Adsorption of Phosphates onto Mg/Al-Oxide/Hydroxide/Sulfate-Impregnated Douglas Fir Biochar
Abstract
1. Introduction
2. Materials and Methods
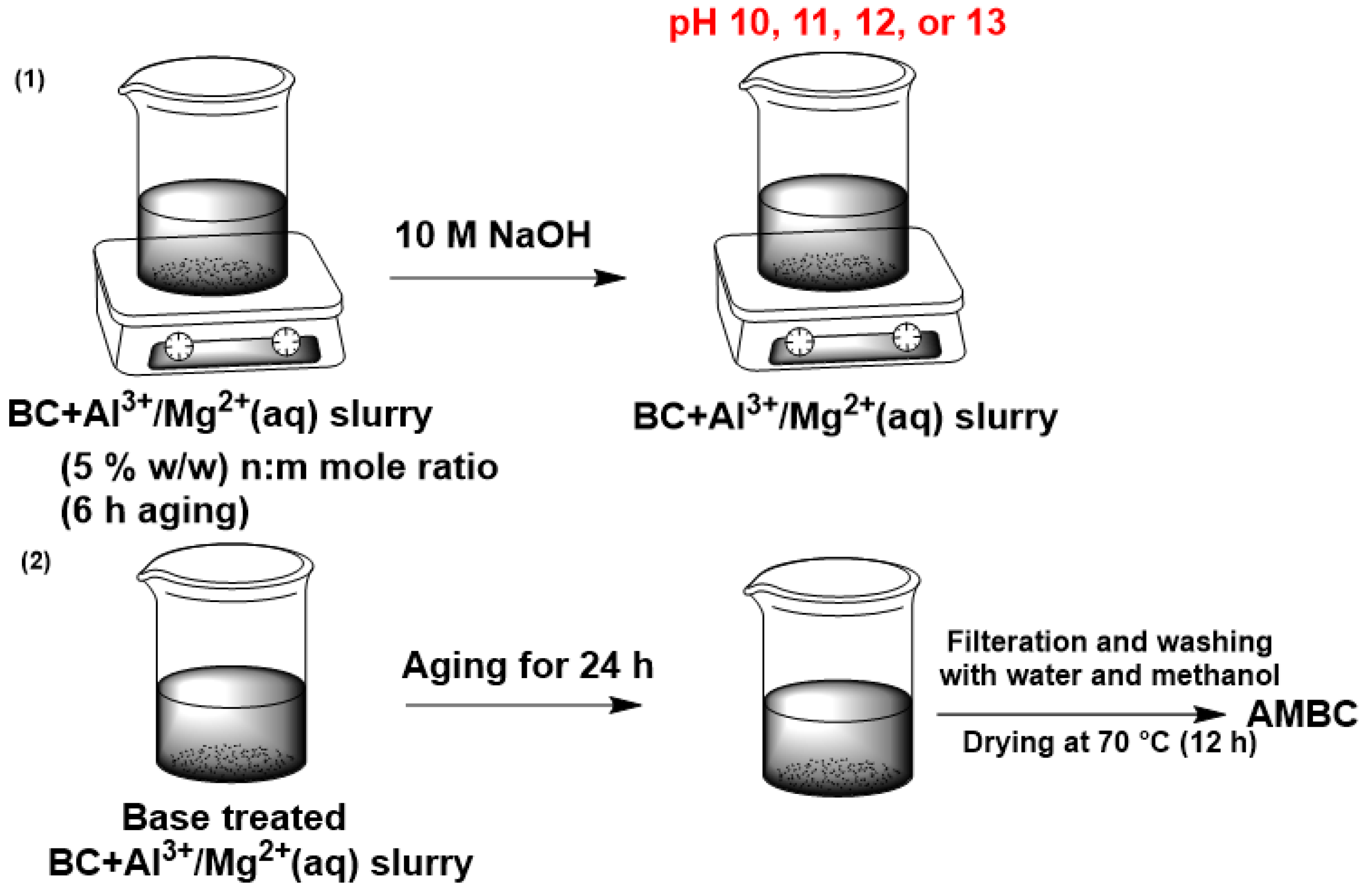
2.1. Preparation of AMBC
2.2. Sorption Kinetics and Isotherms
Adsorbent Characterization
3. Results
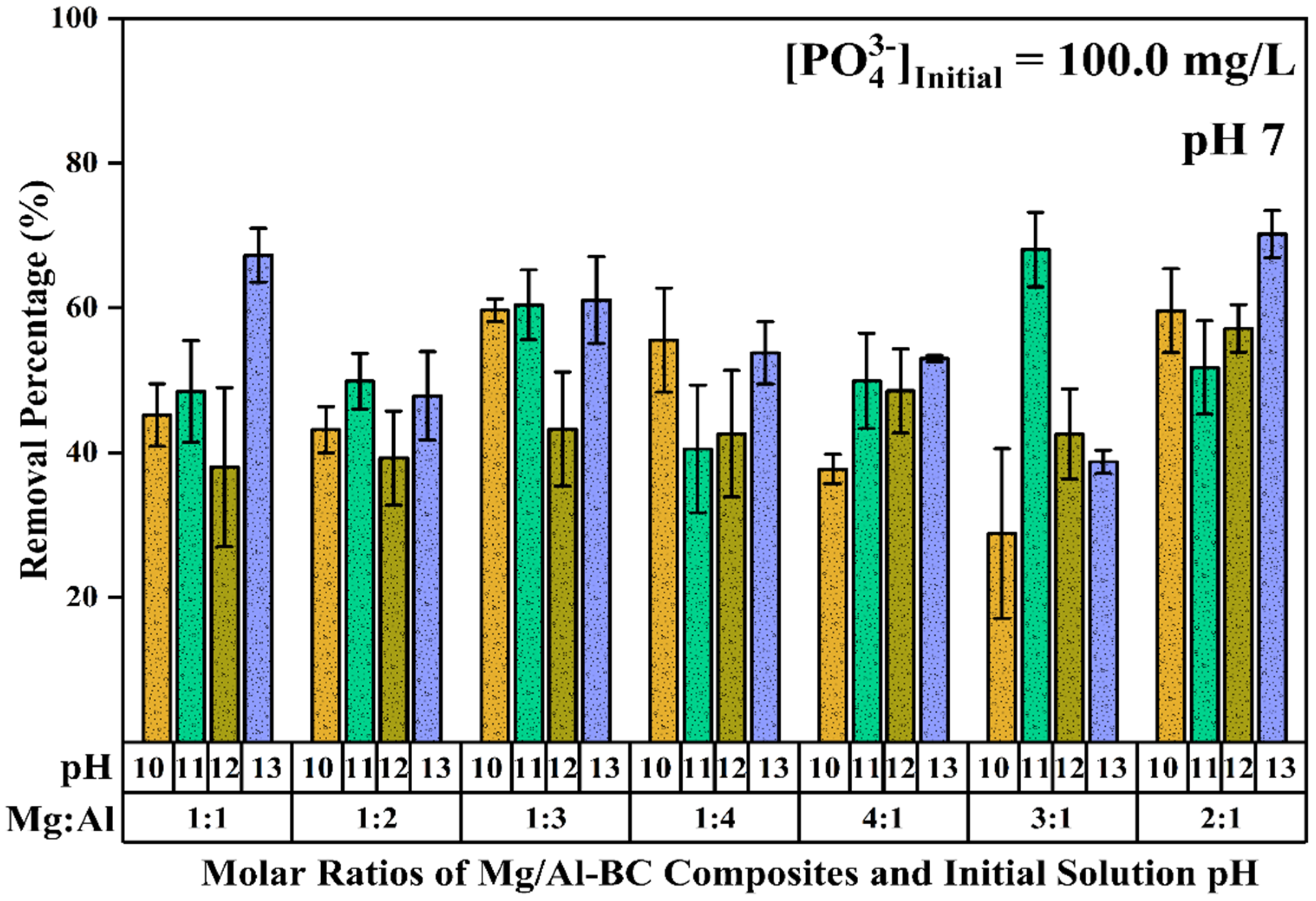
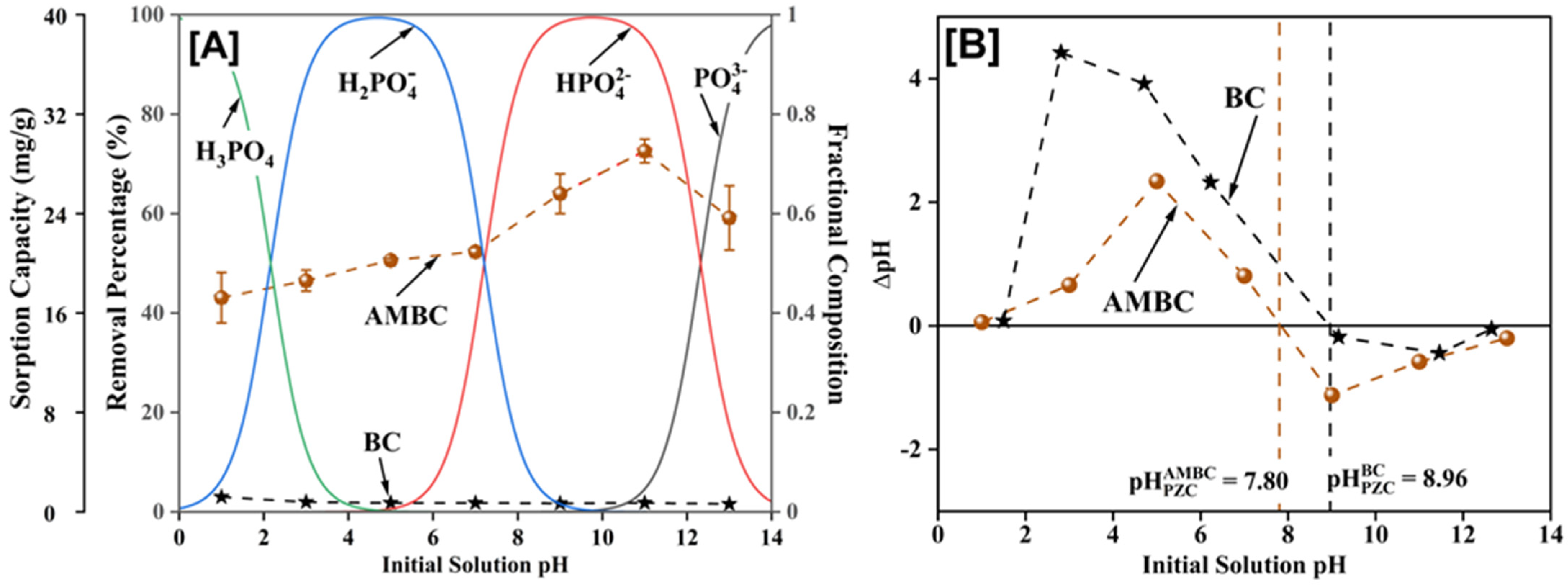
3.1. Preliminary Screening, pH Optimization, and Point of Zero Charge
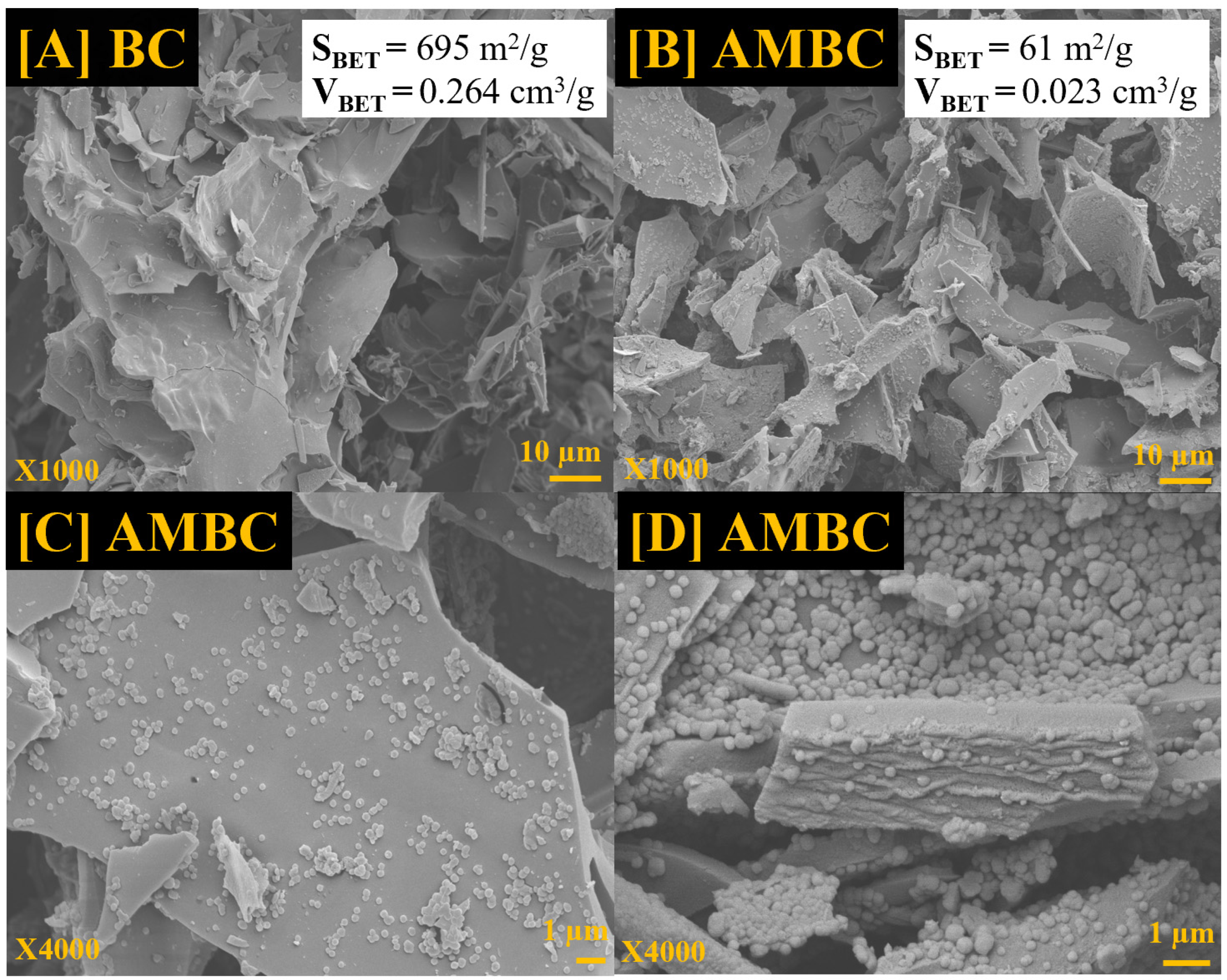
3.2. The Surface Area and Morphological Architecture of the Biochar
3.3. X-ray Diffraction (XRD) and X-ray Photoelectron Spectroscopy
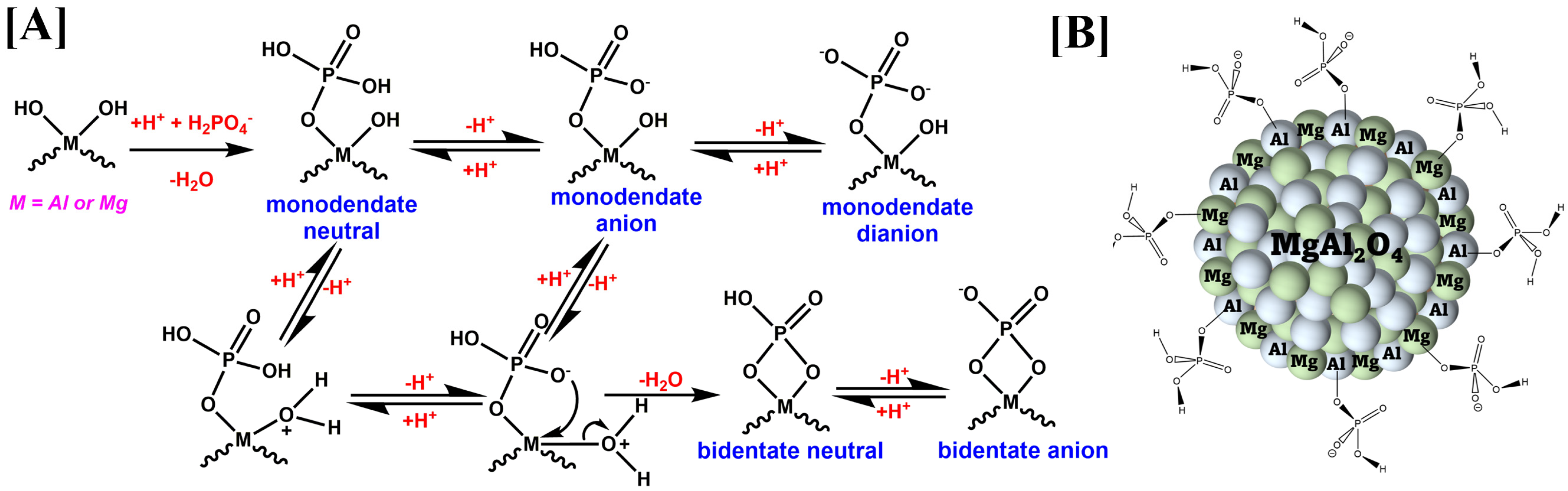
3.4. Adsorption Mechanism/Interactions
3.5. Adsorption Kinetics
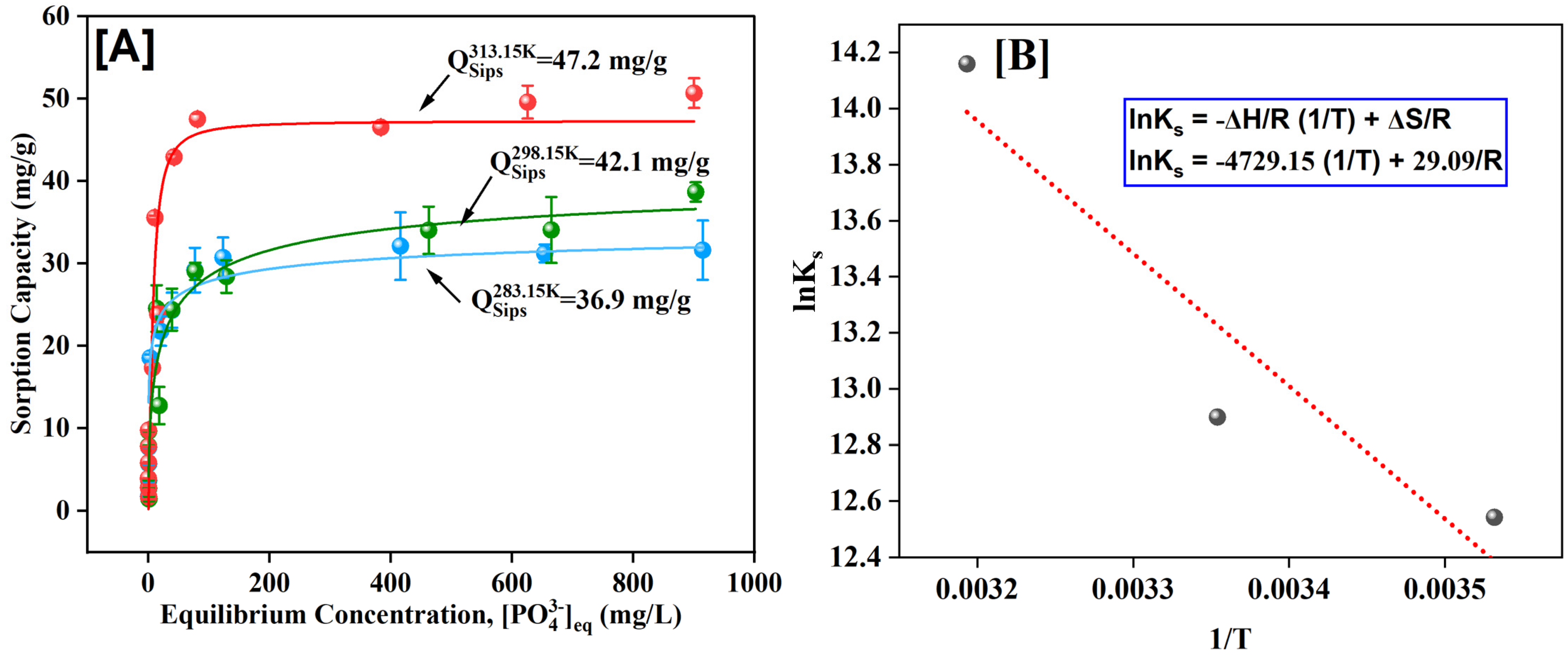
3.6. Adsorption Isotherms and Thermodynamics
3.7. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambers, H.; Raven, J.A.; Shaver, G.R.; Smith, S.E. Plant nutrient-acquisition strategies change with soil age. Trends Ecol. Evol. 2008, 23, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.; Armstrong, F.; Holmgren, S.; Brunskill, G. Eutrophication of Lake 227, Experimental Lakes Area, northwestern Ontario, by addition of phosphate and nitrate. J. Fish. Board Can. 1971, 28, 1763–1782. [Google Scholar] [CrossRef]
- Knud-Hansen, C. Historical perspective of the phosphate detergent conflict. In Proceedings of the Natural Resources and Environmental Policy Seminar, University of Colorado, Boulder, CO, USA, February 1994; Available online: http://johnhogan.info/hogan/CHEM_1002/Notes/PhosphateDetergentConflict.pdf (accessed on 12 December 2022).
- Lucassen, E.C.; Smolders, A.J.; Lamers, L.P.; Roelofs, J.G. Water table fluctuations and groundwater supply are important in preventing phosphate-eutrophication in sulphate-rich fens: Consequences for wetland restoration. Plant Soil 2005, 269, 109–115. [Google Scholar] [CrossRef]
- Bricker, S.B.; Clement, C.G.; Pirhalla, D.E.; Orlando, S.P.; Farrow, D.R. National Estuarine Eutrophication Assessment: Effects of Nutrient Enrichment in the Nation’s Estuaries; US National Oceanographic and Atmospheric Administration, National Ocean Service, Special Projects Office and the National Center for Coastal Ocean Science: Silver Spring, MD, USA, 1999.
- Schindler, D.W.; Vallentyne, J.R. The Algal Bowl: Overfertilization of the World’s Freshwaters and Estuaries; University of Alberta Press: Edmonton, AB, Canada, 2008. [Google Scholar]
- Bonsdorff, E.; Blomqvist, E.; Mattila, J.; Norkko, A. Coastal eutrophication: Causes, consequences and perspectives in the archipelago areas of the northern Baltic Sea. Estuar. Coast. Shelf Sci. 1997, 44, 63–72. [Google Scholar] [CrossRef]
- Rabalais, N.N.; Turner, R.E.; Gupta, B.K.S.; Platon, E.; Parsons, M.L. Sediments tell the history of eutrophication and hypoxia in the northern Gulf of Mexico. Ecol. Appl. 2007, 17, S129–S143. [Google Scholar] [CrossRef]
- Mino, T.; Van Loosdrecht, M.; Heijnen, J. Microbiology and biochemistry of the enhanced biological phosphate removal process. Water Res. 1998, 32, 3193–3207. [Google Scholar] [CrossRef]
- Blaney, L.M.; Cinar, S.; SenGupta, A.K. Hybrid anion exchanger for trace phosphate removal from water and wastewater. Water Res. 2007, 41, 1603–1613. [Google Scholar] [CrossRef]
- Özacar, M.; Şengil, I.A. Enhancing phosphate removal from wastewater by using polyelectrolytes and clay injection. J. Hazard. Mater. 2003, 100, 131–146. [Google Scholar] [CrossRef]
- Karunanayake, A.G.; Navarathna, C.M.; Gunatilake, S.R.; Crowley, M.; Anderson, R.; Mohan, D.; Perez, F.; Pittman, C.U., Jr.; Mlsna, T. Fe3O4 nanoparticles dispersed on Douglas fir biochar for phosphate sorption. ACS Appl. Nano Mater. 2019, 2, 3467–3479. [Google Scholar] [CrossRef]
- Rahman, S.; Navarathna, C.M.; Das, N.K.; Alchouron, J.; Reneau, P.; Stokes, S.; Thirumalai, R.V.; Perez, F.; Hassan, E.B.; Mohan, D. High capacity aqueous phosphate reclamation using Fe/Mg-layered double hydroxide (LDH) dispersed on biochar. J. Colloid Interface Sci. 2021, 597, 182–195. [Google Scholar] [CrossRef]
- Das, J.; Patra, B.; Baliarsingh, N.; Parida, K. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Seida, Y.; Nakano, Y. Removal of phosphate by layered double hydroxides containing iron. Water Res. 2002, 36, 1306–1312. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Henrist, C.; Mathieu, J.-P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhou, B.; Awasthi, M.K.; Ali, A.; Zhang, Z.; Gaston, L.A.; Lahori, A.H.; Mahar, A. Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ. 2016, 559, 121–129. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Yao, Y.; Inyang, M. Phosphate removal ability of biochar/MgAl-LDH ultra-fine composites prepared by liquid-phase deposition. Chemosphere 2013, 92, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, A.; Arbestain, M.C.; Sevilla, M.; Maciá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.; Aitkenhead, W.; Arce, F.; Macìas, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Burevski, D. The application of the Dubinin-Astakhov equation to the characterization of microporous carbons. Colloid Polym. Sci. 1982, 260, 623–627. [Google Scholar] [CrossRef]
- Lopez-Ramon, M.V.; Stoeckli, F.; Moreno-Castilla, C.; Carrasco-Marin, F. On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 1999, 37, 1215–1221. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Inyang, M.; Zimmerman, A.R.; Cao, X.; Pullammanappallil, P.; Yang, L. Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard. Mater. 2011, 190, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Sverjensky, D.A. Zero-point-of-charge prediction from crystal chemistry and solvation theory. Geochim. Cosmochim. Acta 1994, 58, 3123–3129. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D.; Bertrand, G.; Soulié, J. 1.11 Bioactive Calcium Phosphate Compounds: Physical Chemistry☆. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 244–290. [Google Scholar]
- Bafrooei, H.B.; Ebadzadeh, T. MgAl2O4 nanopowder synthesis by microwave assisted high energy ball-milling. Ceram. Int. 2013, 39, 8933–8940. [Google Scholar] [CrossRef]
- Molefe, D.M.; Labuschagne, J.; Focke, W.W.; Van der Westhuizen, I.; Ofosu, O. The effect of magnesium hydroxide, hydromagnesite and layered double hydroxide on the heat stability and fire performance of plasticized poly (vinyl chloride). J. Fire Sci. 2015, 33, 493–510. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, J.; Chen, Z.; Yang, J.; Liu, C. Study on kinetics of the pyrolysis process of aluminum sulfate. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 285–292. [Google Scholar] [CrossRef]
- Saoud, K.M.; Saeed, S.; Al-Soubaihi, R.M.; Bertino, M.F. Microwave assisted preparation of magnesium hydroxide nano-sheets. Am. J. Nanomater. 2014, 2, 21–25. [Google Scholar]
- Prorok, R.; Ramult, J.; Nocun-Wczelik, W.; Madej, D. The Effect of Chelate Compounds on the Hydration Process of MgO–Al2O3 Phase System under Hydrothermal Conditions. Appl. Sci. 2021, 11, 2834. [Google Scholar] [CrossRef]
- Wignall, G.; Rothon, R.; Longman, G.; Woodward, G. The structure of amorphous aluminium phosphate by radial distribution functions derived from X-ray diffraction. J. Mater. Sci. 1977, 12, 1039–1049. [Google Scholar] [CrossRef]
- Abbona, F.; Baronnet, A. A XRD and TEM study on the transformation of amorphous calcium phosphate in the presence of magnesium. J. Cryst. Growth 1996, 165, 98–105. [Google Scholar] [CrossRef]
- Konno, H.; Kobayashi, S.; Takahashi, H.; Nagayama, M. The hydration of barrier oxide films on aluminium and its inhibition by chromate and phosphate ions. Corros. Sci. 1982, 22, 913–923. [Google Scholar] [CrossRef]
- Chong, K.Z.; Shih, T.S. Conversion-coating treatment for magnesium alloys by a permanganate–phosphate solution. Mater. Chem. Phys. 2003, 80, 191–200. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Q.; Huang, C.; Javed, M.S.; Aslam, M.K.; Chen, C. Influence of additives fluoride and phosphate on the electrochemical performance of Mg–MnO2 battery. J. Appl. Electrochem. 2017, 47, 767–775. [Google Scholar] [CrossRef]
- Akolekar, D.B. Novel, crystalline, large-pore magnesium aluminophosphate molecular sieve of type 50: Preparation, characterization, and structural stability. Zeolites 1995, 15, 583–590. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Shoval, Y.; Froumin, N.; Hayun, S. Effect of structure and composition of non-stoichiometry magnesium aluminate spinel on water adsorption. Materials 2020, 13, 3195. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wu, C.; Wang, J.; Mo, S.; Zou, Z.; Zhou, B.; Long, F. Space-confined effect one-pot synthesis of γ-AlO (OH)/MgAl-LDH heterostructures with excellent adsorption performance. Nanoscale Res. Lett. 2019, 14, 281. [Google Scholar] [CrossRef] [PubMed]
- Herath, A.; Navarathna, C.; Warren, S.; Perez, F.; Pittman, C.U., Jr.; Mlsna, T.E. Iron/titanium oxide-biochar (Fe2TiO5/BC): A versatile adsorbent/photocatalyst for aqueous Cr (VI), Pb2+, F− and methylene blue. J. Colloid Interface Sci. 2022, 614, 603–616. [Google Scholar] [CrossRef]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Jung, K.-W.; Ahn, K.-H. Fabrication of porosity-enhanced MgO/biochar for removal of phosphate from aqueous solution: Application of a novel combined electrochemical modification method. Bioresour. Technol. 2016, 200, 1029–1032. [Google Scholar] [CrossRef]
- Jack, J.; Huggins, T.M.; Huang, Y.; Fang, Y.; Ren, Z.J. Production of magnetic biochar from waste-derived fungal biomass for phosphorus removal and recovery. J. Clean. Prod. 2019, 224, 100–106. [Google Scholar] [CrossRef]
- Seftel, E.M.; Ciocarlan, R.G.; Michielsen, B.; Meynen, V.; Mullens, S.; Cool, P. Insights into phosphate adsorption behavior on structurally modified ZnAl layered double hydroxides. Appl. Clay Sci. 2018, 165, 234–246. [Google Scholar] [CrossRef]
- Yan, L.-G.; Yang, K.; Shan, R.-R.; Yan, T.; Wei, J.; Yu, S.-J.; Yu, H.-Q.; Du, B. Kinetic, isotherm and thermodynamic investigations of phosphate adsorption onto core–shell Fe3O4@LDHs composites with easy magnetic separation assistance. J. Colloid Interface Sci. 2015, 448, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, R.; Tezuka, S.; Sonoda, A.; Sakane, K.; Ooi, K.; Hirotsu, T. Adsorption of phosphate from seawater on calcined MgMn-layered double hydroxides. J. Colloid Interface Sci. 2005, 290, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Daou, T.; Begin-Colin, S.; Greneche, J.-M.; Thomas, F.; Derory, A.; Bernhardt, P.; Legaré, P.; Pourroy, G. Phosphate adsorption properties of magnetite-based nanoparticles. Chem. Mater. 2007, 19, 4494–4505. [Google Scholar] [CrossRef]



| Phosphate Concentration (mg/L) | R2 | qcal (mg/g) | qexp (mg/g) | k2 (g/mg·min) |
|---|---|---|---|---|
| pH 11, 12.5 | 0.99 | 5.05 | 4.94 | 0.2548 |
| 25 | 0.99 | 4.66 | 4.57 | 0.7797 |
| 50 | 0.96 | 16.88 | 16.44 | 0.0319 |
| pH 7, 100 | 0.98 | 18.85 | 21.91 | 0.0238 |
| Temperature (°C) | Temperature (K) | 1/T (K−1) | Ks (L/mg) | Ks (unitless) | ln Ks | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (kJ/mol·K) |
|---|---|---|---|---|---|---|---|---|
| 10 | 283.15 | 0.003532 | 0.28 | 280,000 | 12.54 | −29.52 | 39.3 | 0.24 |
| 25 | 298.15 | 0.003354 | 0.4 | 400,000 | 12.89 | −31.97 | ||
| 40 | 313.15 | 0.003193 | 1.41 | 1,410,000 | 14.15 | −36.86 |
| Adsorbent | Temp (°C) | Equilibrium Time | pH | BET Surface Area (m2/g) | Adsorption Capacity (mg/g) | Ref. |
|---|---|---|---|---|---|---|
| Marine macroalgae BC | 20 | 48 h | 2.4 | 3.3 | [44] | |
| Waste-derived fungal biomass magnetite BC | 25 | 24 h | 53.0 | 23.9 | [45] | |
| 2:1 Mg/Al-LDHs sugar cane leaf BC composite | 25 | 1 h | 3 | 10.17 | 53.4 | [18] |
| 3:1 Mg/Al-LDHs sugar cane leaf BC composite | 11.41 | 72.1 | ||||
| 4:1 Mg/Al-LDHs sugar cane leaf BC composite | 12.25 | 81.8 | ||||
| Magnetic biochar (MBC) | 25 | 2 min | 3 | 312.6 | 91.3 | [12] |
| 35 | 91.0 | |||||
| 45 | 90.0 | |||||
| Zn-Al LDH | 25 | 72 h | 35.9 | [46] | ||
| 30 | 58.2 | |||||
| 40 | 79.1 | |||||
| 50 | 92.6 | |||||
| Fe3O4 Zn/Al-LDH | 25 | 1 h | 133 | 36.9 | [47] | |
| Fe3O4 Mg/Al-LDH | 71.9 | 31.7 | ||||
| Fe3O4 Ni/Al-LDH | 50.9 | 26.5 | ||||
| Mg/Mn Layered double hydroxides | 10 | 3 d | 6.2 | [48] | ||
| 25 | 7.3 | |||||
| 40 | 7.5 | |||||
| Magnetite based nanoparticles | 24 | 3 | 31 | 5.2 | [49] | |
| Al/Mg oxide/hydroxide/sulfate impregnated Douglas fir biochar | 10 | 15 mins | 11, 7 | 61 | 36.9 | This work |
| 25 | 42.1 (21.9 @ pH 7) | |||||
| 40 | 47.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarathna, C.M.; Pennisson, J.E.; Dewage, N.B.; Reid, C.; Dotse, C.; Jazi, M.E.; Rodrigo, P.M.; Zhang, X.; Farmer, E.; Watson, C.; et al. Adsorption of Phosphates onto Mg/Al-Oxide/Hydroxide/Sulfate-Impregnated Douglas Fir Biochar. Processes 2023, 11, 111. https://doi.org/10.3390/pr11010111
Navarathna CM, Pennisson JE, Dewage NB, Reid C, Dotse C, Jazi ME, Rodrigo PM, Zhang X, Farmer E, Watson C, et al. Adsorption of Phosphates onto Mg/Al-Oxide/Hydroxide/Sulfate-Impregnated Douglas Fir Biochar. Processes. 2023; 11(1):111. https://doi.org/10.3390/pr11010111
Chicago/Turabian StyleNavarathna, Chanaka M., Jaylen E. Pennisson, Narada Bombuwala Dewage, Claudia Reid, Charles Dotse, Mehdi Erfani Jazi, Prashan M. Rodrigo, Xuefeng Zhang, Erin Farmer, Colton Watson, and et al. 2023. "Adsorption of Phosphates onto Mg/Al-Oxide/Hydroxide/Sulfate-Impregnated Douglas Fir Biochar" Processes 11, no. 1: 111. https://doi.org/10.3390/pr11010111
APA StyleNavarathna, C. M., Pennisson, J. E., Dewage, N. B., Reid, C., Dotse, C., Jazi, M. E., Rodrigo, P. M., Zhang, X., Farmer, E., Watson, C., Craig, D. O., Ramirez, A., Walker, M., Madduri, S., Mohan, D., & Mlsna, T. E. (2023). Adsorption of Phosphates onto Mg/Al-Oxide/Hydroxide/Sulfate-Impregnated Douglas Fir Biochar. Processes, 11(1), 111. https://doi.org/10.3390/pr11010111









