Effect of the Processing Conditions on the Supercritical Extraction and Impregnation of Rosemary Essential Oil in Linear Low-Density Polyethylene Films
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Method
2.2.1. Design of Experiments
2.2.2. Supercritical Fluid Extraction Procedure
2.2.3. Supercritical Solvent Impregnation Process
2.2.4. Gas Chromatography-Mass Spectrometry (GC-MS)
2.2.5. Antioxidant Activity of Impregnated Films
2.3. Physical Characterization of the Impregnated Samples
2.4. Mechanical Properties
3. Results and Discussion
3.1. Supercritical Fluid Extraction of EO from Rosemary
3.2. Gas Chromatography Results
3.3. Supercritical Solvent Impregnation of Rosemary Essential Oil in LLDPE Films
3.4. Effect of the Operational Conditions on the Impregnation of Rosemary Essential Oil in LLDPE Films
3.5. Characterization of the Rosemary Essential Oil and Impregnated LLDPE Films
3.5.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.5.2. Thermal Properties
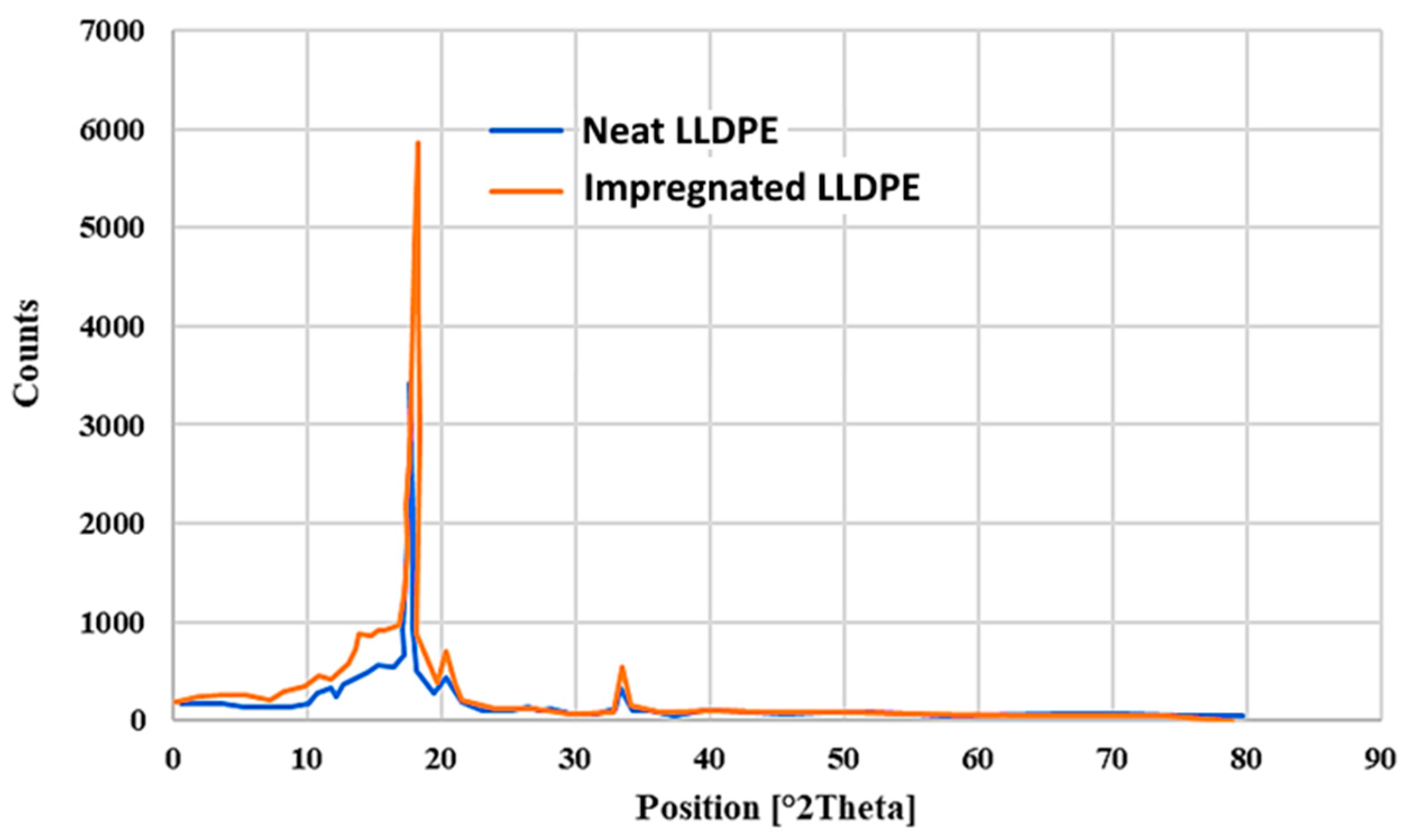
3.5.3. X-ray Diffraction
3.5.4. Scanning Electron Microscopy (SEM)
3.6. Antioxidant Activity of Impregnated Films
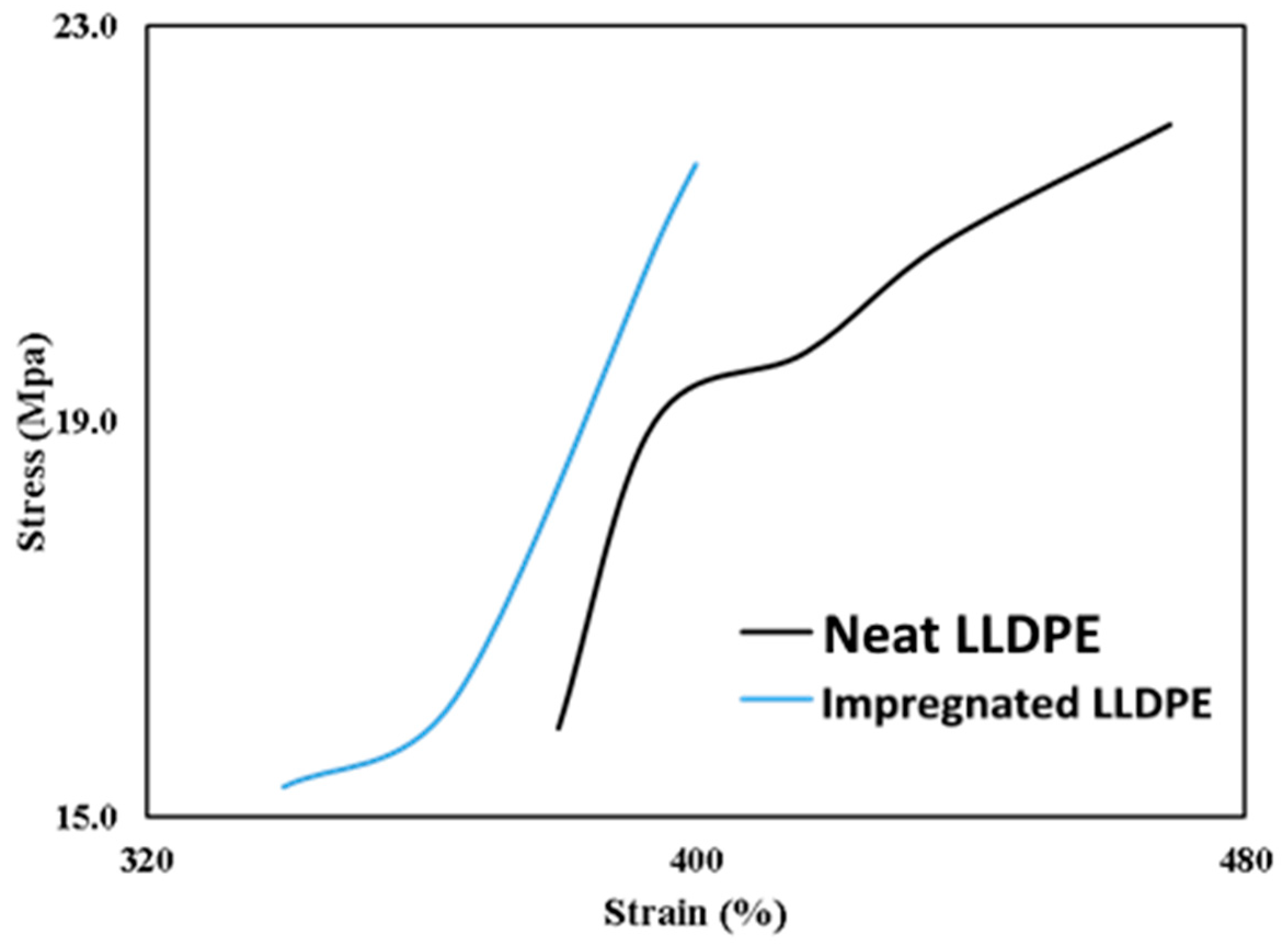
3.7. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrentino, G.; Morozova, K.; Horn, C.; Scampicchio, M. Extraction of Essential Oils from Medicinal Plants and their Utilization as Food Antioxidants. Curr. Pharm. Des. 2020, 26, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against Salmonella typhimurium during refrigerated storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Turasan, H.; Sahin, S.; Sumnu, G. Encapsulation of rosemary essential oil. LWT Food Sci. Technol. 2015, 64, 112–119. [Google Scholar] [CrossRef]
- Đilas, S.; Knez, Ž.; Četojević-Simin, D.; Tumbas, V.; Škerget, M.; Čanadanović-Brunet, J.; Ćetković, G. In vitro antioxidant and antiproliferative activity of three rosemary (Rosmarinus officinalis L.) extract formulations. Int. J. Food Sci. Technol. 2012, 47, 2052–2062. [Google Scholar] [CrossRef]
- Sánchez-Escalante, A.; Djenane, D.; Torrescano, G.; Beltrán, J.A.; Roncalés, P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001, 58, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Esfandiari, N.; Sajadian, S.A. Solubility of Lacosamide in supercritical carbon Dioxide: An experimental analysis and thermodynamic modeling. J. Mol. Liq. 2022, 360, 119467. [Google Scholar] [CrossRef]
- Esfandiari, N.; Ghoreishi, M.S. Optimal thermodynamic conditions for ternary system (CO2, DMSO, ampicillin) in supercritical CO2 antisolvent process. J. Taiwan Inst. Chem. Eng. 2015, 50, 31–36. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A.; Ardestani, N.S. Extraction of Dracocephalum kotschyi Boiss using supercritical carbon dioxide: Experimental and optimization. J. Supercrit. Fluids 2016, 107, 137–144. [Google Scholar] [CrossRef]
- Khodaie, F.; Ghoreishi, S.M. Experimental extraction of gallic acid from brown sumac seed (Rhus coriaria) using supercritical carbon dioxide and ethanol as co-solvent: Modeling and optimization. J. Supercrit. Fluids 2021, 175, 105266. [Google Scholar] [CrossRef]
- Bimakr, M.; Ghoreishi, S.M.; Ganjloo, A.; Mousavi, M. Modified Supercritical Carbon Dioxide Extraction of Biologically Active Compounds from Feijoa Sellowiana Leaves. Int. J. Food Eng. 2019, 15, 0180342. [Google Scholar] [CrossRef]
- Ahmed, Z.; Abdeslam-Hassan, M.; Ouassila, L.; Danielle, B. Extraction and Modeling of Algerian Rosemary Essential Oil Using Supercritical CO2: Effect of Pressure and Temperature. Energy Procedia 2012, 18, 1038–1046. [Google Scholar] [CrossRef] [Green Version]
- Bensebia, O.; Barth, D.; Bensebia, B.; Dahmani, A. Supercritical CO2 extraction of rosemary: Effect of extraction parameters and modelling. J. Supercrit. Fluids 2009, 49, 161–166. [Google Scholar] [CrossRef]
- Conde-Hernández, L.A.; Espinosa-Victoria, J.R.; Trejo, A.; Guerrero-Beltrán, J.Á. CO2-supercritical extraction, hydrodistillation and steam distillation of essential oil of rosemary (Rosmarinus officinalis). J. Food Eng. 2017, 200, 81–86. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, B.; Zheng, Z.; You, X.; Pu, Y.; Li, Q. Preparation of liposomes entrapping essential oil from Atractylodes macrocephala Koidz by modified RESS technique. Chem. Eng. Res. Des. 2010, 88, 1102–1107. [Google Scholar] [CrossRef]
- Yan, T.; Tao, Y.; Wang, X.; Lv, C.; Miao, G.; Wang, S.; Wang, D.; Wang, Z. Preparation, characterization and evaluation of the antioxidant capacity and antitumor activity of myricetin microparticles formated by supercritical antisolvent technology. J. Supercrit. Fluids 2021, 175, 105290. [Google Scholar] [CrossRef]
- Esfandiari, N.; Sajadian, S.A. CO2 utilization as gas antisolvent for the pharmaceutical micro and nanoparticle production: A review. Arab. J. Chem. 2022, 15, 104164. [Google Scholar] [CrossRef]
- Najafi, M.; Esfandiari, N.; Honarvar, B.; Aboosadi, A.Z. Production of Rosuvastatin Calcium Nanoparticles Using Gas Antisolvent Technique: Experimental and Optimization. Period. Polytech. Chem. Eng. 2021, 65, 442–453. [Google Scholar] [CrossRef]
- Tokunaga, S.; Ono, K.; Ito, S.; Sharmin, T.; Kato, T.; Irie, K.; Mishima, K.; Satho, T.; Harada, T.; Aida, T.M.; et al. Microencapsulation of drug with enteric polymer Eudragit L100 for controlled release using the particles from gas saturated solutions (PGSS) process. J. Supercrit. Fluids 2021, 167, 105044. [Google Scholar] [CrossRef]
- Rojas, A.; Torres, A.; Galotto, M.J.; Guarda, A.; Julio, R. Supercritical impregnation for food applications: A review of the effect of the operational variables on the active compound loading. Crit. Rev. Food Sci. Nutr. 2020, 60, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.D.; Mosquera, J.E.; Martini, R.E.; Goñi, M.L.; Gañán, N.A. Supercritical CO2-assisted impregnation of cellulose microparticles with R-carvone: Effect of process variables on impregnation yield. J. Supercrit. Fluids 2022, 188, 105671. [Google Scholar] [CrossRef]
- Liparoti, S.; Franco, P.; Pantani, R.; de Marco, I. Supercritical CO2 impregnation of caffeine in biopolymer films to produce anti-cellulite devices. J. Supercrit. Fluids 2022, 179, 105411. [Google Scholar] [CrossRef]
- Fathi, M.; Sodeifian, G.; Sajadian, S.A. Experimental study of ketoconazole impregnation into polyvinyl pyrrolidone and hydroxyl propyl methyl cellulose using supercritical carbon dioxide: Process optimization. J. Supercrit. Fluids 2022, 188, 105674. [Google Scholar] [CrossRef]
- Sutil, G.A.; Andrade, K.S.; Rebelatto, E.A.; Lanza, M. Effects of incorporation of pure or multicomponent active agents in biopolymers for food packaging using supercritical CO2. Trends Food Sci. Technol. 2022, 120, 349–362. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Nabi Dar, B.; Wani, A.A.; Gamai, S.A.; Nishad, J. Supercritical Impregnation of Active Components into Polymers for Food Packaging Applications. Food Bioprocess. Tech. 2017, 10, 1749–1754. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Ferreira, S.R.S.; Carciofi, B.A.M. High pressure carbon dioxide for impregnation of clove essential oil in LLDPE films. Innov. Food Sci. Emerg. Technol. 2017, 41, 206–215. [Google Scholar] [CrossRef]
- Sepulveda, J.; Villegas, C.; Torres, A.; Vargas, E.; Rodriguez, F.; Baltazar, S.; Prada, A.; Rojas, A.; Romero, J.; Faba, S.; et al. Effect of functionalized silica nanoparticles on the mass transfer process in active PLA nanocomposite films obtained by supercritical impregnation for sustainable food packaging. J. Supercrit. Fluids 2020, 161, 104844. [Google Scholar] [CrossRef]
- Milovanovic, S.; Hollermann, G.; Errenst, C.; Pajnik, J.; Frerich, S.; Kroll, S.; Rezwan, K.; Ivanovic, J. Supercritical CO2 impregnation of PLA/PCL films with natural substances for bacterial growth control in food packaging. Food Res. Int. 2018, 107, 486–495. [Google Scholar] [CrossRef]
- Aredo, V.; Passalacqua, E.S.; Pratavieira, S.; de Oliveira, A.L. Formation of lycopene-loaded hydrolysed collagen particles by supercritical impregnation. LWT 2019, 110, 158–167. [Google Scholar] [CrossRef]
- Varona, S.; Rodríguez-Rojo, S.; Martín, Á.; Cocero, M.J.; Duarte, C.M.M. Supercritical impregnation of lavandin (Lavandula hybrida) essential oil in modified starch. J. Supercrit. Fluids 2011, 58, 313–319. [Google Scholar] [CrossRef]
- Gañan, N.; Bordón, M.G.; Ribotta, P.D.; González, A. Study of chia oil microencapsulation in soy protein microparticles using supercritical CO2-assisted impregnation. J. CO2 Util. 2020, 40, 101221. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Mohammed, J.K.; Mahdi, A.A.; Al-Ansi, W.; Zhang, M.; Al-Adeeb, A.; Wei, M.; Phyo, H.M.; Yao, W. Physicochemical properties, microstructure, and storage stability of Pulicaria jaubertii extract microencapsulated with different protein biopolymers and gum arabic as wall materials. Int. J. Biol. Macromol. 2021, 187, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Goñi, M.L.; Gañán, N.A.; Strumia, M.C.; Martini, R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids 2016, 111, 28–35. [Google Scholar] [CrossRef]
- Miranda-Villa, P.P.; Gañán, N.A.; Martini, R.E.; Goñi, M.L. Supercritical CO2-assisted impregnation of polylactic acid films with R-carvone: Effect of processing on loading, mass transfer kinetics, and final properties. J. CO2 Util. 2022, 61, 102029. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Herrera, J.M.; Strumia, M.C.; Andreatta, A.E.; Martini, R.E. Supercritical CO2 iof LDPE films with terpene ketones as biopesticides against corn weevil (Sitophilus zeamais). J. Supercrit. Fluids 2017, 122, 18–26. [Google Scholar] [CrossRef]
- de Souza, A.C.; Dias, A.M.A.; Sousa, H.C.; Tadini, C.C. Impregnation of cinnamaldehyde into cassava starch biocomposite films using supercritical fluid technology for the development of food active packaging. Carbohydr. Polym. 2014, 102, 830–837. [Google Scholar] [CrossRef] [Green Version]
- Goñi, M.L.; Gañán, N.A.; Barbosa, S.E.; Strumia, M.C.; Martini, R.E. Supercritical CO2-assisted impregnation of LDPE/sepiolite nanocomposite films with insecticidal terpene ketones: Impregnation yield, crystallinity and mechanical properties assessment. J. Supercrit. Fluids 2017, 130, 337–346. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa-Fernández, E.J.M. Supercritical impregnation of antioxidant mango polyphenols into a multilayer PET/PP food-grade film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Bastante, C.C.; Cardoso, L.C.; Ponce, M.T.F.; Serrano, C.M.; de la Ossa-Fernández, E.J.M. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Mosquera, J.E.; Goñi, M.L.; Martini, R.E.; Gañán, N.A. Supercritical carbon dioxide assisted impregnation of eugenol into polyamide fibers for application as a dental floss. J. CO2 Util. 2019, 32, 259–268. [Google Scholar] [CrossRef]
- Goñi, M.L.; Gañán, N.A.; Martini, R.E.; Andreatta, A.E. Carvone-loaded LDPE films for active packaging: Effect of supercritical CO2-assisted impregnation on loading, mechanical and transport properties of the films. J. Supercrit. Fluids 2018, 133, 278–290. [Google Scholar] [CrossRef]
- Torres, A.; Ilabaca, E.; Rojas, A.; Rodríguez, F.; Galotto, M.J.; Guarda, A.; Villegas, C.; Romero, J. Effect of processing conditions on the physical, chemical and transport properties of polylactic acid films containing thymol incorporated by supercritical impregnation. Eur. Polym. J. 2017, 89, 195–210. [Google Scholar] [CrossRef]
- Hussain, Y.A.; Grant, C.S. Ibuprofen impregnation into submicron polymeric films in supercritical carbon dioxide. J. Supercrit. Fluids 2012, 71, 127–135. [Google Scholar] [CrossRef]
- Gurina, D.L.; Budkov, Y.A.; Kiselev, M.G. A molecular insight into poly(methyl methacrylate) impregnation with mefenamic acid in supercritical carbon dioxide: A computational simulation. J. Mol. Liq. 2021, 337, 116424. [Google Scholar] [CrossRef]
- Satpaeva, A.; Rojas, A.; Tyrka, M.; Ksepko, E.; Galotto, M.J.; Zizovic, I. Supercritical Foaming and Impregnation of Polycaprolactone and Polycaprolactone-Hydroxyapatite Composites with Carvacrol. Processes 2022, 10, 482. [Google Scholar] [CrossRef]
- Ardestani, N.S.; Rojas, A.; Esfandiari, N.; Galotto, M.; Babhadiashar, A.; Sajadian, S.A. Supercritical Fluid Extraction from Zataria multiflora Boiss Impregnation of Bioactive Compounds in PLA for the Development of Materials with Antibacterial Properties. Processes 2022, 10, 1787. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1936, 11, 463–471. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Hedayati, A.; Ghoreishi, S.M. Supercritical carbon dioxide extraction of glycyrrhizic acid from licorice plant root using binary entrainer: Experimental optimization via response surface methodology. J. Supercrit. Fluids 2015, 100, 209–217. [Google Scholar] [CrossRef]
- Bashipour, F.; Ghoreishi, S.M. Response surface optimization of supercritical CO2 extraction of α-tocopherol from gel and skin of Aloe vera and almond leaves. J. Supercrit. Fluids 2014, 95, 348–354. [Google Scholar] [CrossRef]
- Sodeifian, G.; Ardestani, N.S.; Sajadian, S.A. Extraction of seed oil from Diospyros lotus optimized using response surface methodology. J. For. Res. 2019, 30, 709–719. [Google Scholar] [CrossRef]
- Hasanov, J.; Zhang, M.; Ismailov, A.; Zhang, Y.; Liu, C. The influence of particle size on supercritical extraction of dog rose (Rosa canina) seed oil. J. King Saud Univ. Eng. Sci. 2019, 31, 140–143. [Google Scholar]
- Asep, E.K.; Jinap, S.; Tan, T.J.; Russly, A.R.; Harcharan, S.; Nazimah, S.A.H. The effects of particle size, fermentation and roasting of cocoa nibs on supercritical fluid extraction of cocoa butter. J. Food Eng. 2008, 85, 450–458. [Google Scholar] [CrossRef]
- Ishak, I.; Hussain, N.; Coorey, R.; Ghani, M.A. Optimization and characterization of chia seed (Salvia hispanica L.) oil extraction using supercritical carbon dioxide. J. CO2 Util. 2021, 45, 101430. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Eng. Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Szumny, A.; Figiel, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of rosemary essential oil (Rosmarinus officinalis) as affected by drying method. J. Food Eng. 2010, 97, 253–260. [Google Scholar] [CrossRef]
- Kamel, D.G.; Mansour, A.I.A.; El-diin, M.A.H.N.; Hammam, A.R.A.; Mehta, D.; Abdel-Rahman, A.M. Using Rosemary Essential Oil as a Potential Natural Preservative during Stirred-like Yogurt Making. Foods 2022, 11, 1993. [Google Scholar] [CrossRef]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Fragr. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Nowak, A.; Kalemba, D.; Krala, L.; Piotrowska, M.; Czyzowska, A. The effects of thyme (Thymus vulgaris) and rosemary (Rosmarinus officinalis) essential oils on Brochothrix thermosphacta and on the shelf life of beef packaged in high-oxygen modified atmosphere. Food Microbiol. 2012, 32, 212–216. [Google Scholar] [CrossRef]
- Presti, M.L.; Ragusa, S.; Trozzi, A.; Dugo, P.; Visinoni, F.; Fazio, A.; Dugo, G.; Mondello, L. A comparison between different techniques for the isolation of rosemary essential oil. J. Sep. Sci. 2005, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Senatore, F. Isolation of rosemary oil: Comparison between hydrodistillation and supercritical CO2 extraction. Flavour Fragr. J. 1992, 7, 227–230. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Zhalnov, I.; Georgieva, T.D. Method for attaining rosemary essential oil with differential composition from dried or fresh material. J. Oleo Sci. 2015, 64, 485–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avison, S.J.; Gray, D.A.; Davidson, G.M.; Taylor, A.J. Infusion of volatile flavor compounds into low-density polyethylene. J. Agric. Food Chem. 2001, 49, 270–275. [Google Scholar] [CrossRef]
- Medeiros, G.R.; Guimarães, C.; Ferreira, S.R.S.; Carciofi, B.A.M. Thermomechanical and transport properties of LLDPE films impregnated with clove essential oil by high-pressure CO2. J. Supercrit. Fluids 2018, 139, 8–18. [Google Scholar] [CrossRef]
- Li, D.; Han, B. Impregnation of Polyethylene (PE) with Styrene Using Supercritical CO2 as the Swelling Agent and Preparation of PE/Polystyrene Composites. Ind. Eng. Chem. Res. 2000, 39, 4506–4509. [Google Scholar] [CrossRef]
- Almeida, A.P.; Rodríguez-Rojo, S.; Serra, A.T.; Vila-Real, H.; Simplicio, A.L.; Delgadilho, I.; da Costa, S.B.; da Costa, L.B.; Nogueira, I.D.; Duarte, C.M.M. Microencapsulation of oregano essential oil in starch-based materials using supercritical fluid technology. Innov. Food Sci. Emerg. Technol. 2013, 20, 140–145. [Google Scholar] [CrossRef]
- Rojas, A.; Cerro, D.; Torres, A.; Galotto, M.J.; Guarda, A.; Romero, J. Supercritical impregnation and kinetic release of 2-nonanone in LLDPE films used for active food packaging. J. Supercrit. Fluids 2015, 104, 76–84. [Google Scholar] [CrossRef]
- Ismail, H.; Nordin, R.; Ahmad, Z.; Rashid, A. Processability and miscibility of linear low-density polyethylene/poly (vinyl alcohol) blends: In situ compatibilization with maleic acid. Iran. Polym. J. 2010, 19, 297–308. [Google Scholar]
- Rezanejad, R.; Ojagh, S.M.; Heidarieh, M.; Raeisi, M.; Rafiee, G.; Alishahi, A. Characterization of Gamma-Irradiated Rosmarinus officinalis L. (Rosemary). Turk. J. Pharm. Sci. 2019, 16, 43–47. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Missirli, T.; Thanassoulia, I.; Krokida, M. Encapsulation of Rosemary Essential Oil into Biodegradable Polymers for Application in Crop Management. J. Polym. Environ. 2020, 28, 2161–2177. [Google Scholar] [CrossRef]
- Torres, A.; Romero, J.; Macan, A.; Guarda, A.; Galotto, M.J. Near critical and supercritical impregnation and kinetic release of thymol in LLDPE films used for food packaging. J. Supercrit. Fluids 2014, 85, 41–48. [Google Scholar] [CrossRef]
- Marina, R. Characterization and antimicrobial activity studies of polypropylene films with carvacrol and thymol for active packaging. J. Food Eng. 2012, 109, 513–519. [Google Scholar]
- Persico, P.; Ambrogi, V.; Carfagna, C.; Cerruti, P.; Ferrocino, I.; Mauriello, G. Nanocomposite polymer films containing carvacrol for antimicrobial active packaging. Polym. Eng. Sci. 2009, 49, 1447–1455. [Google Scholar] [CrossRef]
- Oliani, W.L.; Fermino, D.M.; Komatsu, L.G.H.; Lugao, A.B.; Rangari, V.K.; Lincopan, N.; Parra, D.F. Preparation and Characterization of Polyethylene Nanocomposites with Clay and Silver Nanoparticles. In Characterization of Minerals, Metals, and Materials; Ikhmayies, S., Li, B., Carpenter, J.S., Li, J., Hwang, J.-Y., Monteiro, S.N., Firrao, D., Zhang, M., Peng, Z., Escobedo-Diaz, J.P., et al., Eds.; Characterization of Minerals, Metals, and Materials; Springer: Cham, Switzerland, 2017; pp. 709–718. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef] [Green Version]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [Green Version]
- Adel, K.; Zied, Z.; Ines, B.C.; Ahmed, B.; Neji, G.; Mohamed, D.; Radhouane, G. Chemical constituents and antioxidant properties of Rosmarinus officinalis L. essential oil cultivated South-Western Tunisia. J. Med. Plants Res. 2011, 5, 5999–6004. [Google Scholar]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant activity of rosemary and thyme by-products and synergism with added antioxidant in a liposome system. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Botsoglou, N.; Christaki, E.; Fletouris, D.; Florou-Paneri, P.; Spais, A. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002, 62, 259–265. [Google Scholar] [CrossRef]
- del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; Marín, M.P.; del Río, J.A.; Ortuño, A.; Ibarra, I. Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Postulation of a biosynthetic pathway. J. Agric. Food Chem. 2004, 52, 4987–4992. [Google Scholar] [CrossRef] [PubMed]
- Inatani, R.; Nakatani, N.; Fuwa, H. Antioxidative effect of the constituents of rosemary (Rosmarinus officinalis L.) and their derivatives. Agric. Biol. Chem. 1983, 47, 521–528. [Google Scholar] [CrossRef]
- Aruoma, O.; Halliwell, B.; Aeschbach, R.; Löligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Souza, L.C.; de Gomes, M.G.; Goes, A.T.; del Fabbro, L.; Filho, B.C.; Boeira, S.P.; Jesse, C.R. Evidence for the involvement of the serotonergic 5-HT1A receptors in the antidepressant-like effect caused by hesperidin in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Hong, C.-O.; Lee, G.P.; Kim, C.-T.; Lee, K.-W. The hepatoprotection of caffeic acid and rosmarinic acid, major compounds of Perilla frutescens, against t-BHP-induced oxidative liver damage. Food Chem. Toxicol. 2013, 55, 92–99. [Google Scholar] [CrossRef]
- Tornuk, F.; Sagdic, O.; Hancer, M.; Yetim, H. Development of LLDPE based active nanocomposite films with nanoclays impregnated with volatile compounds. Food Res. Int. 2018, 107, 337–345. [Google Scholar] [CrossRef]



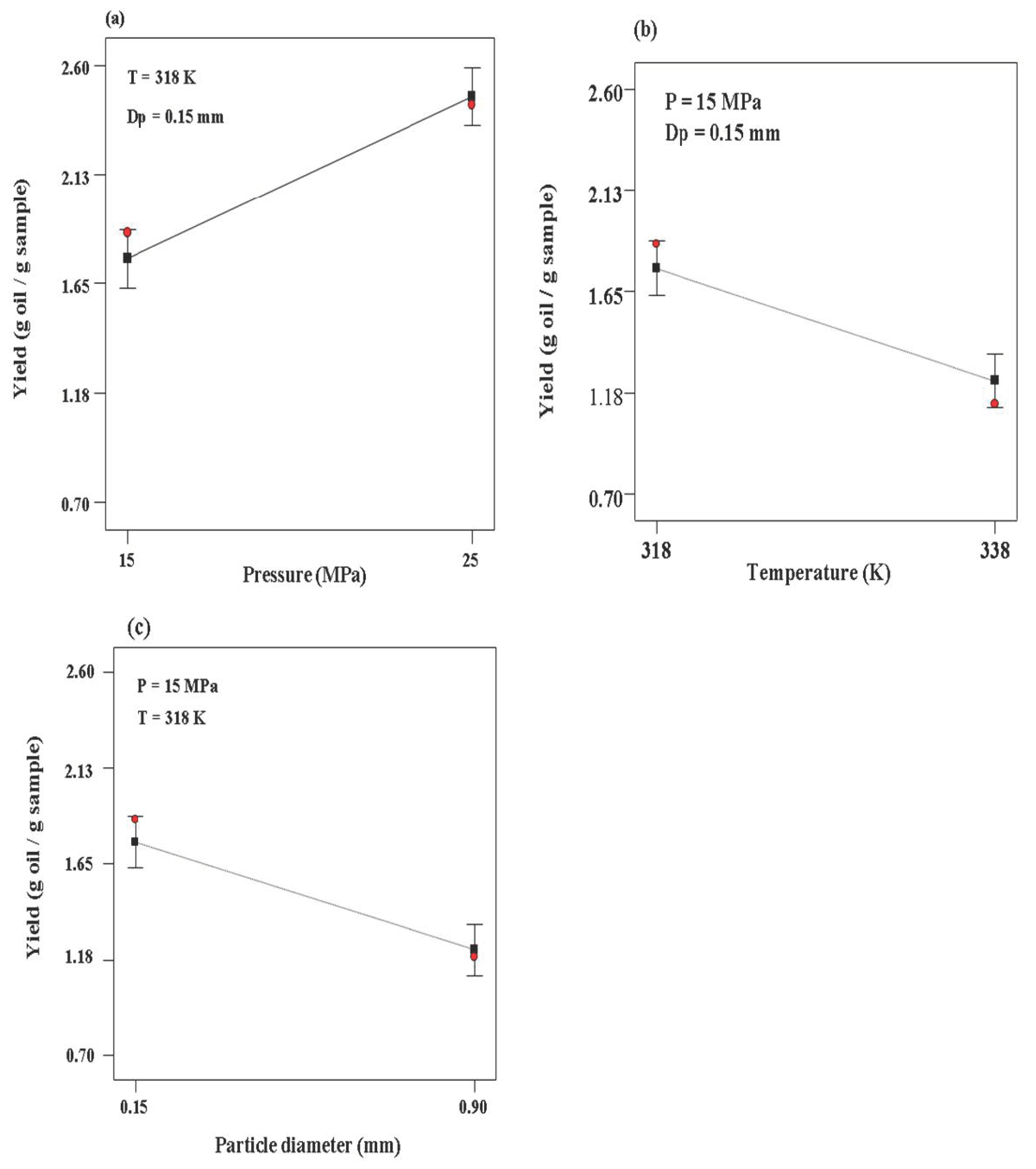
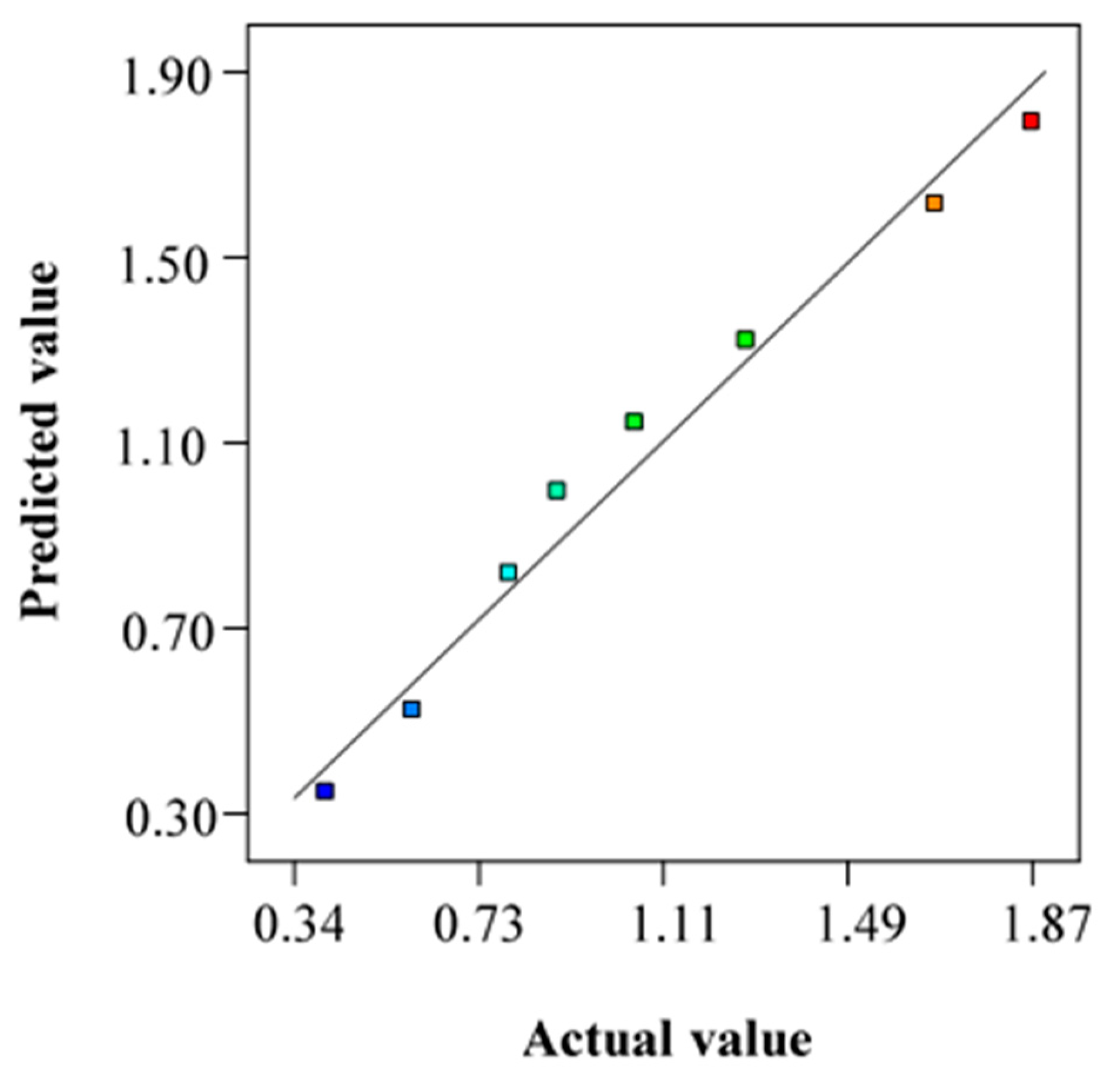
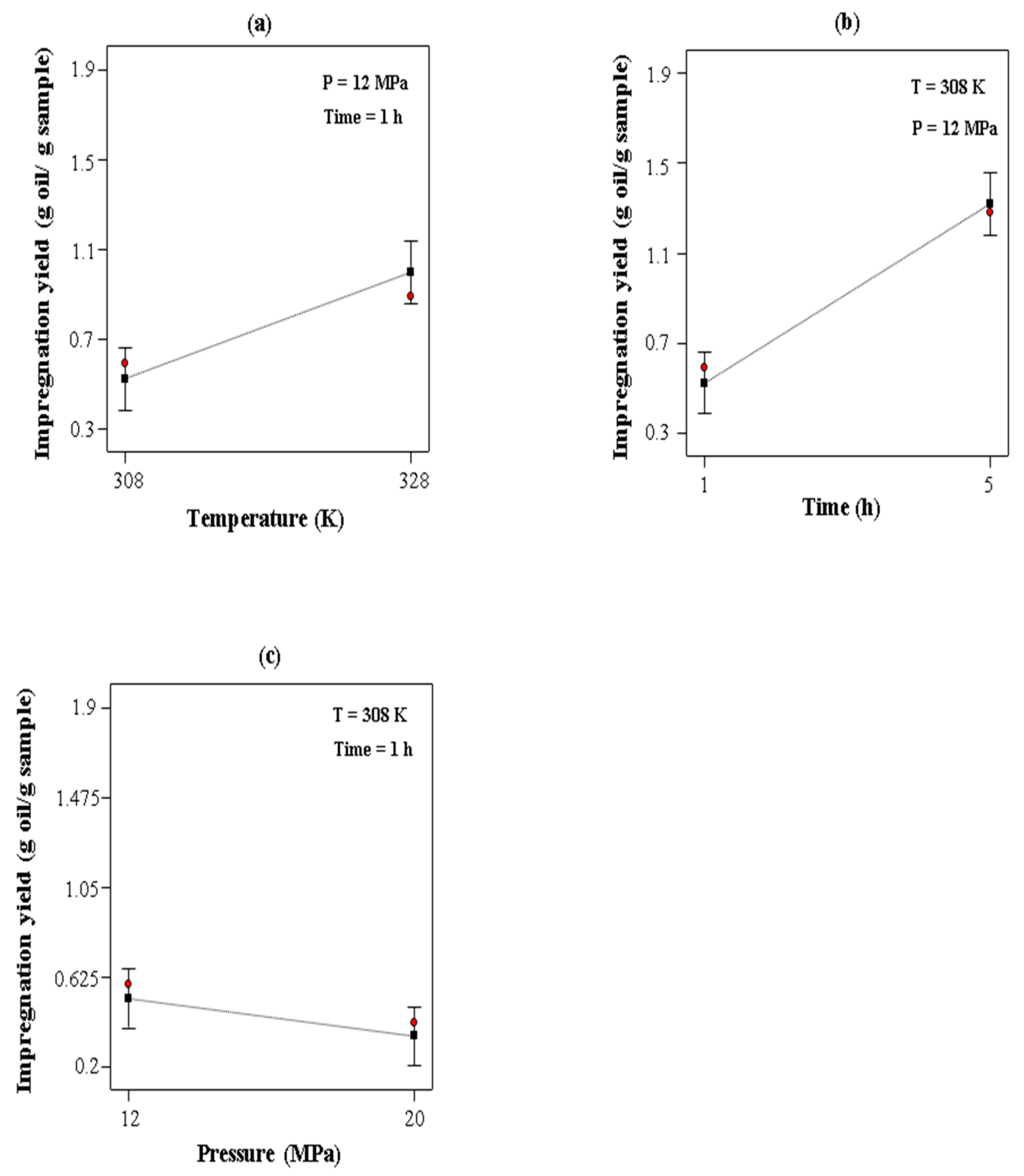
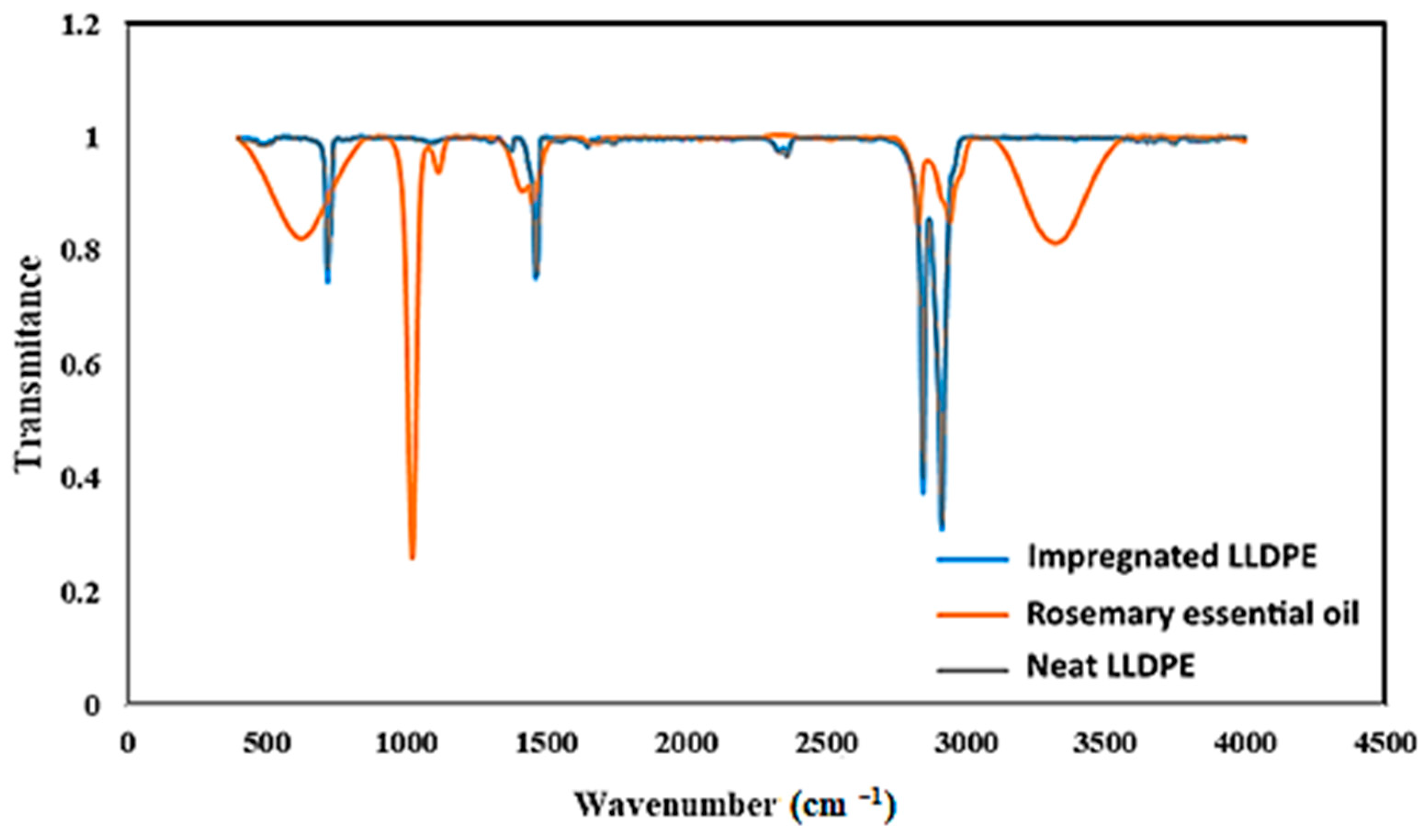


| Run | Pressure (MPa) | Temperature (K) | Particle Diameter (mm) | Extraction Yield (%) |
|---|---|---|---|---|
| 1 | 15 | 318 | 0.15 | 1.87 ± 0.06 |
| 5 | 15 | 338 | 0.15 | 1.12 ± 0.04 |
| 4 | 15 | 318 | 0.90 | 1.19 ± 0.05 |
| 3 | 15 | 338 | 0.90 | 0.73 ± 0.03 |
| 2 | 25 | 318 | 0.15 | 2.43 ± 0.11 |
| 8 | 25 | 338 | 0.15 | 1.97 ± 0.10 |
| 7 | 25 | 318 | 0.90 | 1.89 ± 0.09 |
| 6 | 25 | 338 | 0.90 | 1.45 ± 0.05 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 2.12 | 3 | 0.71 | 85.46 | 0.0004 |
| A-P | 1.00 | 1 | 1.00 | 120.80 | 0.0004 |
| B-T | 0.56 | 1 | 0.56 | 67.15 | 0.0012 |
| C-Dp | 0.57 | 1 | 0.57 | 68.43 | 0.0012 |
| Residual | 0.033 | 4 | 0.0082 | ||
| Cor Total | 2.16 | 7 | |||
| R² | Adjusted R² | Predicted R² | |||
| 0.9846 | 0.9731 | 0.9386 | |||
| No. | Compound | % | Retention Index |
|---|---|---|---|
| 1 | α-Pinene | 22.66 | 941 |
| 2 | Camphene | 6.52 | 953 |
| 3 | Sabinene | 0.25 | 969 |
| 4 | β-Pinene | 5.17 | 979 |
| 5 | β-Myrcene | 0.49 | 987 |
| 6 | α-phellandrene | 0.74 | 1007 |
| 7 | α-Terpinene | 0 | 1016 |
| 8 | p-cymene | 1.47 | 1021 |
| 9 | Limonene | 4.8 | 1030 |
| 10 | 1, 8-Cineole | 16.12 | 1033 |
| 11 | trance-Ocimene | t | 1040 |
| 12 | α-Terpinene | t | 1057 |
| 13 | α-Terpinolene | 0.49 | 1080 |
| 14 | Linalool | 2.7 | 1094 |
| 15 | Chrysthenone | t | 1120 |
| 16 | Camphor | 9.33 | 1136 |
| 17 | Verbenol | t | 1138 |
| 18 | Borneoil | 8.09 | 1162 |
| 19 | Terpine-4-ol | 0.49 | 1179 |
| 20 | α-Terpineol | 2.7 | 1184 |
| 21 | Verbenone | t | 1195 |
| 22 | cis-Myrtanol | 0.1 | 1244 |
| 23 | Trans-Myrtanol | 0.1 | 1251 |
| 24 | Bornyl acetate | 8.33 | 1281 |
| 25 | Methyl eugenol | 0.61 | 1401 |
| 26 | α-Caryophyllene | 0.84 | 1408 |
| 27 | β-Caryophyllene | 5.05 | 1422 |
| 28 | α-Humulene | 0.11 | 1443 |
| 29 | trance-beta-Farnesene | 0.1 | 1457 |
| Run | Pressure (P), X1 (MPa) | Temperature (T), X2 (K) | Impregnation Time, X3 (h) | Actual Impregnation Yield (wt.%) |
|---|---|---|---|---|
| 1 | 20 | 308 | 5 | 1.05 ± 0.04 |
| 2 | 20 | 328 | 1 | 0.79 ± 0.03 |
| 3 | 20 | 308 | 1 | 0.41 ± 0.02 |
| 4 | 20 | 328 | 5 | 1.67 ± 0.09 |
| 5 | 12 | 308 | 1 | 0.59 ± 0.02 |
| 6 | 12 | 328 | 1 | 0.89 ± 0.04 |
| 7 | 12 | 308 | 5 | 1.28 ± 0.08 |
| 8 | 12 | 328 | 5 | 1.87 ± 0.09 |
| Source | Sum of Squares | D.F. (Degree of Freedom) | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.78 | 3 | 0.59 | 59.76 | 0.0009 |
| A-T | 0.45 | 1 | 0.45 | 44.93 | 0.0026 |
| B-P | 0.063 | 1 | 0.063 | 6.34 | 0.0655 |
| C-Time | 1.27 | 1 | 1.27 | 128 | 0.0003 |
| Residual | 0.04 | 4 | 0.009938 | ||
| Cor Total | 1.82 | 7 | |||
| R² | Adjusted R² | Predicted R² | |||
| 0.9782 | 0.9618 | 0.9127 |
| Sample | IC50 |
|---|---|
| Rosemary essential oil | 76.44 ± 2.87 |
| Neat LLDPE | 0.00 |
| Impregnated LLDPE | |
| Run 1 | 69.55 ± 1.80 |
| Run 2 | 58.68 ± 1.24 |
| Run 3 | 60.50 ± 1.29 |
| Run 4 | 69.82 ± 1.72 |
| Run 5 | 60.69 ± 1.92 |
| Run 6 | 75.07 ± 2.34 |
| Run 7 | 75.33 ± 2.65 |
| Run 8 | 75.40 ± 2.56 |
| Yield Strength (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | |
|---|---|---|---|
| Neat LLDPE | 14.9 0.62 a | 19.5 3.21 a | 421 a |
| Impregnated LLDPE film | 14.7 a | 18.5 a | 375 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esfandiari, N.; Rojas, A.; Babhadiashar, A.; Galotto, M.J.; Saadati Ardestani, N.; Sajadian, S.A. Effect of the Processing Conditions on the Supercritical Extraction and Impregnation of Rosemary Essential Oil in Linear Low-Density Polyethylene Films. Processes 2023, 11, 11. https://doi.org/10.3390/pr11010011
Esfandiari N, Rojas A, Babhadiashar A, Galotto MJ, Saadati Ardestani N, Sajadian SA. Effect of the Processing Conditions on the Supercritical Extraction and Impregnation of Rosemary Essential Oil in Linear Low-Density Polyethylene Films. Processes. 2023; 11(1):11. https://doi.org/10.3390/pr11010011
Chicago/Turabian StyleEsfandiari, Nadia, Adrián Rojas, Arman Babhadiashar, María José Galotto, Nedasadat Saadati Ardestani, and Seyed Ali Sajadian. 2023. "Effect of the Processing Conditions on the Supercritical Extraction and Impregnation of Rosemary Essential Oil in Linear Low-Density Polyethylene Films" Processes 11, no. 1: 11. https://doi.org/10.3390/pr11010011
APA StyleEsfandiari, N., Rojas, A., Babhadiashar, A., Galotto, M. J., Saadati Ardestani, N., & Sajadian, S. A. (2023). Effect of the Processing Conditions on the Supercritical Extraction and Impregnation of Rosemary Essential Oil in Linear Low-Density Polyethylene Films. Processes, 11(1), 11. https://doi.org/10.3390/pr11010011








