Biological Profiling of Essential Oils and Hydrolates of Ocimum basilicum var. Genovese and var. Minimum Originated from Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Essential Oil and Hydrolate Obtaining Process
- OBGEO for Ocimum basilicum var. genovese essential oil;
- OBMEO for Ocimum basilicum var. minimum essential oil;
- OBGH for Ocimum basilicum var. genovese hydrolate;
- OBMH for Ocimum basilicum var. minimum hydrolate.
2.3. Analysis of Volatile Compounds
2.4. In Vitro Assessment of Antioxidant Activity
2.5. In Vitro Assessment of Antibacterial Activity
2.5.1. Test Microorganisms
2.5.2. The Disk Diffusion Method
2.5.3. Determination of Minimal Inhibitory Concentration
2.6. Statistical Analysis
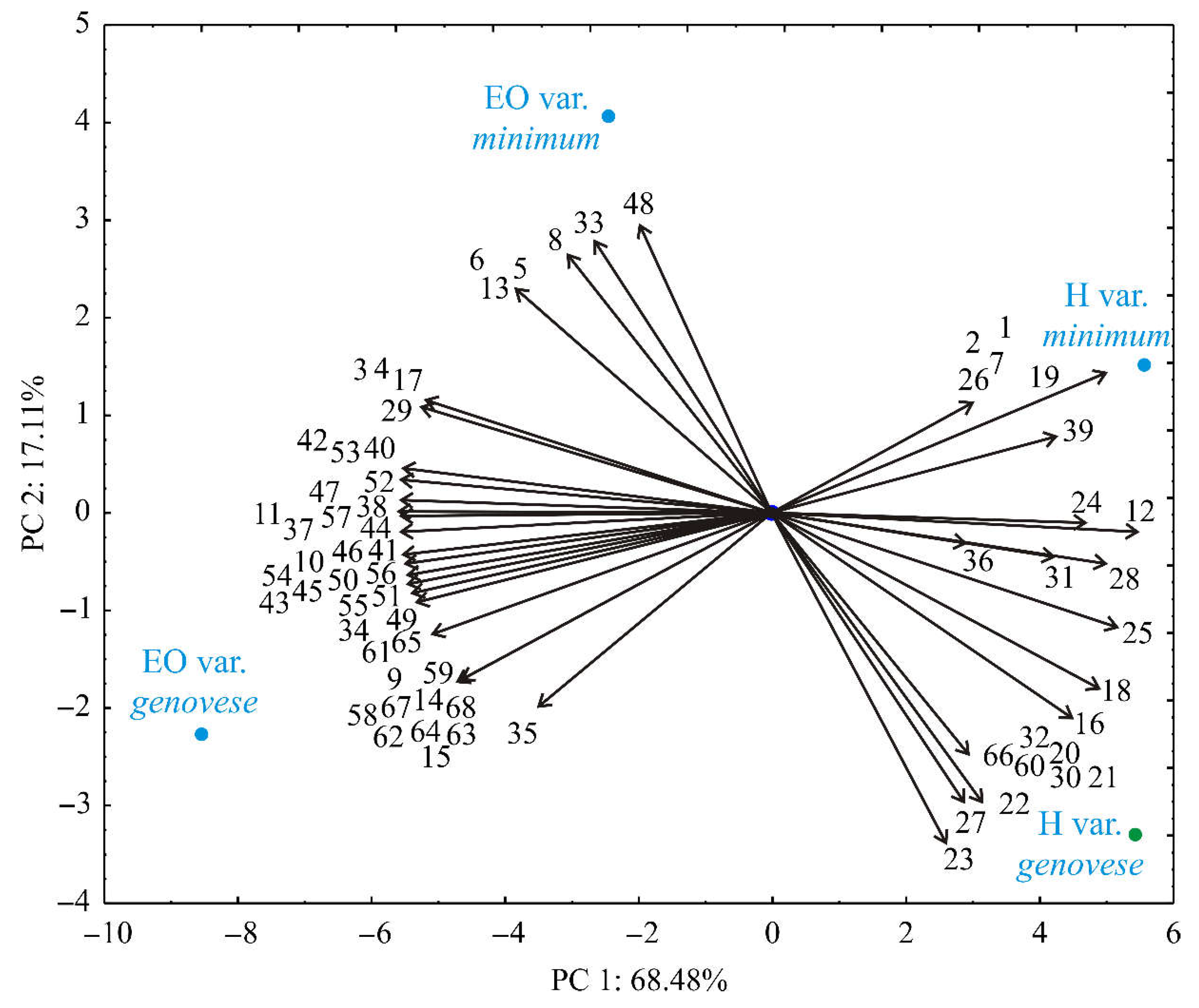
3. Results and Discussion
3.1. Volatile Compounds of Essential Oils and Hydrolates
3.2. In vitro Assessment of Antioxidant Activity
3.3. In vitro Assessment of Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharafati Chaleshtori, R.; Rokni, N.; Rafieian-Kopaei, M.; Deris, F.; Salehi, E. Antioxidant and antibacterial activity of basil (Ocimum basilicum L.) essential oil in beef burger. J. Agric. Sci. Technol. 2015, 17, 817–826. [Google Scholar]
- Kaya, I.; Yigit, N.; Benli, M. Antimicrobial activity of various extracts of Ocimum basilicum L. and observation of the inhibition effect on bacterial cells by use of scanning electron microscopy. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 363–369. [Google Scholar] [CrossRef]
- Barbosa, C.O.; de Morais, S.M.; de Sousa, H.A.; Martins, V.C.; Neto, J.F.C.; Vieira, I.G.P.; Pereira, R.C.A.; Rodrigues, A.L.M.; Carioca, J.O.B. Chemical composition and antioxidant potential of essential oils from different Ocimum species (Basil). Braz. J. Dev. Curitiba 2021, 7, 24422–24442. [Google Scholar] [CrossRef]
- Kumar Sinha, A.; Singh, T.P. A new chemotype of Ocimum basilicum var. purpurascens Benth. from Madhubani district of Bihar, India and phytochemical investigations of their colchiploids. Int. J. Fauna Biol. Stud. 2021, 8, 14–18. [Google Scholar] [CrossRef]
- Mkaddem Mounira, G.; Ahlem, Z.; Abdallah Mariem, B.; Romdhane, M.; Okla, M.K.; Al-Hashimi, A.; Alwase, Y.A.; Madnay, M.M.; AbdElgayed, G.; Asard, H.; et al. Essential Oil Composition and Antioxidant and Antifungal Activities of Two Varieties of Ocimum basilicum L. (Lamiaceae) at Two Phenological Stages. Agronomy 2022, 12, 825. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kabir, M.J.; Salehuddin, S.M.; Rahman, S.M.; Das, A.K.; Singha, S.K.; Alam, M.K.; Rahman, A. Antibacterial properties of essential oils and methanol extracts of sweet basil Ocimum basilicum occurring in Bangladesh. Pharm. Biol. 2010, 48, 504–511. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Fedoul, F.; Meddah, B.; Larouci, M.; Tir Touil, A.; Merazi, Y.; Bekhti, N.; Piras, A.; Falconieri, D.; Cakmak, Y. Medicinal Applications, Chemical Compositions, and Biological Effects of Algerian Ocimum basilicum L. var Genovese with the Conversion of Experimental Doses to Humans. J. Appl. Biotechnol. Rep. 2022, 9, 671–683. [Google Scholar]
- Seyed, M.A.; Ayesha, S.; Azmi, N.; Al-Rabae, F.M.; Al-Alawy, A.I.; Al-Zahrani, O.R.; Hawsaw, Y. The neuroprotective attribution of Ocimum basilicum: A review on the prevention and management of neurodegenerative disorders. Future J. Pharm. Sci. 2021, 7, 139. [Google Scholar] [CrossRef]
- Tašković, J. Basil Extracts (Ocimum basilicum L.) Obtained by Different Extraction Methods. Master’s Thesis, University of Novi Sad, Novi Sad, Serbia, 2016. [Google Scholar]
- Michalak, M. Aromatherapy and methods of applying essential oils. Arch. Physiother. Glob. Res. 2018, 22, 25–31. [Google Scholar]
- Aćimović, M. Essential Oils: Inhalation Aromatherapy—A Comprehensive Review. J. Agron. Technol. Eng. Manag. 2021, 4, 547–557. [Google Scholar]
- Han, H.J. Development of an effective formulation for an acne treatment cream with Ocimum basilicum using invasomes. J. Cosmet. Med. 2018, 2, 69–75. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Bakay, L.; Zagrobelna, E.; Kluz, M.; et al. Assessment of Ocimum basilicum Essential Oil Anti-Insect Activity and Antimicrobial Protection in Fruit and Vegetable Quality. Plants 2022, 11, 1030. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, S.; Xu, Q.; Wang, J.; Yang, Y.; Mi, Y.; Jin, Z.; Desneux, N.; Wang, S. Optimizing the Use of Basil as a Functional Plant for the Biological Control of Aphids by Chrysopa pallens (Neuroptera: Chrysopidae) in Greenhouses. Insects 2022, 13, 552. [Google Scholar] [CrossRef]
- Moura, E.d.S.; Faroni, L.R.D.; Heleno, F.F.; Rodrigues, A.A.Z. Toxicological Stability of Ocimum basilicum Essential Oil and Its Major Components in the Control of Sitophilus zeamais. Molecules 2021, 26, 6483. [Google Scholar] [CrossRef] [PubMed]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil Essential Oil: Composition, Antimicrobial Properties, and Microencapsulation to Produce Active Chitosan Films for Food Packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef]
- Chu, H.T.T.; Vu, T.N.; Dinh, T.T.T.; Do, P.T.; Chu, H.H.; Tien, T.Q.; Tong, Q.C.; Nguyen, M.H.; Ha, Q.T.; Setzer, W.N. Effects of Supplemental Light Spectra on the Composition, Production and Antimicrobial Activity of Ocimum basilicum L. Essential Oil. Molecules 2022, 27, 5599. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, D.; Bojko, K.; Kaymakanova, M.; Berova, M. Salinity-Induced Changes in the Antioxidant Status of Common Basil Plants (Ocimum basilicum L.) Grown under Controlled Conditions. Horticulturae 2022, 8, 775. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, Antioxidant Activity, and Phytochemicals of Basil (Ocimum basilicum L.) Leaves Cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Camlica, M.; Yaldiz, G.; Özen, F. Effects of different basil hydrosol doses on the germination and shoot and root lenghts of basil (Ocimum basilicum) and quinoa (Chenopodium quinoa) seeds. Indian J. Pharm. Educ. Res. 2017, 51, 254–257. [Google Scholar] [CrossRef]
- Moghaddam, A.M.D.; Shayegh, J.; Mikaili, P.; Sharaf, J.D. Antimicrobial activity of essential oil extract of Ocimum basilicum L. leaves on a variety of pathogenic bacteria. J. Med. Plants Res. 2011, 5, 3453–3456. [Google Scholar]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Tmušić, N.; Mastilović, J.; Kevrešan, Ž.; Stanojević, L.; Danilović, B.; Stanojević, J. Efficiency of basil essential oil antimicrobial agents under different shading treatments and harvest times. Agronomy 2021, 11, 1574. [Google Scholar] [CrossRef]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Abewoy, D.; Belew, D.; Zigene, Z.D. Influence of Harvesting Age and Genotype on Growth Parameters and Herbage Yield of Sweet Basil (Ocimum basilicum L.) at Wondo Genet, Southern Ethiopia. Adv. Crop Sci. Technol. 2018, 6, 407. [Google Scholar] [CrossRef]
- Juškevičiene, D.; Radzevičius, A.; Viškelis, P.; Maročkiene, N.; Karkleliene, R. Estimation of Morphological Features and Essential Oil Content of Basils (Ocimum basilicum L.) Grown under Different Conditions. Plants 2022, 11, 1896. [Google Scholar] [CrossRef]
- Copolovici, L.; Copolovici, D.M.; Moisa, C.; Lupitu, A. Antagonist Temperature Variation Affects the Photosynthetic Parameters and Secondary Metabolites of Ocimum basilicum L. and Salvia officinalis L. Plants 2022, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Aćimović, M.; Šovljanski, O.; Šeregelj, V.; Pezo, L.; Zheljazkov, V.D.; Ljujić, J.; Tomić, A.; Ćetković, G.; Čanadanović-Brunet, J.; Miljković, A.; et al. Chemical Composition, Antioxidant, and Antimicrobial Activity of Dracocephalum moldavica L. Essential Oil and Hydrolate. Plants 2022, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Aborus, N.E.; Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Hidalgo, A.; Vulić, J.; Šeregelj, V. Sprouted and freeze-dried wheat and oat seeds—Phytochemical profile and in vitro biological activities. Chem. Biodivers. 2018, 15, e1800119. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction–antioxidant activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Aćimović, M.; Šeregelj, V.; Šovljanski, O.; Tumbas Šaponjac, V.; Švarc Gajić, J.; Brezo-Borjan, T.; Pezo, L. In vitro antioxidant, antihyperglycemic, anti-inflammatory and antimicrobial activity of Satureja kitaibelii Wierzb. ex Heuff. subcritical water extract. Ind. Crops Prod. 2021, 169, 113672. [Google Scholar] [CrossRef]
- Micić, D.; Đurović, S.; Riabov, P.; Tomić, A.; Šovljanski, O.; Filip, S.; Tosti, T.; Dojčinović, B.; Božović, R.; Jovanović, D.; et al. Rosemary Essential Oils as a Promising Source of Bioactive Compounds: Chemical Composition, Thermal Properties, Biological Activity, and Gastronomical Perspectives. Foods 2021, 10, 2734. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Toraldo, G.; Cavella, S.; Masi, P. Description of leavening of bread dough with mathematical modelling. J. Food Eng. 2007, 83, 142–148. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Edris, A.E. Identification and absolute quantification of the major water-soluble aroma components isolated from the hydrosols of some aromatic plants. J. Essent. Oil Bear. Plants 2009, 12, 155–161. [Google Scholar] [CrossRef]
- Smigielski, K.B.; Prusinowska, R.; Bemska, J.E. Comparison of the chemical composition of essential oils and hydrolates from basil (Ocimum basilicum L.). J. Essent. Oil Bear. Plants 2016, 19, 492–498. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151–156. [Google Scholar] [CrossRef]
- Vieira, P.R.N.; de Morais, S.M.; Bezerra, F.H.Q.; Travassos Ferreira, P.A.; Oliveira, Í.R.; Silva, M.G.V. Chemical composition and antifungal activity of essential oils from Ocimum species. Ind. Crops Prod. 2014, 55, 267–271. [Google Scholar] [CrossRef]
- Martins, A.; Salgueiro, L.; Vila, R.; Tomi, F.; Cañigueral, S.; Casanova, J.; da Cunha, A.P.; Adzet, T. Composition of the Essential Oils of Ocimum canum, O. gratissimum and O. minimum. Planta Med. 1999, 65, 187–189. [Google Scholar] [CrossRef]
- Stefan, M.; Zamfirache, M.; Padurariu, C.; Trută, E.; Gostin, I. The composition and antibacterial activity of essential oils in three Ocimum species growing in Romania. Open Life Sci. 2013, 8, 600–608. [Google Scholar] [CrossRef]
- Da Costa, A.S.; Arrigoni-Blank, M.d.F.; de Carvalho Filho, J.L.S.; de Santana, A.D.D.; Santos, D.d.A.; Alves, P.B.; Blank, A.F. Chemical Diversity in Basil (Ocimum sp.) Germplasm. Sci. World J. 2015, 2015, 352638. [Google Scholar] [CrossRef]
- Ilić, A.S.; Antić, M.P.; Jelačić, S.C.; Šolevič Knudsen, T.M. Chemical Composition of the Essential Oils of Three Ocimum basilicum L. Cultivars from Serbia. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 347–351. [Google Scholar] [CrossRef]
- Danesi, F.; Elementi, S.; Neri, R.; Maranesi, M.; D’Antuono, L.F.; Bordoni, A. Effect of cultivar on the protection of cardiomyocytes from oxidative stress by essential oils and aqueous extracts of basil (Ocimum basilicum L.). J. Agric. Food Chem. 2008, 56, 9911–9917. [Google Scholar] [CrossRef]
- Beatović, D.; Krstić-Milošević, D.; Trifunović, S.; Šiljegović, J.; Glamočlija, J.; Ristić, M.; Jelačić, S. Chemical Composition, Antioxidant and Antimicrobial Activities of the Essential Oils of Twelve Ocimum basilicum L. Cultivars Grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oyeleye, S.I.; Oboh, G. Biological activities, antioxidant properties, and phytoconstituents of essential oil from sweet basil (Ocimum basilicum L.) leaves. Comp. Clin. Pathol. 2016, 25, 169–176. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant activity of eight hydrosols from Morocco. Asian J. Plant Sci. 2012, 11, 137–142. [Google Scholar] [CrossRef][Green Version]
- De Miranda, C.A.S.F.; Cardoso, M.D.G.; de Carvalho, M.L.M.; Machado, S.M.F.; Gomes, M.D.S.; Andrade, J.D.; Teixeira, M.L. Allelopathic activity of medicinal plant essential oils on seed germination and vigor of lettuce achenes. Semina Ciências Agrárias 2015, 36 (Suppl. 1), 1783–1798. [Google Scholar]
- Carović-Stanko, K.; Orlić, S.; Politeo, O.; Strikić, F.; Kolak, I.; Milos, M.; Satovic, Z. Composition and antibacterial activities of essential oils of seven Ocimum taxa. Food Chem. 2010, 119, 196–201. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 49, 5–16. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef]

| No | Compound | RI | OBGEO | OBGH | OBMEO | OBMH |
|---|---|---|---|---|---|---|
| 1. | 3E-Hexenol | 851 | - | tr | - | 0.5 |
| 2. | n-Hexanol | 864 | - | - | - | 0.1 |
| 3. | α-Pinene | 931 | 0.2 | - | 0.2 | - |
| 4. | Camphene | 946 | 0.1 | - | 0.1 | - |
| 5. | Sabinene | 970 | 0.1 | - | 0.2 | - |
| 6. | β-Pinene | 974 | 0.2 | - | 0.4 | - |
| 7. | 1-Octen-3-ol | 977 | - | tr | - | 0.1 |
| 8. | Myrcene | 988 | 0.1 | tr | 0.3 | - |
| 9. | α-Terpinene | 1014 | 0.1 | tr | - | - |
| 10. | p-Cymene | 1022 | 0.6 | - | 0.2 | - |
| 11. | Limonene | 1025 | 0.4 | - | 0.2 | - |
| 12. | 1,8-Cineole | 1028 | 2.4 | 5.5 | 3.4 | 5.9 |
| 13. | trans-β-Ocimene | 1044 | 0.1 | - | 0.2 | - |
| 14. | γ-Terpinene | 1055 | 0.2 | 0.1 | 0.1 | - |
| 15. | cis-Sabinene hydrate (IPP vs OH) | 1063 | 0.3 | tr | - | - |
| 16. | cis-Linalool oxide (furanoid) | 1069 | 0.1 | 1.5 | 0.1 | 0.7 |
| 17. | Terpinolene | 1086 | 0.1 | - | 0.1 | - |
| 18. | trans-Linalool oxide (furanoid) | 1088 | - | 1.3 | - | 0.8 |
| 19. | Linalool | 1100 | 40.5 | 75.5 | 71.5 | 77.1 |
| 20. | cis-p-Menth-2-en-1-ol | 1121 | - | 0.1 | - | - |
| 21. | trans-Pinocarveol | 1137 | - | 0.1 | - | - |
| 22. | Camphor | 1141 | 1.1 | 2.7 | 0.6 | 1.2 |
| 23. | Borneol | 1163 | 0.1 | 0.2 | tr | 0.1 |
| 24. | δ-Terpineol | 1163 | - | 0.1 | - | 0.2 |
| 25. | cis-Linalool oxide (pyanoid) | 1171 | - | 0.2 | - | 0.2 |
| 26. | trans-Linalool oxide (pyanoid) | 1172 | - | - | - | 0.2 |
| 27. | Terpinen-4-ol | 1174 | 1.4 | 5.4 | 0.3 | 1.3 |
| 28. | α-Terpineol | 1188 | 0.3 | 1.1 | 0.3 | 1.5 |
| 29. | Estragole | 1196 | 3.2 | 0.5 | 3.1 | 0.5 |
| 30. | Verbenone | 1207 | - | 0.1 | - | - |
| 31. | Geraniol | 1252 | 0.1 | 0.3 | - | 0.6 |
| 32. | Chavicol | 1252 | - | 0.1 | - | - |
| 33. | Linalool acetate | 1253 | 0.1 | - | 0.4 | - |
| 34. | Bornyl acetate | 1284 | 1.5 | 0.1 | 0.5 | tr |
| 35. | Thymol | 1291 | 0.6 | 0.5 | 0.4 | - |
| 36. | Carvacrol | 1301 | 0.1 | 0.1 | - | 0.3 |
| 37. | δ-Elemene | 1335 | 0.4 | - | 0.2 | - |
| 38. | α-Cubebene | 1347 | 0.2 | - | 0.1 | - |
| 39. | Eugenol | 1356 | 0.3 | 2.7 | 1.2 | 7.7 |
| 40. | α-Copaene | 1373 | 0.5 | - | 0.3 | - |
| 41. | β-Bourbonene | 1382 | 1.1 | - | 0.4 | - |
| 42. | β-Cubebene | 1387 | 0.3 | - | 0.2 | - |
| 43. | β-Elemene | 1390 | 5.3 | - | 1.9 | - |
| 44. | trans-Caryophyllene | 1417 | 1.4 | - | 0.6 | - |
| 45. | β-Copaene | 1427 | 0.3 | - | 0.1 | - |
| 46. | β-Gurjunene | 1430 | 0.5 | - | 0.2 | - |
| 47. | trans-α-Bergamotene | 1434 | 3.1 | - | 1.7 | - |
| 48. | α-Guaiadiene | 1441 | 0.1 | - | 0.8 | - |
| 49. | α-Humulene | 1452 | 2.2 | - | 0.6 | - |
| 50. | trans-β-Farnesene | 1455 | 0.3 | - | 0.1 | - |
| 51. | epi-Bicyclosesquiphellandrene | 1461 | 1.0 | - | 0.3 | - |
| 52. | Germacrene D | 1480 | 5.3 | - | 3.0 | - |
| 53. | Bicyclogermacrene | 1496 | 0.7 | - | 0.5 | - |
| 54. | α-Bulnesene | 1505 | 3.4 | - | 1.2 | - |
| 55. | γ-Cadinene | 1513 | 5.2 | - | 1.6 | - |
| 56. | trans-Calamenene | 1521 | 0.6 | - | 0.2 | - |
| 57. | δ-Cadinene | 1522 | 0.2 | - | 0.1 | - |
| 58. | α-Cadinene | 1536 | 0.1 | - | tr | - |
| 59. | trans-Nerolidol | 1562 | 0.3 | - | - | - |
| 60. | Maaliol | 1567 | - | 0.1 | - | - |
| 61. | Spathulenol | 1575 | 0.6 | tr | 0.1 | - |
| 62. | Caryophyllene oxide | 1581 | 0.3 | - | - | - |
| 63. | Humulene epoxide II | 1606 | 0.4 | - | - | - |
| 64. | 1,10-di-epi-Cubenol | 1612 | 1.1 | tr | - | - |
| 65. | epi-α-Cadinol (=τ-cadinol) | 1639 | 6.1 | - | 1.0 | 0.1 |
| 66. | 10-epi-α-Cadinol | 1641 | - | 0.2 | - | - |
| 67. | β-Eudesmol | 1648 | 0.3 | - | - | - |
| 68. | α-Cadinol | 1653 | 0.4 | tr | - | - |
| Antioxidant Activity (μmol TE/mL) | Samples | |||
|---|---|---|---|---|
| OBGEO | OBMEO | OBGH | OBMH | |
| DPPH• | 41.77 ± 1.79 | 43.25 ± 2.43 | 2.10 ± 1.47 | 0.94 ± 0.49 |
| ABTS•+ | 478.065 ± 27.30 | 508.36 ± 31.03 | 2.91 ± 0.36 | 1.51 ± 0.17 |
| RP | 21.0 ± 1.51 | 22.71 ± 0.97 | 0.76 ± 0.07 | 0.48 ± 0.02 |
| Bacterial Strain | Inhibition Zones * (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| OBGEO | OBMEO | OBGH | OBMH | Water | Tetracycline | 5% DMSO | ||
| Gram-negative bacteria | E. coli ATCC 25922 | 38.00 ± 0.00 | 40.00 ± 0.00 | nd | nd | nd | 15.00 ± 0.00 | nd |
| P. aureginosa ATCC 27853 | 12.00 ± 0.00 | 12.00 ± 0.00 | nd | nd | nd | 11.00 ± 0.00 | nd | |
| S. Typhimurium ATCC 13311 | 32.00 ± 0.00 | 40.00 ± 0.00 | nd | nd | nd | 21.00 ± 1.00 | nd | |
| Gram-positive bacteria | B. cereus ATCC 11778 | 14.67 ± 1.15 | nd | nd | nd | nd | 17 ± 0.00 | nd |
| L. monocytogenes ATCC 35152 | 19.00± 1.00 | nd* | nd | nd | nd | 23.33 ± 0.58 | nd | |
| S. aureus ATCC 25923 | 35.67± 0.58 | 38.00 ± 2.00 | nd | nd | nd | 28.33 ± 0.58 | nd | |
| Bacterial Type | MIC (%*) | |||
|---|---|---|---|---|
| OBGEO | OBMEO | OBGH | OBMH | |
| Pseudomonas aureginosa ATCC 27853 | >100 | >100 | >100 | |
| Escherichia coli ATCC 25922 | 6.25 | 3.125 | ||
| Staphylococcus aureus ATCC 25923 | 1.56 | 0.78 | ||
| Listeria monocytogenes ATCC 35152 | >100 | >100 | ||
| Salmonella Typhimurium ATCC 13311 | 25.0 | 12.5 | ||
| Bacillus cereus ATCC 11778 | >100 | >100 | ||
| Bacteria | E. coli | S. aureus | S. Typhimurium | ||||
|---|---|---|---|---|---|---|---|
| Sample | OBGEO | OBMH | OBGEO | OBMH | OBGEO | OBMH | |
| MIC | d | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| a | 5.51 | 5.63 | 5.91 | 5.83 | 6.00 | 5.68 | |
| b | 5.29 | 3.00 | 2.71 | 1.35 | 2.10 | 4.20 | |
| c | 7.14 | 5.70 | 2.67 | 3.47 | 7.88 | 6.24 | |
| 2 × MIC | d | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| a | 5.40 | 5.64 | 5.90 | 5.93 | 5.72 | 5.52 | |
| b | 5.47 | 2.80 | 3.28 | 2.30 | 4.88 | 4.02 | |
| c | 7.22 | 5.38 | 2.69 | 2.28 | 6.16 | 6.18 | |
| Parameters | χ2 | RMSE | MBE | MPE | r2 | Skew | Kurt | SD | Var. |
|---|---|---|---|---|---|---|---|---|---|
| MIC | |||||||||
| E. coli | |||||||||
| OBGEO | 0.10 | 0.30 | -0.03 | 4.17 | 0.98 | 0.79 | -0.55 | 0.32 | 0.10 |
| OBMEO | 0.11 | 0.30 | -0.05 | 4.18 | 0.98 | 0.17 | -0.42 | 0.32 | 0.10 |
| S. aureus | |||||||||
| OBGEO | 0.11 | 0.32 | -0.05 | 6.36 | 0.98 | -0.06 | 0.51 | 0.33 | 0.11 |
| OBMEO | 0.34 | 0.54 | -0.07 | 10.20 | 0.92 | -0.09 | -0.97 | 0.58 | 0.33 |
| S. Typhimurium | |||||||||
| OBGEO | 0.08 | 0.27 | -0.03 | 4.26 | 0.98 | -0.14 | 1.02 | 0.29 | 0.08 |
| OBMEO | 0.05 | 0.20 | -0.04 | 2.68 | 0.99 | 0.60 | 0.06 | 0.21 | 0.05 |
| 2x MIC | |||||||||
| E. coli | |||||||||
| OBGEO | 0.12 | 0.32 | -0.03 | 4.59 | 0.98 | 1.13 | 0.33 | 0.34 | 0.12 |
| OBMEO | 0.11 | 0.31 | -0.05 | 4.40 | 0.98 | -0.13 | -1.25 | 0.32 | 0.10 |
| S. aureus | |||||||||
| OBGEO | 0.16 | 0.38 | -0.06 | 6.12 | 0.97 | 0.22 | 0.57 | 0.40 | 0.16 |
| OBMEO | 0.34 | 0.55 | -0.06 | 9.22 | 0.93 | 0.74 | 0.84 | 0.58 | 0.34 |
| S. Typhimurium | |||||||||
| OBGEO | 0.03 | 0.16 | -0.02 | 1.54 | 1.00 | 0.35 | 0.90 | 0.16 | 0.03 |
| OBMEO | 0.08 | 0.26 | -0.04 | 3.29 | 0.99 | 0.93 | 0.75 | 0.27 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šovljanski, O.; Saveljić, A.; Aćimović, M.; Šeregelj, V.; Pezo, L.; Tomić, A.; Ćetković, G.; Tešević, V. Biological Profiling of Essential Oils and Hydrolates of Ocimum basilicum var. Genovese and var. Minimum Originated from Serbia. Processes 2022, 10, 1893. https://doi.org/10.3390/pr10091893
Šovljanski O, Saveljić A, Aćimović M, Šeregelj V, Pezo L, Tomić A, Ćetković G, Tešević V. Biological Profiling of Essential Oils and Hydrolates of Ocimum basilicum var. Genovese and var. Minimum Originated from Serbia. Processes. 2022; 10(9):1893. https://doi.org/10.3390/pr10091893
Chicago/Turabian StyleŠovljanski, Olja, Anja Saveljić, Milica Aćimović, Vanja Šeregelj, Lato Pezo, Ana Tomić, Gordana Ćetković, and Vele Tešević. 2022. "Biological Profiling of Essential Oils and Hydrolates of Ocimum basilicum var. Genovese and var. Minimum Originated from Serbia" Processes 10, no. 9: 1893. https://doi.org/10.3390/pr10091893
APA StyleŠovljanski, O., Saveljić, A., Aćimović, M., Šeregelj, V., Pezo, L., Tomić, A., Ćetković, G., & Tešević, V. (2022). Biological Profiling of Essential Oils and Hydrolates of Ocimum basilicum var. Genovese and var. Minimum Originated from Serbia. Processes, 10(9), 1893. https://doi.org/10.3390/pr10091893











