Antioxidative Properties and Phenolic Profile of the Core, Pulp and Peel of Commercialized Kiwifruit by LC-ESI-QTOF-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Extraction of Phenolic Compounds
2.3. Estimation of the Phenolic Contents and Antioxidant Assays
2.3.1. Determination of the Total Phenolic Content (TPC)
2.3.2. Determination of the Total Flavonoid Content (TFC)
2.3.3. Determination of the Total Tannin Content (TTC)
2.3.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Antioxidant Assay
2.3.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.3.6. 2,2-Azino-bis-3ethylbenzothiazoline-6-sulfonic Acid (ABTS) Radical Scavenging Assay
2.3.7. Reducing Power Assay (RPA)
2.3.8. Hydroxyl Radical Scavenging Activity (•OH-RSA)
2.3.9. Ferrous Ion Chelating Activity (FICA)
2.3.10. Total Antioxidant Capacity (TAC) Assay
2.4. LC-ESI-QTOF-MS/MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Estimation (TPC, TFC and TTC)
3.2. Antioxidant Activity (DPPH, FRAP, ABTS, FICA, •OH-RSA, RPA, and TAC)
3.3. LC-ESI-QTOF-MS/MS Characterization
3.3.1. Phenolic Acids
- Hydroxybenzoic acids
- Hydroxycinnamic acids
3.3.2. Flavonoids
- Dihydrochalcones, Dihydroflavonols, and Anthocyanins
- Flavanols, Flavones, and Flavaones
- Flavonols
- Isoflavonoids
3.3.3. Lignans, Stilbenes and Other Polyphenols
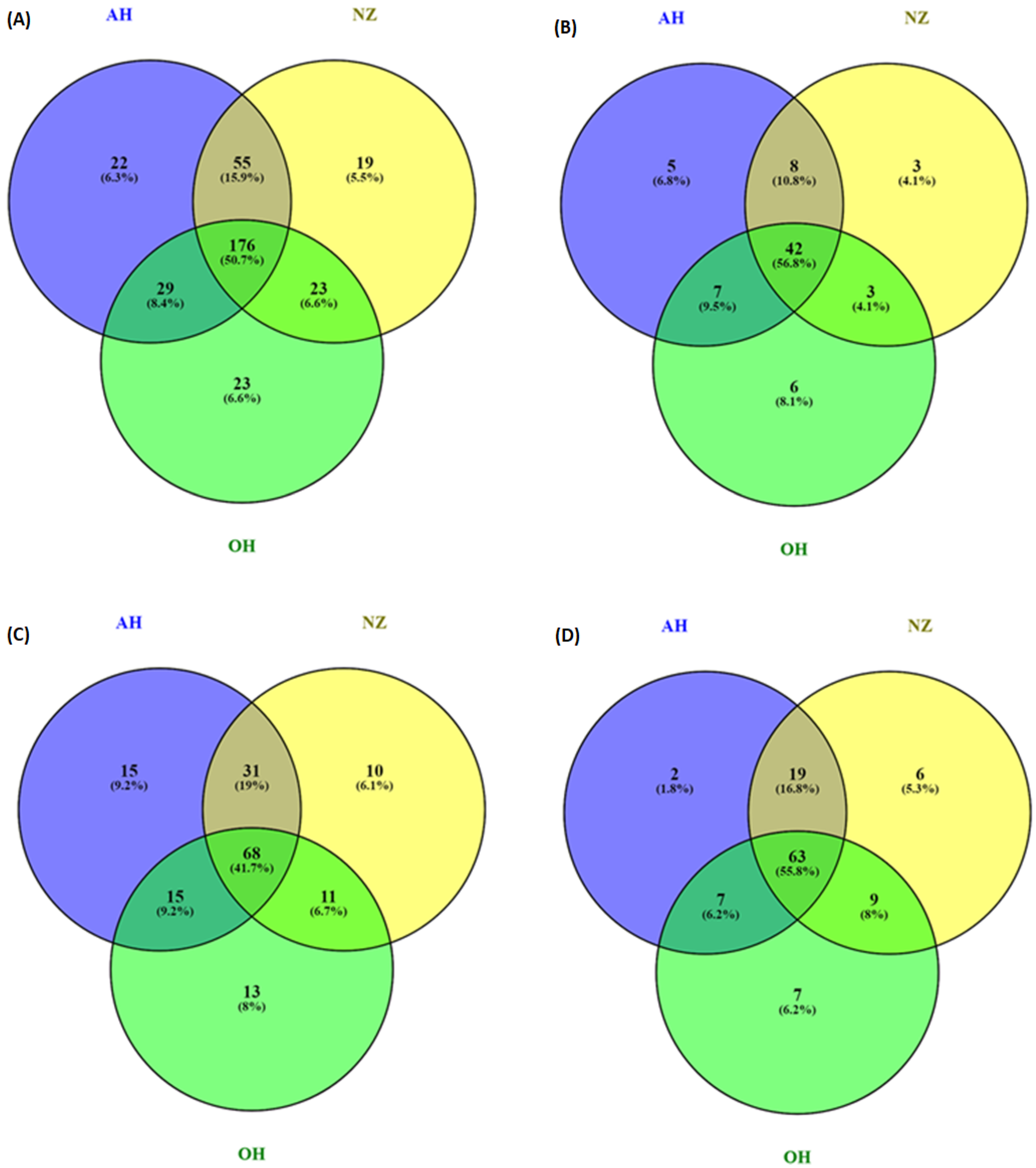
3.4. Distribution of Phenolic Compounds—Venn Diagram
3.5. Correlation between the Antioxidant Assays and Phenolic Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, V.; López-Hortas, L.; Torres, M.; Domínguez, H. Trends in kiwifruit and byproducts valorization. Trends Food Sci. Technol. 2021, 107, 401–414. [Google Scholar] [CrossRef]
- Ferguson, A.R. Kiwifruit: The wild and the cultivated plants. Adv. Food Nutr. Res. 2013, 68, 15–32. [Google Scholar] [PubMed]
- Vissers, M.C.; Carr, A.C.; Pullar, J.M.; Bozonet, S.M. The bioavailability of vitamin C from kiwifruit. Adv. Food Nutr. Res. 2013, 68, 125–147. [Google Scholar] [PubMed]
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S.; Drummond, L. The nutritional composition of Zespri® SunGold Kiwifruit and Zespri® Sweet Green Kiwifruit. Food Chem. 2018, 238, 195–202. [Google Scholar] [CrossRef]
- Tyagi, S.; Nanher, A.; Sahay, S.; Kumar, V.; Bhamini, K.; Nishad, S.; Ahmad, M. Kiwifruit: Health benefits and medicinal importance. Rashtriya Krishi 2015, 10, 98–100. [Google Scholar]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of kiwi agricultural waste and industry by-products by recovering bioactive compounds and applications as food additives: A circular economy model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.-S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa ‘Hayward’and Actinidia eriantha ‘Bidan’. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef]
- Drummond, L.; Gearry, R.B. Kiwifruit modulation of gastrointestinal motility. Adv. Food Nutr. Res. 2013, 68, 219–232. [Google Scholar]
- Carr, A.C.; Bozonet, S.M.; Pullar, J.M.; Vissers, M.C. Mood improvement in young adult males following supplementation with gold kiwifruit, a high-vitamin C food. J. Nutr. Sci. 2013, 2, e24. [Google Scholar] [CrossRef]
- Lin, H.-H.; Tsai, P.-S.; Fang, S.-C.; Liu, J.-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac. J. Clin. Nutr. 2011, 20, 169–174. [Google Scholar] [PubMed]
- Skinner, M.A.; Bentley-Hewitt, K.; Rosendale, D.; Naoko, S.; Pernthaner, A. Effects of kiwifruit on innate and adaptive immunity and symptoms of upper respiratory tract infections. Adv. Food Nutr. Res. 2013, 68, 301–320. [Google Scholar] [PubMed]
- Beck, K.; Conlon, C.A.; Kruger, R.; Coad, J.; Stonehouse, W. Gold kiwifruit consumed with an iron-fortified breakfast cereal meal improves iron status in women with low iron stores: A 16-week randomised controlled trial. Br. J. Nutr. 2011, 105, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Szwajgier, D. Characteristics and pro-health properties of mini kiwi (Actinidia arguta). Hortic. Environ. Biotechnol. 2019, 60, 217–225. [Google Scholar] [CrossRef]
- Pratt, H.K.; Reid, M.S. Chinese gooseberry: Seasonal patterns in fruit growth and maturation, ripening, respiration and the role of ethylene. J. Sci. Food Agric. 1974, 25, 747–757. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Raghavan, V. High-intensity ultrasound processing of kiwifruit juice: Effects on the ascorbic acid, total phenolics, flavonoids and antioxidant capacity. LWT 2019, 107, 299–307. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2021, 373, 131455. [Google Scholar] [CrossRef]
- Guroo, I.; Wani, S.; Wani, S.; Ahmad, M.; Mir, S.; Masoodi, F. A review of production and processing of kiwifruit. J. Food Processing Technol. 2017, 8, 10. [Google Scholar]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural residues as a source of bioactive natural products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Latocha, P.; Krupa, T.; Wołosiak, R.; Worobiej, E.; Wilczak, J. Antioxidant activity and chemical difference in fruit of different Actinidia sp. Int. J. Food Sci. Nutr. 2010, 61, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Talukder, P.; Talapatra, S.; Ghoshal, N.; Sen Raychaudhuri, S. Antioxidant activity and high-performance liquid chromatographic analysis of phenolic compounds during in vitro callus culture of Plantago ovata Forsk. and effect of exogenous additives on accumulation of phenolic compounds. J. Sci. Food Agric. 2016, 96, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Soquetta, M.B.; Stefanello, F.S.; da Mota Huerta, K.; Monteiro, S.S.; da Rosa, C.S.; Terra, N.N. Characterization of physiochemical and microbiological properties, and bioactive compounds, of flour made from the skin and bagasse of kiwi fruit (Actinidia deliciosa). Food Chem. 2016, 199, 471–478. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, S.; Chae, D.; Hyun, J.W.; Kang, H.K.; Koh, Y.-S.; Kim, Y.H. Anti-inflammatory and antioxidant activities of phenolic compounds from Desmodium caudatum leaves and stems. Arch. Pharmacal Res. 2014, 37, 721–727. [Google Scholar] [CrossRef]
- Ali Asgar, M. Anti-diabetic potential of phenolic compounds: A review. Int. J. Food Prop. 2013, 16, 91–103. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Liu, H.; Zhao, T.; Meng, C.; Liu, Z.; Liu, X. Bioactive compounds and in vitro antioxidant activities of peel, flesh and seed powder of kiwi fruit. Int. J. Food Sci. Technol. 2018, 53, 2239–2245. [Google Scholar] [CrossRef]
- LIsa, M.; Holcapek, M.; Bohac, M. Statistical evaluation of triacylglycerol composition in plant oils based on high-performance liquid chromatography−atmospheric pressure chemical ionization mass spectrometry data. J. Agric. Food Chem. 2009, 57, 6888–6898. [Google Scholar] [CrossRef]
- Deng, J.; Sun, T.; Cao, W.; Fan, D.; Cheng, N.; Wang, B.; Gao, H.; Yang, H. Extraction optimization and functional properties of proteins from kiwi fruit (Actinidia chinensis Planch.) seeds. Int. J. Food Prop. 2014, 17, 1612–1625. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Y.; Dou, P.; Yue, T. Multivariate statistical analysis of the polyphenolic constituents in kiwifruit juices to trace fruit varieties and geographical origins. Food Chem. 2017, 232, 552–559. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, H.; Yan, F.; Yan, C.; Wei, S.; Lai, J.; Wang, Y.; Zhang, B. Morphology and molecular identification of twelve commercial varieties of kiwifruit. Molecules 2019, 24, 888. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Im, S.; Jin, C.-R.; Heo, H.J.; Cho, Y.-S.; Baik, M.-Y.; Kim, D.-O. Effect of maturity stage at harvest on antioxidant capacity and total phenolics in kiwifruits (Actinidia spp.) grown in Korea. Hortic. Environ. Biotechnol. 2015, 56, 841–848. [Google Scholar] [CrossRef]
- Faller, A.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- Lim, Y.J.; Oh, C.-S.; Park, Y.-D.; Eom, S.H.; Kim, D.-O.; Kim, U.-J.; Cho, Y.-S. Physiological components of kiwifruits with in vitro antioxidant and acetylcholinesterase inhibitory activities. Food Sci. Biotechnol. 2014, 23, 943–949. [Google Scholar] [CrossRef]
- Park, Y.-S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Suhaj, M.; Cvikrová, M.; Martincová, O.; Weisz, M.; Gorinstein, S. Comparison of the contents of bioactive compounds and the level of antioxidant activity in different kiwifruit cultivars. J. Food Compos. Anal. 2011, 24, 963–970. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof/ms characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Zhu, C.; Chou, O.; Lee, F.Y.; Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Characterization of phenolics in rejected kiwifruit and their antioxidant potential. Processes 2021, 9, 781. [Google Scholar] [CrossRef]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. A comparative investigation on phenolic composition, characterization and antioxidant potentials of five different australian grown pear varieties. Antioxidants 2021, 10, 151. [Google Scholar] [CrossRef]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Haile, M.; Kang, W.H. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation 2019, 5, 29. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-X.; Feng, H.-L.; Ding, Y.-M.; Chai, W.-M.; Xiang, Z.-H.; Shi, Y.; Chen, Q.-X. Structure characterization of proanthocyanidins from Caryota ochlandra Hance and their bioactivities. Food Chem. 2014, 155, 1–8. [Google Scholar] [CrossRef]
- Severo, J.; Tiecher, A.; Chaves, F.C.; Silva, J.A.; Rombaldi, C.V. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chem. 2011, 126, 995–1000. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Dinis, T.C.; Madeira, V.M.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Screening of phenolic compounds in australian grown berries by lc-esi-qtof-ms/ms and determination of their antioxidant potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yoo, M.-S.; Whang, Y.-J.; Jin, Y.-J.; Hong, M.-H.; Pyo, Y.-H. Vitamin C, total polyphenol, flavonoid contents and antioxidant capacity of several fruit peels. Korean J. Food Sci. Technol. 2012, 44, 540–544. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Q.; Zhang, C.; Cao, W.; Fan, D.; Yang, H. Extraction Optimization of Polyphenols from Waste Kiwi Fruit Seeds (Actinidia chinensis Planch.) and Evaluation of Its Antioxidant and Anti-Inflammatory Properties. Molecules 2016, 21, 832. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.; Ons, M.; Yosra, E.T.; Rayda, S.; Neji, G.; Moncef, N. Chemical composition and antioxidant and radical-scavenging activities of Periploca laevigata root bark extracts. J. Sci. Food Agric. 2009, 89, 897–905. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof/ms profiling of australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Yang, D.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterization of Australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Processing Preserv. 2020, 44, e14497. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; von Baer, D.; Vallverdú-Queralt, A. Phenolic profile of grape canes: Novel compounds identified by lc-esi-ltq-orbitrap-ms. Molecules 2019, 24, 3763. [Google Scholar]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, C.; Chakraborty, S. Study of dietary polyphenols from natural herbal sources for providing protection against human degenerative disorders. Biocatal. Agric. Biotechnol. 2021, 33, 101956. [Google Scholar] [CrossRef]
- Ma, C.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof/ms characterization of phenolic compounds in palm fruits (jelly and fishtail palm) and their potential antioxidant activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhu, Z.; Zuo, L.; Chen, L.; Yuan, Q.; Shan, G.; Luo, S.-Z. A strategy for rapid structural characterization of saponins and flavonoids from the testa of Camellia oleifera Abel seeds by ultra-high-pressure liquid chromatography combined with electrospray ionization linear ion trap-orbitrap mass spectrometry. Anal. Methods 2015, 7, 5942–5953. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Liao, J.; Fan, X.; Cheng, Y. An ultra-robust fingerprinting method for quality assessment of traditional chinese medicine using multiple reaction monitoring mass spectrometry. J. Pharm. Anal. 2021, 11, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Jahan, N.; Rahman, K.-U.; Sultana, B.; Jamil, S. Identification of hypotensive biofunctional compounds of Coriandrum sativum and evaluation of their angiotensin-converting enzyme (ACE) inhibition potential. Oxidative Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Wan, M.; Zhang, Y.; Yang, Y.; Liu, X.; Jia, L.; Yang, X. Analysis of the chemical composition of Angelicae Pubescentis Radix by ultra-performance liquid chromatography and quadrupole time-of-flight tandem mass spectrometry. J. Chin. Pharm. Sci. 2019, 28, 145. [Google Scholar]


| Assays | Peel | Pulp | Core | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NZ | AH | OH | NZ | AH | OH | NZ | AH | OH | |
| TPC (mg GAE/g) | 6.61 ± 0.24 c | 7.99 ± 0.10 b | 9.65 ± 0.44 a | 0.58 ± 0.05 a | 0.45 ± 0.03 ab | 0.33 ± 0.02 b | 0.66 ± 0.05 a | 0.45 ± 0.01 b | 0.44 ± 0.03 b |
| TFC (mg QE/g) | 0.21 ± 0.00 b | 0.42 ± 0.04 a | - | - | - | - | 0.01 ± 0.00 | - | - |
| TTC (mg CE/g) | 10.99 ± 0.12 a | 7.00 ± 0.56 b | 7.67 ± 0.34 b | - | - | - | - | - | - |
| DPPH (mg AAE/g) | 12.33 ± 1.18 a | 7.88 ± 0.11 b | 6.69 ± 0.09 b | 0.25 ± 0.00 a | 0.13 ± 0.00 b | 0.08 ± 0.00 c | 1.04 ± 0.06 a | 0.31 ± 0.00 b | 0.22 ± 0.00 b |
| FRAP (mg AAE/g) | 2.64 ± 0.03 b | 3.12 ± 0.11 a | 0.03 ± 0.00 c | 0.32 ± 0.01 a | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.04 ± 0.00 b | 0.10 ± 0.00 a | 0.03 ± 0.00 b |
| ABTS (mg AAE/g) | 18.31 ± 0.79 a | 17.01 ± 0.81 ab | 13.25 ± 0.35 b | 0.71 ± 0.01 a | 0.21 ± 0.02 b | 0.16 ± 0.00 b | 0.85 ± 0.02 a | 0.15 ± 0.00 c | 0.32 ± 0.02 b |
| FICA (mg EDTA/g) | 0.31 ± 0.07 a | 0.12 ± 0.02 b | 0.09 ± 0.01 c | 0.14 ± 0.04 a | 0.08 ± 0.02 b | - | 0.09 ± 0.01 a | 0.03 ± 0.01 b | - |
| •OH-RSA (mgAAE/g) | 0.37 ± 0.04 a | 0.09 ± 0.02 c | 0.21 ± 0.07 b | 0.04 ± 0.01 a | 0.05 ± 0.03 a | - | 0.12 ± 0.09 a | 0.09 ± 0.01 b | 0.04 ± 0.02 c |
| RPA (mg AAE/g) | 1.24 ± 0.01 a | 0.98 ± 0.07 b | 0.74 ± 0.02 c | 0.18 ± 0.08 b | 0.24 ± 0.03 a | 0.04 ± 0.07 c | 0.72 ± 0.04 a | 0.51 ± 0.01 b | 0.19 ± 0.03 c |
| TAC (mg AAE/g) | - | - | 4.43 ± 0.11 | 0.37 ± 0.01 a | 0.03 ± 0.00 c | 0.12 ± 0.00 b | 0.51 ± 0.02 a | 0.11 ± 0.00 c | 0.28 ± 0.00 b |
| No. | Proposed Compounds | Molecular Formula | RT (min) | Ionization (ESI+/ESI−) | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Error (ppm) | MS2 Product Ions | Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||||
| Hydroxybenzoic acids | ||||||||||
| 1 | Gallic acid | C7H6O5 | 6.956 | ** [M−H]− | 170.0215 | 169.0142 | 169.0148 | 3.5 | 125 | * OHL, AHL |
| 2 | Gallic acid 4-O-glucoside | C13H16O10 | 10.236 | ** [M−H]− | 332.0743 | 331.0670 | 331.0655 | −4.5 | 169, 125 | * OHL, OHC |
| 3 | 3-O-Methylgallic acid | C8H8O5 | 11.694 | [M+H]+ | 184.0372 | 185.0445 | 185.0444 | −0.5 | 170, 142 | * OHC, AHL |
| 4 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 12.539 | ** [M−H]− | 316.0794 | 315.0721 | 315.0707 | −4.4 | 153 | * OHL, OHC, AHL |
| 5 | 2,3-Dihydroxybenzoic acid | C7H6O4 | 15.580 | [M−H]− | 154.0266 | 153.0193 | 153.0195 | 1.3 | 109 | * AHL, OHL |
| 6 | 3,4-O-Dimethylgallic acid | C9 H10 O5 | 17.965 | ** [M+H]+ | 198.0528 | 199.0601 | 199.0602 | 0.5 | 153, 139, 125, 111 | AHL |
| 7 | 2-Hydroxybenzoic acid | C7H6O3 | 22.943 | ** [M−H]− | 138.0317 | 137.0244 | 137.0242 | −1.5 | 93 | * OHL, AHL, NZL, OHP |
| 8 | Paeoniflorin | C23H28O11 | 34.151 | ** [M−H]− | 480.1632 | 479.1559 | 479.1578 | 4.0 | 449, 357, 327 | * AHL, OHL, OHP |
| Hydroxycinnamic acids | ||||||||||
| 9 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 4.106 | [M−H]− | 516.1268 | 515.1195 | 515.1198 | 0.6 | 353, 335, 191, 179 | * OHL, OHP, OHC |
| 10 | 3-Caffeoylquinic acid | C16H18O9 | 4.458 | ** [M−H]− | 354.0951 | 353.0878 | 353.0873 | −1.4 | 253, 190, 144 | * NZC, NZP, AHL, OHL, NZL, AHP, OHP |
| 11 | 3-Feruloylquinic acid | C17H20O9 | 4.653 | ** [M−H]− | 368.1107 | 367.1034 | 367.1038 | 1.1 | 298, 288, 192, 191 | * OHL, NZC, NZP, AHP, OHP, NZL |
| 12 | Ferulic acid 4-sulfate | C10H10O7S | 7.041 | [M−H]− | 274.0147 | 273.0074 | 273.0081 | 2.6 | 193, 178 | NZL |
| 13 | Caffeoyl glucose | C15H18O9 | 7.589 | [M−H]− | 342.0951 | 341.0878 | 341.0874 | −1.2 | 179, 161 | * NZC, AHL |
| 14 | Ferulic acid 4-O-glucuronide | C16H18O10 | 18.512 | ** [M−H]− | 370.0900 | 369.0827 | 369.0831 | 1.1 | 193 | * AHL, OHL |
| 15 | Caffeic acid 3-O-glucuronide | C15H16O10 | 22.273 | ** [M−H]− | 356.0743 | 355.0670 | 355.0671 | 0.3 | 179 | * AHL, OHL |
| 16 | m-Coumaric acid | C9H8O3 | 22.306 | ** [M−H]− | 164.0473 | 163.0400 | 163.0403 | 1.8 | 119 | * AHL, OHL, NZL |
| 17 | Rosmarinic acid | C18H16O8 | 22.323 | ** [M−H]− | 360.0845 | 359.0772 | 359.0755 | −4.7 | 179 | * AHL, OHC, NZL |
| 18 | Ferulic acid | C10H10O4 | 23.366 | ** [M−H]− | 194.0579 | 193.0506 | 193.0505 | −0.5 | 178, 149, 134 | * AHL, OHC, OHP |
| 19 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 23.764 | ** [M−H]− | 326.1002 | 325.0929 | 325.0924 | −1.5 | 163 | * AHL, NZC, NZP |
| 20 | Sinapic acid | C11H12O5 | 26.166 | ** [M−H]− | 224.0685 | 223.0612 | 223.0604 | −3.6 | 205, 163 | * AHL, NZL |
| 21 | 1-Sinapoyl-2,2′-diferuloylgentiobiose | C43H48O21 | 26.763 | [M−H]− | 900.2688 | 899.2615 | 899.2579 | −4.0 | 613, 201 | AHL |
| 22 | Caffeic acid | C9H8O4 | 28.724 | ** [M−H]− | 180.0423 | 179.0350 | 179.0349 | −0.6 | 143, 133 | * OHL, OHC, NZP |
| 23 | 1,2,2′-Triferuloylgentiobiose | C42H46O20 | 31.127 | [M−H]− | 870.2582 | 869.2509 | 869.2506 | −0.3 | 693, 517 | OHL |
| 24 | 3-p-Coumaroylquinic acid | C16H18O8 | 32.031 | ** [M−H]− | 338.1002 | 337.0929 | 337.0923 | −1.8 | 265, 173, 162 | * AHL, NZC, NZP, OHL, AHC |
| 25 | Ferulic acid 4-O-glucoside | C16H20O9 | 35.526 | [M−H]− | 356.1107 | 355.1034 | 355.1040 | 1.7 | 193, 178, 149, 134 | AHL |
| 26 | 5-5′-Dehydrodiferulic acid | C20 H18 O8 | 39.819 | ** [M+H]+ | 386.1002 | 387.1075 | 387.1064 | −2.8 | 369 | * OHC, AHL, OHL |
| Hydroxyphenylacetic acids | ||||||||||
| 27 | 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 24.874 | [M−H]− | 168.0423 | 167.0350 | 167.0349 | −0.6 | 149, 123 | * AHL, OHL |
| 28 | 2-Hydroxy-2-phenylacetic acid | C8H8O3 | 88.387 | ** [M−H]− | 152.0473 | 151.0400 | 151.0397 | −2.0 | 136, 92 | * AHC, OHL, AHL |
| Hydroxyphenylpropanoic acids | ||||||||||
| 29 | Dihydrocaffeic acid 3-O-glucuronide | C15H18O10 | 12.340 | [M−H]− | 358.0900 | 357.0827 | 357.0818 | −2.5 | 181 | OHL |
| 30 | Dihydroferulic acid 4-O-glucuronide | C16H20O10 | 33.025 | [M−H]− | 372.1056 | 371.0983 | 371.0990 | 1.9 | 195 | AHL |
| Flavnoids | ||||||||||
| Flavanols | ||||||||||
| 31 | 3′-O-Methylcatechin | C16H16O6 | 11.736 | ** [M−H]− | 304.0947 | 303.0874 | 303.0873 | −0.3 | 271, 163 | * OHC, NZC, NZL, OHC, AHC |
| 32 | (-)-Epigallocatechin | C15H14O7 | 14.222 | ** [M−H]− | 306.0740 | 305.0667 | 305.0674 | 2.3 | 261, 219 | * AHL, NZL, OHP |
| 33 | (-)-Epicatechin | C15H14O6 | 24.211 | ** [M−H]− | 290.0790 | 289.0717 | 289.0722 | 1.7 | 245, 205, 179 | * AHL, OHL |
| 34 | (+)-Gallocatechin 3-O-gallate | C22H18O11 | 25.099 | ** [M−H]− | 458.0849 | 457.0776 | 457.0786 | 2.2 | 305, 169 | * NZL, AHP |
| 35 | Procyanidin dimer B1 | C30H26O12 | 26.498 | ** [M−H]− | 578.1424 | 577.1351 | 577.1338 | −2.3 | 451 | * AHL, OHL, NZL |
| 36 | 4′-O-Methyl-(-)-epigallocatechin 7-O-glucuronide | C22H24O13 | 27.607 | [M−H]− | 496.1217 | 495.1144 | 495.1163 | 3.8 | 451, 313 | AHL |
| 37 | Cinnamtannin A2 | C60H50O24 | 35.444 | ** [M−H]− | 1154.2692 | 1153.2619 | 1153.2629 | 0.9 | 739 | * AHL, NZL |
| 38 | Procyanidin trimer C1 | C45H38O18 | 36.239 | ** [M−H]− | 866.2058 | 865.1985 | 865.2002 | 2.0 | 739, 713, 695 | * AHL, OHL, OHC, NZL |
| 39 | Prodelphinidin dimer B3 | C30 H26 O14 | 42.775 | ** [M+H]+ | 610.1323 | 611.1396 | 611.1397 | 0.2 | 469, 311, 291 | * NZC, AHL, OHL, AHC, AHP |
| Flavones | ||||||||||
| 40 | Chrysoeriol 7-O-glucoside | C22 H22 O11 | 7.398 | ** [M+H]+ | 462.1162 | 463.1235 | 463.1254 | 4.1 | 445, 427, 409, 381 | * AHP, AHL |
| 41 | Apigenin 7-O-glucuronide | C21H18O11 | 15.812 | [M+H]+ | 446.0849 | 447.0922 | 447.0930 | 1.8 | 271, 253 | * AHL, NZL |
| 42 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 25.520 | [M−H]− | 594.1585 | 593.1512 | 593.1489 | −3.9 | 503, 473 | AHL |
| 43 | Cirsilineol | C18H16O7 | 26.744 | [M+H]+ | 344.0896 | 345.0969 | 345.0962 | −2.0 | 330, 312, 297, 284 | AHL |
| 44 | Rhoifolin | C27H30O14 | 42.644 | [M−H]− | 578.1636 | 577.1563 | 577.1583 | 3.5 | 413, 269 | NZC |
| 45 | Apigenin 6-C-glucoside | C21H20O10 | 51.563 | ** [M−H]− | 432.1056 | 431.0983 | 431.0996 | 3.0 | 413, 341, 311 | * AHL, NZL |
| 46 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 51.811 | ** [M−H]− | 448.1006 | 447.0933 | 447.0946 | 2.9 | 301 | * AHL, OHL, NZL |
| Flavanones | ||||||||||
| 47 | Narirutin | C27H32O14 | 4.189 | ** [M−H]− | 580.1792 | 579.1719 | 579.1707 | −2.1 | 271 | * OHL, NZL |
| 48 | 8-Prenylnaringenin | C20H20O5 | 4.431 | [M+H]+ | 340.1311 | 341.1384 | 341.1382 | −0.6 | 323, 137 | * NZC, OHC, NZL |
| 49 | Neoeriocitrin | C27H32O15 | 13.168 | ** [M−H]− | 596.1741 | 595.1668 | 595.1674 | 1.0 | 431, 287 | * OHL, NZL |
| 50 | Hesperetin 3′,7-O-diglucuronide | C28H30O18 | 21.163 | [M−H]− | 654.1432 | 653.1359 | 653.1360 | 0.2 | 477, 301, 286, 242 | AHL |
| 51 | Hesperidin | C28 H34 O15 | 37.033 | ** [M+H]+ | 610.1898 | 611.1971 | 611.1982 | 1.8 | 593, 465, 449, 303 | * NZL, OHP, OHC, AHC |
| 52 | Hesperetin 3′-O-glucuronide | C22H22O12 | 52.673 | [M−H]− | 478.1111 | 477.1038 | 477.1039 | 0.2 | 301, 175, 113, 85 | AHL |
| Flavonols | ||||||||||
| 53 | Myricetin 3-O-rhamnoside | C21H20O12 | 11.810 | ** [M−H]− | 464.0955 | 463.0882 | 463.0893 | 2.4 | 317 | * OHL, OHC, NZL |
| 54 | Myricetin 3-O-galactoside | C21H20O13 | 12.754 | ** [M−H]− | 480.0904 | 479.0831 | 479.0841 | 2.1 | 317 | * OHL, AHC |
| 55 | 3-Methoxysinensetin | C21 H22 O8 | 15.633 | ** [M+H]+ | 402.1315 | 403.1388 | 403.1384 | −1.0 | 388, 373, 355, 327 | * OHL, OHP, OHC |
| 56 | Quercetin 3-O-(6”-malonyl-glucoside) | C24H22O15 | 16.395 | [M+H]+ | 550.0959 | 551.1032 | 551.1020 | −2.2 | 303 | NZL |
| 57 | Patuletin 3-O-glucosyl-(1->6)-[apiosyl(1->2)]-glucoside | C33H40O22 | 24.452 | ** [M−H]− | 788.2011 | 787.1938 | 787.1949 | 1.4 | 625, 463, 301, 271 | NZL |
| 58 | Quercetin 3-O-(6”-malonyl-glucoside) 7-O-glucoside | C30H32O20 | 28.900 | [M+H]+ | 712.1487 | 713.1560 | 713.1561 | 0.1 | 551, 303 | NZL |
| 59 | Kaempferol 3,7-O-diglucoside | C27H30O16 | 29.820 | ** [M−H]− | 610.1534 | 609.1461 | 609.1477 | 2.6 | 447, 285 | * NZL, OHC, OHL |
| 60 | Myricetin 3-O-arabinoside | C20H18O12 | 30.606 | [M−H]− | 450.0798 | 449.0725 | 449.0742 | 3.8 | 317 | AHL |
| 61 | Quercetin 3-O-xylosyl-rutinoside | C32 H38 O20 | 41.157 | ** [M+H]+ | 742.1956 | 743.2029 | 743.1992 | −5.0 | 479, 317 | * NZL, OHL |
| 62 | Quercetin 3-O-xylosyl-glucuronide | C26 H26 O17 | 43.207 | ** [M+H]+ | 610.1170 | 611.1243 | 611.1255 | 2.0 | 479, 303, 285, 239 | * AHL, OHP, AHC |
| 63 | Kaempferol 3-O-xylosyl-glucoside | C26H28O15 | 51.675 | ** [M+H]+ | 580.1428 | 581.1501 | 581.1503 | 0.3 | 419, 401, 383 | * NZL, OHL, AHL |
| 64 | Kaempferol 3-O-(2″-rhamnosyl-galactoside) 7-O-rhamnoside | C33H40O19 | 60.187 | ** [M−H]− | 740.2164 | 739.2091 | 739.2072 | −2.6 | 593, 447, 285 | * NZL, OHL |
| Dihydrochalcones | ||||||||||
| 65 | Dihydroquercetin | C15H12O7 | 12.382 | ** [M−H]− | 304.0583 | 303.0510 | 303.0508 | −0.7 | 285, 275, 151 | * OHC, AHL, NZL |
| 66 | 3-Hydroxyphloretin 2′-O-glucoside | C21H24O11 | 24.659 | ** [M−H]− | 452.1319 | 451.1246 | 451.1249 | 0.7 | 289, 273 | * AHL, OHL, NZC, NZL, OHC |
| 67 | Phloridzin | C21H24O10 | 56.168 | [M−H]− | 436.1369 | 435.1296 | 435.1295 | −0.2 | 273 | * AHL, OHL, NZC, OHP |
| Dihydroflavonols | ||||||||||
| 68 | Dihydromyricetin 3-O-rhamnoside | C21H22O12 | 23.549 | ** [M−H]− | 466.1111 | 465.1038 | 465.1032 | −1.3 | 301 | * AHL, OHL |
| 69 | Dihydroquercetin 3-O-rhamnoside | C21H22O11 | 53.650 | ** [M−H]− | 450.1162 | 449.1089 | 449.1095 | 1.3 | 303 | * AHL, NZL |
| Anthocyanins | ||||||||||
| 70 | Cyanidin 3-O-(6″-p-coumaroyl-glucoside) | C30 H27 O13 | 20.618 | ** [M+H]+ | 595.1452 | 596.1525 | 596.1524 | −0.2 | 287 | * NZL, AHP, OHL, AHL, AHC |
| 71 | Isopeonidin 3-O-arabinoside | C21H21O10 | 22.324 | [M+H]+ | 433.1135 | 434.1208 | 434.1196 | −2.8 | 271, 253, 243 | NZL |
| 72 | Delphinidin 3-O-glucosyl-glucoside | C27H31O17 | 25.952 | [M+H]+ | 627.1561 | 628.1634 | 628.1607 | −4.3 | 465, 303 | NZL |
| 73 | Pelargonidin 3-O-rutinoside | C27 H31 O14 | 26.267 | ** [M+H]+ | 579.1714 | 580.1787 | 580.1775 | −2.1 | 271, 433 | * NZL, NZC, AHC, NZP, AHP, OHC, OHL |
| 74 | Delphinidin 3-O-glucoside | C21 H21 O12 | 45.278 | ** [M+H]+ | 465.1033 | 466.1106 | 466.1113 | 1.5 | 303 | * AHL, NZL |
| 75 | Cyanidin 3,5-O-diglucoside | C27 H31 O16 | 88.305 | ** [M+H]+ | 611.1612 | 612.1685 | 612.1693 | 1.3 | 449, 287 | * AHC, AHL, OHL, NZL |
| Isoflavonoids | ||||||||||
| 76 | 6″-O-Malonylglycitin | C25 H24 O13 | 11.939 | ** [M+H]+ | 532.1217 | 533.1290 | 533.1299 | 1.7 | 285, 270, 253 | * NZL, OHC, OHC |
| 77 | Violanone | C17H16O6 | 12.572 | ** [M−H]− | 316.0947 | 315.0874 | 315.0872 | −0.6 | 300, 285, 135 | * OHL, AHC, OHC, NZL |
| 78 | 3′-O-Methylviolanone | C18H18O6 | 13.790 | [M−H]− | 330.1103 | 329.1030 | 329.1027 | −0.9 | 314, 299, 284, 256 | * OHC, NZC, NZP, AHC, OHL |
| 79 | 5,6,7,3′,4′-Pentahydroxyisoflavone | C15 H10 O7 | 15.260 | ** [M+H]+ | 302.0427 | 303.0500 | 303.0497 | −1.0 | 285, 257 | * AHC, AHL, NZL, OHC, NZL |
| 80 | 2-Dehydro-O-desmethylangolensin | C15H12O4 | 17.749 | ** [M−H]− | 256.0736 | 255.0663 | 255.0657 | −2.4 | 135, 119 | * OHC, OHL, AHP |
| 81 | Dihydrobiochanin A | C16H14O5 | 22.255 | [M+H]+ | 286.0841 | 287.0914 | 287.0925 | 3.8 | 269, 203, 201, 175 | AHL |
| 82 | 6″-O-Malonyldaidzin | C24H22O12 | 22.772 | [M+H]+ | 502.1111 | 503.1184 | 503.1202 | 3.6 | 255 | NZL |
| 83 | 6″-O-Acetylglycitin | C24H24O11 | 23.815 | [M+H]+ | 488.1319 | 489.1392 | 489.1385 | −1.4 | 285, 270 | NZL |
| 84 | 2′,7-Dihydroxy-4′,5′-dimethoxyisoflavone | C17 H14 O6 | 33.008 | **[M+H]+ | 314.0790 | 315.0863 | 315.0849 | −4.4 | 300, 282 | *NZL, OHL |
| 85 | 3′-Hydroxygenistein | C15H10O6 | 33.575 | [M+H]+ | 286.0477 | 287.0550 | 287.0548 | −0.7 | 269, 259 | OHC |
| 86 | 2′-Hydroxyformononetin | C16H12O5 | 37.908 | [M+H]+ | 284.0685 | 285.0758 | 285.0755 | −1.1 | 270, 229 | AHP |
| 87 | 3′-Hydroxydaidzein | C15H10O5 | 42.979 | [M+H]+ | 270.0528 | 271.0601 | 271.0606 | 1.8 | 253, 241, 225 | NZL |
| Other polyphenols | ||||||||||
| Hydroxycoumarins | ||||||||||
| 88 | Scopoletin | C10H8O4 | 7.678 | ** [M−H]− | 192.0423 | 191.0350 | 191.0355 | 2.6 | 176 | * NZP, AHP, OHP, NZL |
| 89 | Esculin | C15H16O9 | 21.132 | [M+H]+ | 340.0794 | 341.0867 | 341.0863 | −1.2 | 179, 151 | * NZL, OHL, AHC |
| 90 | Esculetin | C9H6O4 | 27.267 | [M−H]− | 178.0266 | 177.0193 | 177.0190 | −1.7 | 149, 133, 89 | * OHL, AHL |
| Hydroxybenzaldehydes | ||||||||||
| 91 | p-Anisaldehyde | C8 H8 O2 | 13.770 | ** [M+H]+ | 136.0524 | 137.0597 | 137.0595 | −1.5 | 122, 109 | * AHC, AHL, OHL, NZL |
| Curcuminoids | ||||||||||
| 92 | Demethoxycurcumin | C20H18O5 | 20.648 | [M−H]− | 338.1154 | 337.1081 | 337.1091 | 3.0 | 217 | OHC |
| 93 | Bisdemethoxycurcumin | C19H16O4 | 32.721 | [M+H]+ | 308.1049 | 309.1122 | 309.1126 | 1.3 | 291, 263 | NZC |
| Furanocoumarins | ||||||||||
| 94 | Isopimpinellin | C13H10O5 | 27.757 | [M+H]+ | 246.0528 | 247.0601 | 247.0607 | 2.4 | 232, 217, 205, 203 | * NZL, OHL |
| Phenolic terpenes | ||||||||||
| 95 | Rosmanol | C20 H26 O5 | 10.856 | ** [M+H]+ | 346.1780 | 347.1853 | 347.1853 | 0.0 | 301, 241, 231 | * OHP, OHL |
| Tyrosols | ||||||||||
| 96 | Hydroxytyrosol 4-O-glucoside | C14H20O8 | 20.180 | ** [M−H]− | 316.1158 | 315.1085 | 315.1088 | 1.0 | 153, 123 | * NZC, AHL, AHC |
| 97 | 3,4-DHPEA-AC | C10H12O4 | 25.537 | ** [M−H]− | 196.0736 | 195.0663 | 195.0658 | −2.6 | 135 | * AHL, NZL |
| Other polyphenols | ||||||||||
| 98 | Arbutin | C12H16O7 | 4.148 | ** [M−H]− | 272.0896 | 271.0823 | 271.0824 | 0.4 | 109 | * OHC, NZL |
| Lignans | ||||||||||
| 99 | Enterolactone | C18 H18 O4 | 4.234 | ** [M+H]+ | 298.1205 | 299.1278 | 299.1279 | 0.3 | 281, 187, 165 | * AHL, OHP, OHC |
| 100 | Pinoresinol | C20H22O6 | 11.189 | [M−H]− | 358.1416 | 357.1343 | 357.1331 | −3.4 | 342, 327, 313, 221 | * OHC, AHC |
| 101 | Schisandrin | C24H32O7 | 14.899 | [M+H]+ | 432.2148 | 433.2221 | 433.2221 | 0.0 | 415, 361 | NZP |
| 102 | Episesamin | C20H18O6 | 15.348 | ** [M−H]− | 354.1103 | 353.1030 | 353.1029 | −0.3 | 338, 163 | * NZP, AHC, AHP, NZC, OHL, OHC, OHP |
| 103 | 7-Hydroxymatairesinol | C20H22O7 | 15.773 | [M−H]− | 374.1366 | 373.1293 | 373.1294 | 0.3 | 343, 313, 298, 285 | * NZC, AHC, OHL |
| 104 | 7-Oxomatairesinol | C20 H20 O7 | 27.502 | ** [M+H]+ | 372.1209 | 373.1282 | 373.1296 | 3.8 | 358, 343, 328, 325 | * OHP, OHC, OHL, NZP |
| 105 | Schisandrin C | C22 H24 O6 | 32.561 | ** [M+H]+ | 384.1573 | 385.1646 | 385.1651 | 1.3 | 370, 315, 300 | * NZL, OHP |
| 106 | Schisantherin A | C30 H32 O9 | 37.579 | ** [M+H]+ | 536.2046 | 537.2119 | 537.2115 | −0.7 | 519, 415, 385, 371 | NZL |
| Stilbenes | ||||||||||
| 107 | 4′-Hydroxy-3,4,5-trimethoxystilbene | C17H18O4 | 41.207 | [M+H]+ | 286.1205 | 287.1278 | 287.1275 | −1.0 | 271, 241, 225 | * NZL, NZC |
| TPC | TFT | TTC | DPPH | FRAP | ABTS | FICA | •OH-RSA | RAP | |
|---|---|---|---|---|---|---|---|---|---|
| TFT | −0.614 | ||||||||
| TTC | −0.703 | −0.13 | |||||||
| DPPH | −0.904 | 0.217 | 0.940 | ||||||
| FRAP | −0.885 | 0.911 | 0.291 | 0.600 | |||||
| ABTS | −1.000 * | 0.611 | 0.705 | 0.905 | 0.883 | ||||
| FICA | −0.948 | 0.330 | 0.893 | 0.993 | 0.690 | 0.949 | |||
| •OH-RSA | −0.769 | −0.032 | 0.995 | 0.969 | 0.383 | 0.771 | 0.933 | ||
| RAP | −0.998 * | 0.661 | 0.658 | 0.876 | 0.912 | 0.998 * | 0.927 | 0.729 | |
| TAC | 0.847 | −0.940 | −0.217 | −0.537 | −0.997 * | −0.845 | −0.633 | −0.311 | −0.878 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shi, L.; Qi, Y.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Antioxidative Properties and Phenolic Profile of the Core, Pulp and Peel of Commercialized Kiwifruit by LC-ESI-QTOF-MS/MS. Processes 2022, 10, 1811. https://doi.org/10.3390/pr10091811
Liu Z, Shi L, Qi Y, Barrow CJ, Dunshea FR, Suleria HAR. Antioxidative Properties and Phenolic Profile of the Core, Pulp and Peel of Commercialized Kiwifruit by LC-ESI-QTOF-MS/MS. Processes. 2022; 10(9):1811. https://doi.org/10.3390/pr10091811
Chicago/Turabian StyleLiu, Zifan, Linghong Shi, Yunyang Qi, Colin J. Barrow, Frank R. Dunshea, and Hafiz A. R. Suleria. 2022. "Antioxidative Properties and Phenolic Profile of the Core, Pulp and Peel of Commercialized Kiwifruit by LC-ESI-QTOF-MS/MS" Processes 10, no. 9: 1811. https://doi.org/10.3390/pr10091811
APA StyleLiu, Z., Shi, L., Qi, Y., Barrow, C. J., Dunshea, F. R., & Suleria, H. A. R. (2022). Antioxidative Properties and Phenolic Profile of the Core, Pulp and Peel of Commercialized Kiwifruit by LC-ESI-QTOF-MS/MS. Processes, 10(9), 1811. https://doi.org/10.3390/pr10091811








