Validation of Two Theoretically Derived Equations for Predicting pH in CO2 Biomethanisation
Abstract
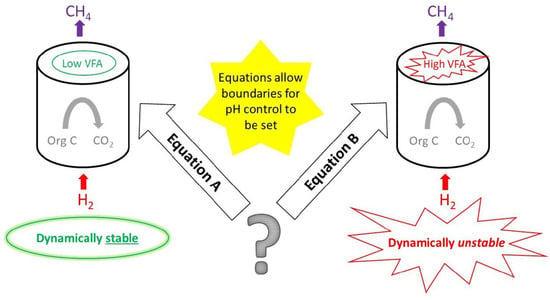
1. Introduction
2. Materials and Methods
2.1. Equations Relating pH and pCO2
2.2. Literature Data
2.3. Digester Set-Up and Operation
2.4. Analytical Methods
2.5. Performance Assessment and Statistical Analyses
3. Results
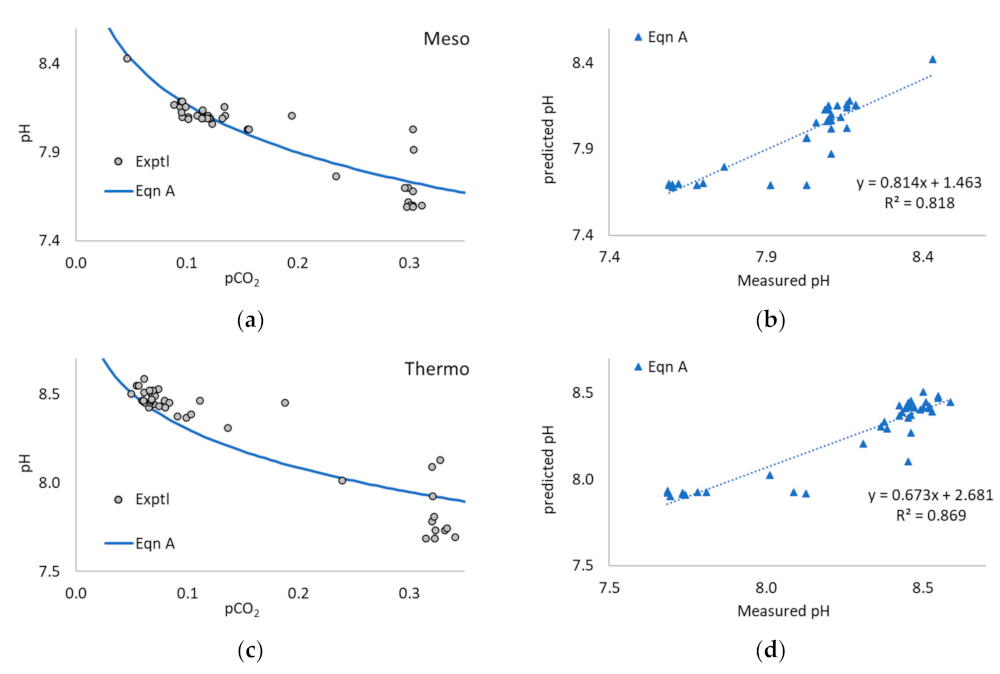
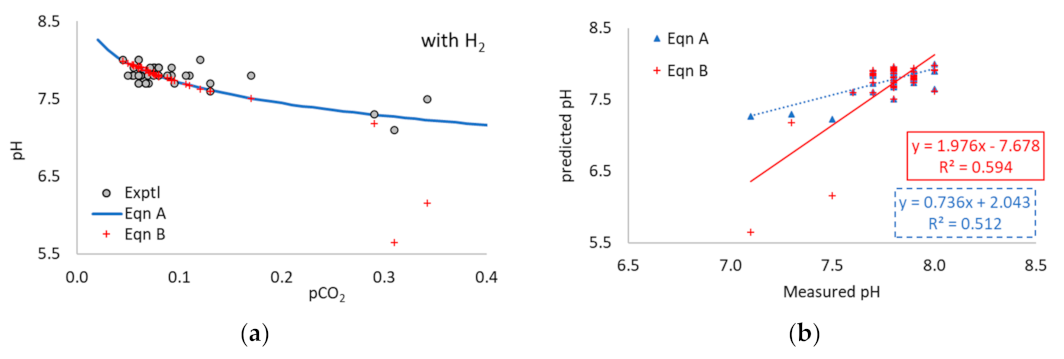
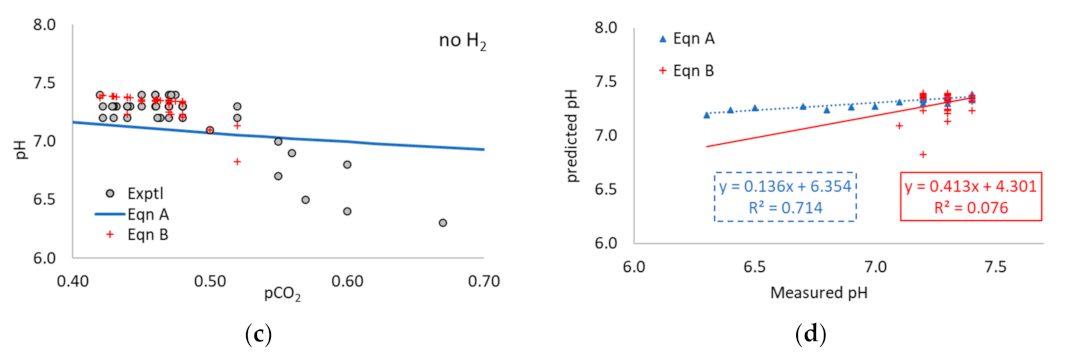
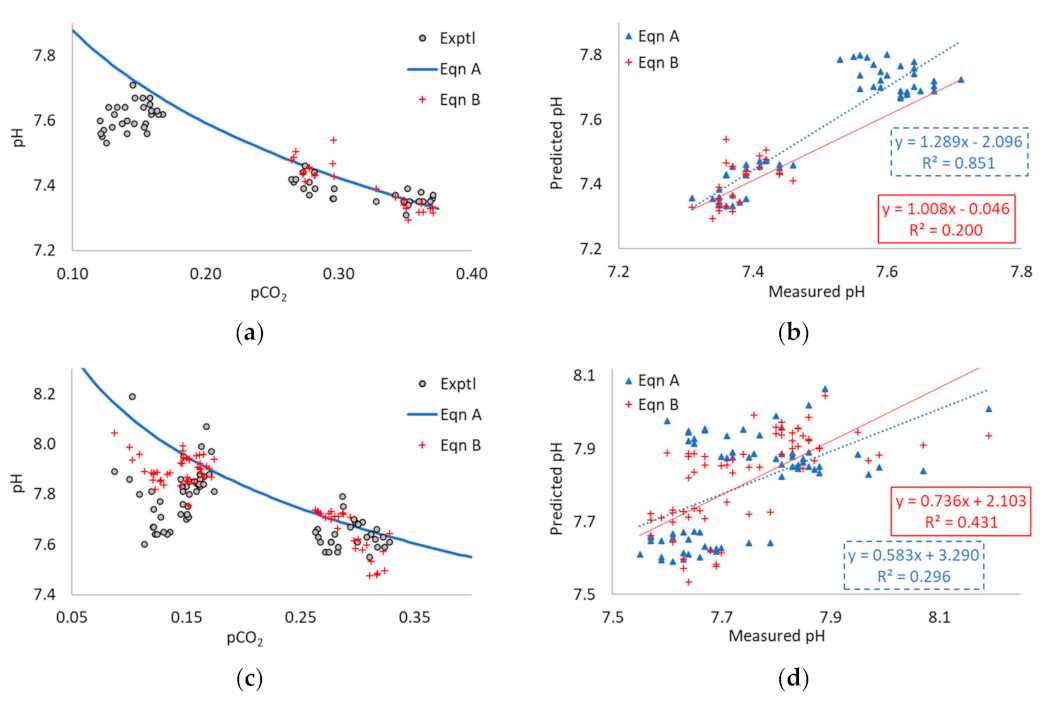
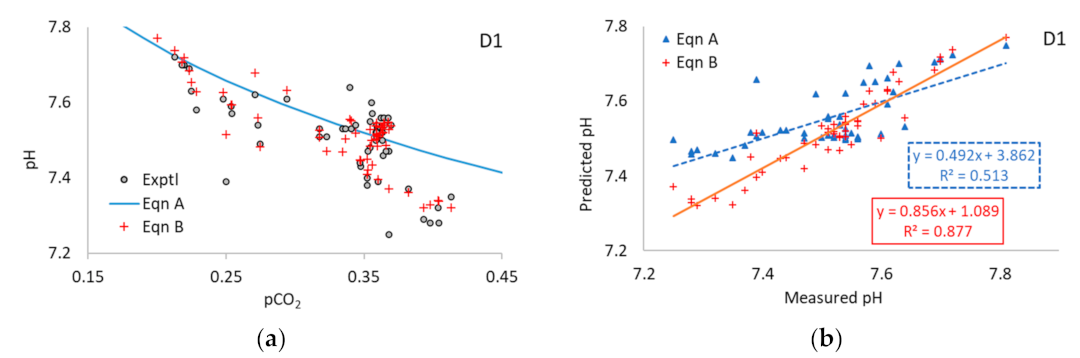
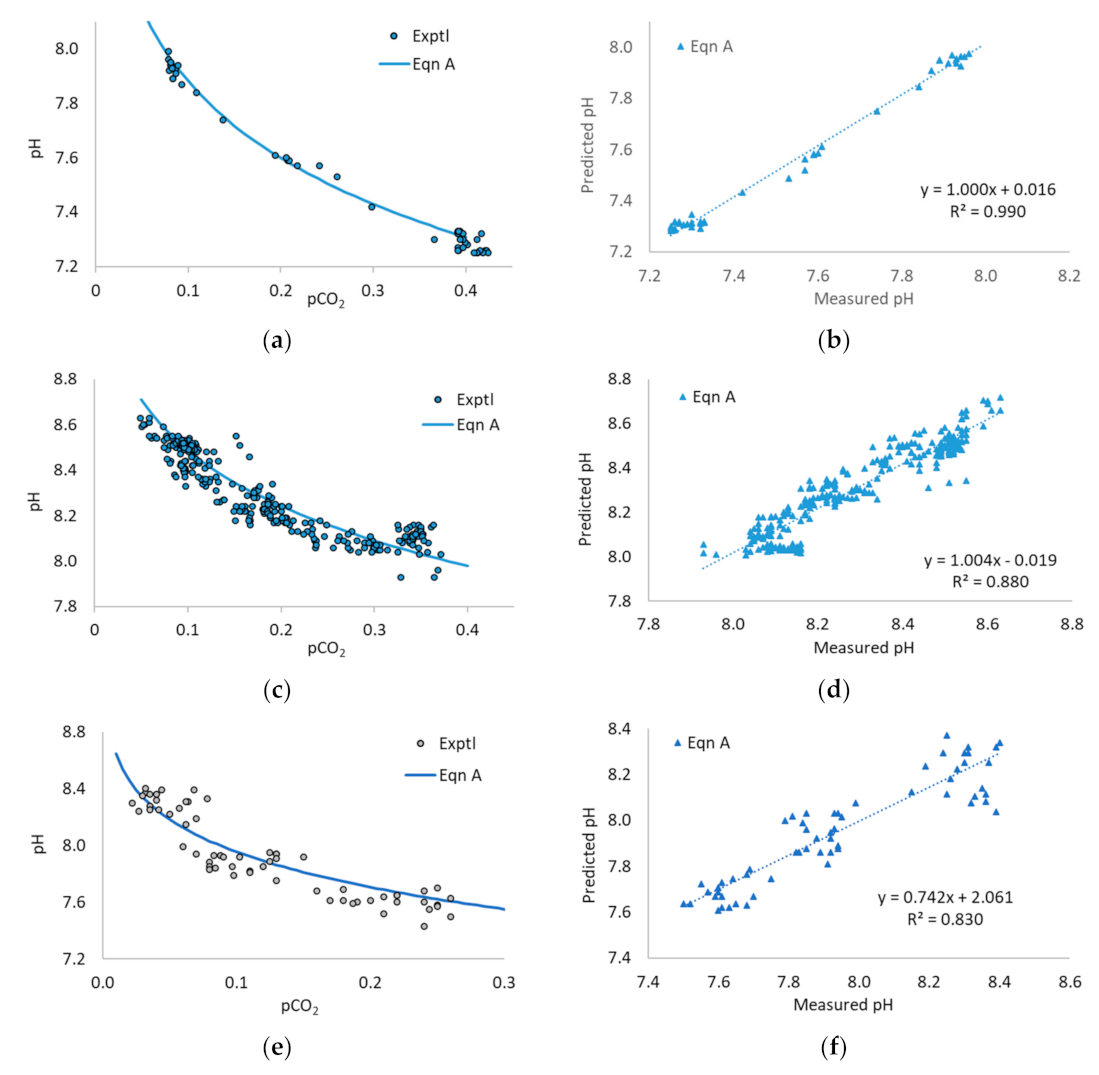
3.1. Application of Equation A to the Literature Data
3.2. Application of Equation B to the Literature Data
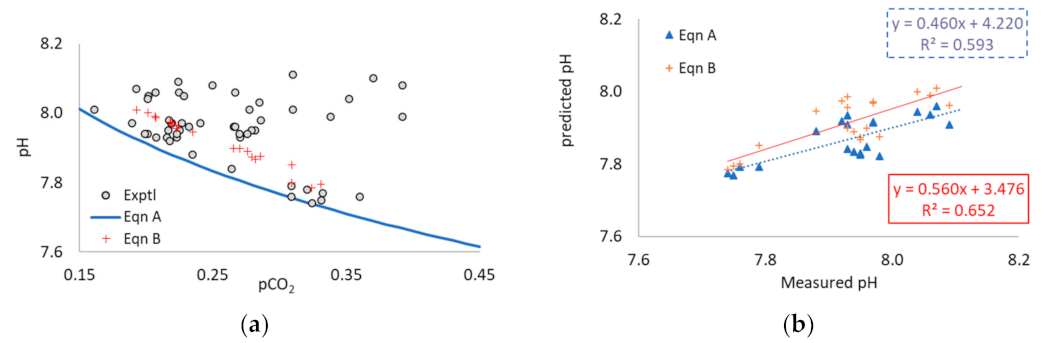
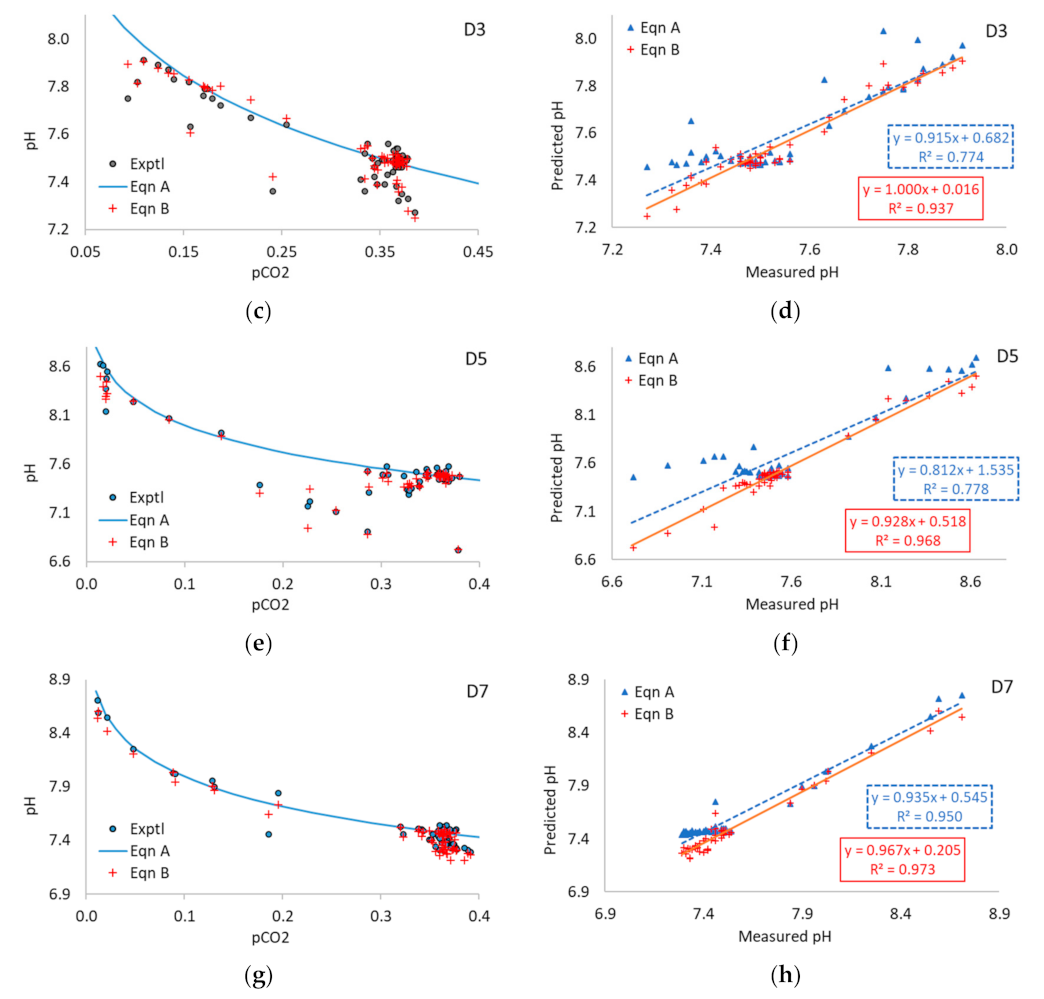
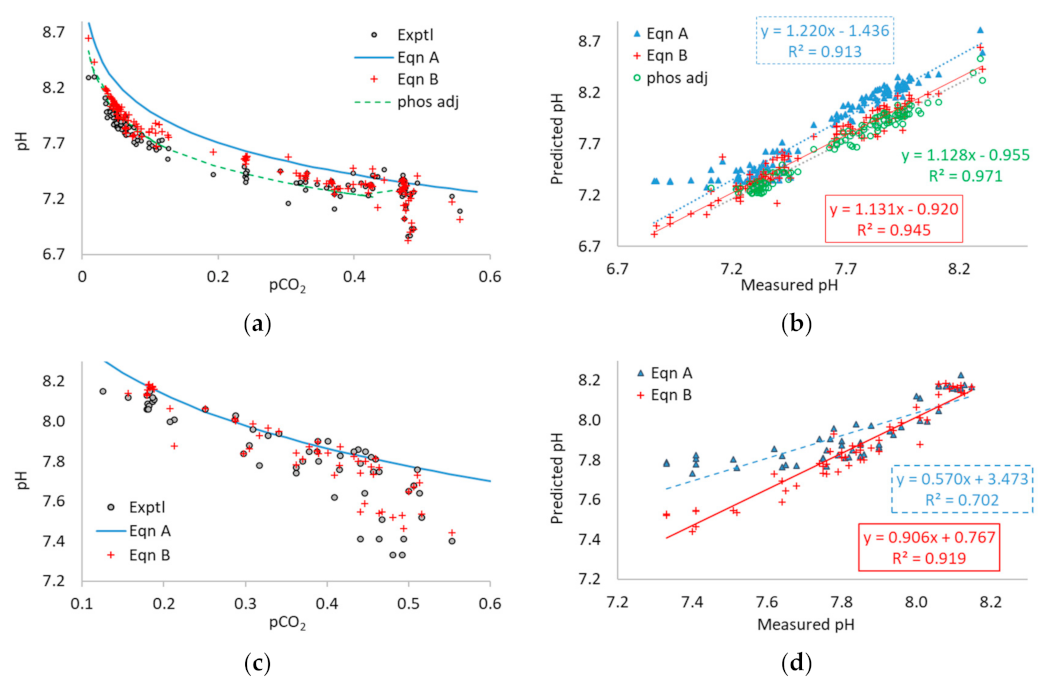
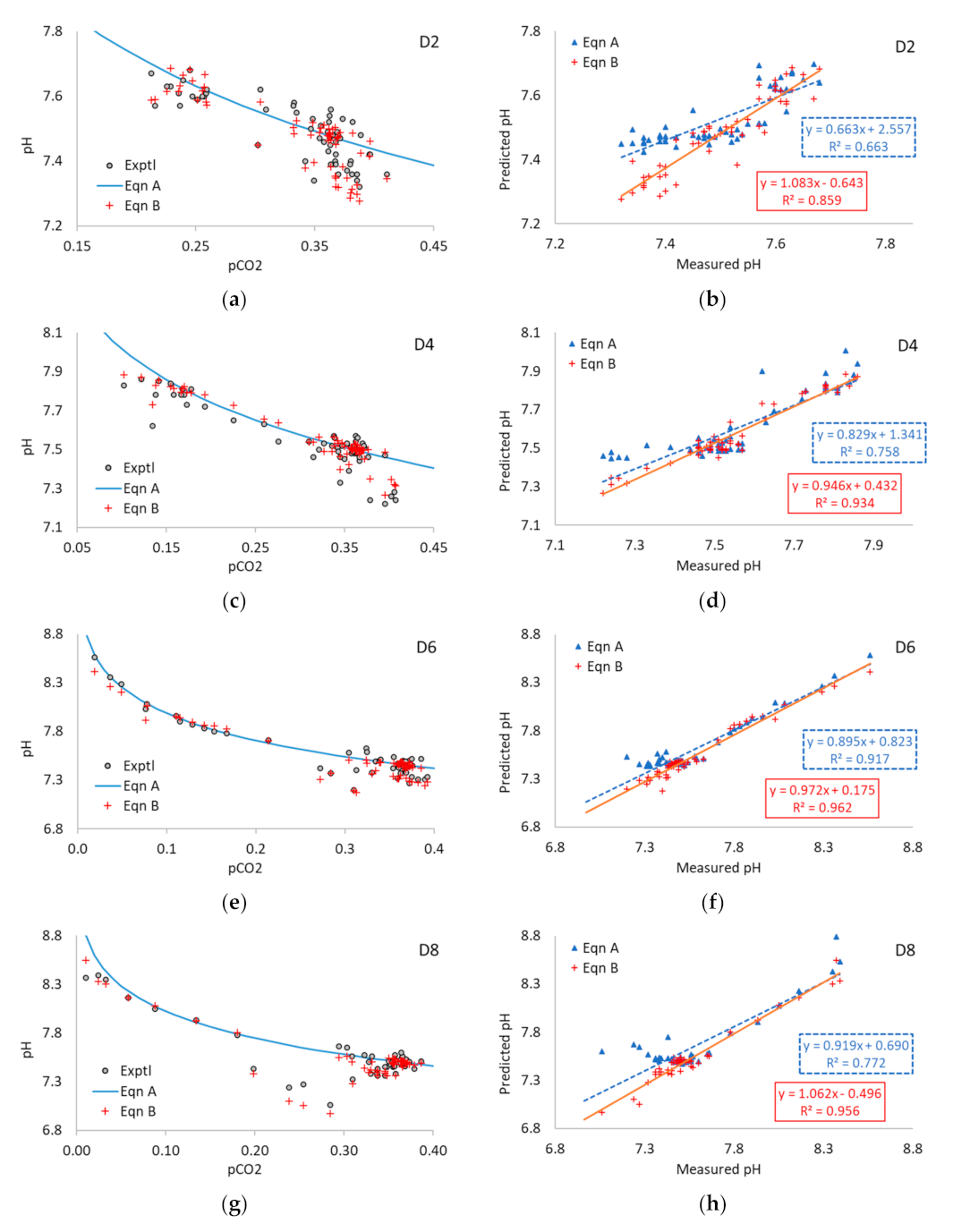
3.3. Application of Equations A and B to Multi-Point Data
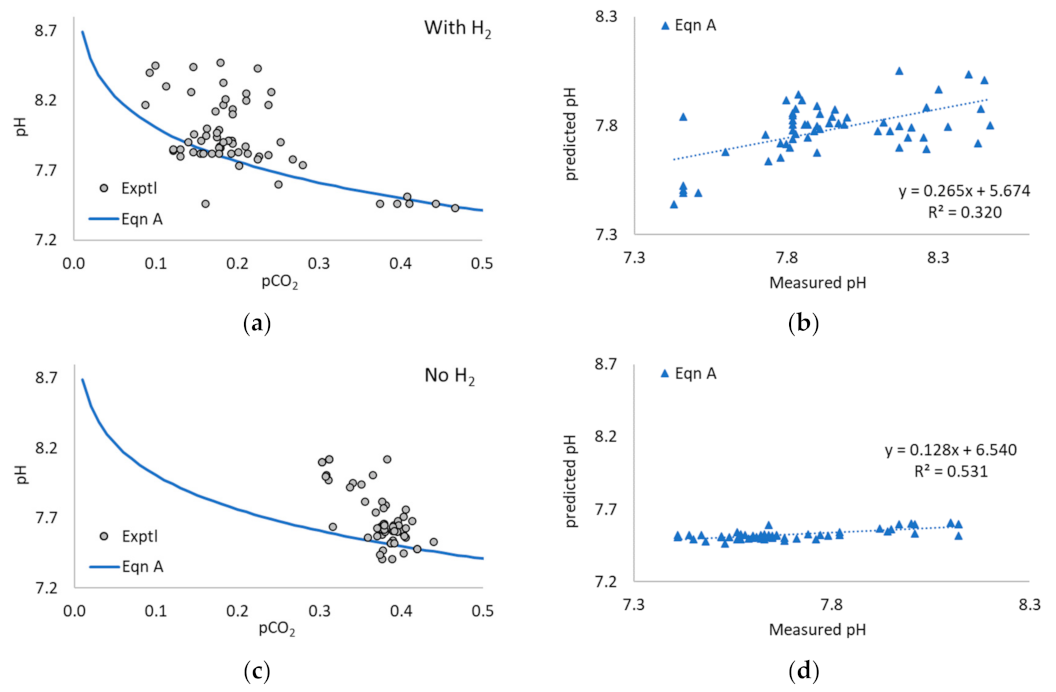
3.4. Experimental Trial of H2 Addition to Induce Digestion Instability
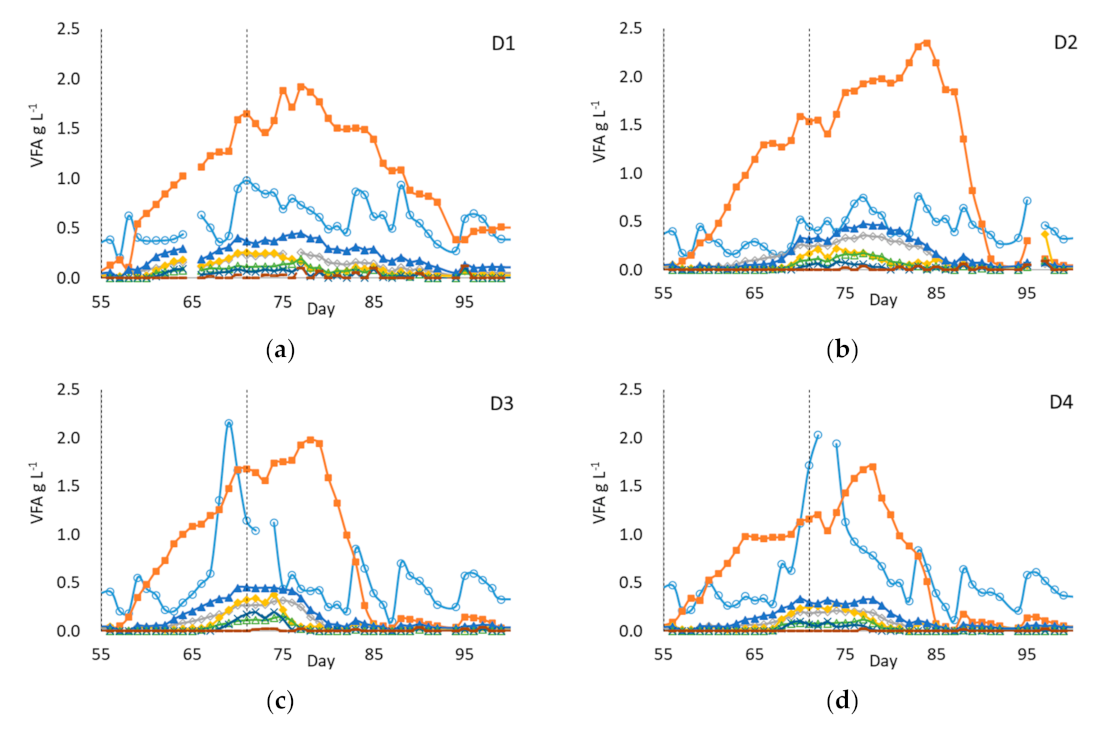
3.4.1. Digestion Performance
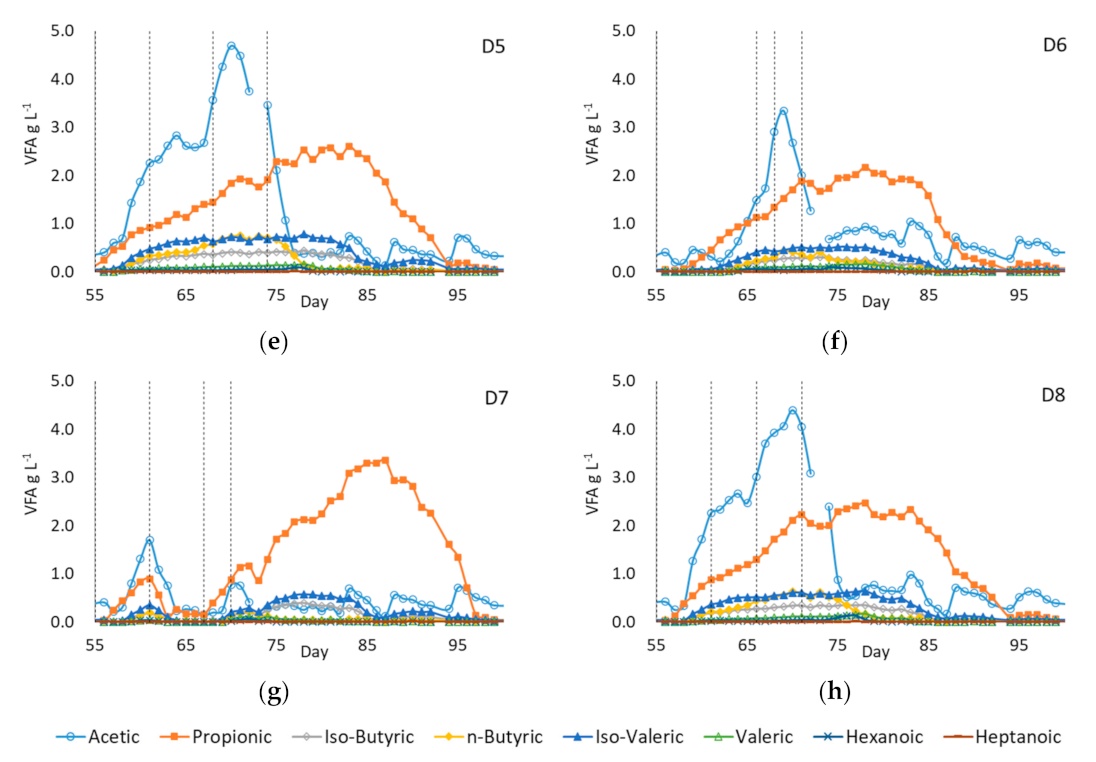
3.4.2. VFA Profiles
3.5. Application of Equations A and B to Experimental Data
4. Discussion
4.1. Methodological Issues with Data in Literature Studies
4.2. General Research-Related Issues
4.3. Considerations for Large-Scale Operation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Zeikus, J. The biology of methanogenic bacteria. Bacteriol. Rev. 1977, 41, 514–541. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Angelidaki, I. Integrated biogas upgrading and hydrogen utilization in an anaerobic reactor containing enriched hydrogenotrophic methanogenic culture. Biotechnol. Bioeng. 2012, 109, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2020, 12, 5259–5282. [Google Scholar] [CrossRef]
- Zavarkó, M.; Imre, A.R.; Pörzse, G.; Csedő, Z. Past, Present and Near Future: An Overview of Closed, Running and Planned Biomethanation Facilities in Europe. Energies 2021, 14, 5591. [Google Scholar] [CrossRef]
- Aryal, N.; Kvist, T.; Ammam, F.; Pant, D.; Ottosen, L.D. An overview of microbial biogas enrichment. Bioresour. Technol. 2018, 264, 359–369. [Google Scholar] [CrossRef]
- Tao, B.; Zhang, Y.; Heaven, S.; Banks, C.J. Predicting pH rise as a control measure for integration of CO2 biomethanisation with anaerobic digestion. Appl. Energy 2020, 277, 115535. [Google Scholar] [CrossRef]
- Andreides, D.; Stransky, D.; Bartackova, J.; Pokorna, D.; Zabranska, J. Syngas biomethanation in countercurrent flow trickle-bed reactor operated under different temperature conditions. Renew. Energy 2022, 199, 1329–1335. [Google Scholar] [CrossRef]
- Ghofrani-Isfahani, P.; Tsapekos, P.; Peprah, M.; Kougias, P.; Zervas, A.; Zhu, X.; Yang, Z.; Jacobsen, C.S.; Angelidaki, I. Ex-Situ biogas upgrading in thermophilic trickle bed reactors packed with micro-porous packing materials. Chemosphere 2022, 296, 133987. [Google Scholar] [CrossRef]
- Paniagua, S.; Lebrero, R.; Muñoz, R. Syngas biomethanation: Current state and future perspectives. Bioresour. Technol. 2022, 358, 127436. [Google Scholar] [CrossRef]
- Dong, D.; Choi, O.K.; Lee, J.W. Influence of the continuous addition of zero valent iron (ZVI) and nano-scaled zero valent iron (nZVI) on the anaerobic biomethanation of carbon dioxide. Chem. Eng. J. 2022, 430, 132233. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; Ter Heijne, A. Bioelectrochemical power-to-gas: State of the art and future perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef]
- Bywater, A.; Heaven, S.; Zhang, Y.; Banks, C.J. Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the United Kingdom. Processes 2022, 10, 1202. [Google Scholar] [CrossRef]
- D’Silva, T.C.; Isha, A.; Chandra, R.; Vijay, V.K.; Subbarao, P.M.V.; Kumar, R.; Chaudhary, V.P.; Singh, H.; Khan, A.A.; Tyagi, V.K. Enhancing methane productionh in anaerobic digestion through hydrogen assisted pathways—A state-of-the-art review. Renew. Sustain. Energy Rev. 2021, 151, 111536. [Google Scholar] [CrossRef]
- Lai, C.S.; McCulloch, M.D. Sizing of stand-alone solar PV and storage system with anaerobic digestion biogas power plants. IEEE Trans. Ind. Electron. 2016, 64, 2112–2121. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Jin, R.; Li, G.; Zhou, M.; Xia, Q. Integrating biogas in regional energy systems to achieve near-zero carbon emissions. Appl. Energy 2022, 322, 119515. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Co-digestion of manure and whey for in situ biogas upgrading by the addition of H2: Process performance and microbial insights. Appl. Microbiol. Biotechnol. 2013, 97, 1373–1381. [Google Scholar] [CrossRef]
- Luo, G.; Johansson, S.; Boe, K.; Xie, L.; Zhou, Q.; Angelidaki, I. Simultaneous hydrogen utilization and in situ biogas upgrading in an anaerobic reactor. Biotechnol. Bioeng. 2012, 109, 1088–1094. [Google Scholar] [CrossRef]
- Drosg, B. Process Monitoring in Biogas Plants; IEA Bioenergy: Paris, France, 2013. [Google Scholar]
- Gaida, D.; Wolf, C.; Bongards, M. Feed control of anaerobic digestion processes for renewable energy production: A review. Renew. Sustain. Energy Rev. 2017, 68, 869–875. [Google Scholar] [CrossRef]
- Nguyen, D.; Gadhamshetty, V.; Nitayavardhana, S.; Khanal, S.K. Automatic process control in anaerobic digestion technology: A critical review. Bioresour. Technol. 2015, 193, 513–522. [Google Scholar] [CrossRef]
- Wade, M.J. Not just numbers: Mathematical modelling and its contribution to anaerobic digestion processes. Processes 2020, 8, 888. [Google Scholar] [CrossRef]
- Tao, B.; Alessi, A.M.; Zhang, Y.; Chong, J.P.; Heaven, S.; Banks, C.J. Simultaneous biomethanisation of endogenous and imported CO2 in organically loaded anaerobic digesters. Appl. Energy 2019, 247, 670–681. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Ripley, L.E.; Boyle, W.C.; Converse, J.C. Improved alkalimetric monitoring for anaerobic digestion of high-strength wastes. J. (Water Pollut. Control Fed.) 1986, 58, 406–411. [Google Scholar]
- Walker, M.; Zhang, Y.; Heaven, S.; Banks, C. Potential errors in the quantitative evaluation of biogas production in anaerobic digestion processes. Bioresour. Technol. 2009, 100, 6339–6346. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Agneessens, L.M.; Ottosen, L.D.M.; Andersen, M.; Olesen, C.B.; Feilberg, A.; Kofoed, M.V.W. Parameters affecting acetate concentrations during in-situ biological hydrogen methanation. Bioresour. Technol. 2018, 258, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Agneessens, L.M.; Ottosen, L.D.M.; Voigt, N.V.; Nielsen, J.L.; de Jonge, N.; Fischer, C.H.; Kofoed, M.V.W. In-situ biogas upgrading with pulse H2 additions: The relevance of methanogen adaption and inorganic carbon level. Bioresour. Technol. 2017, 233, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. H2 addition through a submerged membrane for in-situ biogas upgrading in the anaerobic digestion of sewage sludge. Bioresour. Technol. 2019, 280, 1–8. [Google Scholar] [CrossRef]
- Andreides, D.; Pokorna, D.; Zabranska, J. Assessing the syngas biomethanation in anaerobic sludge digestion under different syngas loading rates and homogenisation. Fuel 2022, 320, 123929. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Treu, L.; Angelidaki, I. Biogas upgrading via hydrogenotrophic methanogenesis in two-stage continuous stirred tank reactors at mesophilic and thermophilic conditions. Environ. Sci. Technol. 2015, 49, 12585–12593. [Google Scholar] [CrossRef]
- Corbellini, V.; Catenacci, A.; Malpei, F. Hydrogenotrophic biogas upgrading integrated into WWTPs: Enrichment strategy. Water Sci. Technol. 2019, 79, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Corbellini, V.; Feng, C.; Bellucci, M.; Catenacci, A.; Stella, T.; Espinoza-Tofalos, A.; Malpei, F. Performance Analysis and Microbial Community Evolution of In Situ Biological Biogas Upgrading with Increasing H2/CO2 Ratio. Archaea 2021, 2021, 8894455. [Google Scholar] [CrossRef] [PubMed]
- Díaz, I.; Fdz-Polanco, F.; Mutsvene, B.; Fdz-Polanco, M. Effect of operating pressure on direct biomethane production from carbon dioxide and exogenous hydrogen in the anaerobic digestion of sewage sludge. Appl. Energy 2020, 280, 115915. [Google Scholar] [CrossRef]
- Khan, A.; Akbar, S.; Okonkwo, V.; Smith, C.; Khan, S.; Shah, A.A.; Adnan, F.; Ijaz, U.Z.; Ahmed, S.; Badshah, M. Enrichment of the hydrogenotrophic methanogens for, in-situ biogas up-gradation by recirculation of gases and supply of hydrogen in methanogenic reactor. Bioresour. Technol. 2022, 345, 126219. [Google Scholar] [CrossRef]
- Kim, S.; Mostafa, A.; Im, S.; Lee, M.-K.; Kang, S.; Na, J.-G.; Kim, D.-H. Production of high-calorific biogas from food waste by integrating two approaches: Autogenerative high-pressure and hydrogen injection. Water Res. 2021, 194, 116920. [Google Scholar] [CrossRef]
- Lebranchu, A.; Blanchard, F.; Fick, M.; Pacaud, S.; Olmos, E.; Delaunay, S. Pilot-scale biomethanation of cattle manure using dense membranes. Bioresour. Technol. 2019, 284, 430–436. [Google Scholar] [CrossRef]
- Lovato, G.; Alvarado-Morales, M.; Kovalovszki, A.; Peprah, M.; Kougias, P.G.; Rodrigues, J.A.D.; Angelidaki, I. In-situ biogas upgrading process: Modeling and simulations aspects. Bioresour. Technol. 2017, 245, 332–341. [Google Scholar] [CrossRef]
- Luo, G.; Angelidaki, I. Hollow fiber membrane based H2 diffusion for efficient in situ biogas upgrading in an anaerobic reactor. Appl. Microbiol. Biotechnol. 2013, 97, 3739–3744. [Google Scholar] [CrossRef]
- Luo, G.; Wang, W.; Angelidaki, I. Anaerobic digestion for simultaneous sewage sludge treatment and CO biomethanation: Process performance and microbial ecology. Environ. Sci. Technol. 2013, 47, 10685–10693. [Google Scholar] [CrossRef]
- Treu, L.; Kougias, P.; de Diego-Díaz, B.; Campanaro, S.; Bassani, I.; Fernández-Rodríguez, J.; Angelidaki, I. Two-year microbial adaptation during hydrogen-mediated biogas upgrading process in a serial reactor configuration. Bioresour. Technol. 2018, 264, 140–147. [Google Scholar] [CrossRef]
- Treu, L.; Tsapekos, P.; Peprah, M.; Campanaro, S.; Giacomini, A.; Corich, V.; Kougias, P.G.; Angelidaki, I. Microbial profiling during anaerobic digestion of cheese whey in reactors operated at different conditions. Bioresour. Technol. 2019, 275, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Voelklein, M.; Rusmanis, D.; Murphy, J. Biological methanation: Strategies for in-situ and ex-situ upgrading in anaerobic digestion. Appl. Energy 2019, 235, 1061–1071. [Google Scholar] [CrossRef]
- Wahid, R.; Horn, S.J. Impact of operational conditions on methane yield and microbial community composition during biological methanation in in situ and hybrid reactor systems. Biotechnol. Biofuels 2021, 14, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wahid, R.; Horn, S.J. The effect of mixing rate and gas recirculation on biological CO2 methanation in two-stage CSTR systems. Biomass Bioenergy 2021, 144, 105918. [Google Scholar] [CrossRef]
- Wahid, R.; Mulat, D.G.; Gaby, J.C.; Horn, S.J. Effects of H2:CO2 ratio and H2 supply fluctuation on methane content and microbial community composition during in-situ biological biogas upgrading. Biotechnol. Biofuels 2019, 12, 1–15. [Google Scholar] [CrossRef]
- Wang, W.; Xie, L.; Luo, G.; Zhou, Q.; Angelidaki, I. Performance and microbial community analysis of the anaerobic reactor with coke oven gas biomethanation and in situ biogas upgrading. Bioresour. Technol. 2013, 146, 234–239. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Zhang, J.; Mao, K.; Kurbonova, M.; Liu, G.; Zhang, R.; Wang, W. Improvement of biofuel recovery from food waste by integration of anaerobic digestion, digestate pyrolysis and syngas biomethanation under mesophilic and thermophilic conditions. J. Clean. Prod. 2020, 256, 120594. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Chen, Y.; Sun, X.; Liu, X.; Li, D. Effects of mixing and sodium formate on thermophilic in-situ biogas upgrading by H2 addition. J. Clean. Prod. 2019, 216, 373–381. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Differences of methanogenesis between mesophilic and thermophilic in situ biogas-upgrading systems by hydrogen addition. J. Ind. Microbiol. Biotechnol. 2019, 46, 1569–1581. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Chen, Y.; Cao, Q.; Liu, X.; Li, D. Effect of H2 addition on the microbial community structure of a mesophilic anaerobic digestion system. Energy 2020, 198, 117368. [Google Scholar] [CrossRef]
- Bassani, I.; Kougias, P.G.; Angelidaki, I. In-situ biogas upgrading in thermophilic granular UASB reactor: Key factors affecting the hydrogen mass transfer rate. Bioresour. Technol. 2016, 221, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Campanaro, S.; Kougias, P.; Treu, L.; Angelidaki, I.; Zhang, S.; Luo, G. Anaerobic granular sludge for simultaneous biomethanation of synthetic wastewater and CO with focus on the identification of CO-converting microorganisms. Water Res. 2017, 126, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, L.; Imatoukene, N.; Lemaire, J.; Mounkaila, M.; Filali, R.; Lopez, M.; Theoleyre, M.-A. In-situ biogas upgrading by bio-methanation with an innovative membrane bioreactor combining sludge filtration and H2 injection. Bioresour. Technol. 2021, 337, 125444. [Google Scholar] [CrossRef]
- Hafuka, A.; Fujino, S.; Kimura, K.; Oshita, K.; Konakahara, N.; Takahashi, S. In-situ biogas upgrading with H2 addition in an anaerobic membrane bioreactor (AnMBR) digesting waste activated sludge. Sci. Total Environ. 2022, 828, 154573. [Google Scholar] [CrossRef] [PubMed]
- Illi, L.; Lecker, B.; Lemmer, A.; Müller, J.; Oechsner, H. Biological methanation of injected hydrogen in a two-stage anaerobic digestion process. Bioresour. Technol. 2021, 333, 125126. [Google Scholar] [CrossRef]
- Thapa, A.; Park, J.-G.; Yang, H.-M.; Jun, H.-B. In-situ biogas upgrading in an anaerobic trickling filter bed reactor treating a thermal post-treated digestate. J. Environ. Chem. Eng. 2021, 9, 106780. [Google Scholar] [CrossRef]
- Corbellini, V.; Kougias, P.G.; Treu, L.; Bassani, I.; Malpei, F.; Angelidaki, I. Hybrid biogas upgrading in a two-stage thermophilic reactor. Energy Convers. Manag. 2018, 168, 1–10. [Google Scholar] [CrossRef]
- Andreides, D.; Quispe, J.I.B.; Bartackova, J.; Pokorna, D.; Zabranska, J. A novel two-stage process for biological conversion of syngas to biomethane. Bioresour. Technol. 2021, 327, 124811. [Google Scholar] [CrossRef]
- Scholten, J.C.; Conrad, R. Energetics of syntrophic propionate oxidation in defined batch and chemostat cocultures. Appl. Environ. Microbiol. 2000, 66, 2934–2942. [Google Scholar] [CrossRef]
- Fontana, A.; Kougias, P.G.; Treu, L.; Kovalovszki, A.; Valle, G.; Cappa, F.; Morelli, L.; Angelidaki, I.; Campanaro, S. Microbial activity response to hydrogen injection in thermophilic anaerobic digesters revealed by genome-centric metatranscriptomics. Microbiome 2018, 6, 194. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.; Pavlostathis, S.; Rozzi, A.; Sanders, W.; Siegrist, H.; Vavilin, V. The IWA anaerobic digestion model no 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Heaven, S.; Zhang, Y.; Bywater, A.; Banks, C. Dataset for ‘Potential for Biomethanisation of CO2 from Anaerobic Digestion of Organic Wastes in the UK’. 2022. Available online: https://eprints.soton.ac.uk/467644/1/processes_10_01202_1_.pdf (accessed on 6 June 2022).
- Palù, M.; Peprah, M.; Tsapekos, P.; Kougias, P.; Campanaro, S.; Angelidaki, I.; Treu, L. In-situ biogas upgrading assisted by bioaugmentation with hydrogenotrophic methanogens during mesophilic and thermophilic co-digestion. Bioresour. Technol. 2022, 348, 126754. [Google Scholar] [CrossRef] [PubMed]

| (i) | (ii) | (iii) | (iv) | (v) | (vi) | RMSD | R2 | ||
|---|---|---|---|---|---|---|---|---|---|
| No H2 | With H2 | No H2 | With H2 | No H2 | With H2 | ||||
| Temp | °C | 55 | 55 | 55 | 55 | 55 | 55 | - | - |
| OLR | g VS L−1 day−1 | 1.67 | 1.67 | 1.67 | 1.67 | 1.67 | 1.67 | - | - |
| HRT | days | 15 | 15 | 15 | 15 | 15 | 15 | - | - |
| H2 input | L L−1 day−1 | 0 | 0.93 | 0 | 1.44 | 0 | 1.76 | - | - |
| SMPtot | L CH4 g−1 VS | 0.275 | 0.407 | 0.271 | 0.478 | 0.287 | 0.515 | - | - |
| CH4 | % | 54.2 | 78.4 | 53.1 | 90.2 | 55.4 | 96.1 | - | - |
| TAN | M | n/r | n/r | n/r | n/r | n/r | n/r | - | - |
| VFA | M | 0.002 | 0.004 | 0.002 | 0.012 | 0.002 | 0.038 | - | - |
| pH | - | 7.29 | 7.61 | 7.28 | 7.9 | 7.3 | 8.31 | - | - |
| pCO2 | - | 0.458 | 0.216 | 0.469 | 0.098 | 0.446 | 0.039 | - | - |
| a × 108 | 10.41 | 9.81 | 10.42 | 9.81 | 10.43 | 6.99 | - | - | |
| Difference between experimental and predicted pH based on Equation A with coefficient a(x) | |||||||||
| a(i) | - | - | 0.02 | 0.00 | 0.02 | 0.00 | 0.12 | 0.06 | 0.997 |
| a(ii) | - | 0.02 | - | 0.02 | 0.00 | 0.02 | 0.10 | 0.05 | 0.997 |
| a(iii) | - | 0.00 | 0.02 | - | 0.02 | 0.00 | 0.12 | 0.06 | 0.997 |
| a(iv) | - | 0.02 | 0.00 | 0.02 | - | 0.02 | 0.10 | 0.05 | 1.000 |
| a(v) | - | 0.00 | 0.02 | 0.00 | 0.02 | - | 0.12 | 0.06 | 0.997 |
| a(vi) | - | 0.16 | 0.13 | 0.16 | 0.12 | 0.16 | - | 0.15 | 1.000 |
| (i) | (ii) | (iii) | (iv) | (v) | (vi) | (vii) | RMSD | R2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 2 | 3a | 3b | 3c | 3d | 3e | |||
| H2/CO2 | 0 | 2 | 4 | 4 | 4 | 4 | 4 | |||
| Mixing | Rpm | 80 | 80 | 80 | 120 | 140 | 170 | 200 | - | - |
| Temp | °C | 55 | 55 | 55 | 55 | 55 | 55 | 55 | - | - |
| OLR | g VS L−1 day−1 | 3.09 | 2.69 | 2.30 | 2.36 | 2.19 | 2.37 | 2.32 | - | - |
| HRT | Days | 20 | 20 | 20 | 20 | 20 | 20 | 20 | - | - |
| H2 input | L L−1 day−1 | 0.0 | 0.8 | 1.6 | 1.7 | 1.7 | 1.7 | 1.7 | - | - |
| SMP total | L CH4 g−1 VS | 0.217 | 0.246 | 0.305 | 0.331 | 0.411 | 0.321 | 0.315 | - | - |
| CH4 | % | 62.2 | 37.8 | 31.6 | 38.3 | 40.3 | 41.9 | 40.2 | - | - |
| TAN | M | 0.10 | 0.11 | 0.13 | 0.15 | 0.14 | 0.14 | 0.14 | - | - |
| VFA | M | 0.005 | 0.006 | 0.008 | 0.011 | 0.012 | 0.016 | 0.015 | - | - |
| pH | - | 7.86 | 8.07 | 8.25 | 8.37 | 8.41 | 8.43 | 8.41 | - | - |
| pCO2 | - | 0.378 | 0.207 | 0.137 | 0.124 | 0.119 | 0.116 | 0.120 | - | - |
| a × 108 | - | 2.85 | 2.82 | 2.43 | 1.81 | 1.63 | 1.56 | 1.62 | - | - |
| b × 108 | - | 0.255 | 0.277 | 0.278 | 0.233 | 0.193 | 0.162 | 0.171 | - | - |
| Difference between experimental and predicted pH based on Equation A with coefficient a(x) | ||||||||||
| a(i) | - | - | 0.00 | 0.05 | 0.14 | 0.17 | 0.18 | 0.17 | 0.14 | 0.949 |
| a(ii) | - | 0.00 | - | 0.05 | 0.13 | 0.16 | 0.18 | 0.16 | 0.13 | 0.966 |
| a(iii) | - | 0.06 | 0.05 | - | 0.09 | 0.12 | 0.13 | 0.12 | 0.10 | 0.978 |
| a(iv) | - | 0.16 | 0.14 | 0.09 | - | 0.03 | 0.04 | 0.03 | 0.10 | 0.955 |
| a(v) | - | 0.19 | 0.17 | 0.12 | 0.03 | - | 0.01 | 0.00 | 0.12 | 0.956 |
| a(vi) | - | 0.21 | 0.19 | 0.13 | 0.04 | 0.01 | - | 0.01 | 0.13 | 0.958 |
| a(vii) | - | 0.19 | 0.18 | 0.12 | 0.03 | 0.00 | 0.01 | - | 0.12 | 0.957 |
| Difference between experimental and predicted pH based on Equation B with coefficient b(x) | ||||||||||
| b(i) | - | - | 0.03 | 0.03 | 0.03 | 0.08 | 0.12 | 0.11 | 0.08 | 0.853 |
| b(ii) | - | 0.03 | - | 0.00 | 0.05 | 0.10 | 0.15 | 0.13 | 0.09 | 0.940 |
| b(iii) | - | 0.03 | 0.00 | - | 0.05 | 0.10 | 0.15 | 0.13 | 0.09 | 0.963 |
| b(iv) | - | 0.03 | 0.06 | 0.05 | - | 0.05 | 0.10 | 0.08 | 0.07 | 0.941 |
| b(v) | - | 0.10 | 0.11 | 0.11 | 0.05 | - | 0.05 | 0.03 | 0.08 | 0.929 |
| b(vi) | - | 0.16 | 0.17 | 0.16 | 0.10 | 0.05 | - | 0.01 | 0.12 | 0.947 |
| b(vii) | - | 0.14 | 0.15 | 0.14 | 0.09 | 0.03 | 0.01 | - | 0.11 | 0.942 |
| OLR 3 | (i) | (ii) | (iii) | (iv) | (v) | (vi) | RMSD | R2 | |
|---|---|---|---|---|---|---|---|---|---|
| Phase | 1 | 2 | 3 | ||||||
| No H2 | No H2 | With H2 | With H2 | H2 + CO2 | H2 + CO2 | ||||
| Temp | °C | 37 | 37 | 37 | 37 | 37 | 37 | - | - |
| OLR | g VS L−1 day−1 | 3 | 3 | 3 | 3 | 3 | 3 | - | - |
| HRT | days | 15 | 15 | 15 | 15 | 15 | 15 | - | - |
| H2 input | L L−1 day−1 | 0.0 | 0.0 | 2.9 | 2.9 | 2.9 | 7.4 | - | - |
| SMPtot | L CH4 g−1 VS | 0.287 | 0.289 | 0.496 | 0.490 | 0.452 | 0.804 | - | - |
| CH4 | % | 50.0 | 50.1 | 93.7 | 90.9 | 75.0 | 76.6 | - | - |
| TAN | M | 0.075 | 0.074 | 0.045 | 0.045 | 0.049 | 0.044 | - | - |
| VFA | M | 0.000 | 0.000 | 0.001 | 0.002 | 0.007 | 0.006 | - | - |
| pH | - | 7.34 | 7.35 | 8.00 | 7.93 | 7.82 | 7.81 | - | - |
| pCO2 | - | 0.474 | 0.473 | 0.040 | 0.046 | 0.071 | 0.077 | - | - |
| a × 108 | - | 9.29 | 9.23 | 22.08 | 23.03 | 19.45 | 18.67 | - | - |
| b × 108 | - | 0.69 | 0.68 | 0.97 | 1.00 | 0.81 | 0.70 | - | - |
| Temp | °C | 37 | 37 | 37 | 37 | 37 | 37 | - | - |
| Difference between experimental and predicted pH based on Eqn A with coefficient a(x) | |||||||||
| a(i) | - | - | 0.00 | 0.32 | 0.35 | 0.29 | 0.27 | 0.28 | 0.995 |
| a(ii) | - | 0.00 | - | 0.33 | 0.35 | 0.29 | 0.28 | 0.28 | 0.996 |
| a(iii) | - | 0.37 | 0.37 | - | 0.02 | 0.05 | 0.07 | 0.24 | 1.000 |
| a(iv) | - | 0.39 | 0.39 | 0.02 | - | 0.07 | 0.09 | 0.25 | 0.998 |
| a(iv) | - | 0.31 | 0.32 | 0.05 | 0.07 | - | 0.02 | 0.20 | 0.998 |
| a(vi) | - | 0.30 | 0.30 | 0.06 | 0.08 | 0.02 | - | 0.19 | 0.998 |
| Difference between experimental and predicted pH based on Equation B with coefficient b(x) | |||||||||
| b(i) | - | - | 0.01 | 0.13 | 0.14 | 0.06 | 0.00 | 0.09 | 0.980 |
| b(ii) | - | 0.01 | - | 0.14 | 0.15 | 0.07 | 0.01 | 0.10 | 0.979 |
| b(iii) | - | 0.14 | 0.15 | - | 0.01 | 0.07 | 0.13 | 0.11 | 0.983 |
| b(iv) | - | 0.16 | 0.17 | 0.01 | - | 0.09 | 0.15 | 0.13 | 0.990 |
| b(iv) | - | 0.06 | 0.07 | 0.07 | 0.08 | - | 0.06 | 0.07 | 0.987 |
| b(vi) | - | 0.00 | 0.01 | 0.13 | 0.14 | 0.06 | - | 0.09 | 0.997 |
| Difference between experimental and predicted pH for Equation A with a(x) and phosphate adjustment | |||||||||
| Period (i) | - | - | 0.01 | 0.05 | 0.07 | 0.06 | 0.01 | 0.05 | 0.993 |
| Period (ii) | - | 0.01 | - | 0.06 | 0.08 | 0.07 | 0.01 | 0.06 | 0.993 |
| Period (iii) | - | 0.06 | 0.07 | - | 0.01 | 0.00 | 0.05 | 0.05 | 0.995 |
| Period (iv) | - | 0.09 | 0.10 | 0.03 | - | 0.02 | 0.07 | 0.07 | 0.996 |
| Period (iv) | - | 0.07 | 0.08 | 0.01 | 0.01 | - | 0.06 | 0.06 | 0.997 |
| Period (vi) | - | 0.01 | 0.02 | 0.04 | 0.06 | 0.05 | - | 0.04 | 0.999 |
| (i) | (ii) | (iii) | (iv) | ||
|---|---|---|---|---|---|
| Before H2 Addition | Baseline Period 1 | Baseline Period 2 | After Recovery | ||
| Day 0–54 | Day 0–24 | Day 25–54 | Day 110–125 | ||
| pH | - | 7.50 ± 0.04 | 7.48 ± 0.04 | 7.50 ± 0.04 | 7.46 ± 0.04 |
| TAN | M | 0.131 ± 0.005 | 0.128 ± 0.004 | 0.134 ± 0.003 | 0.133 ± 0.004 |
| Total VFA | M | 0.008 ± 0.005 | 0.006 ± 0.005 | 0.010 ± 0.004 | 0.006 ± 0.003 |
| TA | g CaCO3 L−1 | 9.38 ± 0.32 | 9.26 ± 0.34 | 9.51 ± 0.22 | 9.37 ± 0.28 |
| PA | g CaCO3 L−1 | 6.33 ± 0.25 | 6.20 ± 0.18 | 6.46 ± 0.22 | 6.40 ± 0.23 |
| IA | g CaCO3 L−1 | 3.05 ± 0.24 | 3.05 ± 0.30 | 3.05 ± 0.17 | 2.97 ± 0.15 |
| TS | %WW | 3.86 ± 0.11 | 3.85 ± 0.11 | 3.88 ± 0.12 | 3.88 ± 0.07 |
| VS | %WW | 2.59 ± 0.09 | 2.59 ± 0.08 | 2.58 ± 0.09 | 2.60 ± 0.06 |
| SMP | L CH4 g−1 VS | 0.358 ± 0.017 | 0.355 ± 0.017 | 0.360 ± 0.017 | 0.322 ± 0.027 |
| pCH4 | - | 0.616 ± 0.008 | 0.617 ± 0.009 | 0.616 ± 0.008 | 0.620 ± 0.009 |
| pCO2 | - | 0.365 ± 0.009 | 0.362 ± 0.010 | 0.367 ± 0.008 | 0.369 ± 0.008 |
| R2 | Slope | Int’cept | RMSE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a × 108 | b × 108 | n | Equation A | Equation B | Equation A | Equation B | Equation A | Equation B | Equation A | Equation B | |
| D1 | 8.29 | 0.962 | 50 | 0.513 | 0.877 | 0.492 | 0.856 | 3.86 | 1.09 | 0.06 | 0.04 |
| D2 | 8.85 | 1.052 | 51 | 0.663 | 0.859 | 0.663 | 1.083 | 2.56 | −0.64 | 0.05 | 0.04 |
| D3 | 8.70 | 1.043 | 51 | 0.774 | 0.937 | 0.915 | 1.000 | 0.68 | 0.02 | 0.08 | 0.04 |
| D4 | 8.45 | 1.023 | 50 | 0.758 | 0.934 | 0.829 | 0.946 | 1.34 | 0.43 | 0.08 | 0.04 |
| D5 | 8.87 | 1.103 | 47 | 0.778 | 0.968 | 0.812 | 0.928 | 1.54 | 0.52 | 0.18 | 0.07 |
| D6 | 9.18 | 1.118 | 50 | 0.917 | 0.962 | 0.895 | 0.972 | 0.82 | 0.17 | 0.08 | 0.06 |
| D7 | 8.96 | 1.134 | 46 | 0.950 | 0.973 | 0.935 | 0.967 | 0.54 | 0.21 | 0.07 | 0.06 |
| D8 | 8.35 | 1.039 | 45 | 0.772 | 0.956 | 0.919 | 1.062 | 0.69 | -0.50 | 0.14 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Heaven, S.; Banks, C.J. Validation of Two Theoretically Derived Equations for Predicting pH in CO2 Biomethanisation. Processes 2023, 11, 113. https://doi.org/10.3390/pr11010113
Zhang Y, Heaven S, Banks CJ. Validation of Two Theoretically Derived Equations for Predicting pH in CO2 Biomethanisation. Processes. 2023; 11(1):113. https://doi.org/10.3390/pr11010113
Chicago/Turabian StyleZhang, Yue, Sonia Heaven, and Charles J. Banks. 2023. "Validation of Two Theoretically Derived Equations for Predicting pH in CO2 Biomethanisation" Processes 11, no. 1: 113. https://doi.org/10.3390/pr11010113
APA StyleZhang, Y., Heaven, S., & Banks, C. J. (2023). Validation of Two Theoretically Derived Equations for Predicting pH in CO2 Biomethanisation. Processes, 11(1), 113. https://doi.org/10.3390/pr11010113