Potential Use of Grape Stems and Pomaces from Two Red Grapevine Cultivars as Source of Oligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Sample Preparation
2.2. Chemical Composition of Pomaces and Stems
2.3. Autohydrolysis Experiments and Modelling
2.4. Sugar Analysis for Autohydrolysis Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of the Winery Wastes
3.1.1. Grape Pomace
3.1.2. Grape Stems
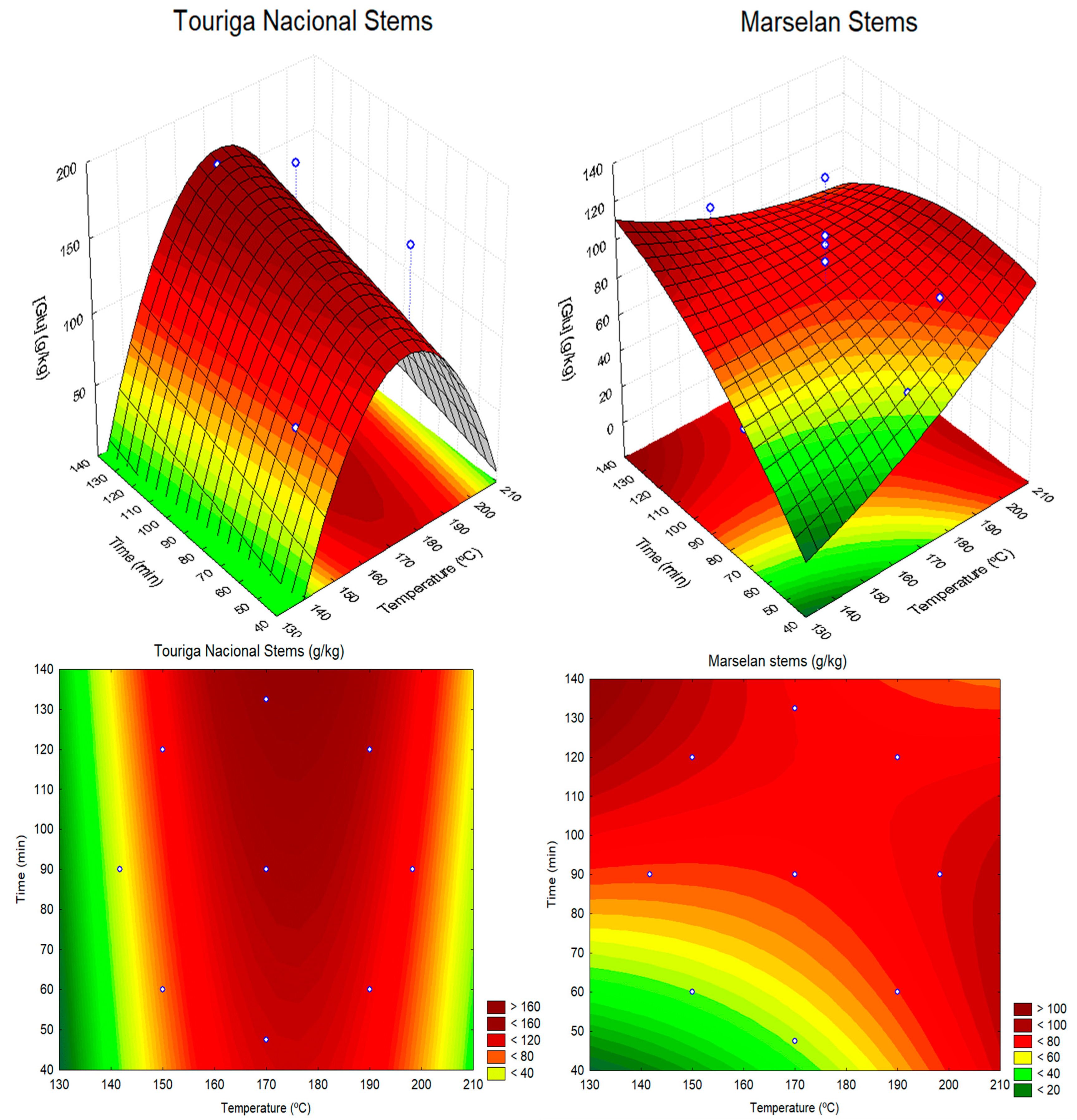
3.2. Autohydrolysis Experiments and Modelling
3.3. Monomeric and Oligosaccharides Quantification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. State of the World Vine and Wine Sector 2021. 2022. Available online: https://www.oiv.int/public/medias/8778/eng-state-of-the-world-vine-and-wine-sector-april-2022-v6.pdf (accessed on 3 June 2022).
- OIV. OIV Collective Expertise: Managing by-Products of Vitivinicultural Origin. International Organisation of Vine and Wine. Paris, France. 2018. Available online: https://www.oiv.int/public/medias/6267/managing-viticulture-by-products-web.pdf (accessed on 3 June 2022).
- Teissedre, P.-L.; Catarino, S.; Comuzzo, P. Wine quality production and sustainability. In Improving Sustainable Viticulture and Winemaking Practices; Costa, J.M., Catarino, S., Escalona, J.M., Comuzzo, P., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2022; pp. 187–196. [Google Scholar]
- Garcia-Perez, J.V.; Blasco, M.; Carcel, J.A.; Clemente, G.; Mulet, A. Drying Kinetics of Grape Stalk. In Defect and Diffusion Forum; Trans Tech Publications Ltd.: Baech, Switzerland, 2006; Volumes 258–260, pp. 225–230. [Google Scholar]
- Prozil, S.O.; Costa, E.V.; Evtuguin, D.V.; Cruz Lopes, L.P.; Domingues, M.R.M. Structural characterization of polysaccharides isolated from grape stems of Vitis vinifera L. Carbohydr. Res. 2012, 356, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Cruz Lopes, L.P. Chemical composition of grape stems of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.A.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stems waste. Ind. Crops Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of wine production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity–A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional characterization of different industrial white and red grape pomaces in Virginia and the potential valorization of the major components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Pedras, B.M.; Regalin, G.; Sá-Nogueira, I.; Simões, P.; Pavia, A.; Barreiros, S. Fractionation of red wine grape pomace by subcritical water extraction/hydrolysis. J. Supercrit. Fluids 2020, 160, 104793. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; Neto, P.O.; Fernades da Silva, D.; Pastore, C.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J. Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood. J. Chem. Technol. Biotechnol. 1999, 74, 1101–1109. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.; Egües, I.; Serrano, L.; Labidi, J.; Spigno, G. Autohydrolysis and organosolv process for recovery of hemicelluloses, phenolic compounds and lignin from grape stalks. Bioresour. Technol. 2012, 107, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Spigno, G.; Moncalvo, A.; de Faveri, D.M.; Silva, A. Valorisation of stalks from different grape cultivars for sugars recovery. Chem. Eng. Trans. 2014, 37, 745–750. [Google Scholar]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S. Optimization of a method to extract polysaccharides from white grape pomace by-products. Food Chem. 2021, 365, 130445. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S.; Doco, T. Characterization of polysaccharide extracts recovered from different grape and winemaking products. Food Res. Int. 2022, 157, 111480. [Google Scholar] [CrossRef]
- TAPPI 211 om-93. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C; TAPPI Press: Atlanta, GA, USA, 1993. [Google Scholar]
- TAPPI 222 om-02. Acid-Insoluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 2002. [Google Scholar]
- TAPPI UM 250. Acid-Soluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 1991. [Google Scholar]
- Haaland, P.D. Experimental Design in Biotechnology; Marcel Dekker Inc.: New York, NY, USA, 1989. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. 1987, A321, 523–536. [Google Scholar]
- Zhang, Y.H.P.; Hong, J.; Ye, X. Cellulase assays. Methods Mol. Biol. 2009, 581, 213–231. [Google Scholar]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- Freitas, L.; Simões, R.; Miranda, I.; Peres, F.; Ferreira-Dias, S. Optimization of autohydrolysis of olive pomaces to obtain bioactive oligosaccharides: The effect of cultivar and fruit ripening. Catalysts 2022, 12, 788. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Carvalheiro, F.; Moura, P.; Ballesteros, I.; Castro, E.; Gírio, F. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind. Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordevíć, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stems and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crops Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Spigno, G.; Maggi, L.; Amendola, D.; Dragoni, M.; de Faveri, D.M. Influence of cultivar on the lignocellulosic fractionation of grape stems. Ind. Crops Prod. 2013, 46, 283–289. [Google Scholar] [CrossRef]
- Spigno, G.; Pizzorno, T.; de Faveri, D.M. Cellulose and hemicelluloses recovery from grape stems. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape Stalk Valorization for Fermentation Purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Cruz Lopes, L.P. Towards comprehensive utilization of winemaking residues: Characterization of grape skins from red grape pomaces of variety Touriga Nacional. Ind. Crops Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Manufacture of xylose-based fermentation media from corncobs by posthydrolysis of autohydrolysis liquors Appl. Biochem. Biotechnol. 2001, 95, 195–207. [Google Scholar] [CrossRef]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of suitability of vine shoots for hemicellulosic oligosaccharides production through aqueous processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef]
- Gullón, B.; Gemma, E.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal treatment of chestnut shells (Castanea sativa) to produce oligosaccharides and antioxidant compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef] [PubMed]

| Experiment No. | Temperature (Coded Value) | Time (Coded Value) | Temperature (Decoded Value; ℃) | Time (Decoded Value; min) | S.F. (logR0) |
|---|---|---|---|---|---|
| 1 | (−1) | (−1) | 150 | 60 | 3.3 |
| 2 | (−1) | 1 | 150 | 120 | 3.6 |
| 3 | 1 | (−1) | 190 | 60 | 4.4 |
| 4 | 1 | 1 | 190 | 120 | 4.7 |
| 5 | ) | 0 | 142 | 90 | 3.2 |
| 6 | 0 | 198 | 90 | 4.8 | |
| 7 | 0 | 170 | 48 | 3.7 | |
| 8 | 0 | 170 | 132 | 4.2 | |
| 9 | 0 | 0 | 170 | 90 | 4.0 |
| 10 | 0 | 0 | 170 | 90 | 4.0 |
| 11 | 0 | 0 | 170 | 90 | 4.0 |
| 12 | 0 | 0 | 170 | 90 | 4.0 |
| 13 | 0 | 0 | 170 | 90 | 4.0 |
| Pomace | Stems | |||
|---|---|---|---|---|
| Component | Marselan | Touriga Nacional | Marselan | Touriga Nacional |
| Ash | 6.75 a ± 1.75 | 8.14 a ± 0.24 | 5.11 c ± 0.00 | 4.49 c ± 0.86 |
| Extractives, total: | 63.87 a ± 2.69 | 64.40 a ± 1.93 | 46.19 c ± 0.28 | 59.51 d ± 0.32 |
| -Dichloromethane | 25.37 a ± 0.57 | 26.56 a ± 0.58 | 2.16 c ± 0.02 | 1.05 d ± 0.03 |
| -Ethanol | 12.71 a ± 1.27 | 15.61 b ± 0.70 | 24.15 c ± 0.71 | 39.22 d ± 0.78 |
| -Water | 25.79 a ± 1.74 | 22.23 a ± 2.35 | 19.87 c ± 0.58 | 19.23 c ± 0.69 |
| Lignin, total: | 20.35 a ± 0.30 | 19.36 a ± 0.31 | 26.47 c ± 0.45 | 17.25 d ± 0.53 |
| -Klason lignin | 20.20 a ± 0.31 | 19.21 a ± 0.33 | 26.21 c ± 0.44 | 17.10 d ± 0.55 |
| -Soluble lignin | 0.15 a ± 0.02 | 0.15 a ± 0.02 | 0.26 c ± 0.01 | 0.15 d ±0.03 |
| Lignin (extractive free) | 55.6 | 53.6 | 49.06 | 42.73 |
| Polysaccharides | 8.98 a ± 0.0 | 8.16 a ± 0.0 | 22.32 a ± 2.03 | 18.73 b ± 0.22 |
| Polysaccharides (extractive free) | 24.63 | 23.03 | 41.48 | 46.26 |
| Experiment | Autohydrolysis | Pomace | Stems | ||
|---|---|---|---|---|---|
| No. | (T/t; °C/min) | Marselan | Touriga Nacional | Marselan | Touriga Nacional |
| (g Glu/kg) | (g Glu/kg) | (g Glu/kg) | (g Glu/kg) | ||
| 1 | 150/60 | 26.6 | 30.1 | 34.3 | 82.9 |
| 2 | 150/120 | 36.5 | 38.9 | 116.3 | 104.0 |
| 3 | 190/60 | 57.5 | 24.6 | 82.9 | 162.3 |
| 4 | 190/120 | 68.2 | 43.1 | 102.2 | 162. 6 |
| 5 | 142/90 | 19.0 | 15.8 | 28.0 | 38.3 |
| 6 | 198/90 | 31.1 | 43.5 | 38.0 | 50.8 |
| 7 | 170/48 | 31.2 | 55.0 | 58.4 | 109.7 |
| 8 | 170/132 | 81.2 | 76.3 | 55.0 | 168.4 |
| 9 | 170/90 | 58.7 | 70.9 | 103.8 | 135.0 |
| 10 | 170/90 | 56.7 | 68.6 | 108.0 | 143.9 |
| 11 | 170/90 | 56.4 | 71.1 | 94.9 | 127.2 |
| 12 | 170/90 | 58.1 | 69.3 | - | 137.6 |
| 13 | 170/90 | 53.4 | 69.4 | - | 144.0 |
| Material | Model Equation | R2 | R2adj |
|---|---|---|---|
| Pomaces: | |||
| Touriga Nacional | [Glu] = −1736.1 + 20.18T − 0.06T2 + 1.33t − 0.01t2 | 0.882 | 0.823 |
| Marselan | [Glu] = −1096.94 + 12.68T − 0.04T2 + 0.38t | 0.827 | 0.770 |
| Stems: | |||
| Touriga Nacional | [Glu] = −2859.37 + 33.90T − 0.10T 2 + 0.43t | 0.751 | 0.627 |
| Marselan | [Glu] = −141.51 + 0.005T2+ 3.99t − 0.006t 2 − 0.015Txt | 0.545 | 0.241 |
| Material | Autohydrolysis | Total Sugars Released | Monomeric Sugars (% Total Sugars) (g/kg d.w.) | OS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (T/t; ℃/min) | (g/kg d.w.) | Rha | Ara | Gal | Glu | Xyl | Total | (g/kg) | Yield (%) | |
| Pomaces: | ||||||||||
| Touriga Nacional | 170/90 | 69.6 | 0.00 | 0.00 | 0.10 | 0.45 | 0.27 | 0.82 | 68.76 | 98.82 |
| Touriga Nacional | 198/90 | 43.5 | 1.09 | 2.76 | 1.12 | 0.80 | 2.92 | 8.68 | 34.86 | 80.06 |
| Marselan | 170/90 | 56.6 | 0.00 | 0.00 | 0.08 | 0.32 | 0.23 | 0.63 | 56.02 | 98.88 |
| Marselan | 198/90 | 31.1 | 0.49 | 1.45 | 0.82 | 0.68 | 2.14 | 5.59 | 25.55 | 82.05 |
| Stems: | ||||||||||
| Touriga Nacional | 170/90 | 138.1 | 0.00 | 0.00 | 0.18 | 0.60 | 0.38 | 1.16 | 136.98 | 99.16 |
| Touriga Nacional | 198/90 | 50.8 | 1.10 | 2.81 | 1.17 | 0.85 | 2.92 | 8.86 | 41.89 | 82.54 |
| Marselan | 170/90 | 102.4 | 0.01 | 0.01 | 0.24 | 0.58 | 0.40 | 1.24 | 101.16 | 98.79 |
| Marselan | 198/90 | 35.8 | 1.46 | 4.69 | 1.88 | 11.90 | 3.61 | 23.55 | 12.25 | 34.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangione, R.; Simões, R.; Pereira, H.; Catarino, S.; Ricardo-da-Silva, J.; Miranda, I.; Ferreira-Dias, S. Potential Use of Grape Stems and Pomaces from Two Red Grapevine Cultivars as Source of Oligosaccharides. Processes 2022, 10, 1896. https://doi.org/10.3390/pr10091896
Mangione R, Simões R, Pereira H, Catarino S, Ricardo-da-Silva J, Miranda I, Ferreira-Dias S. Potential Use of Grape Stems and Pomaces from Two Red Grapevine Cultivars as Source of Oligosaccharides. Processes. 2022; 10(9):1896. https://doi.org/10.3390/pr10091896
Chicago/Turabian StyleMangione, Roberta, Rita Simões, Helena Pereira, Sofia Catarino, Jorge Ricardo-da-Silva, Isabel Miranda, and Suzana Ferreira-Dias. 2022. "Potential Use of Grape Stems and Pomaces from Two Red Grapevine Cultivars as Source of Oligosaccharides" Processes 10, no. 9: 1896. https://doi.org/10.3390/pr10091896
APA StyleMangione, R., Simões, R., Pereira, H., Catarino, S., Ricardo-da-Silva, J., Miranda, I., & Ferreira-Dias, S. (2022). Potential Use of Grape Stems and Pomaces from Two Red Grapevine Cultivars as Source of Oligosaccharides. Processes, 10(9), 1896. https://doi.org/10.3390/pr10091896








