Abstract
Salvia Miltiorrhizae Radix (SMR) is a widely-used herbal medicine for the treatment of various blood stasis-related diseases (mainly circulatory system). It has been extensively studied in the field of pharmacology over the last few decades. In addition, several reviews concerning the effect of SMR are available. The purpose of this study was to review the pharmacological activities of SMC based on the 10th revision of the international disease classification (ICD-10). After an analysis of the literatures in the Medline database between January 1988 and August 2018, 691 eligible articles were chosen and 971 results were obtained (395 in vitro, 536 in vivo, and 40 human). The extracted data were categorized into the disease chapters of the ICD-10 and the major chapters were: IX Diseases of the circulatory system, II Neoplasms, XI Diseases of the digestive system, XIX Injury, poisoning and certain other consequences of external causes, IV Endocrine, nutritional, and metabolic diseases, VI Diseases of the nervous system, V Mental and behavioral disorders, etc. The major diseases and the pharmacological results of each chapter of the ICD-10 were described assiduously, along with the statistical details. The current study provided a comprehensive understanding and insight of SMR in terms of pharmacological activities.
1. Introduction
Salvia Miltiorrhizae Radix (SMR), commonly referred to as “Red Sage” or “Dansam” in Korean, “Danshen” in Chinese, and “Danzin” in Japanese, is a herb consisting of dried Salvia miltiorrhiza Bunge roots, and is widely used in Korea, China, and Japan [1,2,3]. SMR has been used for the treatment of various blood stasis-related diseases, including gynecological diseases, musculoskeletal diseases, psychiatric diseases, and skin diseases. SMR exerts its effects through blood-activation and stasis-dispelling, quietening of the heart, heat-clearing and blood-cooling, nourishing the blood and tonifying qi, activating the blood to resolve aggregation (mass), moving qi to relieve pain, removing impediment and strengthening bone, regulating menstruation and stopping blood flow, and detoxifying and expelling pus [1,2].
Extensive pharmacological research on SMR has been conducted over the last few decades. Under scientific methodology, researchers have not only shown the preventative and curative efficacy of SMR against a single specific disease and pathological mechanism. There are several reviews concerning the pharmacological activities of SMR and its components on specific disease, such as diseases of the central nervous system [4], diseases of the cardiovascular system [5], cancer [6], Alzheimer’s disease (AD) [7], liver diseases [8], and osteoporosis [9]. These reviews highlight the pharmacological effects, molecular mechanisms of actions, and active components of the herb. In addition, there are some reviews that categorized the pharmacological effects and mechanisms of SMR and provided comprehensive insights into the herb [3,10]. However, the scope of the reviews published to date has been limited by several factors. First, the topic of the study is limited to pharmacological effects toward certain diseases. Second, there is a lack of interpretation of the actual clinical application of SMR since emphasis has been placed on a mechanism-based microscopic approach to pharmacological efficacy. Third, descriptions provided by authors are often inconsistent due to a lack of guidelines for classification criteria based on pharmacological activities. To overcome these limitations, it is necessary to analyze the overall pharmacological activity using specific criteria that can be accepted worldwide.
The international statistical classification of diseases and related health problems (ICD), published by the World Health Organization (WHO), is regarded internationally as a standard diagnostic classification system for human diseases, and subsequently, was used as a common classification criteria in the present study [11]. Moreover, ICD has been used to classify health problems and the causes of mortality in several fields of healthcare, including insurance, health-related financial sectors, health policy and government planning, as well as the field of clinical medicine [11].
Therefore, the purpose of the present study was to review the pharmacological activities of SMR by classifying and analyzing research based on the common classification criteria (ICD-10). In addition, the review aims to provide a general yet comprehensive understanding of the use of SMR in the treatment of diseases, while utilizing the scientific evidence related to the treatment of specific diseases and the progress of research into the potential use of SMR to treat certain diseases. To achieve this, we collected pharmacological papers related to the use of SMR from the Medline database (NIH, USA) and extracted all the results. Then, we classified and statistically analyzed the results using ICD-10. Terms related to traditional medicine were expressed according to International Standard Terminologies on Traditional Medicine published by the WHO [12]. Moreover, the reference for each result is accompanied by a reference number (e.g., Suppl. 1–Suppl. 2) in the Excel sheet of “Supplementary Materials”.
2. Methods
2.1. Collection of Articles
Articles were collected from Medline. A comprehensive literature search was conducted on the PubMed database using the keyword “Salvia miltiorrhiza”. Studies from January 1988 to August 2018 were included and no language restrictions were placed. An initial search yielded 2511 studies that were further filtered by various selection criteria.
2.2. Selection Criteria and Data Extraction
Appropriate articles were selected by applying the selection criteria, described below, after reviewing the titles, abstracts, and full-text; a total of 691 articles were selected. Eventually, 971 datasets consisting of 395 in vitro, 536 in vivo, and 40 pertaining to human application were extracted (Figure 1).
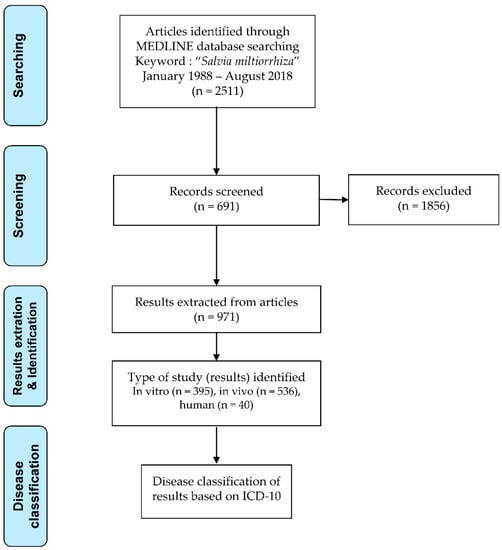
Figure 1.
Flowchart of research article selection, result extraction, and classification.
Inclusion criteria: Studies detailing positive results of pharmacological experiments (in vitro, in vivo, and human), case reports, and clinical trials using SMR, SMR extracts, SMR fractions or SMR compounds irrespective of the extraction method (collectively, Salvia miltiorrhiza components; SMC) were included in this review.
Exclusion criteria: (1) Reviews; (2) standardization (instrumental analysis); (3) studies of other parts (e.g., leaf, flower, stem) of SMR in addition to roots; (4) formulae, prescriptions, and recipes containing other agents; (5) chemical synthesis (analogues and derivatives); (6) combined therapy (SMC with Western medicine/acupuncture/moxibustion); (7) simple mechanistic studies without the mention of a specific disease (ROS scavenging); (8) negative and non-effective results (toxicology); (9) experimental results with disease-free subjects/cells (normal cells, healthy subjects, and healthy humans); (10) no relation to pharmacology (agricultural process, consumption statistics, and pharmacokinetics).
2.3. Result Classification Using ICD-10
We prepared a datasheet containing standardized data extracted from each study, including the names of the authors, the title of the manuscript, PubMed ID (PMID), disease, and the type of result, i.e., in vitro, in vivo, and human (see additional data). We analyzed every result extracted from the selected articles. Wherever feasible, studies with multiple results were classified independently into the ICD-10. The basic classification process was as follows: (1) Find the target disease of a result; (2) find the corresponding ICD-10 block that best matches the disease; and (3) identify the chapter and section of that block.
Four experts participated in the process of classifying the results using ICD-10. To ensure precise classification, ICD-10 clinical modification (CM), which is a clinical modification of ICD-10 developed by The National Center for Health Statistics (NCHS) in the United States of America, was also used. In addition, we used an abbreviation for each ICD-10 chapter to improve readability (Table 1). For example, to obtain the cerebral ischemia rat model results, we searched “cerebral ischemia” using both ICD-10 [13] and ICD-10-CM online [14] and found the classification result “I67.82 Cerebral ischemia” in chapter “IX Diseases of the circulatory system (IX CIRCULATORY).” Then, we categorized it into “IX CIRCULATORY, I60-I69 Cerebrovascular diseases, I67 Other cerebrovascular diseases.” In cases where the category of the result was not evident, the experts discussed and made a classification criterion specifically for that result.

Table 1.
Abbreviation of ICD-10 chapters.
3. Results
The number of pharmacological results of each type of experiment according to the publication year of the articles is depicted in Figure 2. In vivo and in vitro results were similar to each other throughout the years and a smaller proportion of results applicable to humans were present. Studies prior to the year 2000 were insignificant. Subsequently, the number of publications increased rapidly and by the year 2010, an average of 60 results per year were obtained (Figure 2). The number of results for the year 2018 was low, as information was collected only until August 2018.
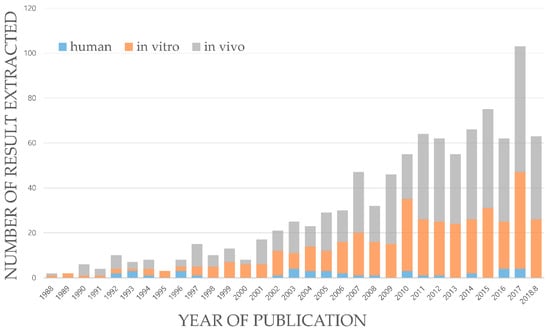
Figure 2.
Number of extracted pharmacological results of SMC, published per year, from January 1988 to August 2018.
The results of the pharmacological effects of SMC were analyzed and classified into chapters. The major chapters that occupied more than 4% of the review were as follows: The first major chapter was IX CIRCULATORY (271 results, 28%), the second major chapter was II NEOPLASMS (168 results, 17%), followed by XI DIGESTIVE (132 results, 13%), XIX EXTERNAL (85 results, 9%), IV ENDO AND META (53 results, 5%), XIV GENITOURINARY (51 results, 5%), VI NERVOUS (48 results, 5%), XIII MUSCULOSKELETAL (38 results, 4%), and V MENTAL (34 results, 4%). Minor chapters that made up less than 4% were I INFECTIOUS, X RESPIRATORY, XII SKIN, II BLOOD AND IMMUNE, VII EYE, and VII EAR (Figure 3). The summary of the results with major diseases in each chapter are listed in Table 2.
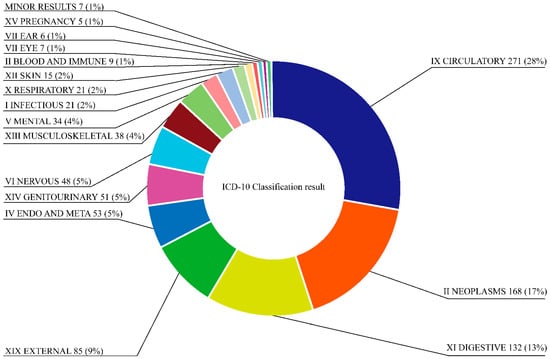
Figure 3.
Overall disease classification statistics on pharmacological results of SMC.

Table 2.
Overall pharmacological results of SMC.
3.1. Chapter IX Circulatory
This chapter classifies the dysfunction of the blood circulatory system in various organs (e.g., brain, heart, lung), diseases of the heart or blood vessels, and abnormality of blood flow kinetics, including hypertension. This chapter specifically discusses various heart diseases, including angina pectoris, myocardial infarction, myocarditis, fibrillation, and heart failure. Diseases of peripheral circulation, such as atherosclerosis, aneurysm, embolism or thrombosis and peripheral angiopathy, along with various types of hypertensive diseases, such as primary hypertension, pulmonary hypertension, and renovascular hypertension were included. Furthermore, rheumatic diseases, including rheumatic fever and rheumatic valve disorder, cerebrovascular disorders, including hemorrhage, infarction, and their sequelae, as well as pulmonary heart disease, including pulmonary embolism were discussed [11]. In IX CIRCULATORY, 117 in vitro datasets, 142 in vivo datasets, and 12 human applications, totaling 271 results, were included (Table 3).

Table 3.
The statistics of pharmacological results and its major diseases of SMC by experimental type in Chapter IX Diseases of the circulatory system of ICD-10.
3.1.1. I70-I79 Diseases of Arteries, Arterioles, and Capillaries
This block classifies damage to blood vessels and diseases related to circulatory failure, including atherosclerosis, aneurysm, dissection, Raynaud’s syndrome, thromboangiitis obliterans, embolism, thrombosis, arteriovenous fistula, stricture and rupture, as well as telangiectasia and naevus [11].
Datasets pertaining to atherosclerosis consisted of 32 in vitro and 19 in vivo results (Suppl. 1–Suppl. 51). Vascular protective effects of SMC were demonstrated against homocysteine, oxidized low-density lipoprotein (oxLDL), tumor necrosis factor-alpha (TNF-a), and lipopolysaccharides (LPS) in vascular smooth muscle cells (VSMCs), endothelial progenitor cells and human umbilical vein endothelial cells (HUVECs). Vascular endothelial growth factor (VEGF)-induced hyperpermeability in bovine aortic endothelial cells and TNF-a, oxLDL, and peroxidized low-density lipoprotein (pox-LDL)-induced activation of macrophages were also inhibited by SMC. In vivo, SMC demonstrated anti-atherosclerotic effects through various mechanisms, including the inhibition of intimal thickening in animals fed a high fat diet (HFD) or apolipoprotein E-deficient mice. Moreover, it inhibited oxysterol-induced endothelial cell apoptosis in rats.
Datasets pertaining to arterial embolism and thrombosis consisted of 13 in vitro and 4 in vivo results (Suppl. 52–Suppl. 68). In the in vitro studies, SMC inhibited platelet aggregation induced by arachidonic acid, adenosine diphosphate, collagen or thrombin and U46619. Moreover, SMC inhibited the aggregation of platelets, which were isolated from patients with acute coronary heart disease, including acute myocardial infarction and unstable angina pectoris through the inhibition of phosphodiesterase and P2Y12 antagonism. In vivo, SMC demonstrated anti-thrombotic effects in animal models, in which thrombosis was induced photochemically or with collagen, adenosine diphosphate (ADP), an arteriovenous shunt, and γ-radiation. In addition, SMC prolonged bleeding even in normal mice.
Datasets pertaining to peripheral angiopathy consisted of seven in vitro and five in vivo results, and three cases in which SMC was applied to humans (Suppl. 69–Suppl. 83). The induction of endothelial cell damage and dysfunction, specifically in EA.hy926, HUVECs, human aortic endothelial cells, and endothelial progenitor cells, by advanced glycation end-products (AGEs) or high glucose-media, were inhibited by SMC. In vivo, SMC improved the vascular function of aorta rings in rats fed a HFD, streptozotocin (STZ)-induced diabetic rats, and Otsuka Long-Evans Tokushima Fatty (OLETF) rats. SMC ameliorated endothelial cell damage and endothelial dysfunction by reducing the levels of AGEs, the von Willebrand factor (vWF), and malondialdehyde in rats fed a HFD, STZ-induced diabetic rats, and OLETF rats. In patients with diabetes mellitus (DM), SMC improved blood circulation via endothelium-dependent vasodilation and by regulating platelet aggregation. In addition, it reduced the levels of soluble VCAM-1, vWF, and oxLDL even in diabetic patients with coronary heart disease.
The effects of SMC on aneurysm and fistula have also been investigated (Suppl. 84–Suppl. 86). SMC attenuated aortic aneurysm formation by inhibiting matrix metalloproteinase (MMPs) in apolipoprotein E-deficient mice and an elastase-perfused experimental abdominal aortic aneurysm rat model. Administration of SMC to patients with chronic kidney disease resulted in reduction in the time required for maturation of the arteriovenous fistula.
3.1.2. I20-I25 Ischemic Heart Diseases
This block classifies heart diseases related to ischemic episodes, especially in heart muscles, including angina pectoris, myocardial infarction, chronic ischemic heart disease, and associated complications within the heart and aneurysm or thrombosis of heart [11].
Datasets pertaining to acute myocardial infarction consisted of 18 in vitro and 26 in vivo results and 1 human application (Suppl. 87–Suppl. 131). SMC attenuated hypoxia or ischemia/reperfusion (I/R)-induced cell death by prompting anti-apoptotic effects in cardiomyocytes, including H9c2. Moreover, it reduced tissue damage, metabolic imbalance, cardiac dysfunction, and oxidative stress in an isolated animal heart. In experimental animal models, SMC attenuated acute myocardial infarction induced by coronary artery ligation, I/R or isoproterenol by reducing cardiac dysfunction, inflammatory reactions, oxidative stress, apoptosis, metabolic imbalances, and neovascularization. In a human study, SMC decreased the level of plasma asymmetric dimethylarginine in patients with non-ST elevation myocardial infarction after percutaneous coronary intervention (PCI).
Studies pertaining to chronic ischemic heart disease, including those related to atherosclerosis, cardiovascular aneurysm, and old infarctions, consisted of one in vivo result and two human applications (Suppl. 132–Suppl. 134). SMC alleviated intimal thickening, smooth muscle cell proliferation, arterial damage, and cardiac dysfunction in an animal model of artery stenosis or restenosis induced by ligation or balloon injury. In patients with coronary heart disease, SMC reduced the risk of coronary heart disease and serum lipid peroxide level, while simultaneously increasing SOD activity.
Studies related to angina pectoris consisted of five in vitro results and one human application (Suppl. 135–Suppl. 140). SMC induced vaso-relaxation and prevented contraction in coronary artery rings by antagonizing 5-hydroxytryptamine, CaCl2, and U46619. In patients with heart diseases, SMC dilated blood vessels, reduced the pressure of the blood flowing into the left ventricle, and increased the cardiac output.
3.1.3. I60-I69 Cerebrovascular Diseases
This block classifies intracranial bleeding, cerebrovascular diseases, and their sequelae, including subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infarction, sequelae of cerebrovascular disease, and occlusion and stenosis of precerebral or cerebral arteries not resulting in cerebral infarction [11].
Cerebral infarction results consisted of 3 in vitro and 27 in vivo results and 1 human application (Suppl. 141–Suppl. 171). SMC inhibited the adhesion of erythrocytes to cultured HUVECs, the Na+, K+-ATPase function in the plasma membrane isolated from rat brains, and cell damage induced by staurosporine in rat cortical neurons. SMC reduced infarct volume, thrombosis formation, oxidative stress, inflammation, blood-brain barrier (BBB) damage, vascular damage, neuronal damage, and metabolic imbalance and increased the blood flow and neovascularization in various animal models, exhibiting cerebral ischemia induced by FeCl3-induced thrombogenesis or occlusion of the cerebral artery. In patients with traumatic cerebral infarction, SMC alleviated the symptoms and blood coagulation disorders by reducing the levels of plasma P-selectin, vWF, and D-dimer.
Studies pertaining to intracranial hemorrhage consisted of one in vitro and four in vivo results (Suppl. 172–Suppl. 176). SMC suppressed oxidative damage, alleviated neuronal degeneration, and improved cell viability in primary cultured cortical neurons. Moreover, SMC inhibited vasoconstriction, oxidative damage, and neural cell apoptosis in the subarachnoid hemorrhage in a rat model. Furthermore, it reduced the occurrence and area of hemorrhage induced by atorvastatin in zebrafish.
Studies related to sequelae of cerebrovascular disease consisted of 15 in vivo results (Suppl. 177–Suppl. 191). SMC attenuated neurological deficits and improved the recovery of motor function in cerebral infarction that occurred in animal models. Moreover, it reduced neurological impairment and restored motor function in atorvastatin-induced hemorrhage or subarachnoid hemorrhage animal models.
Studies pertaining to carotid artery stenosis consisted of five in vivo results (Suppl. 192–Suppl. 196). SMC inhibited intimal thickening in ligation and balloon-induced carotid artery stenosis in rodents.
3.1.4. I30-I52 Other Forms of Heart Disease
This block classifies various types of heart diseases that do not belong to other blocks, including myocarditis, pericarditis, endocarditis, atrial fibrillation, tachycardia, cardiomegaly, cardiac arrest, and heart failure [11].
Myocarditis results, which included acute or chronic myocarditis and cardiac fibrosis, consisted of eight in vitro, six in vivo, and two human applications (Suppl. 197–Suppl. 212). SMC demonstrated anti-fibrotic effects on cardiac fibrosis induced by endothelin-1 (ET-1), phenylephrine, insulin-like growth factor 1 (IGF-1), norepinephrine, MMP-9, isoprenaline, X-rays, and angiotensin (Ang) II in cardiac fibroblasts via inhibition of collagen synthesis and cell proliferation. In animals, SMC attenuated cardiac fibrosis induced by acute myocardial infarction, hyperglycemia, Ang II, isoproterenol, hypertension, and isoprenaline.
Cardiomegaly results consisted of six in vitro and three in vivo results (Suppl. 213–Suppl. 221). SMC suppressed hypertrophy of cardiomyocytes induced by IGF-1, isoproterenol, Leu27IGF-II, and Ang II. In vivo, SMC attenuated cardiomegaly in spontaneously hypertensive rats (SHR) or isoproterenol-treated rats. Furthermore, in a case report of 36 pediatric patients with viral myocarditis, SMC reduced the length of hospitalization, led to the normalization of electrocardiogram, and decreased plasma lipid peroxide and erythrocyte membrane microviscosity.
Additional minor results included cardiomyopathy, congestive heart failure, cardiac arrhythmia, and ventricular or atrial fibrillation (Suppl. 222–Suppl. 235). SMC attenuated cell damage induced by doxorubicin or cardiac glycosides. Moreover, it reduced adriamycin-induced cardiomyopathy in rats by inhibiting oxidative stress and mitochondrial dysfunction. Furthermore, acetylcholine- and isoproterenol-induced atrial fibrillation in isolated rabbit hearts and isoproterenol-induced ventricular fibrillation in rats were alleviated by SMC. SMC demonstrated the ability to reduce the arrhythmogenic status in a canine model of Brugada syndrome. In addition, SMC showed protective effects against cardiac cell damage induced by Ang II or Leu27IGF-II, which is associated with heart failure. Moreover, it protected against heart damage induced by severe acute pancreatitis or obstructive jaundice rat (SAP-OJ) model.
3.1.5. I10-I15 Hypertensive Diseases
This block classifies the hypertensive disease status and associated complications, such as essential (primary) hypertension, secondary hypertension due to disorders of organs, including renal and endocrine disorders, and complications, such as hypertensive heart and renal diseases [11].
Information collected on essential hypertension consisted of 11 in vitro and 4 in vivo results (Suppl. 236–Suppl. 250). Contraction of artery rings or isolated mesenteric arteries by phenylephrine, KCl, Ang I, CaCl2 methoxamine or norepinephrine was alleviated by SMC. Moreover, SMC inhibited the activity of the angiotensin converting enzyme (ACE) in vitro and decreased intracellular calcium concentration and potassium currents in aortic smooth muscle cells induced by phenylephrine, adenosine triphosphate or thapsigargin. Furthermore, the blood pressure in SHR was reduced with the administration of SMC.
Secondary hypertension studies consisted of one in vitro and five in vivo results (Suppl. 251–Suppl. 256). SMC antagonized the mineralocorticoid receptor in human kidney cells after incubation with corticosterone. It decreased left ventricular systolic end pressure and left ventricular developed pressure in STZ-induced diabetic rats. Moreover, it reduced blood pressure via endothelial nitric oxide synthase-dependent vasorelaxation and by reducing ACE activity and serum aldosterone in two-kidney, one-clip renovascular hypertensive animal models. In addition, SMC decreased the blood pressure of hypertensive rats, which was induced by sodium or phenylephrine.
Additional results revealed that SMC inhibited hypertensive heart disease, including ventricular tachycardia and ventricular fibrillation and reduced the blood pressure in SHR (Suppl. 257).
3.1.6. I26-I28 Pulmonary Heart Disease and Diseases of Pulmonary Circulation
This block includes pulmonary embolism, primary pulmonary hypertension, pulmonary hypoxia, pulmonary heart disease, pulmonary arteriovenous fistula, and aneurysm of the pulmonary artery [11].
The pharmacological activities of SMC in this block consisted of three in vitro, six in vivo, and one human result, and were predominantly related to pulmonary hypertension, pulmonary hypoxia, and pulmonary heart diseases that might be associated with pulmonary hypertension due to chronic hypoxia or monocrotaline (Suppl. 258–Suppl. 267). Right ventricular systolic pressure was reduced by SMC in pulmonary arterial hypertensive rats induced by monocrotaline. SMC inhibited hypoxia-induced proliferation and cytoskeleton remodeling in pulmonary arterial smooth muscle cells and arteries. Moreover, it ameliorated media thickening, endothelial cell injury, and hypoxic structural remodeling of arteries in animal models exhibiting pulmonary hypoxia. SMC alleviated pulmonary heart diseases associated with pulmonary hypertension. In pulmonary hypertension animal models, induced by monocrotaline and chronic hypoxia, SMC reduced right ventricular collagen content and hypertrophy and provided a hypotensive effect. In a human study, SMC demonstrated antioxidant effects in patients with chronic cor pulmonale.
Additional minor results show that SMC attenuated the symptoms, including shortness of breath and loss of independent activities in an acute pulmonary embolism mouse model induced by ADP (Suppl. 268).
3.1.7. Other Blocks
SMC alleviated the pathological changes in skeletal muscles in rats, after hind limb I/R injury and protected mesenteric lymph nodes of rats with severe acute pancreatitis or obstructive jaundice. Deep vein thrombosis in rabbits under femoral vein ligation was attenuated by SMC, which allowed for an increase in the prothrombin time and reduction in whole blood viscosity (Suppl. 269–Suppl. 271).
3.2. Chapter II Neoplasms
This chapter classifies neoplasms of various types (malignant, in situ, benign) according to the organ in which the lesion occurs [11]. In II NEOPLASMS, 168 cases consisting of 124 in vitro, 41 in vivo, and 3 human results were obtained (Suppl. 272–Suppl. 439) (Table 4).

Table 4.
The statistics of pharmacological results and its major diseases of SMC by experimental type in II Neoplasms of ICD-10.
SMC induced the apoptosis and inhibited the proliferation of cancer cells. In addition, it suppressed tumor growth in animal xenograft models, while improving body weight, spleen index, the thymus index, and other parameters. Moreover, SMC inhibited tube formation in HUVECs, chick embryo chorioallantoic membranes, and lymphatic endothelial cells. In vivo, SMC attenuated microvessel formation in the mouse Matrigel plug assay model and inhibited metastasis of colon carcinoma. In humans, SMC improved the rate of survival of patients with colon and lung cancers and demonstrated anti-angiogenetic activity in colorectal cancer patients.
ICD-10 classifies neoplasms according to the type and the origin of the lesion [11]. However, due to the limitations of pharmacological studies that predominantly investigate the cytotoxic and anti-cancer effects of SMC using in vitro methods and artificially induced xenograft animal models, respectively, we classified the results of this chapter based on the organ in which the target cell originated. Among 169 results, the major cancers that were investigated included liver (12 in vitro, 6 in vivo, 0 human), colon (13, 3, 1), lung (10, 4, 1), breast (11, 4, 0), prostate (8, 4, 0), stomach (4, 3, 0), pharynx (4, 2, 0), and bile duct (3, 2, 0). In addition, leukemia was thoroughly examined along with myeloid leukemia (15, 2, 0), lymphoid leukemia (6, 0, 0), and monocytic leukemia (3, 0, 0). Other cancers, such as malignant neoplasm of bone (3, 1, 0), skin (3, 0, 0), cervix uteri (3, 1, 0), ovarian (2, 0, 0), pancreatic (3, 0, 0), mouth (1, 1, 0), salivary gland (1, 0, 0), kidney (1, 1, 0), brain (4, 0, 0), head and neck (1, 1, 0), peritoneum (1, 1, 0), and rhabdomyosarcoma (1, 0, 0) cancers were also studied.
3.3. Chapter XI Digestive
This chapter classifies diseases related to dysfunction of the peritoneum, liver, gallbladder, and pancreas, as well as the gastrointestinal tract from the mouth to the anus. This section specifically includes gingivitis, stomatitis, gastro-esophageal reflux disease, appendicitis, hernia, colitis, irritable bowel syndrome, peritonitis, alcoholic liver disease, hepatic failure, toxic liver disease, liver cirrhosis, pancreatitis, and cholelithiasis [11]. In XI DIGESTIVE, 132 results consisting of 45 in vitro, 82 in vivo results, and 5 human applications were noted (Table 5).

Table 5.
The statistics of pharmacological results and its major diseases of SMC by experimental type in XI Diseases of the digestive system of ICD-10.
3.3.1. K70-K77 Diseases of Liver
This block classifies several diseases of the liver, including liver fibrosis and cirrhosis, toxic and alcoholic liver disease, and fatty liver disease, as well as liver infarction and viral hepatitis [11].
Liver fibrosis and cirrhosis consisted of 23 in vitro, 17 in vivo, and 4 human results (Suppl. 440–Suppl. 483). Hepatic stellate cells (HSCs) have been recognized in the pathological process of hepatic fibrosis. Activation of HSCs to fibrogenic myofibroblasts contributes to fibrogenesis, which is driven by connective tissue growth factor and transforming the growth factor (TGF)-β1. In addition, amplified inflammation, immunoregulation, and altered matrix degradation can be attributed to activation of HSCs [15,16]. Moreover, in vitro and in vivo experiments are well established. In vitro studies on SMC predominantly used HSCs to investigate the effects on liver fibrosis. SMC inhibited HSC proliferation, a-smooth muscle actin (SMA) production, synthesis, and deposition of collagen, and expression of transcription factors, as well as oxidative stress and inflammatory reactions. SMC ameliorated liver fibrosis and cirrhosis induced by carbon tetrachloride (CCL4), thioacetamide, dimethylnitrosamine, and bile duct ligation in animals through mechanisms similar to those occurring in the in vitro studies. Four human studies revealed that SMC improved clinical symptoms, liver functional indices, liver fibrosis indices, and liver function in patients with liver cirrhosis and hepatitis B. SMC ameliorated clinical symptoms and reduced portal pressure by regulating portal blood flow in patients with liver cirrhosis.
Studies pertaining to toxic liver disease consisted of 8 in vitro and 20 in vivo results (Suppl. 484–Suppl. 511). Necrosis and apoptosis of hepatocytes induced by CCL4, hydrogen peroxide, TNF-α/d-Galactosamine (TNF-α/D-GalN), and γ-radiation via anti-oxidation was inhibited by SMC. In addition, SMC showed protective effects on the liver, including the amelioration of liver functional indexes, including aspartate transaminase and alanine transaminase, inhibition of oxidative stress, and inflammatory reactions in animal models, in which liver hepatotoxicity was induced by CCL4, iron-overload, acetaminophen, restraint stress, LPS, and Bacille Calmette-Guérin (BCG). Moreover, SMC inhibited liver damage in a mouse model, in which cholestatic liver was induced by lithocholic acid. Furthermore, SMC lowered pathological scores of the liver in an SAP-OJ model.
Studies related to alcoholic liver disease (ALD) comprised one in vitro and five in vivo results (Suppl. 512–Suppl. 517). SMC inhibited liver cell death caused by 4-hydroxynonenal (4-HNE), a known inducer of ALD [17]. In addition, SMC reduced hepatic damage, serum cholesterol level, hepatic lipid accumulation, inflammatory reactions, and 4-HNE formation, while improving liver functional indices in acute and chronic alcoholic animal ALD models.
Studies on non-alcoholic fatty liver disease consisted of two in vitro and four in vivo results (Suppl. 518–Suppl. 523). SMC inhibited the accumulation of hepatic lipids, oxidative stress, and inflammatory reactions in human liver cells (HepG2 cells) induced by palmitic acid. In addition, hepatic lipid accumulation (hepatic steatosis), fibrosis, oxidative stress, and inflammatory reactions were inhibited by SMC in animal models induced by feeding a HFD or a diet deficient in methionine-choline. Similar results were observed in ovariectomized (OVX) rats, which were fed a HFD.
Liver infarction studies consisted of three in vitro and five in vivo results (Suppl. 524–Suppl. 531). SMC reduced hypoxia- or reoxygenation-induced free calcium ion concentration and oxidative stress in hepatocytes, liver sinusoidal endothelial cells, and isolated rat livers. SMC decreased liver damage, liver functional indices, oxidative stress, and inflammatory reactions and improved microcirculatory disturbance in a liver ischemia rat model. In addition, SMC normalized the imbalances in intestinal microflora and reduced structural damage to the ileal mucosa, bacterial translocation, and the concentration of plasma endotoxins in a liver ischemia rat model.
Autoimmune hepatitis studies involved three in vivo results (Suppl. 532–Suppl. 534). SMC inhibited T-lymphocyte subsets, cell apoptosis, and inflammatory reactions in an animal model where autoimmune hepatitis was induced by concanavalin A. Using the same model, researchers revealed that SMC restored the functional indices of the liver.
Additional studies considered the effects of SMC on portal hypertension, wherein it was revealed that SMC lowered the portal blood pressure in an ET-1-induced portal hypertension rat model. SMC reduced cirrhosis induced by bile duct ligation in a rat model. In addition, the administration of SMC prompted a reduction in liver damage, inflammatory reactions, and mortality in a mouse model, in which fulminant hepatic failure was induced by galactosamine or LPS (Suppl. 535–Suppl. 538).
3.3.2. K20-K31 Diseases of Oesophagus, Stomach, and Duodenum
This block classifies diseases of the oesophagus, stomach, and duodenum, including gastric ulcer, gastric ischemia, and oesophageal reflux disease [11].
Studies pertaining to gastric ulcer included two in vitro and five in vivo results (Suppl. 539–Suppl. 545). In vitro SMC inhibited gastric H+, K+-ATPase, and pNPPase in pigs and impeded H. pylori-induced inflammation and cell apoptosis in human gastric cancer cells. In vivo, SMC suppressed the formation of gastric lesions in animal models, in which gastric ulcer was induced by acetic acid, water immersion, and restraint stress.
Other results revealed that SMC protected against gastric mucosal damage induced by hemorrhagic shock or reperfusion. In addition, it ameliorated the function of the oesophageal sphincter isolated from rats (Suppl. 546–Suppl. 548).
3.3.3. K55-K64 Other Diseases of Intestines
This block classifies various diseases of the intestines, which were not categorized elsewhere, including vascular intestinal disorder, irritable bowel syndrome, functional intestinal disorder, anal and rectal polyp, and ulcer of the intestines [11] (Suppl. 549–Suppl. 555). SMC induced tonic contraction in isolated rat ilea and improved jejunum function in the digestive tract of a rat model, wherein congestion was induced by hepatic I/R. In addition, it ameliorated mesenteric microcirculation disturbances in an animal model induced by LPS, I/R, and photochemical reactions. In the SAP-OJ model, SMC exhibited protective effects against intestinal mucosa damage.
3.3.4. K00-K14 Diseases of Oral Cavity, Salivary Glands, and Jaws
This block classifies various diseases occurring in the mouth, including oral mucositis, submucous fibrosis, and gingivitis [11] (Suppl. 556–Suppl. 560). SMC inhibited the arecoline-induced proliferation of oral mucosal fibroblasts and protected human pharyngeal cells against damage induced by 5-fluorouracil (5-FU). In addition, it showed a protective effect against oral mucosal damage induced by 5-FU in hamsters. Moreover, SMC reduced tissue damage and bone loss in a periodontitis rat model and increased fluid secretion in isolated rat submandibular glands.
3.3.5. K50-K52 Non-Infective Enteritis and Colitis
This block classifies diseases of the colon, including colitis, but excludes vascular disorders, irritable bowel syndrome, and other functional disorders [11] (Suppl. 561–Suppl. 564). SMC inhibited LPS-induced inflammation in human Caco-2 intestinal cells. In addition, SMC inhibited symptoms and inflammatory reactions in animal models, in which ulcerative colitis was induced by dextran sodium sulfate or in Chron’s disease induced by 2,4,6-trinitrobenzene sulfonic acid.
3.3.6. K80-K87 Disorders of Gallbladder, Biliary Tract, and Pancreas
This block classifies diseases of the gallbladder, biliary tract, and pancreas, including cholelithiasis, obstruction of the gallbladder and biliary tract, and acute pancreatitis [11] (Suppl. 565–Suppl. 568). SMC ameliorated the pathological changes of various organs, including the pancreas, thymus, spleen, kidney, and lungs in acute pancreatitis rat models. In humans, SMC decreased serum interleukin (IL)-6, IL-8, and TNF-a in patients with severe acute pancreatitis.
3.3.7. K65-K67 Diseases of Peritoneum
This block classifies diseases of the peritoneum, including peritonitis, fibrosis, and infections [11] (Suppl. 569–Suppl. 571). SMC inhibited peritoneal fibrosis, adhesion, and damage by exerting anti-inflammatory effects, while simultaneously inhibiting aquaporin-1 and the increase in creatinine and glucose clearance induced by surgical operation or by dialysis in rats.
3.4. Chapter XIX External
This chapter classifies traumatic and toxic diseases according to the cause and the site at which it occurs. Specifically, this chapter includes traumatic injuries, burn, frostbite, poisoning, and complications of surgical and medical care [11]. In XIX EXTERNAL, 85 results consisting of 16 in vitro, 60 in vivo, and 9 human results were identified (Table 6).

Table 6.
The statistics of pharmacological results and its major diseases of SMC by experimental type in XIX Injury, poisoning, and certain other consequences of external causes of ICD-10.
3.4.1. S00-S89 Injuries
The S-section classifies traumatic injury in different regions of the body, including fracture, organ damage, and spinal cord damages [11] (Suppl. 572–Suppl. 586). SMC promoted bone formation and improved healing in rat models, exhibiting radial fracture and tibial fracture. In addition, SMC improved neurological and urinary function and tissue damage in animal models of spinal cord injury. Moreover, it enhanced axonal regeneration and motor function after sciatic nerve injury and reversed the injuries associated with the muscles and kidneys after traumatic injury in rats. In human studies, SMC improved the symptoms of patients exhibiting traumatic hyphema, by absorption, and improved the pathological scores in patients with traumatic cerebral infarctions.
3.4.2. T36-T50 Poisoning by Drugs, Medicaments, and Biological Substances
This block classifies injuries caused by drugs and biological substances, including antineoplastic agents, steroids, vaccines, and diagnostic reagents. These agents are used to develop experimental models or to investigate the response to the adverse effects of the agent itself [11] (Suppl. 587–Suppl. 611). SMC inhibited the adverse effects of various anti-neoplastic agents, including cisplatin, oxaliplatin, gentamicin, adriamycin, bleomycin, 5-FU, and doxorubicin, which may induce ototoxicity, pulmonary fibrosis, oral fibrosis, neuropathy, hepatic toxicity, and cardiac toxicity. SMC ameliorated toxic damage caused by acetaminophen, steroidal hormones, dexamethasone, arecoline, scopolamine, and sevoflurane, which induce hepatic toxicity, osteonecrosis, oral fibrosis, and neurocognitive deficits. Moreover, SMC showed hepato-protective effects against liver injury induced by the BCG vaccine and a reno-protective effect against renal injury induced by iron, glycerol, and ioversol (contrast agent).
3.4.3. T80-T88 Complications of Surgical and Medical Care, Not Elsewhere Classified
This block classifies complications and adverse effects caused by surgical and medical care, including organ transplant, dialysis, angioplasty, and stenting [11] (Suppl. 612–Suppl. 631). SMC showed ameliorative effects on surgery-induced complications. In rats, intestinal adhesion and epidural fibrosis recognized after abdominal surgery and laminectomy, respectively, were inhibited by SMC. In addition, it reduced the complications of surgical processes on the vascular system, including an improvement in myocardial microperfusion and reduction in the risk of arterial restenosis in open-chest surgery and vascular angioplasty, including carotid artery dissection, ligation or balloon insertion in animals. In human studies, SMC attenuated adverse effects or complications after various medical and surgical procedures, including wound ischemia and necrosis after mastectomy in breast cancer patients, lung and heart damage after cardiopulmonary bypass in congenital heart disease patients, cardiac damage after coronary artery bypass in coronary artery disease patients, and PCI in myocardial infarction patients. SMC showed ameliorating effects on transplant-induced complications. Moreover, SMC attenuated organ damage, transplant rejection and dysfunction in liver, and cardiac transplants performed in rodents. In human studies, SMC increased renal function and blood viscosity in patients who underwent renal transplantation. Furthermore, SMC demonstrated ameliorative effects on the complications associated with medical care. It also inhibited peritoneal fibrosis and pathological damage in rats after peritoneal dialysis. Complications arising in patients undergoing continuous haemodialysis were attenuated by reducing oxidative stress and microinflammation.
3.4.4. T66-T78 Other and Unspecified Effects of External Causes
This block classifies adverse effects caused by various external factors, including radiation, allergic reaction, anaphylactic shock, air pressure, exhaustion, heat, and cold [11] (Suppl. 632–Suppl. 647). SMC attenuated the cell damage and fibrosis induced by radiation in cochlear, liver, and cardiac cells. In addition, it protected guinea pigs against ototoxic cochlear damage induced by radiation. SMC inhibited allergic reactions in mouse bone marrow-derived mast cells, rat basophilic leukemia cells, and rat peritoneal mast cells. Moreover, SMC suppressed passive anaphylactic reactions and inflammation in normal and knock out mice (AMPKα2-/- and Sirt1-/-). Moreover, SMC restricted heat stress-induced apoptosis of HUVECs. It normalized blood pressure and tachycardia induced by hypobaric hypoxia in rats and mitigated the state of fatigue induced by forced swimming in mice.
3.4.5. Other Blocks
Minor classifications of the T-section, include burns, sequelae of injury, and toxic effects of heavy metals, pesticides, and aflatoxins [11] (Suppl. 648–Suppl. 656). SMC showed protective activity against toxins and heavy metals. It improved mitochondrial membrane potential impairments induced by paraquat in neuroblastoma cells and inhibited renal and liver damage induced by aflatoxin-B1 and heavy metals (cadmium and lead) in rodents. In addition, SMC repressed inflammation and pulmonary oedema in acute postburn pulmonary injury and improved the recovery of postburn skin injury in rats. SMC maintained arterial oxygen levels and inhibited lipid peroxidation in a traumatic fat embolism dog model and improved neurological function induced by spinal cord injury (SCI) in rats.
3.5. Chapter IV ENDO and META
This chapter classifies dysfunctions of the components of the endocrine system, including the thyroid, thymus, adrenal glands, and gonads, as well as malnutrition and metabolic disorders. This chapter specifically includes thyrotoxicosis, hypertrophy of thymus, DM, Cushing syndrome, gonadal dysfunction, nutritional deficiency, obesity, and various metabolic disorders [11]. In IV ENDO AND META, 53 results were identified, which consisted of 12 in vitro, 40 in vivo results, and 1 human application (Table 7).

Table 7.
The statistics of pharmacological results and its major diseases of SMC by experimental type in IV Endocrine, nutritional, and metabolic diseases of ICD-10.
3.5.1. E10-E14 Diabetes Mellitus
This block classifies type 1 and type 2 DM, including its complications [11].
Type 1 DM comprised 3 in vitro and 11 in vivo results (Suppl. 657–Suppl. 670). SMC protected pancreatic-beta cells (INS-1 cells) against human islet amyloid polypeptide and high glucose-induced damage. In addition, SMC lowered blood glucose in rodent models, in which type 1 DM was induced by starch, alloxan, and STZ.
Type 2 DM comprised five in vitro and six in vivo results (Suppl. 671–Suppl. 681). SMC inhibited protein tyrosine phosphatase 1B, a negative regulator of insulin signaling, in an in vitro enzyme assay. It enhanced the insulin sensitizing activity of an insulin receptor in Chinese-hamster ovary cells expressing human insulin receptors (CHO/IR cells), as well as in adipocytes (3T3-L1 cells). In addition, it promoted glucose uptake and gluconeogenesis in adipocytes. SMC showed hypoglycaemic effects, decreased fasting blood serum insulin, and improved insulin intolerance in various type 2 DM animal models induced by HFD only, HFD + tert-butyl hydroperoxide or STZ and by genetic modification, including (OLETF) rats and db/db mice.
Furthermore, complications of DM are regarded to be clinically important in patients. However, complications of DM were not included in this category and were assigned to categories where it suited a particular disease [11]. The total number of DM complications were 28 and the specific chapters to which they were assigned are as follows: IX Diseases of the circulatory system (14), XIV Diseases of the genitourinary system (5), VII Diseases of the eye and adnexa (3), V Mental and behavioral disorders (2), VI Diseases of the nervous system (2), and XIII Diseases of the musculoskeletal system and connective tissue (1). Major blocks were I70-I79 Diseases of arteries, arterioles, and capillaries (14) and N00-N08 Glomerular diseases (5).
3.5.2. E70-E90 Metabolic Disorder
This block classifies various metabolic disorders related to important nutrients, including lipids, amino acids, carbohydrates, and water [11] (Suppl. 682–Suppl. 699). SMC inhibited rat liver diacylglycerol acyltransferase in vitro. In addition, SMC lowered serum lipid content, including LDL, triglyceride (TG), and total cholesterol in various animal models induced by HFD, HFD + STZ, HFD + OVX, HFD + balloon injury, and genetic modification in mice (APPswe/PS1dE9 mice). On the contrary, SMC elevated the concentration of high-density lipoprotein cholesterol in a rat model fed a HFD. Moreover, SMC decreased serum urate level against potassium oxonate-induced hyperuricemia in mice. In human studies, SMC reduced TG, total cholesterol, LDL cholesterol, lipoprotein (a), gamma-glutamyl transpeptidase, total bilirubin, uric acid, and homocysteine in patients with coronary heart disease.
3.5.3. E65-E68 Obesity and Other Hyperalimentation
This block includes obesity, hyperalimentation and its sequelae [11] (Suppl. 700–Suppl. 703). In vitro, SMC inhibited lipid droplet accumulation in 3T3-L1 pre-adipocytes. In addition, SMC reduced weight gain in HFD-induced animal models and even in OVX-treated HFD models.
3.5.4. Other Blocks
SMC exerted effects on diseases related to the thyroid and other endocrine glands. SMC showed anti-inflammatory, antioxidant, and antiadipogenic effects in primary orbital fibroblasts from patients with Graves’ orbitopathy. Moreover, SMC expended protective effects against thymus damage in SAP-OJ rats and increased the levels of serum estradiol, luteinizing hormone, and follicle-stimulating hormone in immature and OVX mice (Suppl. 704–Suppl. 709).
3.6. Chapter XIV Genitourinary
This chapter classifies the dysfunction of components of the urinary system, including the kidney, ureter, bladder, and male and female genital tracts. This chapter specifically includes nephritic syndrome, nephritis, renal failure, ureter calcification, prostate hyperplasia, infertility, breast hypertrophy, salpingitis, oophoritis, and endometriosis [11]. In XIV GENITOURINARY, 51 results, consisting of 11 in vitro, 38 in vivo, and 2 human results, were included (Table 8).

Table 8.
The statistics of pharmacological results and its major diseases of SMC by experimental type in XIV Diseases of the genitourinary system of ICD-10.
3.6.1. N17-N19 Renal Failure
This block classifies various types of renal failures dividing them into acute, chronic, and unspecified [11].
Studies pertaining to acute renal failure consisted of five in vivo results (Suppl. 710–Suppl. 714). Acute renal failure is a loss of renal function within 7 days and could occur due to several factors. SMC inhibited renal damage, fibrosis, and dysfunction and reduced mortality in rodent models, in which acute kidney injury was induced by hypoxia, glycerol, folic acid, and sodium.
Chronic kidney disease studies consisted of one in vitro and two in vivo results (Suppl. 715–Suppl. 717). SMC suppressed proliferation and release of endothelin in rat glomerular mesangial cells induced by LPS. Moreover, SMC inhibited renal dysfunction, damage, and fibrotic change in rats, when chronic kidney failure was induced by adenine and 5/6 nephrectomy.
Studies related to unspecified kidney failure consisted of one in vitro and five in vivo results (Suppl. 718–Suppl. 723). In vitro, SMC showed anti-oxidative effects in renal microsomes induced by arachidonic acid and increased the synthesis of prostaglandin E2 in renal slices. In addition, SMC inhibited oxidative damage, inflammation, and renal dysfunction in animal models exhibiting renal damage induced by N(G)-nitro-D-arginine, aging, adenine, and myocardial infarction.
3.6.2. N00-N08 Glomerular Diseases
This block classifies various glomerular diseases, including various types of nephritic syndromes and hematuria [11]. Research pertaining to nephrotic syndrome consisted of one in vitro result and two human cases (Suppl. 724–Suppl. 726). Various symptoms of renal dysfunction are grouped together and are referred to as nephrotic syndrome, which is caused by inflammation and other systematic diseases, such as cancer and lupus erythematosus. SMC inhibited Ang II-induced glomerular sclerosis in rat mesangial cells. Moreover, it inhibited proliferation and induced apoptosis in cultured fibroblast from human kidney with lupus nephritis. In human studies, SMC reduced the levels of serum endothelin and soluble IL-2 receptor in children with primary nephrotic syndrome and hematuria, and reduced urinary protein content in Henoch-Schonlein purpura nephritis.
DM is known to cause chronic damage to the small blood vessels of the glomeruli, resulting in the loss of kidney function [18]. Similarly, in the present review, there were many cases in which adverse effects on glomeruli were associated with DM. Studies consisted of six in vitro and five in vivo results (Suppl. 727–Suppl. 737). SMC inhibited oxidative stress, inflammatory reactions, and fibrotic change induced by high levels of glucose in mesangial cells and renal proximal tubular epithelial cells. In addition, it restricted pathological changes, such as oxidative stress, inflammatory reactions, hypertrophy, fibrosis, and renal dysfunction in diabetic nephropathy induced by STZ.
3.6.3. N25-N29 Other Disorders of Kidney and Ureter
This block classifies various diseases of urinary systems, including impairment of renal tubular function, atrophy, sclerosis, and infarction [11].
Studies pertaining to kidney ischemia consisted of one in vitro and five in vivo results (Suppl. 738–Suppl. 743). SMC inhibited inflammatory reactions and cell damage in an in vitro kidney ischemia model using proximal tubular cells. In addition, it suppressed oxidative stress, inflammatory reactions, cell damage, and renal dysfunction in a rat model of kidney ischemia.
Studies related to renal fibrosis consisted of one in vitro and two in vivo results (Suppl. 744–Suppl. 746). SMC inhibited fibrotic changes, such as elevation of TGF-β1, collagen IV, fibronectin, vimentin, and α-SMA induced by folic acid, adenine, indoxyl sulfate, STZ, and high glucose in renal proximal tubular epithelial cell and mesangial cells. Furthermore, it showed similar effects in animal models of DM, in SAP-OJ, and in adenine- and folic acid-induced chronic kidney disease (Suppl. 747–Suppl. 751).
3.6.4. N10-N16 Renal Tubulo-Interstitial Diseases
This block classifies diseases situated at the renal tubulo-interstitial site and includes nephritis, obstructive uropathy, and nephropathy induced by heavy metals, drugs, and biological substances [11] (Suppl. 752–Suppl. 756). SMC inhibited oxidative stress, inflammatory reactions, cell damage, oedema, and renal dysfunction in rodent models, in which kidney injury was induced by cadmium, lead, iron-overload, ioversol, and adenine.
3.6.5. Other Blocks
SMC inhibited inflammatory reactions in an endometriosis model and inhibited structural changes and fibrosis in a chlamydia trachomatis-infected salpingitis model in female rats. In addition, it suppressed atrophy of reproductive tissues in an OVX mouse model. Moreover, SMC improved the percentage of haploid cells in unilateral testicular torsion in male rats (Suppl. 757–Suppl. 760).
3.7. Chapter VI Nervous
This chapter classifies diseases of the central and peripheral nervous system, as well as the disorders of nervous function. It specifically includes AD, Parkinson’s disease (PD), cerebral oedema, dystonia, Bell’s palsy, trigeminal neuralgia, post zoster neuralgia, multiple sclerosis, encephalopathy, episodic disorders, and sleep disorders [11]. In VI NERVOUS, 48 results were included, which consisted of 17 in vitro and 31 in vivo results (Table 9).

Table 9.
The statistics of pharmacological results and its major diseases of SMC by experimental type in VI Diseases of the nervous system of ICD-10.
3.7.1. G90-G99 Other Disorders of the Nervous System
This block includes autonomic neural disorder, hydrocephalus, cerebral oedema, anoxic brain damage, encephalopathy, and myelopathy [11].
Studies pertaining to cerebral oedema comprised 11 in vivo results (Suppl. 761–Suppl. 771). Cerebral oedema is usually caused by traumatic damage, cerebral ischemia, and infectious brain disease. Despite the fact that results related to cerebral vascular diseases, including cerebral infarction and haemorrhage, were classified into IX CIRCULATORY, results pertaining to cerebral oedema, such as downregulation of brain water contents, were classified into this block. SMC lowered brain water content and inhibited cerebral oedema formation by inhibiting oxidative stress, inflammatory reactions, and BBB damage in transient or permanent ischemia in subarachnoid haemorrhage rodent models.
Anoxic brain damage studies consisted of two in vitro and six in vivo results (Suppl. 772–Suppl. 779). Anoxic brain damage occurs when the entire brain lacks oxygen and blood flow, as would be the case during a heart attack. SMC ameliorated rat hippocampal neuronal damage in an in vitro ischemia model induced by oxygen-glucose deprivation. In addition, neuronal damage was ameliorated in vivo by SMC through the inhibition of excitotoxicity, inflammation, and apoptosis in animal models, in which global cerebral ischemia was induced by bilateral common carotid artery ligation and 4-vessel occlusion.
In addition, studies related to encephalopathy revealed that SMC inhibited cell damage induced by paraquat in neuroblastoma cells and microcirculation disturbance induced by LPS in mice brains. SMC inhibited oedema and damage and improved microcirculation in a rat model of SCI (Suppl. 780–Suppl. 782).
3.7.2. G30-G32 Other Degenerative Diseases of the Nervous System
This block classifies degenerative brain diseases, including AD [11].
Studies pertaining to the effects of SMC on AD comprised 11 in vitro and 4 in vivo results (Suppl. 783–Suppl. 797). SMC inhibited amyloid-beta (Aβ) aggregation, oxidative damage, and apoptosis in neuron cells (PC12 cells, SH-SY5Y cells), as well as in primary rat cortical neuronal cells. In addition, it inhibited Aβ generation in cells overexpressing human Swedish AβPP, including neuroblastoma and cortical neuronal cells. SMC inhibited amyloid plaque deposition and neuronal damage in Aβ-induced animal models of AD and APP/PS1 transgenic mice.
3.7.3. G20-G26 Extrapyramidal and Movement Disorders
This block classifies brain diseases related to movement disorders, including PD and dystonia [11].
Studies related to the effect of SMC on PD consisted of three in vitro and two in vivo results (Suppl. 798–Suppl. 802). SMC increased dopamine release in rat striatal slices and inhibited amyloid-mediated formation of α-synuclein as determined by the protein misfolding cyclic amplification assay. In addition, it inhibited apoptosis induced by 6-hydroxydopamine in human neuroblastoma cells (SH-SY5Y cells). Moreover, SMC exerted protective effects against dopaminergic neuronal damage in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induced PD mouse model and extended the life span of a transgenic Caenorhabditis elegans model of PD.
3.7.4. G50-G59 Nerve, Nerve Root, and Plexus Disorders
This block classifies various neuropathies, including Bell’s palsy, trigeminal neuralgia, post zoster neuralgia, carpal tunnel syndrome, and diabetic mononeuropathy [11].
Studies related to the effect of SMC on diabetic neuropathy consisted of two in vivo results (Suppl. 803–Suppl. 804). SMC improved peripheral nerve function and inhibited nerve pathogenesis in a rat model of STZ-induced DM.
3.7.5. G35-G37 Demyelinating Diseases of the Central Nervous System
This block classifies diseases, including multiple sclerosis and other demyelinating diseases of the central nervous system [11].
Studies pertaining to multiple sclerosis comprised three in vivo results (Suppl. 805–Suppl. 807). SMC reduced Th1 responses, BBB damage, and clinical symptoms in an experimental animal model of autoimmune encephalomyelitis induced by MOG35-55 and Mycobacterium tuberculosis (H37Ra).
3.7.6. Other Blocks
SMC exerted effects on cauda equina syndrome and polyneuropathy, while ameliorating the pathological changes and dysfunction of the lower urinary tract in a rat model of SCI (Suppl. 808).
3.8. Chapter XIII Musculoskeletal
This chapter classifies the dysfunction of components of the musculoskeletal system, including bones, joints, muscles, and connective tissues. In particular, this chapter includes arthropathies, dorsopathies, and soft tissue disorders, including muscles and tendons, connective tissue disorders, including systemic lupus erythematosus and systemic sclerosis, as well as osteopathies and chondropathies [11]. In XIII MUSCULOSKELETAL, 38 results were identified, of which 15 were in vitro and 23 were in vivo (Table 10).

Table 10.
The statistics of pharmacological results and its major diseases of SMC by experimental type in XIII Diseases of the musculoskeletal system and connective tissue of ICD-10.
3.8.1. M00-M25 Arthropathies
This block classifies various joint disorders, including infectious arthritis, rheumatoid arthritis (RA), osteoarthritis (OA), and gout [11] (Suppl. 809–Suppl. 824).
SMC inhibited inflammation induced by IL-1β in human chondrocytes and by LPS in murine chondrogenic cells. In addition, it induced apoptosis in fibroblast-like synoviocytes of RA and inhibited osteoclast differentiation. Moreover, SMC suppressed inflammation, cartilage destruction, and subchondral bone thickening in animal models of OA induced by surgical incision, collagen, carrageenan, and dextrin. Furthermore, it inhibited paw swelling, oedema, thymus, spleen index, and arthritis score in rat models, where RA was induced by adjuvants.
3.8.2. M80-M85 Disorders of Bone Density and Structure
This block classifies diseases related to bone density and structure, including osteoporosis, osteonecrosis, osteomalacia, and other bone density-related diseases [11] (Suppl. 825–Suppl. 837).
SMC inhibited dexamethasone-induced apoptosis and enhanced the differentiation of osteoblast cells. In addition, it repressed osteoclast differentiation and cathepsin K, which is associated with the degradation of collagen and gelatin in bone. SMC improved bone mineral density, mechanical strength, and bone mass in an OVX-induced animal model of osteoporosis. Furthermore, it reversed osteoporosis induced by glucocorticoids and DM.
3.8.3. Other Blocks
Other blocks include the effects of SMC on systemic connective tissue disorder, hyperalgesia, osteonecrosis, and epidural fibrosis (Suppl. 838–Suppl. 846). SMC inhibited IL-17A-induced proliferation, collagen synthesis, and migration of dermal VSMCs derived from patients with systemic sclerosis and inhibited collagen secretion from human skin fibroblasts. In addition, it reduced prolyl hydroxylation in skin collagen in mice. SMC inhibited proliferation of cultured fibroblasts from the kidneys of patients presenting lupus nephritis. Moreover, SMC regulated the transmission of neural electric current in isolated dorsal root ganglion neurons, inhibited neuropathic pain of chronic sciatic nerve injury in rats, and hindered chemotherapy (oxaliplatin)-induced neuropathic pain in mice. SMC impeded steroid-induced osteonecrosis of femoral head and laminectomy-induced epidural fibrosis in rats.
3.9. Chapter V Mental
This chapter classifies disorders of mood, mental health, and behavior. This chapter specifically includes psychiatric disorders, including amnesia, dementia, delirium, schizophrenia, anxiety, depression, and mental/behavioral disorders due to the usage of psychoactive substances [11]. In V MENTAL, 34 results were identified, 6 of which were in vitro and 28 were in vivo (Table 11).

Table 11.
The statistics of pharmacological results and its major diseases of SMC by experimental type in V Mental and behavioral disorders of ICD-10.
3.9.1. F00-F09 Organic, including Symptomatic Mental Disorders
This block includes various types of dementia, delirium, and organic amnesic syndrome, including AD and dementia [11] (Suppl. 847–Suppl. 863).
SMC inhibited acetylcholinesterase (AChE) and butyrylcholinesterase in vitro. In addition, it antagonized gamma-aminobutyric acid (GABA)-induced outward Cl− currents in single hippocampal CA1 neurons. Moreover, SMC attenuated amyloid plaque deposition, reduced the activity of AChE, and cognitive-memory functional deficits in various animal models induced by scopolamine, diazepam, Aβ peptides along with transgenic mice models, including APP/PS1, APPswe/PS1dE9, and senescence-acceleration. Furthermore, SMC improved cognitive functions in a sevoflurane-induced neurotoxic animal model and in a STZ-induced diabetic animal model. It also prevented apoptosis of hippocampal CA1 neurons and reversed the decrease in hippocampal IGF-1 in a rat model exhibiting vascular dementia.
3.9.2. F10-F19 Mental and Behavioral Disorders Due to Psychoactive Substance Use
This block includes mental and behavioral disorders induced by the use of alcohol, opioids, cannabinoids, sedatives or hypnotics, cocaine, stimulants, hallucinogens, and tobacco, among others [11] (Suppl. 864–Suppl. 875).
SMC ameliorated ethanol-induced long-term potentiation and excitatory postsynaptic potential deficits in animal hippocampus slices. In addition, it inhibited diazepam-induced GABA potentiation and upregulation of the GABA-A receptor a4 subunit mRNA in cultured hippocampal neurons. SMC suppressed the binding of flunitrazepam to central benzodiazepine receptors in mice. SMC reversed cognitive impairments exerted by diazepam or ethanol in various animal models. In alcohol-preferring rats, SMC repressed the effects of alcohol deprivation, reduced alcohol intake, and delayed the onset of alcohol consuming behavior.
3.9.3. Other Blocks
SMC showed a high affinity for binding in the GABA-A assay and exerted anxiolytic and anti-depressant effects in animal models (Suppl. 876–Suppl. 880).
3.10. Chapter I Infectious
This chapter classifies various viral and bacterial infections, as well as infectious diseases caused by fungi, protozoa, helminths, and the sequelae of infectious disease. This chapter specifically details the effects of SMC on bacterial diseases, including cholera, typhoid, tuberculosis, and anthrax. In addition, viral diseases, including rabies, viral encephalitis, viral hepatitis, herpes viral infections, and acquired immunodeficiency virus have been examined. Moreover, infectious diseases, including mycoses, protozoal diseases, helminthiases, pediculosis, and acariasis were included [11]. In I INFECTION, 21 results were included, of which 13 were in vitro, 7 were in vivo, and 1 was a human result (Table 12).

Table 12.
The statistics of pharmacological results and its major diseases of SMC by experimental type in I Certain infectious and parasitic diseases of ICD-10.
In bacterial experiments, SMC directly inhibited the activity of Neisseria meningitides, Staphylococcus aureus, and methicillin-resistant Staphylococcus aureus. SMC protected HUVECs against LPS and showed in vivo inhibitory effects against Chlamydia trachomatis-infected salpingitis, LPS-induced endotoxin shock, and Escherichia coli-induced endotoxemia and listeriosis (Suppl. 881–Suppl. 891).
In virus-related experiments, SMC inhibited viral entry of enterovirus 71 and prevented enterovirus 71-induced cytopathic effects in various cells. In addition, it suppressed the transcription of human immunodeficiency virus-1. In human studies, SMC demonstrated favorable effects in children with acute viral myocarditis (Suppl. 892–Suppl. 896).
Additional minor in vitro studies revealed that SMC showed antimicrobial activity against Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Streptococcus mutans, Lactobacillus, Bacillus subtilis, Candida albicans, and Trichophyton mentagrophytes, among others (Suppl. 897–Suppl. 901).
3.11. Chapter X Respiratory
This chapter classifies diseases and dysfunctions of the respiratory system, including upper respiratory tract diseases, such as pharyngitis, sinusitis, tonsillitis, and influenza; lower respiratory tract diseases, including bronchitis, emphysema, asthma, bronchiectasis and pneumonia, and other lung diseases, including pleural effusion and pneumothorax [11]. In X RESPIRATORY, 21 results were included, of which 2 were in vitro, 17 were in vivo, and 2 were human results (Suppl. 902–Suppl. 922) (Table 13).

Table 13.
The statistics of pharmacological results and its major diseases of SMC by experimental type in X Diseases of the respiratory system of ICD-10.
SMC inhibited TGF-β1-induced proliferation of type I collagen, expression of α-SMA in lung fibroblasts, and remodeling of the cytoskeleton. In addition, SMC improved cellular morphology of endothelial cells of the human pulmonary artery. SMC alleviated the pathological changes, including oedema in SAP-OJ and various animal models, in which lung injury was induced by radiation, postburn, LPS, and cigarette smoke. Moreover, it improved pulmonary function in rabbit emphysema model and inhibited fibrotic change in a bleomycin-induced pulmonary fibrosis rat model. In human studies, SMC ameliorated the symptoms and improved pulmonary function in senile chronic asthmatic bronchitis patients and reduced the levels of plasma platelet membrane glycoprotein 140, vWF, and P-selectin/platelet glycoproteins IIIa in chronic obstructive pulmonary disease patients.
3.12. Chapter XII Skin
This chapter classifies the diseases of the skin and subcutaneous tissue, specifically including infections of the skin and subcutaneous tissue, dermatitis, eczema, urticarial, erythema, bullous disorders, including pemphigus and papulosquamous disorders, including psoriasis and lichen planus [11]. Chapter XII SKIN contained 15 results comprising 11 in vitro and 3 in vivo results, in addition to 1 human result (Suppl. 923–Suppl. 937) (Table 14).

Table 14.
The statistics of pharmacological results and its major diseases of SMC by experimental type in XII Diseases of the skin and subcutaneous tissue of ICD-10.
SMC inhibited the population of keratinocytes and alleviated epidermal hyperplasia in an imiquimod-induced psoriatic-like skin mouse model. In addition, SMC suppressed tyrosinase activity in vitro, attenuated α-melanocyte-stimulating hormone-stimulated melanin production in melanoma cells (B16 cells), and enhanced melanocyte adhesion to fibronectin, which is related to vitiligo. Moreover, it prevented hypertrophic scarring by inhibiting TGF-β1, collagen production, and MMP-1 expression in fibroblasts. Furthermore, SMC repressed keloids by reducing prolyl hydroxylation in newly synthesized skin collagen in mice. In addition, SMC suppressed inflammatory reactions in dinitrochlorobenzene-induced atopic dermatitis-like skin mouse model. Moreover, SMC increased the survival rate of skin flap by inhibiting I/R damage after skin flap surgery in rats. In the human study, SMC reduced the rate of wound ischemia and necrosis after mastectomy in breast cancer patients.
3.13. Chapter III Blood and Immune
This chapter classifies various types of anemias, haemorrhagic complications, immunodeficiencies, diseases of blood cells, and dysfunction of the spleen [11]. In III BLOOD AND IMMUNE, nine results were included comprising one in vitro and six in vivo results, in addition to two human results (Suppl. 938–Suppl. 946) (Table 15).

Table 15.
The statistics of pharmacological results and its major diseases of SMC by experimental type in III Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism of ICD-10.
SMC improved human erythrocyte membrane structure and function in vitro. Amelioration of antiphospholipid syndrome induced by beta2 glycoprotein I in mice and the symptoms and mortality in LPS-induced disseminated intravascular coagulation in rabbits were achieved with the application of SMC. Moreover, SMC normalized immune cell imbalances in HFD-induced immunological function disorder in mice and prevented spleen damage in SAP-OJ model. In human studies, SMC raised the level of serum complement component 4 in hyperviscosity syndrome and reduced skin purpura and macroscopic haematuria in patients with Henoch-Schonlein purpura nephritis.
3.14. Chapter VII Eye
This chapter classifies the diseases of the eye and adjacent structures of the eye and includes cataract and glaucoma [11]. In VII EYE, seven results were identified, of which one was in vitro and six were in vivo results (Suppl. 947–Suppl. 953) (Table 16).

Table 16.
The statistics of pharmacological results and its major diseases of SMC by experimental type in VII Diseases of the eye and adnexa of ICD-10.
SMC inhibited macular degeneration, including apoptosis of the retinal pigment epithelium induced by OX-LDL in mice and in diabetic retinopathy induced by alloxan, STZ, and obesity in rats. SMC inhibited cataractogenesis induced by selenite in cultured rat lens, retinal cell loss in a glaucoma rat model, and corneal injury induced by ultraviolet radiation in mice.
3.15. Chapter VIII Ear
This chapter classifies the diseases of the external ear, middle ear, and mastoid, as well as the diseases of the inner ear [11]. In VIII EAR, a total of six results were included, comprising one in vitro and five in vivo results (Suppl. 954–Suppl. 959) (Table 17).

Table 17.
The statistics of pharmacological results and its major diseases of SMC by experimental type in VIII Diseases of the ear and mastoid process of ICD-10.
SMC inhibited cell death induced by radiation in cochlear cells and structural change in cochlea induced by radiation in guinea pigs. In addition, SMC inhibited apoptosis, structural change, and auditory functional deficits induced by chemotherapy agents (cisplatin and gentamicin) in guinea pigs.
3.16. Chapter XV Pregnancy
This chapter classifies conditions related to pregnancy, childbirth, and puerperium, including abortion, oedema, and eclampsia [11]. In XV PREGNANCY, five results were included, comprising three in vitro and one in vivo result, in addition to one human application (Suppl. 960–Suppl. 964) (Table 18).

Table 18.
The statistics of pharmacological results and its major diseases of SMC by experimental type in XV Pregnancy, childbirth, and the puerperium of ICD-10.
SMC increased cell size and aquaporin expression in human amniotic epithelial cells, while ameliorating the secretion of NO and inhibited the secretion of ET-1 in HUVECs cultured with serum from pre-eclampsia patients. Furthermore, SMC increased cell viability and expression of VEGF in HUVECs cultured with intrahepatic cholestasis from the serum of pregnant patients. SMC improved placenta-foetal circulation and decreased foetal distress induced by umbilical artery occlusion in pregnant ewe. A human study revealed that SMC lowered blood viscosity, cholesterol, and lipoprotein pregnant patients with hypertension.
3.17. Minor Chapters
Chapter XVI PERINATAL classifies conditions that originate during the perinatal period, including birth trauma, infections, and transitory diseases [11]. SMC ameliorated 36 infantile acute toxic myocarditis cases (Suppl. 965).
Chapter XVIII SYMPTOMS includes symptoms, signs, and ill-defined conditions, in which no diagnosis has been classified elsewhere [11] (Suppl. 966–Suppl. 971). The administration of SMC to rats attenuated gastric mucosal damage induced by haemorrhagic shock reperfusion, multiple organ oedema induced by hind limb ischemia, tachycardia induced by hypobaric hypoxia, and hypoxemia-induced vascular dysfunction in chronic intermittent hypoxia. Moreover, high blood viscosity was reduced in aging guinea pigs.
4. Discussion
In this study, the pharmacological activities of SMC were categorized into chapters of the ICD-10 by matching the results to specific diseases. The most prominent chapter was IX CIRCULATORY, which included atherosclerosis, thrombosis, myocardial infarction, angina pectoris, cerebral infarction and its sequelae, hypertension, diabetic peripheral angiopathy, cardiac fibrosis, cardiomyopathy, and cardiomegaly as major diseases. II NEOPLASMS comprised various types of cancers. XI DIGESTIVE predominantly covered liver fibrosis, cirrhosis, toxic liver disease, fatty liver disease, alcoholic liver disease, and gastric ulcer as major diseases. XIX EXTERNAL incorporated the adverse effects of medications and radiation, complications of surgery or procedures, allergic reactions, and traumatic injuries to various sites as major diseases. In IV ENDO AND META, major diseases comprised DM, complications of DM, hyperlipidaemia, and obesity. XIV GENITOURINARY included diabetic nephropathy, kidney damage, and renal failure as major diseases. VI NERVOUS included cerebral oedema, anoxic brain damage, AD, and PD as major diseases. In V MENTAL, major diseases were dementia, cognitive disorders, and alcohol addiction, while in XII MUSCULOSKELETAL osteoporosis, OA, RA, and neuropathic pain were the major diseases.
According to the literature, SMR is predominantly implemented in traditional medicine to treat dysmenorrhea, amenorrhea, lower abdominal pain due to postpartum stasis and stagnation, epigastric pain of heart and abdomen, aggregation accumulation, swelling, and pain due to heat impediment, traumatic injury, syndrome of heat entering nutrients and blood, agitation and restlessness, vexation and insomnia, abscess, sore and swelling of skin, joint pain, hemiplegia, and amnesia [1,2,19]. Through the categorization of these indications, based on the ICD-10, XI PREGNANCY, MI MUSCULOSKELETAL, XIX EXTERNAL, IX CIRCULATORY, V MENTAL, and XII SKIN could be the main chapters. These chapters might correspond to the main effect of SMR, which is “blood-activating and stasis-dispelling” of which the target disorder is “blood stasis syndrome” (BSS). Several studies have concorded BSS with diseases of Western medicine. Chinese researchers Liao et al., upon review of 155 papers, suggested that BSS-related diseases were coronary heart disease, cancer, stroke, skin disease, diabetes, hypertension, metabolic diseases, and disorders of the nervous system, among others [20]. Korean researchers Park et al. suggested that coagulopathy, hyperlipidemia, pain, liver injury, traumatic injury, neoplasm, ischemic brain injury, atherosclerosis, hypertension, DM, nephropathy, hematoma, genitourinary, and musculoskeletal disorders were related to BSS [21]. Japanese researcher Goto described BSS, according to Hanawa’s study, as contusions, sprains, bleeding under the skin and mucosa, purpura, varix, thrombosis, complications with menstruation, sterility, postnatal deconditioning, cold, liver dysfunction, metabolic disorders, such as DM, habitual constipation, haemorrhoids, surgical diseases, mental disorders, skin diseases, and autoimmune diseases, among others [22]. In relation to these studies, BSS might be associated with the following disease chapters and representative diseases as mentioned in parentheses, IX CIRCULATORY (coronary heart disease, stroke, hypertension, coagulopathy, atherosclerosis, varix, thrombosis), II NEOPLASMS, XIX EXTERNAL (accident, medical care, surgical procedure), IV ENDO AND META (DM, hyperlipidemia), XI DIGESTIVE (liver injury, liver dysfunction, habitual constipation), V MENTAL, VI NERVOUS, XIV GENITOURINARY (nephropathy), III BLOOD AND IMMUNE (varix), XII SKIN (purpura), and XV PREGNANCY (dysmenorrhea, pre- and post-menstruation syndrome, and postnatal diseases). Subsequently, diseases related to the pharmacological activities of SMR might be consistent with previous studies that described BSS-related diseases [20,21,22]. Although gynecological disease, including menstrual disorders and perinatal diseases, are the main indication of SMR in clinics, there were only two results (endometriosis and immature ovarian dysfunction) that were directly related to those diseases [23,24]. Results indirectly related to diseases included osteoporosis using OVX-induced animal models, which are closely related to menopause [25,26,27,28]. The results pertaining to palpitation and insomnia, related to the sedative effects of SMR, were infinitesimal [29,30].
In this study, we extracted all the pharmacological information relevant to SMR and categorized the results into potential clinical applications based on the ICD-10. It might offer a more comprehensive understanding and insight of the herb in terms of its characteristics, application, and clinical efficacy. The current review presents several advantages as compared to other reviews that were focused on pharmacological studies based on a molecular aspect. We provided pharmacology-based references of SMR with substantial research data (see Supplementary Data), allowing researchers to easily search for and analyze the relevant data. Scholars can easily discern how research has been conducted using SMR in the in vitro, in vivo, and human studies for certain diseases. Therefore, it is possible to reduce the risk of duplication or conduct similar research, while identifying diseases that have not been studied, thereby providing motivation for new investigations, especially for developmental research using SMR.
In addition, since the current study extracted all the pharmacological information and categorized the results according to the ICD-10, clinicians may be able to approach pharmacological data easily as a result of the clinical categorization. Therefore, clinicians who did not use the herb conventionally may utilize this review to gain insight into the potential use of SMR in clinical situations. Clinicians who use traditional medicine regularly may utilize this review to improve their understanding of the modern pharmacological view of the herb, along with its traditional uses. Consequently, this will allow clinicians to offer scientific evidence and to improve the application of the herb in various clinical situations. Moreover, it allows clinicians to elucidate the effects of the herb to patients during clinical sessions or to introduce the herb through public lectures.
Our study has certain limitations that should be addressed. First, we did not discriminate between sample types, including crude extracts, fractions, and compounds, nor did we describe the mode of administration in detail for the purpose of providing a general understanding of the characteristics of SMR. This was due to the fact that it was difficult to comprehensively analyze the results of individual studies during the process of analysis of each sample and mode of administration. Information pertaining to this may be acquired from the additional data included for each result. In addition, further reviews based on each sample type and mode of administration are required to provide a more detailed understanding of the use of SMR. Moreover, the number of studies and results might be influenced by the accessibility of the experiment, including difficulties in experimental assays, absence/presence of animal models, and researcher’s interest. For instance, in vitro cytotoxicity assays [31] (II NEOPLASMS), in vitro antimicrobial assays [32,33] (I INFECTION), and in vitro liver cell toxicity assays [34] (XI DIGESTIVE) produced a vast array of results due to the simplicity of the procedure. Therefore, this should be considered when analyzing the overall results. Finally, we did not analyze or summarize the interrelated pharmacological mechanisms for each specific disease, as the purpose of the current study was to show a comprehensive understanding of SMR under the ICD-10 classification. Collected data comprise various types of studies, modes of administration, and components making it difficult for evaluation and summarization in the same hierarchy. In addition, summarization of each disease can lead to distorted results between classification of disease. Other research papers should be consulted for detailed mechanisms.
5. Conclusions
Our study revealed that the main chapters of pharmacological activities of SMR and its components were IX CIRCULATORY, II NEOPLASMS, XI DIGESTIVE, XIX EXTERNAL, XIV GENITOURINARY, IV ENDO AND META, VI NERVOUS, V MENTAL, and XII MUSCULOSKELETAL, according to the ICD-10 and the main diseases were cancer, atherosclerosis, myocardial infarction, liver fibrosis and cirrhosis, cerebral infarction, DM (including complications), hypertension, and thrombosis. The results might correspond to the clinical usage of SMR in traditional medicine. However, we found that the results pertaining to gynecological disease, palpitation, and insomnia, which are known to be the main indication of SMR in traditional medicine, were few. Our study might offer a more comprehensive understanding, scientific evidence (despite being basic research), and research progress to clinicians and researchers. Therefore, we suggest the current method of reviewing the pharmacological activities of herbal medicine using ICD for further reviews on other herbs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr10091860/s1, An Excel file: The reference for each result is accompanied by a reference number (e.g., Suppl. 1–Suppl. 2) in the Excel sheet.
Author Contributions
Conceptualization, J.-H.K., J.-W.S. and Y.B.; investigation, J.-H.K., J.-W.S., J.-W.J., E.-J.K., H.J. and J.P.; writing—original draft preparation, J.-H.K., J.-W.S., H.J., J.P. and Y.B.; writing—review and editing, J.-W.P., B.-j.L., J.-H.C., K.L., H.L. and Y.B.; project administration, Y.B.; funding acquisition, Y.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Undergraduate Research Program (URP) grant of Kyung Hee University, College of Korean Medicine and this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No. 2020R1A2C1008603).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
4-HNE, 4-hydroxynonenal; 5-FU, 5-fluorouracil; ACE, the angiotensin converting enzyme; AChE, acetylcholinesterase; ADP, adenosine diphosphate; AGEs, advanced glycation end-products; ALD, alcoholic liver disease; AD, Alzheimer’s disease; Ang, angiotensin; Aβ, amyloid-beta; BBB, blood-brain barrier; BCG, Bacille Calmette-Guérin; BSS, blood stasis syndrome; CCL4, carbon tetrachloride; CM, clinical modification; DM, diabetes mellitus; ET-1, endothelin-1; GABA, gamma-aminobutyric acid; HFD, high fat diet; HSC, hepatic stellate cell; HUVECs, human umbilical vein endothelial cells; I/R, ischemia/reperfusion; ICD, the international statistical classification of diseases and related health problems; ICD-10, the international disease classification 10th revision; ICD-10-CM, ICD-10 clinical modification; IGF, insulin-like growth factor; IL, IX CIRCULATORY, IX Diseases of the circulatory system; IL, interleukin; LPS, lipopolysaccharides; MMPs, matrix metalloproteinases; NCHS, The National Center for Health Statistics; NRF, National Research Foundation of Korea; OA, osteoarthritis; OLETF rats, Otsuka Long-Evans Tokushima Fatty rats; OVX, ovariectomized; oxLDL, oxidized low-density lipoprotein; PCI, percutaneous coronary intervention; PD, Parkinson’s disease; PMID, PubMed ID; pox-LDL, peroxidized low-density lipoprotein; RA, rheumatoid arthritis; SAP-OJ, severe acute pancreatitis or obstructive jaundice rat; SCI, spinal cord injury; SHR, spontaneously hypertensive rat; SMR, Salvia Miltiorrhizae Radix; SMA, smooth muscle actin; SMC, Salvia Miltiorrhizae Radix component; STZ, streptozotocin; TG, triglyceride; TGF, transforming growth factor; TNF-a, tumor necrosis factor-alpha; TNF-α/D-GalN, TNF-α/d-galactosamine; URP, undergraduate research program; VEGF, vascular endothelial growth factor; VSMCs, vascular smooth muscle cells; vWF, von Willebrand factor; WHO, World Health Organization.
References
- The Boncho Hak Textbook Committee. Boncho Hak, 3rd ed.; Publication Yunglim: Seoul, Korea, 2009; pp. 458–459. [Google Scholar]
- Editorial Committee of Zhong Hua Ben Cao. Zhong Hua Ben Cao, 1st ed.; Shanghai Science and Technology Publications: Shanghai, China, 1999; pp. 169–186. [Google Scholar]
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Ji, X.Y.; Tan, B.; Zhu, Y.Z. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol. Sin. 2000, 21, 1089–1094. [Google Scholar]
- Chang, C.C.; Chang, Y.C.; Hu, W.L.; Hung, Y.C. Oxidative Stress and Salvia miltiorrhiza in Aging-Associated Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 4797102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, J.; Bao, J.; Lu, J.; Wang, Y. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Qian, S.S.; Zhang, Y.J.; Wang, R.Q. Salvia miltiorrhiza: A source for anti-Alzheimer’s disease drugs. Pharm. Biol. 2016, 54, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, Q.; Shi, J.; Chen, X. Advance in studies on hepatoprotective effect of Salvia miltiorrhiza and its main components. Zhongguo Zhong Yao Za Zhi 2015, 40, 588–593. [Google Scholar]
- Guo, Y.; Li, Y.; Xue, L.; Severino, R.P.; Gao, S.; Niu, J.; Qin, L.P.; Zhang, D.; Brömme, D. Salvia miltiorrhiza: An ancient Chinese herbal medicine as a source for anti-osteoporotic drugs. J. Ethnopharmacol. 2014, 155, 1401–1416. [Google Scholar] [CrossRef]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organization Regional Office for the Western Pacific. WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region; WHO Regional Office for the Western Pacific: Manila, Philippines, 2007. [Google Scholar]
- International Statistical Classification of Diseases and Related Health Problems 10th Revision International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2016/en/ (accessed on 30 October 2018).
- The Web’s Free 2022 ICD-10-CM/PCS Medical Coding Reference. Available online: https://www.icd10data.com/ (accessed on 31 October 2018).
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397. [Google Scholar] [CrossRef]
- Puche, J.E.; Saiman, Y.; Friedman, S.L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 2013, 3, 1473–1492. [Google Scholar] [CrossRef]
- Dey, A.; Cederbaum, A.I. Alcohol and oxidative liver injury. Hepatology 2006, 43, S63–S74. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit Dahm, K.A.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef]
- Du, G.H.; Zhang, J.T. The General Situation and Progress of the Modern Research of Red Sage Root (Radix Salviae Miltiorrhizae). Her. Med. 2004, 23, 355–361. [Google Scholar]
- Liao, J.; Wang, J.; Liu, Y.; Li, J.; Duan, L.; Chen, G.; Hu, J. Modern researches on Blood Stasis syndrome 1989-2015: A bibliometric analysis. Medicine 2016, 95, e5533. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; You, S.; Jung, J.; Lee, J.A.; Yun, K.J.; Lee, M.S. Korean Studies on Blood Stasis: An Overview. Evid. Based Complement. Alternat. Med. 2015, 2015, 316872. [Google Scholar] [CrossRef] [PubMed]
- Goto, H. Blood stasis syndrome in Japan and its molecular biological analysis. Chin. J. Integr. Med. 2014, 20, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, T.; Li, X.; Qu, Y.K.; An, J.N.; Zheng, H.X.; Zhang, Z.J.; Lin, N. Salvia miltiorrhiza bunge increases estrogen level without side effects on reproductive tissues in immature/ovariectomized mice. Aging 2017, 9, 156. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Weng, Q.; Zhou, J.H.; Zhou, J. Extracts of Salvia miltiorrhiza bunge on the cytokines of rat endometriosis models. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 303–314. [Google Scholar] [CrossRef][Green Version]
- Chae, H.; Chae, S.; Yun, D.; Keum, K.; Yoo, S.; Kim, H.R. Prevention of bone loss in ovariectomized rats: The effect of Salvia miltiorrhiza extracts. Immunopharmacol. Immunotoxicol. 2004, 26, 135–144. [Google Scholar] [CrossRef]
- Cui, Y.; Bhandary, B.; Marahatta, A.; Lee, G.-H.; Li, B.; Kim, D.-S.; Chae, S.-W.; Kim, H.-R.; Chae, H.-J. Characterization of Salvia Miltiorrhiza ethanol extract as an anti-osteoporotic agent. BMC Complement. Altern. Med. 2011, 11, 120. [Google Scholar] [CrossRef]
- Dong, X.; Yu, W.; Li, C.; He, S.; Zhou, L.; Poon, C.; Wong, M. Danshen (Salvia miltiorrhiza) protects ovariectomized rats fed with high-saturated fat-sucrose diet from bone loss. Osteoporos. Int. 2018, 29, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Song, H.S.; Kwon, J.E.; Cho, S.M.; Jang, S.A.; Kim, M.Y.; Kang, S.C. Effects of Salvia miltiorrhiza extract with supplemental liquefied calcium on osteoporosis in calcium-deficient ovariectomized mice. BMC Complement. Altern. Med. 2017, 17, 545. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, W.; Mikolajczak, P.L.; Krajewska Patan, A.; Dreger, M.; Gorska Paukszta, M.; Szulc, M.; Polcyn, P.; Piorunska Mikolajczak, A.; Mielcarek, S.; Czerny, B.; et al. Involvement of the different extracts from roots of Salvia miltiorrhiza Bunge on acute hypobaric hypoxia-induced cardiovascular effects in rats--preliminary report. Pol. J. Vet. Sci. 2012, 15, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Shimizu, M.; Lee, C.J.; Han, D.S.; Jung, C.K.; Jo, J.H.; Kim, Y.M. Hypnotic effects and binding studies for GABAA and 5-HT2C receptors of traditional medicinal plants used in Asia for insomnia. J. Ethnopharmacol. 2010, 132, 225–232. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.-L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Terras, F.R.G.; Cammue, B.P.A.; Vanderleyden, J. An automated quantitative assay for fungal growth inhibition. FEMS Microbiol. Lett. 1990, 69, 55–59. [Google Scholar] [CrossRef]
- Iqbal, J.; Siddiqui, R.; Kazmi, S.U.; Khan, N.A. A simple assay to screen antimicrobial compounds potentiating the activity of current antibiotics. Biomed. Res. Int. 2013, 2013, 927323. [Google Scholar] [CrossRef]
- Otoguro, K.; Komiyama, K.; Omura, S.; Tyson, C. An in vitro cytotoxicity assay using rat hepatocytes and MTT and Coomassie blue dye as indicators. Altern. Lab. Anim. 1991, 19, 352–360. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).