermal decomposition at non-isothermal conditions for WPCBs, CS, and their mixture was investigated. A residue of WPCBs at 700 °C was found to be relatively very high (~60%) compared to 6% for CS and 20% for CS:WPCBs. This high residue of WPCBs is due to the content of high inorganic compounds. The degradation profiles of CS:WPCBs samples were initiated at a lower temperature compared to WPCBs and CS. The total mass loss for CS was not affected by an increased heating rate. However, the mass loss for WPCBs at 15 K/min and for CS:PCB at 10 K/min were significantly high. The weight losses at the final temperature were 42–50 wt%, 94–95 wt%, and 73–76 wt% for WPCBs, CS, and CS:WPCBs, respectively. Total weight loss was higher in samples which had a higher volatile matter and lower ash content.
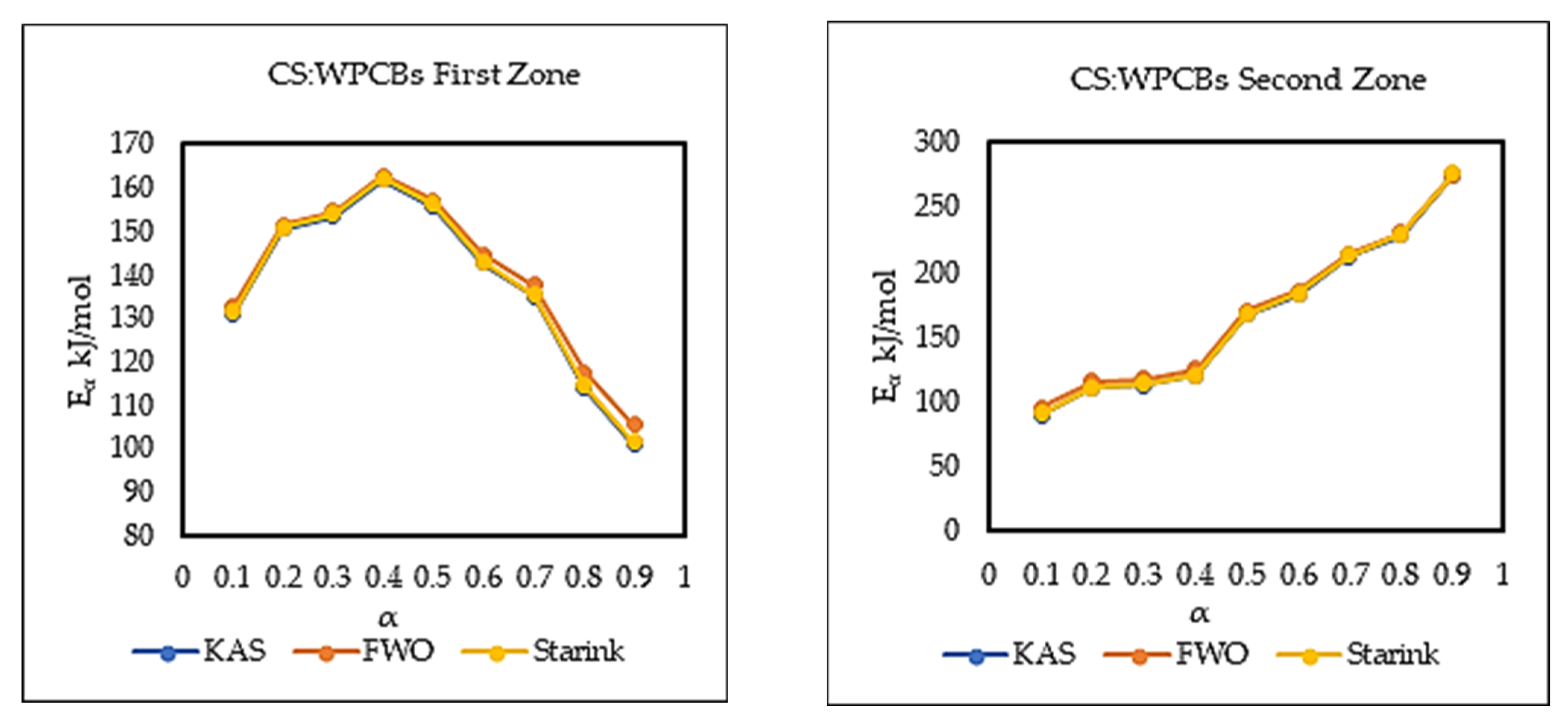
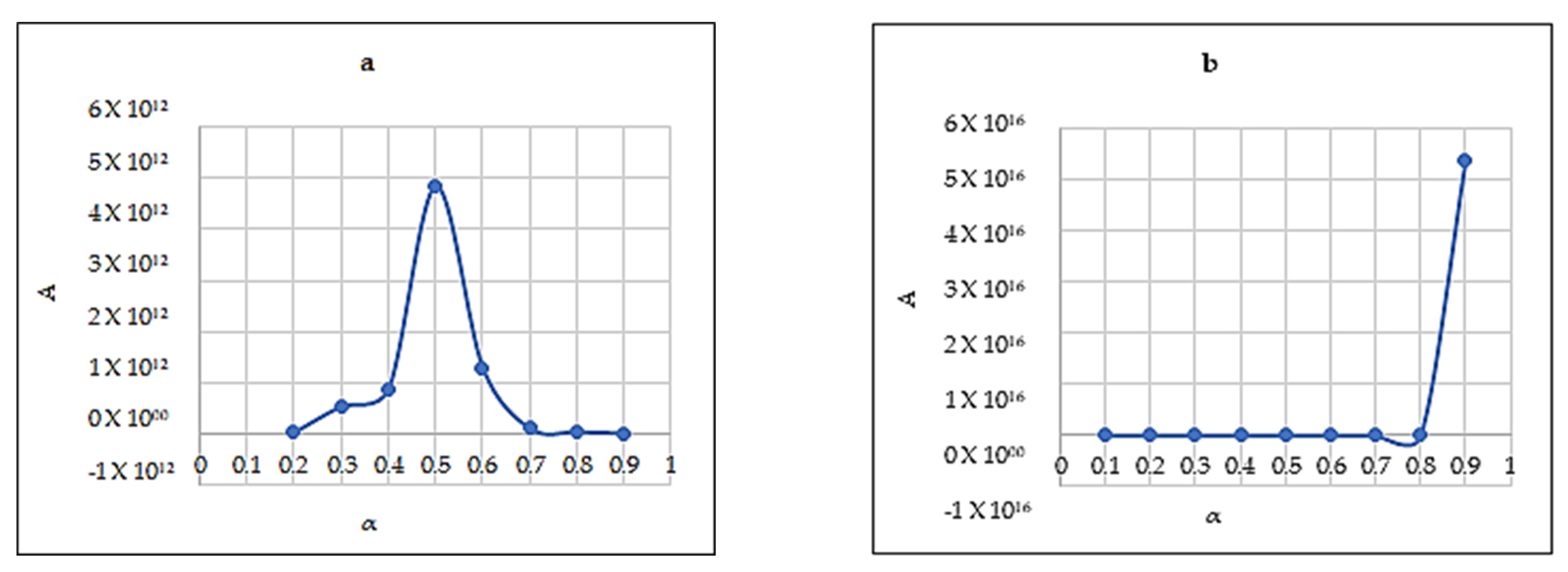
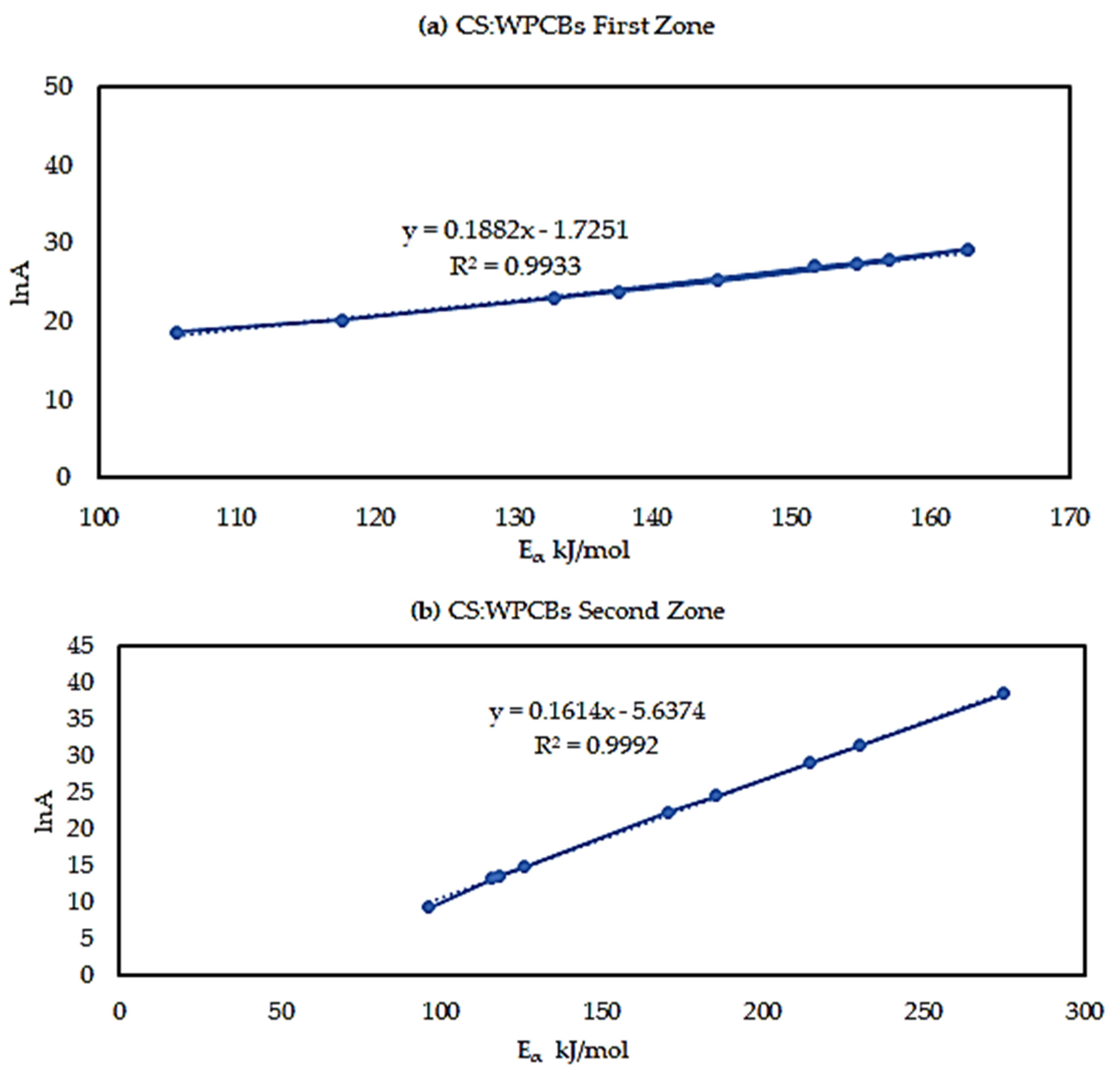
The Eα from three model-free methods were found to vary significantly. This exhibits the existence of a complex multi-reaction mechanism within the solid matrix, showing that the activation energy is dependent on the conversion mechanism. The kinetic parameters calculated using the Starink method displayed the same trend as those derived from the KAS method. Whereas, the Eα and R2 values from the FWO method are higher compared to those of KAS and Starink. FWO method was considered the most reliable method for the reaction mechanism. The average activation energy for all three sample was observed to be between 120 and 200 kJ/mol. Most importantly, PCB second zone has shown a negative value for apparent activation energy Eα. Moreover, the decomposition mechanism was found by the Criado method and it was best described by a third-order reaction, for all three samples. The study of the kinetic compensation effect was used to characterize the dependence of E and lnA. The results indicated that a compensation effect existed between the apparent activation energy and the pre-exponential factor during the pyrolysis process.