Abstract
Research aimed at finding alternative fuels to replace petroleum diesel (petrodiesel) used in controlled combustion engines (CCEs) has identified biodiesel as one of the main candidates, due to its sustainability and potential for use in energy matrices. In this study, the gas emissions from a diesel CCE were investigated, with a focus on total hydrocarbons (THC) and carbon monoxide (CO). Biodiesel (B100) samples derived from the transesterification of frying oil, produced applying conventional chemical catalysis (CC) or non-thermal plasma (NTP) technology, were tested as alternative fuels. Three engine rotation speeds were investigated (900, 1500, and 2500 rpm) and biodiesel samples obtained from the residual frying oil were compared with conventional road diesel (S-500) without biodiesel added, acquired from a gas station. Blends were also prepared with S-500 and B100 obtained applying NTP for 15 or 30 min, in mixes containing 2, 12, 20, and 50% of biodiesel. These blends showed reductions in THC and CO emissions of 62% and 80%, respectively, compared with the emissions for 100% S-500. Thus, biodiesel produced from frying oil offers low emissions of CO and THC, highlighting the potential for reductions using biodiesel produced applying the NTP technology.
1. Introduction
The search for renewable energy sources presents an important challenge to world leaders and is addressed in the UN Sustainable Development Goals of the 2030 agenda [1]. The limited oil reserves and global dependence on fossil fuels has led to the need for solutions to obtain alternative fuels. Biofuels could reduce the environmental impact of combustion gases emitted through the burning of fossil fuels derived from petroleum [2]. The configuration of the energy matrix means that Brazil has significant potential in the world context for the production of renewable energy. In Brazil, 42.9% of the energy originates from biomass, hydro-electric power, and other renewable sources, which compares favorably with the world average of 14% [3]. According to data from the Energy Research Company—EPE, associated with the Brazilian Ministry of Mines and Energy [3], the production of biodiesel B100 (100% biodiesel fuel) in Brazil increased by 24.7% from 2017 to 2018, with the production of 5,350,036 m3 of biodiesel. Biodiesel has great potential to replace petroleum diesel or petrodiesel, being comprised of mono-alkyl esters, obtained from oils of plant and/or animal origin, having properties similar to diesel, and it can therefore be used directly in cyclodiesel engines [4].
Promising results have been observed for the performance, combustion, and emissions of diesel engines running on biodiesel, with little or no modification in the mechanical components, such as valves, piston, compression ratio, and fuel injection system [5,6,7,8]. In addition, biodiesel is associated with low emissions of toxic pollutants, especially sulfur, and is also notable for its biodegradability and the fact that it originates from renewable sources [9].
The production of biodiesel from residual frying oil has been studied based on the conventional process using the transesterification reaction with chemical catalysis (CC), with a view to its application in diesel engines [10]. The technologies developed for the transformation of residual frying oil into biofuels show interesting results, such as a positive environmental impact due to the reuse of residues, and have advanced research on renewable energy sources [11].
The thermal efficiency of fuels based on vegetable oils is equal to or higher than that of petrodiesel, and the temperature of the exhaust gases and the emission values are equal to or better than results for road diesel or petrodiesel. Ref. [12] verified that biodiesel from residual frying oil is a viable alternative to diesel fuel in regions with a cold climate, due to the low density of the combustion gases that provide ignition under these conditions.
Data on carbon monoxide (CO) and total hydrocarbon (THC) emissions in exhaust gases are directly related to the good performance of the engines, as they provide information on the combustion reactions of biodiesel or diesel, the ignition point of the air/fuel mixture in the combustion chamber, and the properties of the mixture. The emission of toxic elements from cargo vehicles with controlled combustion engines (CCEs) in the exhaust gases (EG) has led to research aimed at developing ways to reduce these gas emissions to acceptable levels, mainly with regard to carbon monoxide, carbon dioxide, nitrous oxide, and solid particles [13].
Refs. [14,15] studied the pollutants emitted in the exhaust of diesel engines, highlighting: hydrocarbons (HC)—unburnt fuel; CO—lack of oxygen; and the number of ketones in the combustion mixture.
A study on the use of biodiesel in CCEs showed reductions in exhaust gases of 0.05 to 8.78% for CO, 0.82 to 1.75% for CO2, and 1.19 to 7.49% for THC, along with increased speed through the ducts and eddies in the combustion chamber, improving the stoichiometry of the air/fuel mixture combustível [16,17] observed a reduction in the emissions of CO, HC, and smoke in tests carried out with different mixtures of diesel oil and biodiesel from residual frying oil produced by the transesterification process with CC, in which carbonization of the piston and test engine valves was not observed. A reduction in emissions of CO, THC, and smoke was observed for biodiesel mixtures compared with 100% diesel; however, the NOx emissions increased.
The need to find sustainable technologies for the production of alternative fuels to replace petroleum diesel has led to studies on the production of biofuels using innovative approaches, such as the application of non-thermal plasma (NTP) technology. This technology has been shown to be promising due to the shorter reaction time, easier product separation, and reduced glycerin formation compared to the conventional technology through transesterification with CC [18].
NTP technology consists of the application of an intense electric field that leads to electronic self-propagation, with the ionization of the gas generating free radicals, including reactive oxygen species (ROSs), reactive nitrogen species (RNS), and ions [19]. These species act as catalysts for the transesterification reaction, accelerating the reaction and consequently reducing the biodiesel production time. The time gained with the use of NTP in synergy with heterogeny catalysts impregnated with carbon powder and active carbon, coating the reactor wall, can reach 92.39% in the production of biodiesel, along with a considerable reduction in residues such as glycerol [20,21,22].
Studies of possible reactions of the non-thermal plasma térmico [18] suggest that the high-energy atmosphere of plasma facilitates the breakage of the double bonds and hydroxyl groups. Besides the formation of the methylated esters, there is the formation of H2O present in a small percentage of methanol, which does not react with triglyceride and appears with biodiesel formed in the end of the process. Ref. [23] suggested the case of compounds having aliphatic hydrocarbons such as waste cooking oil, in which the hydrogen abstraction is the primary mechanism for producing methylated esters and water.
Biodiesel produced applying the NTP technology has some characteristics that are superior to those of biodiesel produced with CC, such as lower viscosity, lower glycerin generation, and less waste products [18,22] noted that the production of biodiesel using plasma can reduce the particulate material to one tenth of that in biodiesel obtained by CC. NTP acts as a catalyst for the transesterification reaction through active species (positive ions and radicals) and UV light generated in the plasma jet through a dielectric barrier, enabling the use of high-acid vegetable oils for the production of biofuels with parameters similar to commercial biodiesel [9]. Table 1 shows the main findings of the literature on the production of biodiesel by plasma.

Table 1.
References about the production of biodiesel by plasma.
The aim of this study was to demonstrate the benefits, in terms of reduced THC and CO emissions from compression combustion engines (CCE), of using biodiesel produced by NTP. The biodiesel was produced from used residual frying oil employing NTP technology, and engine speeds of 900, 1500, and 2500 rpm were applied in the tests. For comparison purposes, the same tests were performed using biodiesel produced by chemical catalysis and conventional road diesel S-500.
2. Materials and Methods
2.1. Biodiesel Production
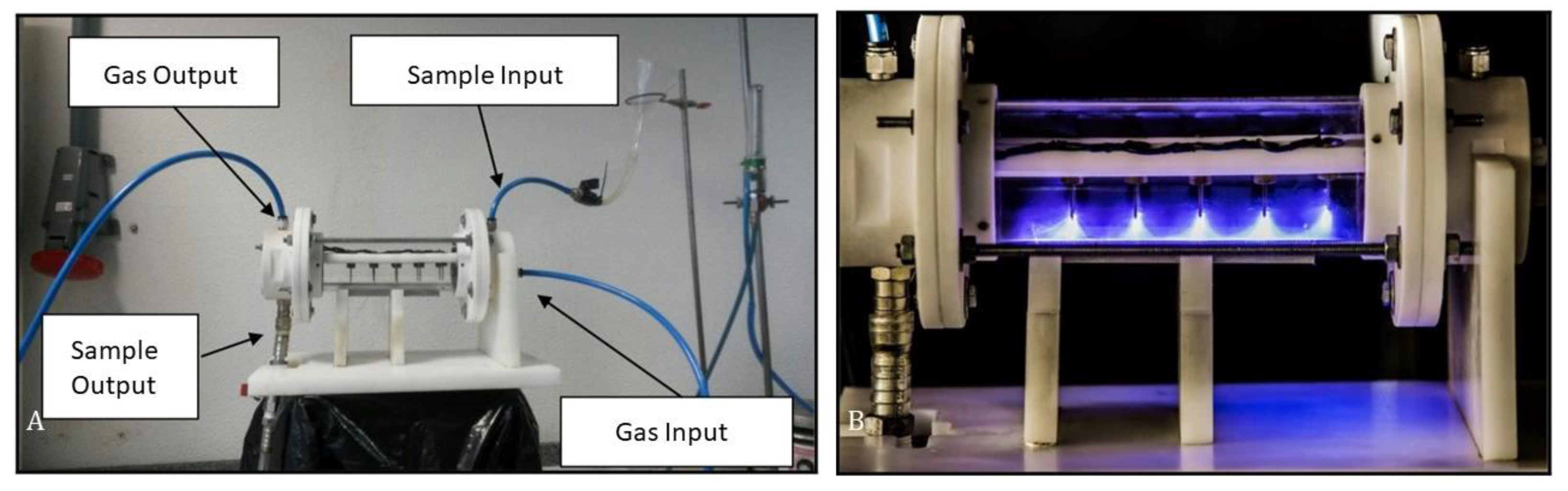
The biodiesel samples produced by NTP technology were obtained from a mixture of pre-treated (heating and filtration) frying oil obtained from the university restaurant at the University of Southern Santa Catarina State (UNISUL), SC, Brazil. The following reagents were used for the plasma formation: KOH catalyst (85%; from Supelco–Germany), methanol (99.8%; Audaz Brasil), and argon gas (99.99%; from White Martins–Brazil). The mixture was placed in the NTP reactor with a capacity of 500 mL of sample (Figure 1), with a dielectric barrier formed by tungsten electrodes in a cylindrical geometry and an aluminum electrode in the form of a plate, with a hybrid gas–liquid discharge at atmospheric pressure. A high voltage (±17 kV) and 30 mA alternating current (AC) source were used to generate the plasma [18].
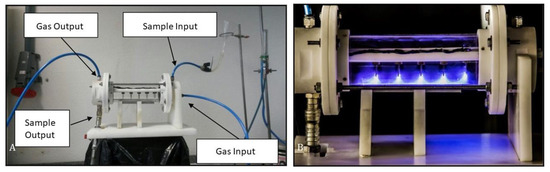
Figure 1.
Non-thermal plasma reactor ((A)—NTP system; (B)—NTP in operation). Source: The authors.
Biodiesel was produced applying two different NTP treatment times. Based on previous experiments and reports in the literature, the best NTP times have already been determined in previous work by our team [25]: 15 min (B100-NTP15) and 30 min (B100-NTP30). For comparison, biodiesel was also produced by CC (B100), with the proportions of 1:6 oil:methanol and 0.75% KOH in relation to the oil mass, with heating at 50 °C under stirring for 2 h [26]. In both processes, the glycerol was separated, and the biodiesel was purified applying the heated water washing process to remove excess KOH and salt (NaCl) present in the oil due to its prior use for food frying.
The engine tests were carried out with mixtures of the biodiesel and automotive diesel (S-500) in different proportions. This diesel, known as common diesel, contains a maximum sulfur content of 500 mg/kg with a cetane number of at least 42 and a reddish color. Different volumetric mixtures (v/v) were prepared under constant stirring at room temperature for use in the engine tests as follows:
- Biodiesel produced by CC (B100)—100% biodiesel
- Biodiesel produced through CC added to common diesel in the proportions (v/v): 2% (B2), 12% (B12), 20% (B20), and 50% (B50) of biodiesel.
- Biodiesel produced by NTP with 15 min of treatment (B100-NTP15) added to common diesel in the proportions (v/v): 2% (B2-NTP15), 12% (B12-NTP15), 20% (B20-NTP15), 50% (B50-NTP15), and 100% (B100-NTP15) of biodiesel.
- Biodiesel produced by NTP with 30 min of treatment (B100-NTP 30) added to common diesel in the proportions (v/v): 2% (B2-NTP30), 12% (B12NTP30), 20% (B20-NTP30), 50% (B50-NTP30), and 100% (B100-NTP30) of biodiesel.
- Common diesel—S-500 (100% petroleum derived diesel, with no additives and without the presence of biodiesel).
Analysis of Biodiesel Produced
Physical-chemical analysis to determine the acidity and saponification index was performed on samples of biodiesel produced by the NTP process (B100-NTP15 and B100-NTP30) and on biodiesel produced through CC (B100), according to the [27] methods n.940.28 for the acidity index and n.920.16. for the saponification index.
Fatty acids were quantified by gas chromatography using a gas chromatograph, model GC2014 (Shimadzu, Kyoto, Japan), equipped with a flame ionization detector, a capillary column (length—105 m; ID = 0.25 mm) filled with 0.25 mL of 10% cyanopropyl phenyl and 90% biscanoane propylsiloxane. The injector and detector temperatures were both 260 °C. The oven temperature was initially 140 °C (5 min), followed by ramping at 2.5 °C min−1. The qualitative composition was determined by comparing the peak times with those for the respective fatty acid standards (Sigma, St. Louis, MO, USA). The quantitative composition was obtained by normalizing the area and expressed as percentage of mass, aimed at qualitatively evaluating the use of residual frying oil for the production of biodiesel.
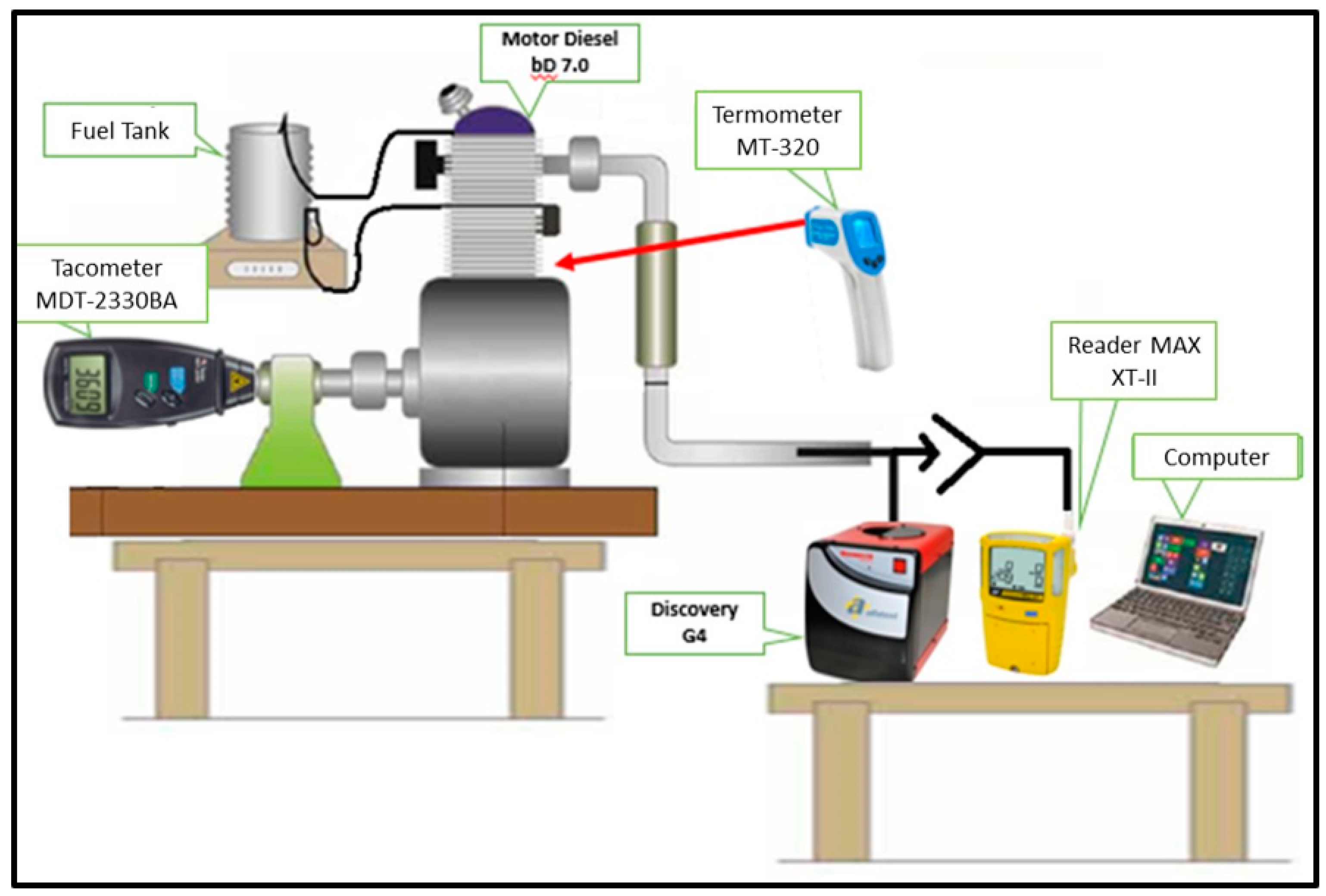
2.2. Test Bench and Instrumentation
The test bench (Figure 2), was composed of a Discovery G-4 vehicle analyzer coupled to a computer with Discovery G-4 software, a Gas Alert MAX XT-II gas detector, Minipa MDT-2338 A digital tachometer, infrared thermometer (model MT-320 A) and a WHITE diesel engine (model BD-7.0, G2), fixed by means of vibration absorbing dampers to allow changes in the idle speed (900 rpm), intermediate speed (1500 rpm), and high-speed rotation (2500 rpm).
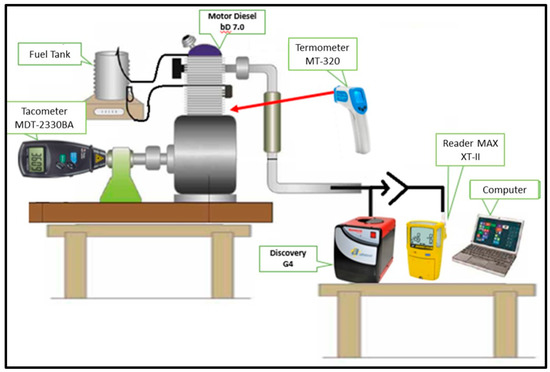
Figure 2.
Test bench and instrumentation.
2.2.1. Discovery G-4 Vehicle Analyzer
The Discovery G-4 vehicle gas analysis equipment was connected via an exhaust probe inserted directly into the engine. The MAX XT-II analyzer/detector was located at a distance of 1 m from the exhaust outlet, as recommended by ABNT NBR 7027, in accordance with CONAMA Resolution 315/2002, under the test conditions to measure CO emissions from heavy and loaded diesel vehicles. The stability of the engine working temperature was monitored with the infrared thermometer (MT-320 A). Figure 2 shows the layout of the test bench and instrumentation used.
The arrangement of the equipment, the instrumentation, and the data collection regime adhered to the recommendations of ABNT NBR 7027 to ensure the reliability of the results.
2.2.2. Diesel or Biodiesel Engine—WHITE BD-7.0 (G2)
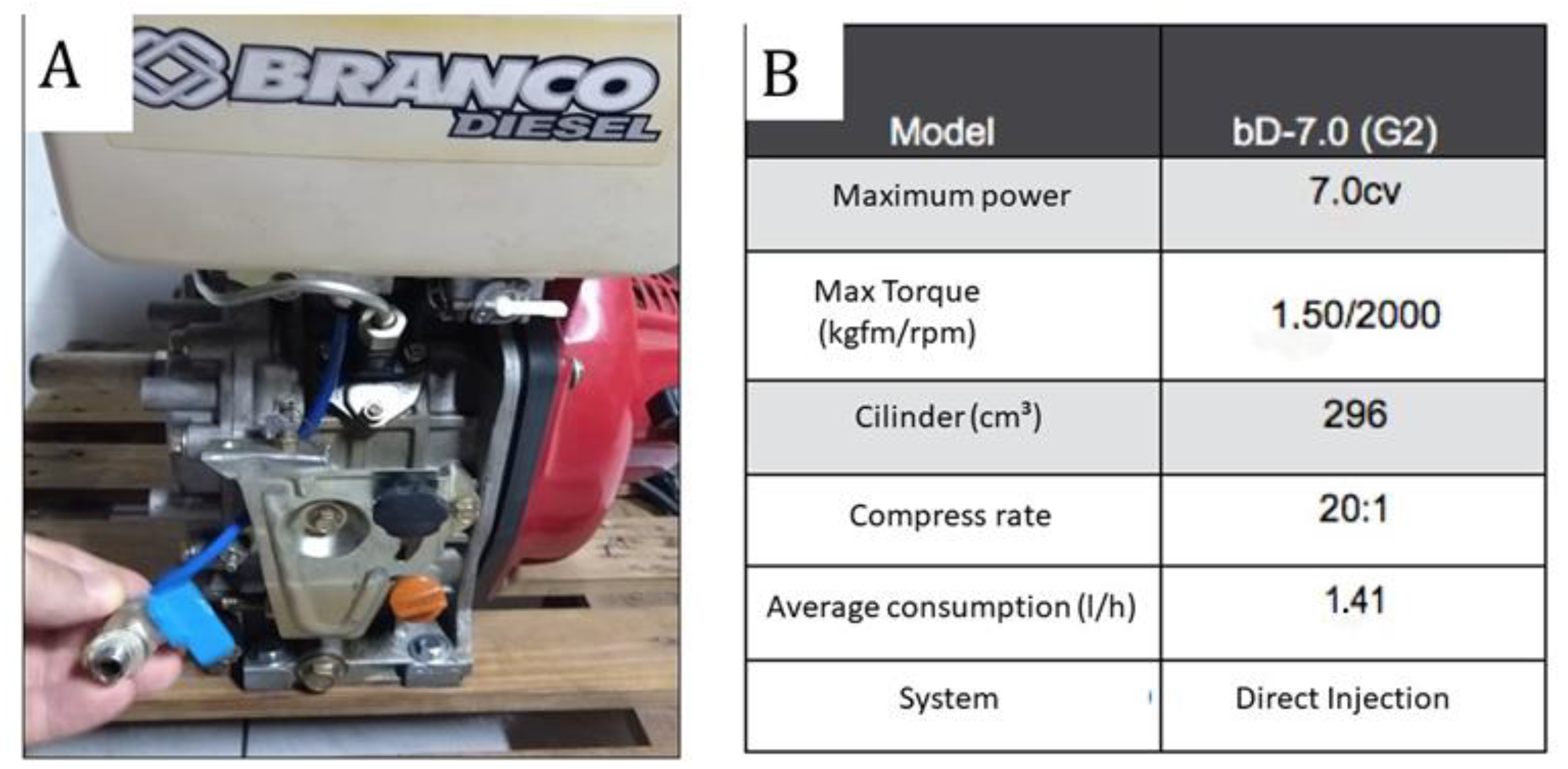
Emission tests were carried out on gases emitted from a diesel or biodiesel engine (WHITE, model BD-7.0, G2- Brasil) (Figure 3), with a mono horizontal cylinder, 12-volt electric start, and air ventilation. Figure 3B shows the technical specifications of the engine. The engine fuel tank was modified with the installation of a drain valve and fuel change check.
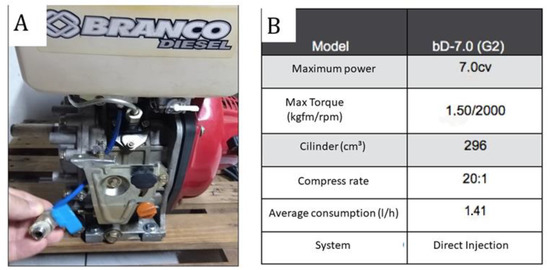
Figure 3.
(A)—Photograph of the engine; (B)—Technical specifications of the engine (source: the authors).
2.2.3. Vehicle Gas Analyzer for THC Determination (% v/v)
The content of total hydrocarbons (THC) in the emissions result from the burning of samples of diesel and biodiesel: diesel mixtures with different proportions were determined with the gas analyzer (Discovery G4—Alfatest Brasil). The results are given as the THC originating from unburned fuel in the combustion process of the engine. The gases were captured by a probe inserted in the discharge duct, separated from the water by means of filters and directed to the infrared measurement bench at specific wavelengths from 3.0 × 1012 to 4.2 × 1014 Hz. The equipment was connected via USB to data acquisition and storage software compatible with Windows 07 or Windows XP operating system.
2.2.4. Gas Alert MAX XT-II Gas Analyzer and Detector
The gas detector/analyzer Gas Alert MAX XT-II (BW Technologies) is a portable device that monitors up to four hazardous gases (H2S, CO, O2, and LEL) by suction from ambient air. This instrument detects levels (ppm) of toxic gases dispersed in the environment, providing greater precision with the dilution of the toxic agent.
The Gas Alert MAX XT-II was also used for more accurate CO readings (in ppm) by dilution, considering that the values provided by the Discovery G4—Alfatest are related to the percentage by gas and exhaust volume, a value that shows little variation with changes in the proportion of S-500 fuel and biodiesel mixtures. The sensitivity and detection at ppm level for CO make it a useful tool in conjunction with the Discovery G4—Alfatest.
2.2.5. Measurement of Engine Speed and Temperature
The engine speed was measured using the digital tachometer (Minipa MDT-2338A), composed of a dedicated microprocessor with a crystal time base and a wide measurement range from 0.5 to 20,000 rpm (contact mode) and from 2.5 to 100,000 rpm. The acceleration was performed manually until stable rotation was obtained.
The motor temperature was measured with an infrared thermometer (MT-320A) that monitors the infrared energy radiated in the spectrum of 8–14 μm, with an operating temperature range of −50 °C to ~580 °C.
2.3. Emission Testing
The filling of the tank was performed by opening the motor feed register and manually activating the air removal from the feed ducts of the injection pump and fuel injection nozzle. A waiting period for temperature stabilization (average 3 min) was then applied. This is also the time required for the warm-up of the Discovery G-4 analyzer and the zeroing of the MAX XT-II, which emits visual and audible signals. With the MT-320 thermometer, temperature stability was monitored, and after the respective times had passed the rotation was adjusted with the MDT-2338A tachometer. In the next step, the readings were triggered with the insertion and positioning of the respective probes, and the CO (ppm) and THC (%, v/v) emissions were recorded as average values.
The time required to change the fuel was sufficient for the engine to cool. When restarted, during the reheating the Discovery G-4 was warmed up, the MAX XT-II was zeroed, and other readings were taken at an average speed of 2000 rpm, which is needed for the elimination of residual fuels from previous measurements, a volume of 4.5 mL being sufficient for the exhaust gas stabilization.
The fuel tests were conducted firstly with the S-500 fuel, and then the samples were prepared with the biodiesel obtained by CC (B2, B12, B20, B50, and B100).
The procedure was then conducted with the samples prepared with the biofuel obtained applying NTP for 15 min (B2-NTP15, B12-NTP15, B20-NTP15, B50-NTP15, and B100-NTP15) and applying NTP for 30 min (B2-NTP30, B12-NTP30, B20-NTP30, B50-NTP30, and B100-NTP30).
The time applied for each fuel test was 900 s. During the first 240 s, the engine remained at 900 rpm, from 300 to 540 s the rotation was 1500 rpm, and from 600 to 900 s the engine was maintained at 2500 rpm. The speed was varied to investigate the response of the engine performance to acceleration and to meet the air demand of the engine. The variations in the emissions were observed after a stabilization period of 60 s. All tests were performed in quadruplicate for each speed range.
The MT-320 thermometer was used to check the engine temperature stability at each rotation based on the average temperature at idle speed (900 rpm) from 73 °C to 82 °C at intermediate speed (1500 rpm) and at 94 °C at high speeds (2500 rpm). The temperature was checked during cold starts, the heating ramp, and at each fuel change, so that the engine speed and temperature stability regime was standardized equally for each fuel tested.
2.4. Investigation of Stabilizing Action of NTP
The reduction in the levels of gaseous emissions in the exhaust of a CCE, commonly associated with the use of antioxidants and additives applied in biodiesel and biodiesel:diesel mixtures, was also investigated.
The observed reductions in CO and THC emissions compared with common diesel reflect differences in the composition of biodiesel produced from vegetable and animal oils with additives. Since the biodiesel investigated in this study was produced from residual frying oil and tested without additives, the results obtained for the emissions of the biodiesel produced by NTP provide information on the stabilizing and antioxidant action of NTP technology applied to biodiesel production.
3. Results and Discussion
3.1. Characterization of Biodiesel Produced Applying NTP and CC
The physico-chemical characteristics of the biodiesel samples produced by the CC method (B100) and applying NTP technology (B100-NTP15 and B100-NTP30) are shown in Table 2. The acidity index of the biodiesel produced is within the standard established by the Brazilian regulatory agency (ANP) in Resolution nº 45 of 08/25/2014, which is 0.5 mg KOH g−1, guaranteeing the lubricity conditions of the biodiesel.

Table 2.
Physico-chemical analysis results for biodiesel samples produced by CC and NTP.
The acid value indicates the oxygen content of the biodiesel. ANP Resolution No. 30, of 06/23/2016, which provides the official specifications for diesel oil BX to B30, establishes a limit of 0.3 mg KOH g−1 for fuels S10, S500, and S1800. In Table 1, we can see that this index varied from 0.378 mg KOH g−1 (B100-CC) to 0.298 KOH g−1 (B100-NTP15) and 0.300 mg KOH g−1 (B100-NTP30), remaining within the above-mentioned limit and evidencing the improvement in the indicators of free fatty acids with the use of the NTP technology.
The saponification index indicates the number of saponifiable units (acyl groups) per unit weight of oil. A high saponification index indicates a higher proportion of low molecular weight fatty acids in the oil [28]. It also indicates the degree of oil unsaturation (biodiesel raw material), which is an important parameter, because as it increases the fuel stability decreases [29]. This index is used to measure the average molecular weight of the oil, and ANP Resolution No. 45 does not establish a standard for this characteristic.
The content of methyl esters (%, v/v), shown in Table 1, was higher when the biodiesel was produced by the CC process, adhering to the standard established in ANP Resolution No. 45 of 96.5% (v/v), while the biodiesel obtained applying NTP for 15 and 30 min provided lower levels, with reductions of 32.70% and 41.19%, respectively. Studies show that the species formed by the plasma are unstable [22] and the breakage of double bonds and hydroxyl groups can be reversible, that is, the increase in the time of the samples in the plasma reactor can cause the esters to formed methylates reverted to aliphatic hydrocarbons.
The results obtained in the chromatographic analysis of the biodiesel samples B100, B100-NTP15, and B100-NTP30 are reported in Table 3, based on the definitions of methyl/ethyl esters of fatty acids highlighted in the characterization according to ANP Resolution No. 45 of 2024.

Table 3.
Properties of biodiesel samples-indices of methyl/ethyl esters of fatty acids.
The fatty acid composition of the biodiesel samples includes the following fatty acids: palmitic acid with 16 carbons and stearic acid with 18 carbons (saturated fatty acids), linoleic acid with 18 carbons with 2 unsaturations (polyunsaturated fatty acid), and the oleic acid (monounsaturated fatty acid).
Ref. [29] noted that as the length of the carbon chain of the alkaliester increases, so does the density (until reaching stability), and an increase in the number of unsaturations is associated with a decrease in density. The values for palmitic and oleic acids (saturated fatty acids) are lower compared to those for the biodiesel obtained by CC. With regard to the NTP methods (15 and 30 min), there were high levels of linoleic acid (polyunsaturated fatty acid) and oleic acid (monounsaturated fatty acid) for the B100-NTP15 biodiesel. Biodiesel samples with higher levels of mono and polyunsaturated fatty acids have lower viscosity and greater fluidity.
The viscosity of the fuel, influenced by the presence of free fatty acids, had an effect on the cold start conditions of the BD 7.0 engine. The mixtures B2, B12, B20, B2-NTP15, B12-NTP15, B20-NTP15, B2-NTP30, B12-NTP30, and B20-NTP30 required less time to start up, due to the lower viscosity of the fuel and the higher number of ketones in the S-500, facilitating the start of ignition in the combustion chamber of the CCE when compared to the biodiesel produced by the CC process. Mixtures with more fatty acids or a higher level of degradation, which in turn are more viscous, hinder cold starting, requiring more rotations until ignition [15].
3.2. GCC Pollutant Emission Analysis
3.2.1. Emission of CO in Exhaust
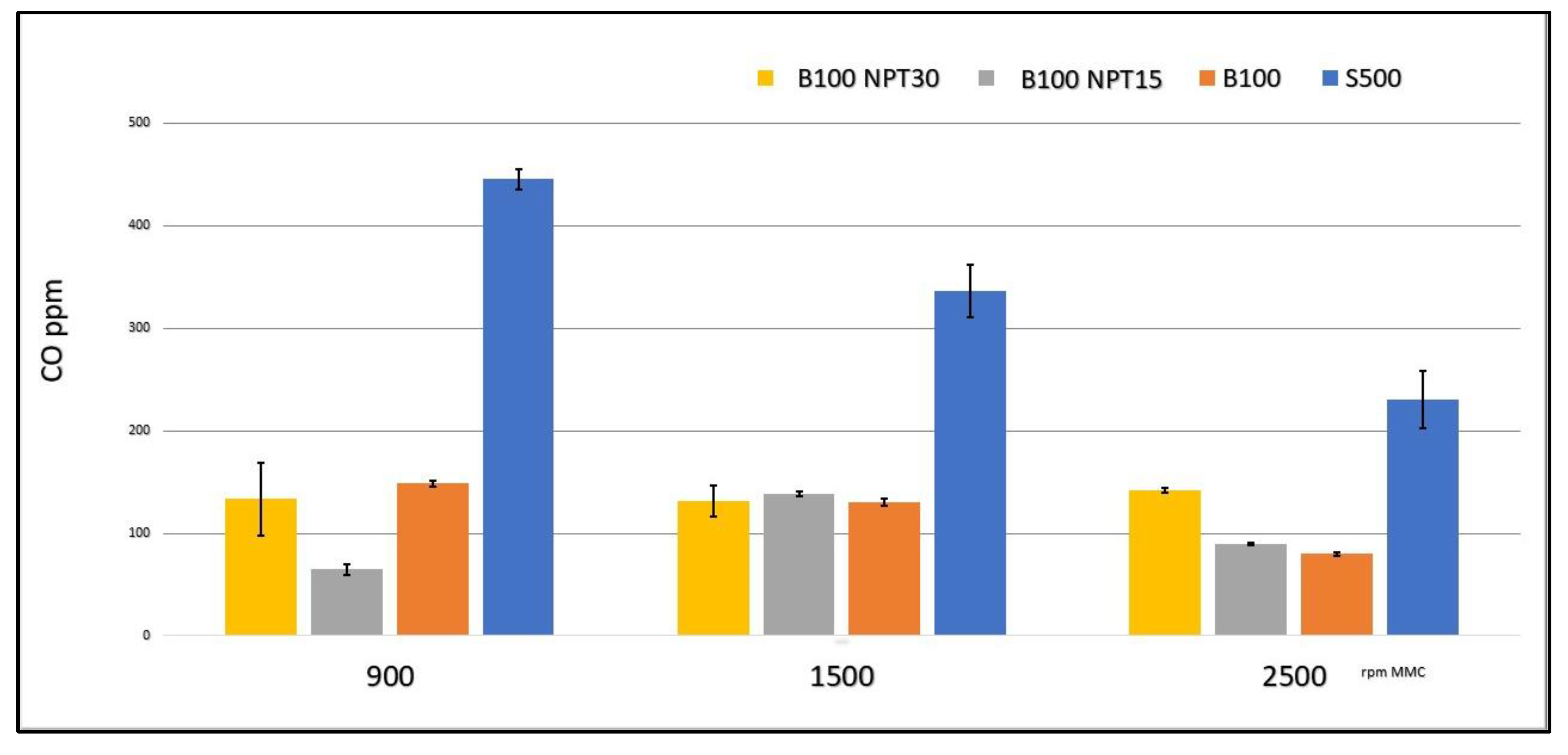
The emission of CO (ppm) was much lower with the use of the B100 biodiesel when compared to the value using the S-500 diesel, according to the results reported in Figure 4 for each engine speed.
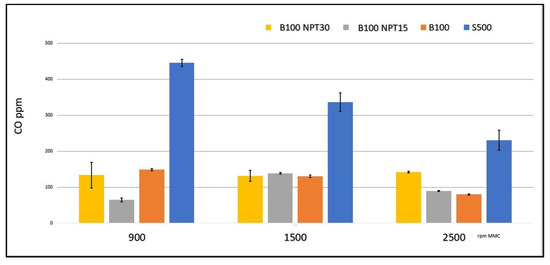
Figure 4.
CO emissions (ppm) during fuel burning (S-500, B100-NTP15, and B100-NTP30) at different engine speeds (rpm).
The greatest reductions in the values for the CO emission in the tests were obtained with B100 and B100-NTP30 at 900 rpm, with a reduction of approximately 77% and 80%, respectively, when compared to the CO value for the S-500 at the same speed. A 61% reduction at 1500 rpm was observed for both of these fuel samples, and reductions of 65% and 48% at 2500 rpm were observed for the biodiesel samples B100 and B100-NTP30. Ref. [30] reported reductions of around 35% in CO emissions using biodiesel produced from residual frying oil using the CC method compared with common diesel.
The B100-NTP15 biodiesel showed even greater reductions than B100 and B100-NTP30. The engine speeds of 900 rpm and 2500 rpm did not result in favorable emissions. The improvement in the reduction of CO emissions, with the use of B100 biofuels obtained by applying the technology at the NTP for 30 min, was from 80% to 48% in relation to the use of S-500 in the CCE.
The low level of CO in CCE emissions using biodiesel is due to the power delivery at low engine speeds [13], where the higher viscosity of the S500 shows an enrichment, causing high CO emissions for common diesel.
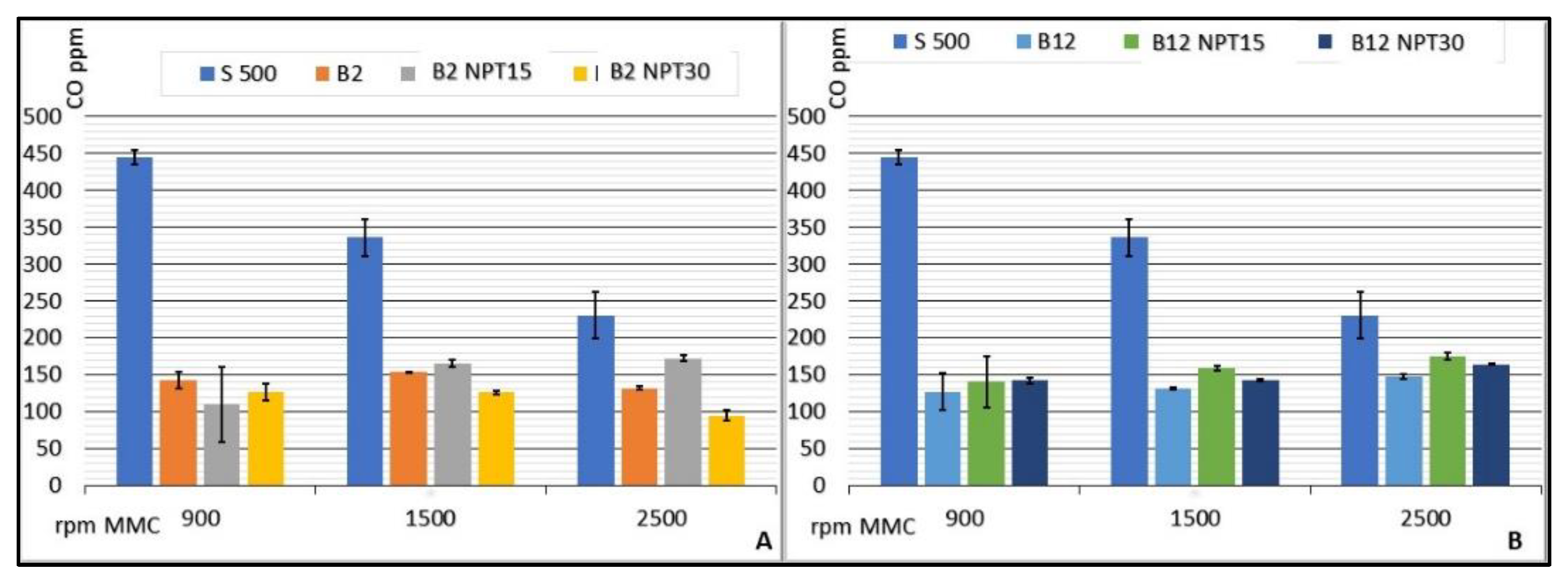
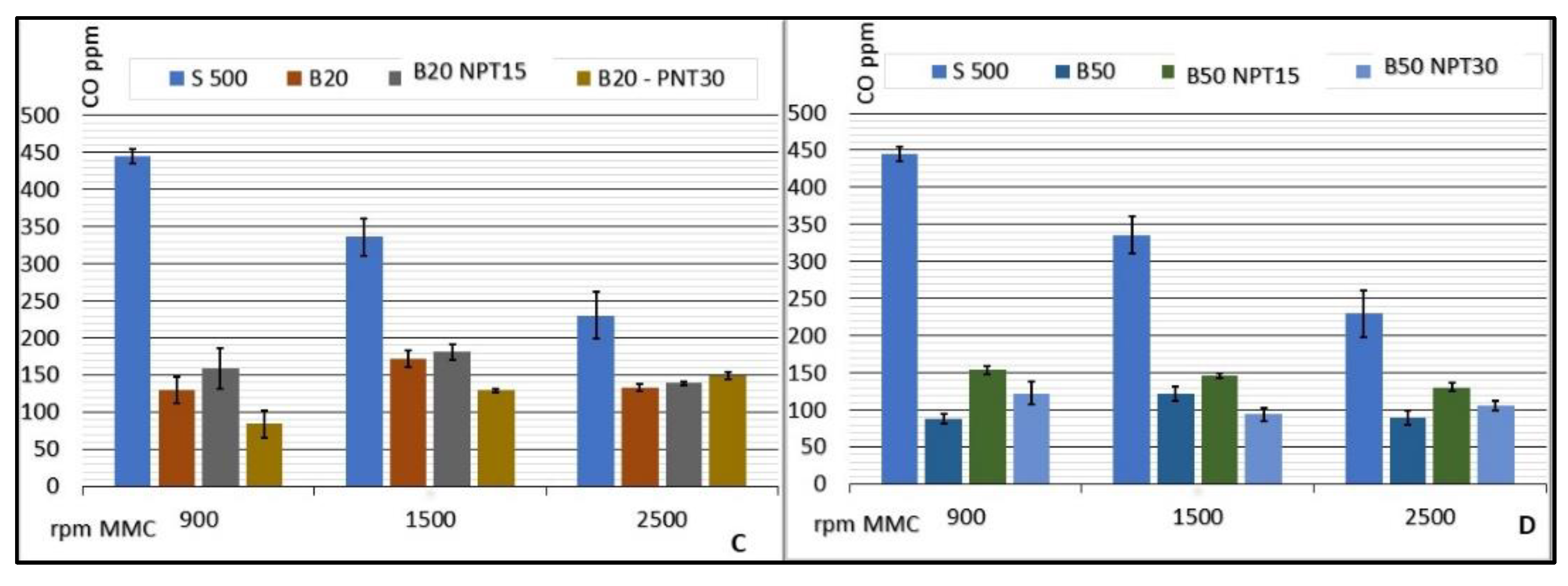
The investigation of biodiesel: diesel mixtures demonstrated a significant reduction in the CO emissions compared with 100% common diesel, presenting stable values with minimum emission for the CCE running at 900 rpm using B50 (87.50 ± 6.45 CO ppm) and maximum emission for B20-NTP15 at 1500 rpm (181.20 ± 10.76 CO ppm). Figure 5 lists the rates of CO emission (ppm) for CCE rotations of 900, 1500, and 2500 rpm, considering the average values for each rotation speed. The value obtained for S500 is used as a reference, since this fuel is processed adhering to the standards required by the ANP and sold as petrodiesel without the addition of biodiesel.
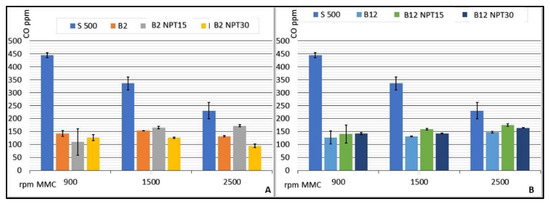
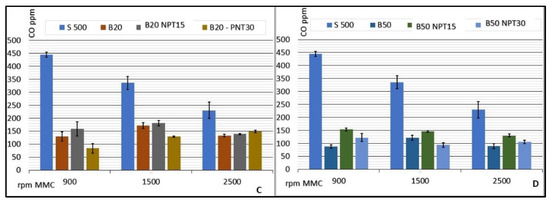
Figure 5.
Results for CO emissions (ppm) from the CCE for biodiesel added to common diesel in the proportions of 2% (A), 12% (B), 20% (C), and 50% (D) for biodiesel obtained by CC, and for B100-NTP15, B100-NTP30, and S-500 at different engine speeds (rpm).
Similar reductions in CO emissions were observed for the biodiesel samples obtained using the NTP technology with increased rotation and air demand in the CCE. Sample B2-NTP30 showed emissions of 126.50 ± 11.09, 126.00 ± 2.83, 95.20 ± 7.43 CO ppm, and B50-NTP15 provided values of 154.25 ± 5.56, 146.80 ± 3.35, 131.00 ± 5.92 CO ppm at rotations of 900, 1500, and 2500 rpm, respectively. Ref. [31] reported that the reduction in CO emission (in ppm) for a CCE running on biodiesel:diesel mixtures was 35% on average when compared to petrodiesel.
The indices obtained in the study reported herein show that, in addition to a reduction in CO emission in the combustion gases of the CCE, the production of biodiesel by NTP guarantees stability in the reduction with an increase in the engine rotation, as observed for a standardized fuel used in the CCE.
The viscosity and the number of ketones following the application of NTP contribute to the reduction in CO emissions with increased demand on the engine and, as reported by [32], this behavior is related to the quantitative degree of unsaturation of the biodiesel esters. According to [5], biodiesel containing fatty acids with a greater number of unsaturations, for instance, 3 unsaturations per fatty acid molecule, has a higher viscosity, a lower number of ketones, a longer ignition period, and leads to higher levels of HC, CO, and smoke emissions. The biodiesel samples produced by the CC and NTP methods do not present fatty acids with a high degree of unsaturation (i.e., >2), and this can also explain the low CO emissions.
3.2.2. Total Hydrocarbons (THC) in the Exhaust Gas
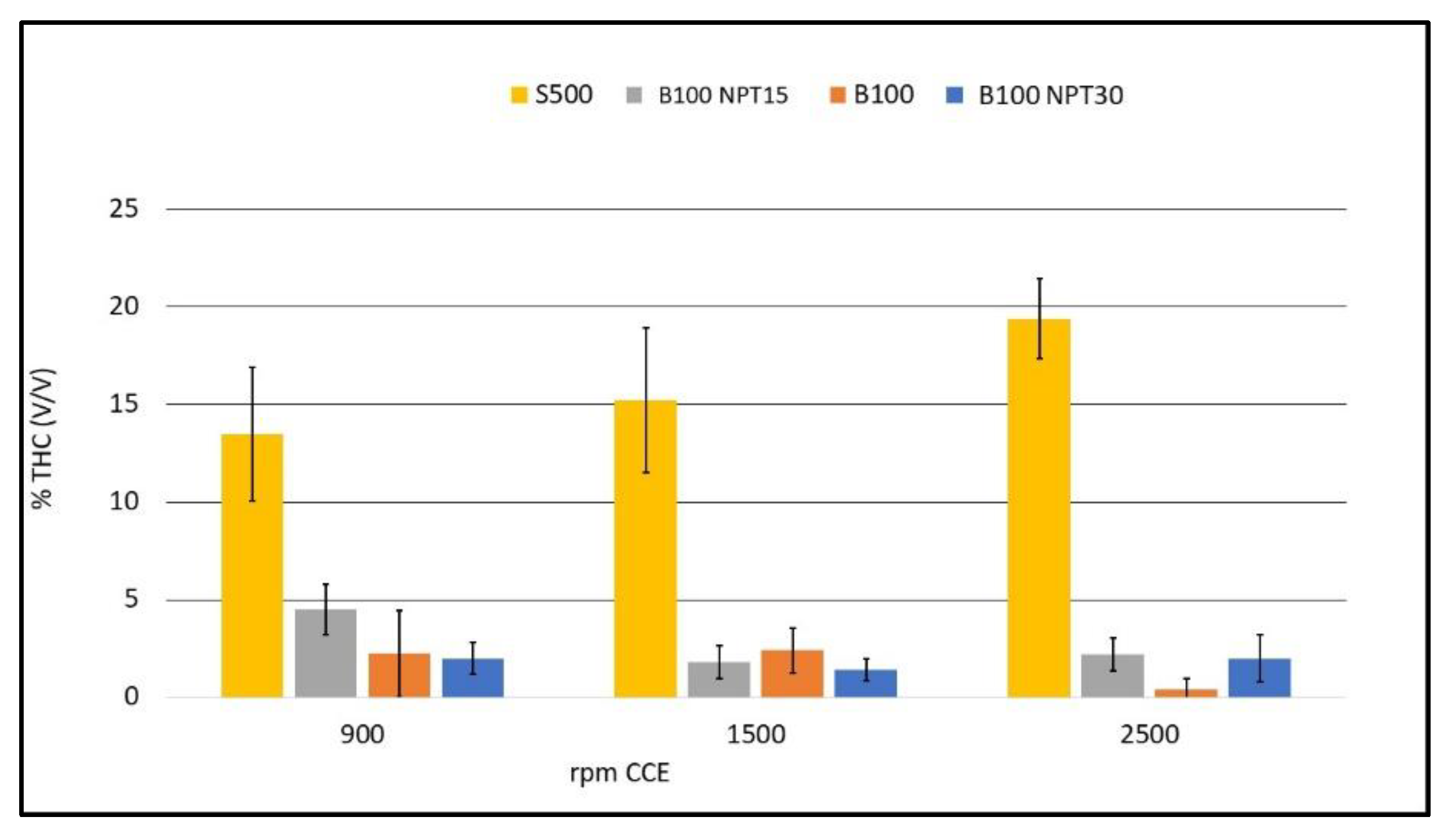
The low levels of THC in the burning gases of biodiesel are associated with reductions in NOx and CO, resulting from the adequate burning time of the mixture [17]; DIESEL, 2018). These values are determined by residues of unburned fuel or enrichment of the mixture injected into the combustion chamber, with more biodiesel than air. Since the biodiesel produced by NTP has a low level of residual glycerol, which indicates a greater predominance of monounsaturated fatty acids, it is expected to have a better performance [25].The level of THC is high with the use of S-500 in the CCE due to the low viscosity when compared to the biodiesel samples B100, B100-NTP15, and B100-NTP30. Figure 6 shows the THC values in % (v/v) for the fuels B100, B100-NTP15, and B100-NTP30, and S500 for CCE rotation speeds of 900, 1500, and 2500 rpm. It can be observed that during combustion, the biodiesel samples generate lower THC emissions.
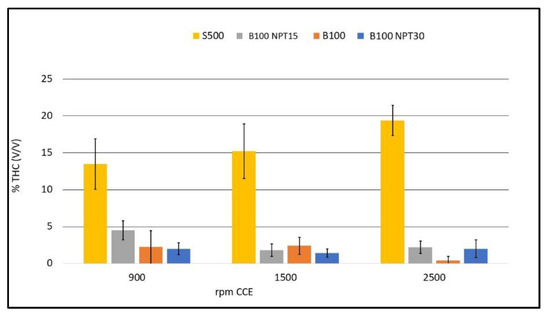
Figure 6.
Emissions of THC (%, v/v) with different engine rotations (rpm) for the fuels B100, B100-NTP15, B100-NTP30, and S500.
The use of NTP technology in the production of biodiesel from residual frying oil reduces the THC emission rate from 13.5 ± 3.41% to 2 ± 0.82% (v/v) at 900 rpm, from 15.2 ± 3.70% to 1.4 ± 0.55% at 1500 rpm, and from 19.4 ± 2.07% for 2 ± 1.22% at 2500 rpm, with the use of B100-NTP30 compared to S-500.
The low value of 0.4 ± 0.55 THC% at 2500 rpm for biodiesel B100 can be attributed to the high viscosity of biodiesel produced by transesterification with CC. High viscosity hinders the fluidity in the fuel injection system, leading to a poor air to fuel ratio (low proportion of fuel). The influence of the NTP, providing a reduction of saturated fatty acids and, consequently, lower viscosity of the fuel, guarantees better combustion efficiency [25]. The low THC emissions for fuels obtained using NTP leads to better efficiency with their use, while the peak reduction for biodiesel produced by CC (B100) indicates low energy efficiency due to the mixture being depleted in biofuel. The use of more viscous biofuels is associated with low energy efficiency, and the axle power required will not be delivered. Additionally, there will be a considerable increase in NOx emissions [16]. The lowest THC emission value was obtained with the use of B12-NTP15 (0.25 ± 0.50%), showing the best burning efficiency at 900 rpm (idling speed) and the best burning time for the mixture.
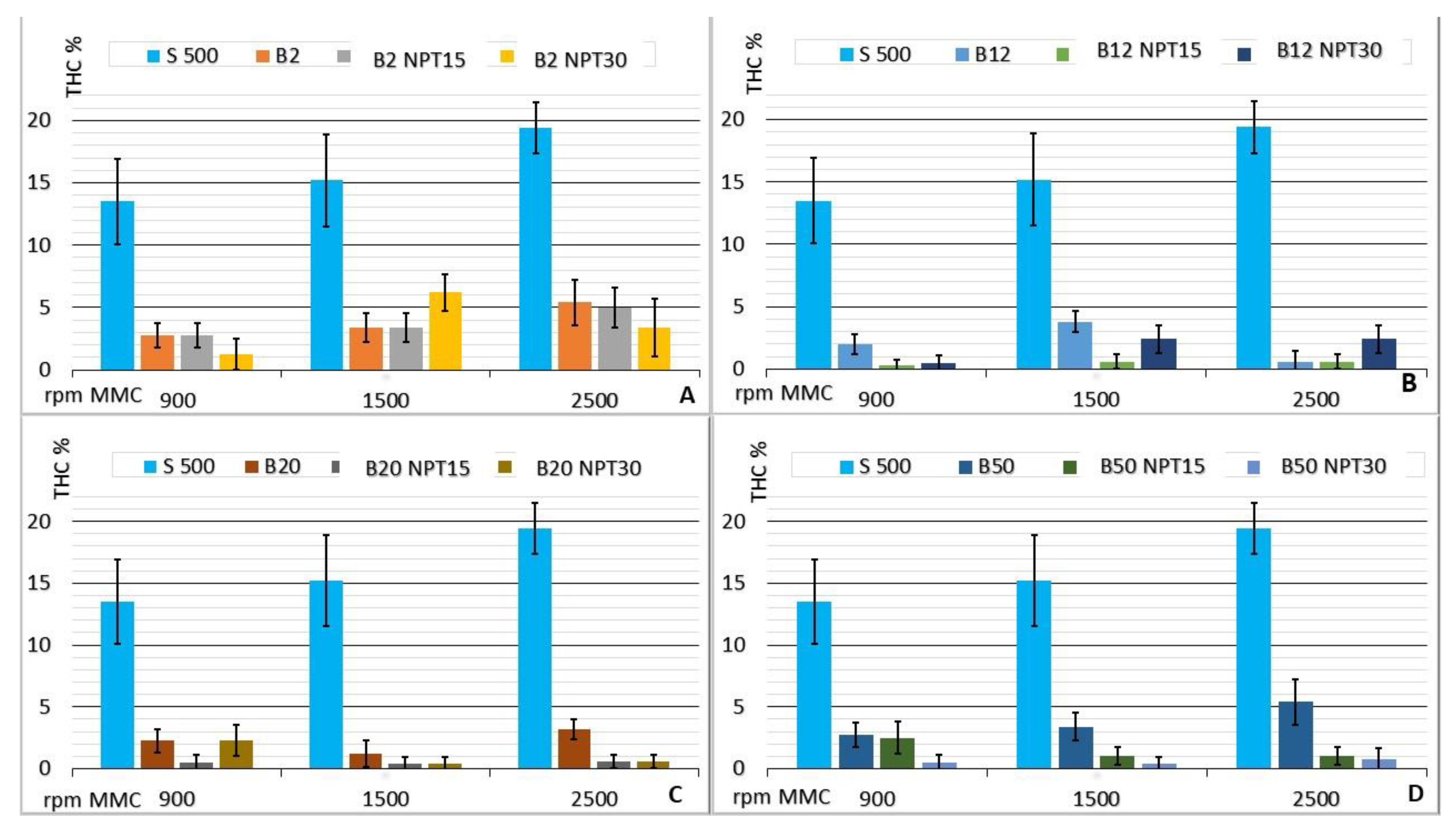
The behavior of the CCE with mixtures of biodiesel produced by the NTP and CC processes in comparison with the use of common diesel (or petrodiesel) is shown in Figure 7, where the average values for the THC emissions (% v/v) at 900, 1500, and 2500 rpm are reported. The use of plasma promotes reductions in the THC emission (%). It can be observed that, in general, lower THC values were obtained for mixtures with biodiesel produced by NTP.
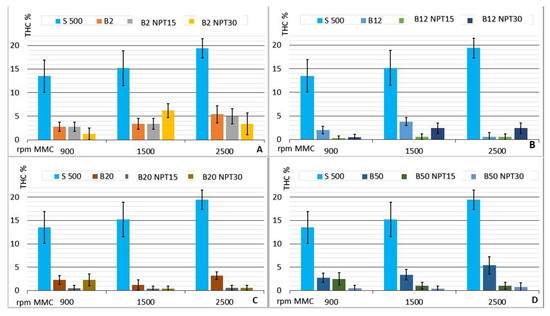
Figure 7.
Emissions of THC (% v/v) from the CCE running on the biodiesel:diesel mixtures in the proportions of 2% (A); 12% (B); 20% (C); and 50% (D) biodiesel obtained by CC, B100-NTP15, B100-NTP30, and S-500 at different engine speeds (rpm).
The constructive design of a CCE provides the delivery of power at low revs, as highlighted by [15]. Os The results showed low THC emissions at low engine rotation speeds for all fuel mixtures when compared to THC emissions observed using S-500.
The response of the engine to the throttle drive normally increases with the demand for air, providing a greater fuel volume injection in the CCE. This guarantees the stoichiometry and air/fuel ratio, which will be favored by the spraying of the fuel in the chamber, promoting the separation and atomization from the constituents, as described by [33]. The greater fluid flow obtained with the injection of biodiesel leads to greater efficiency in the burning of the air/fuel mixture.
The results obtained with the use of biodiesel produced by NTP showed very low levels of THC emissions and little variation in the percentage when the engine rotation speed was changed. The opposite behavior to the expected increase with the rotation of the CCE occurred in the case of B50-NTP15, with values of 2.5 ± 1.3, 1.0 ± 0.7, and 1.0 ± 0.7 for 900, 1500, and 2500 rpm, respectively. The uniform behavior observed for the mixtures of biodiesel produced applying 15 min of NTP with S 500 demonstrates that this biodiesel (B100-NTP15) produced from residual frying oil has a better burning efficiency, reducing the residual THC in the exhaust gas escaping from the CCE. The biodiesel samples produced in this study show a lower conversion to esters, as also observed by [25], who reported that the biodiesel produced with NTP technology showed lower ester conversion compared to biodiesel produced by CC. A high concentration of esters has been observed for biodiesel with 10–20% octanol added (by volume), guaranteeing lower THC emissions, as described by [34], which indicates the effectiveness of the higher concentration of esters obtained with the use of NTP technology.
3.2.3. Stability of Biodiesel Obtained from NTP
The results for the reduction in CO and THC emissions in the exhaust gas of the CCE running on biodiesel obtained by the NTP process applied to residual frying oil reveal a notable difference when compared to the S-500 fuel, since this biodiesel had lower values for toxic gas emissions. However, when compared with biodiesel obtained by CC, the difference is much smaller, with the biodiesel obtained by NTP showing lower emissions of CO and THC, despite being associated with a lower conversion rate to methyl esters.
Commercial synthetic additives, such as BHT and BHA, commonly used in commercial biodiesel produced by the conventional process, provide reductions of 23.81% in the THC levels in the exhaust gas, according to [14]. This behavior of reduced levels of CO and THC in the exhaust gas was also observed for the biodiesel produced by NTP and is related to the antioxidation conditions of the biodiesel produced from residual frying oil by NTP.
The presence of oxygen, which is common for conventional biodiesel, guarantees a reduction in residual THC as a result of a more efficient combustion. With regard to the biodiesel obtained applying NTP, the result for the emission of THC at 900 rpm for B100-NTP30 is 11% lower compared with B100 (obtained by CC). At 1500 rpm, the intermediate rotation condition, the corresponding THC reduction is 62% for B100-NTP30. High THC emissions for biodiesel with oxidation and the presence of saturated fatty acids have been reported by [35], and this was addressed by providing antioxidant effects by mixing petrodiesel with conventional biodiesel. Similar effects are observed with the use of NTP technology in the combustion of biodiesel:diesel mixtures.
The results for the CO emissions show a reduction for the biodiesel samples produced by CC and by NTP. B100-NTP30 at 900 rpm and at 2500 rpm showed reductions of 10% and 64%, respectively, compared with B100. B100-NTP30 has an antioxidative effect similar to that observed for biodiesel with AL-Mg added, which showed a CO reduction of up to 66% compared to biodiesel obtained from jatropha without additives [14].
The results for the THC levels in the exhaust gas of the CCE DB 7.0 using mixtures of biodiesel obtained applying NTP treatment for 30 min revealed a reduction of up to 75% at 900 rpm compared to the S 500 sample. This behavior is similar to that of biodiesel B20 produced from palm oil using the synthetic antioxidants BHA and BHT, which provided reductions above 20% compared to biodiesel without additives [14].
4. Conclusions
The effectiveness of reducing the CO emissions of biodiesel fuels obtained by applying 30 min of NTP reached values of 80% to 48% in relation to the S-500 used in the CCE. Considering the low levels of CO emissions at 1500 and 2500 rpm with the use of the B50-NTP30 and the stability of the emissions when accelerating, this mixture provided the best performance, with 122.5 ppm, 94 ppm, and 106 ppm of CO emissions at 900, 1500, and 2500 rpm, respectively.
The use of NTP technology for the production of biodiesel from residual frying oil led to low THC emissions (%), which showed little variation at the different engine rotation speeds. In the case of B100-NTP30, the reductions in THC emissions were from 13.5 to 2.00 at 900 rpm, from 15.2 to 1.4 at 1500 rpm, and from 19.4 to 2.00 at 2500 rpm using the DB 7.0 engine, compared with common diesel (S-500). Low THC emissions at low speeds were observed for all fuel mixtures containing biodiesel, obtained applying the NTP technology.
In relation to the objectives of this study—reduced THC and CO emissions from compression combustion engines (CCE)—of using biodiesel produced by NTP, we observed a reduction in CO and THC emissions in the exhaust gas for the biodiesel produced by NTP. This is attributed to the antioxidation conditions of the biodiesel produced from residual frying oil provided by NTP. The biodiesel produced by NTP technology, despite having lower concentrations of methyl esters when compared to the biodiesel obtained from CC, shows a reduction in CO and THC emissions.
This is an important point, since biodiesel is produced by NTP in a shorter time and at room temperature. However, further studies with biodiesel produced by NTP are needed to assess the power, torque, start up, and fuel consumption.
The energy performance of the CCE, combined with lower emissions of pollutants, shows that NTP is a useful tool for achieving sustainability and environmental gain, transforming liabilities associated with environmental losses into the generation of energy and the correct disposal of waste.
The improved performance of biofuels treated with NTP technology, observed on adding increasing proportions of biodiesel to diesel for use in a CCE, suggests that further studies should be conducted on the conditions of the biofuel mixtures and additives to reduce emissions of other GHGs and minimize harmful effects to users of CCEs.
Author Contributions
A.L.V.C.: Supervision and Validation, E.H.S.M.: Conceptualization, Methodology, Data curation, F.M.F.: Visualization, Investigation, F.d.S.O.: Writing—Reviewing and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors of this article have read and approved the final version submitted.
Data Availability Statement
All the data used for the research was described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- PNUD. Transformando Nosso Mundo: A Agenda 2030 Para o Desenvolvimento Sustentável. A/Res/70/. Available online: http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E20151-49 (accessed on 1 July 2022).
- Brito, F.D.O. Desenvolvimento de Usina de Produção de Biodiesel a Partir de Òleo de Fritura Usando Simuladores de Processo: Aspectos Operacionais. Master’s Thesis, Postgraduation in Civil Engineering. Centro de Tecnologia da Universidade Federal do Ceará, Fortaleza, Brazil, 2013. [Google Scholar]
- Ministério de Minas e Energia; Empresa de Pesquisa Energética. MME-EPE Balanço Energético Nacional 2019; MME: Brasilia, Brazil; EPE: Rio de Janeiro, Brazil, 2019; p. 303.
- Agência Nacional do Petróleo Gás Natural e Biocombustíveis. ANP Ranp 45-2014. Available online: https://www.gov.br/anp/pt-br/assuntos/producao-e-fornecimento-de-biocombustiveis/biodiesel/especificacao-do-biodiesel (accessed on 1 July 2022).
- Puhan, S.; Vedaraman, N.; Sankaranarayanan, G.; Ram, B.V.B. Desempenho e estudo de emissão de óleo de Mahua (óleo madhuca indica) éster etílico em um motor diesel de injeção direta aspirado natural de 4 tempos. Renov. Energ. 2005, 30, 1269–1278. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, J.; Zeng, G.; Shi, J.; Tong, J.; Huang, G. Otimização da conversão de resíduos de óleo de colza com alta FFA para biodiesel usando metodologia de superfície de resposta. Renov. Energ. 2005, 33, 1678–1684. [Google Scholar] [CrossRef]
- Ulusoy, Y. Alibaßs, K. Investigação tecnológica e econômica do uso de biodiesel como combustível alternativo. R. Fac. Agric. Uludag Univ. 2002, 16, 37–50. [Google Scholar]
- Van Gerpen, J.H. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Abdul-Majeed, W.S.; Aal-Thani, G.S.; Al-Sabahi, J.N. Aplicação de Plasma a jato voador para produção de combustível de biodiesel a partir de óleo vegetal desperdiçado. Plasma Chem. Processo Plasma 2016, 36, 1517–1531. [Google Scholar] [CrossRef]
- Moecke, E.H.S.; Feller, R.; Santos, H.A.D.; Machado, M.D.M.; Cubas, A.L.V.; Dutra, A.R.D.A.; Santos, L.L.V.; Soares, S.R. Produção de biodiesel a partir de resíduos de óleo de cozinha para uso como combustível em barcos de pesca artesanais: Integração de aspectos ambientais, econômicos e sociais. J. Clean. Prod. 2016, 135, 679–688. [Google Scholar] [CrossRef]
- Canakci, M. O potencial dos lipídios de resíduos de restaurantes como matérias-primas de biodiesel. Bioresour. Technol. 2007, 98, 183–190. [Google Scholar] [CrossRef]
- Çetinkaya, M.; Ulusoy, Y.; Tekìn, Y.; Karaosmanoǧlu, F. Engine and winter road test performances of used cooking oil originated biodiesel. Energy Convers. Manag. 2005, 46, 1279–1291. [Google Scholar] [CrossRef]
- Cancelli, D.M.; Dias, N.L. BRevê: Uma metodologia objetiva de cálculo de emissões para a frota brasileira de veículos. Eng. Sanit. Ambient. 2014, 19, 13–20. [Google Scholar] [CrossRef][Green Version]
- Kumar, M.V.; Babu, A.V.; Kumar, P.R. Os impactos na combustão, desempenho e emissões de biodiesel usando aditivos em motor diesel de injeção direta. Alex. Eng. J. 2018, 57, 509–516. [Google Scholar]
- Reis, E.F.; Cunha, J.P.B.; Mateus, D.L.S.; Delmond, J.G.; Couto, R.F. Desempenho e emissões de um motor-gerador ciclo diesel sob diferentes concentrações de biodiesel de soja. Rev. Bras. Eng. Agrícola Ambient. 2013, 2013, 565–571. [Google Scholar] [CrossRef]
- Alam, S. Imece2015-51900 Runner of a Diesel Engine Run on Biodiesel to Improve the Air-Fuel. In Proceedings of the International Mechanical Engineering Congress and Exposition (IMECE2015), Houston, TX, USA, 13–19 November 2015; pp. 1–7. [Google Scholar]
- Aydin, S.; Dizendo, C.; Aydin, H. Investigação da usabilidade do biodiesel obtido a partir de óleo de fritura residual em um motor diesel com revestimento de barreira térmica. Appl. Therm. Eng. 2015, 80, 212–219. [Google Scholar] [CrossRef]
- Cubas, A.L.V.; Machado, M.M.; Pinto, C.R.S.C.; Moecke, E.H.S.; Dutra, A.R.A. Biodiesel production using fatty acids from food industry waste using corona discharge plasma technology. Waste Manag. 2016, 47, 149–154. [Google Scholar] [CrossRef]
- Fridman, A.; Gutsol, A.; Gangoli, S.; Ju, Y.; Ombrello, T. Características do arco de deslizamento e sua aplicação em aprimoramento de combustão. J. Propuls. Power 2008, 24, 1216–1228. [Google Scholar] [CrossRef]
- Buchori, L.; Istadi, eu.; Purwanto, P. Tecnologias avançadas de reator químico para produção de biodiesel a partir de óleos vegetais — uma revisão. Bull. Chem. React. Eng. Catal. 2016, 11, 406–430. [Google Scholar] [CrossRef]
- Buchori, L.; Istadi, I.; Purwanto, P. Síntese de Biodiesel em um Reator Catalítico-Plasma híbrido sobre K2O/CaO-ZnO Catalyst. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2017, 18, 303–318. [Google Scholar]
- Istadi, I.; Yudhistira, A.D.; Anggoro, D.D.; Buchori, L. Sistema de eletro-catálise para síntese de biodiesel a partir de óleo de palma sobre reator de plasma de descarga de barreira dielelétrica. Bull. Chem. React. Eng. O Catal. 2014, 9, 111–120. [Google Scholar]
- Hyun, Y.; Mok, Y.; Jang, D. Transesterificação de Óleos Vegetais no Processo de Descarga de Plasma Pulsed-Corona. J. Korean Oil Chem. Soc. 2012, 29, 81–87. [Google Scholar]
- Istadi, I.; Anggoro, D.D.; Marwoto, P.; Suherman, S.; Nugroho, B.T. Biodiesel Produção de Óleo Vegetal sobre Reator de Plasma: Otimização do Rendimento do Biodiesel utilizando a Metodologia de Superfície de Resposta. Bull. Chem. React. Eng. Catal. 2009, 4, 23–31. [Google Scholar] [CrossRef]
- Machado, M.M. Conversão de Óleo Vegetal Residual em Biodiesel Através da Tecnologia de Plasma: Avaliação do Potencial de Sustentabilidade do Processo. Ph.D. Thesis, Postgraduation Program in Environmental Engineering. Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2018. [Google Scholar]
- Meng, X.; Chen, G.; Wang, Y. Biodiesel production from waste cooking oil via alkali catalyst and its engine test. Fuel Process. Technol. 2008, 89, 851–857. [Google Scholar] [CrossRef]
- AOAC—Associação de Químicos Analíticos Oficiais. Métodos Oficiais de Análise da AOAC International, 18th ed.; AOAC: Washington, DC, ESA, 2005. [Google Scholar]
- Diwakar, B.T.; Dutta, P.K.; Lokesh, B.R.; Naidu, K.A. Propriedades Físico-Químicas de Garden Cress (Lepidium sativum L.) Óleo de Sementes. J. Am. Oil. Chem. Soc. 2010, 87, 539–548. [Google Scholar] [CrossRef]
- Corsini, M.S.; Jorge, N. Estabilidade oxidativa de óleos vegetais utilizados em frituras de mandioca palito congelada. Ciência Tecnol. Aliment. 2006, 26, 27–32. [Google Scholar] [CrossRef][Green Version]
- Amaral, B.; Rezende, D.B.; Pasa, V.M.D. Avaliação de envelhecimento e estabilidade de misturas diesel/biodiesel armazenadas em garrafas de polietileno âmbar em diferentes condições de umidade. Combustível 2020, 279, 118289. [Google Scholar] [CrossRef]
- Miranda, G.R.D.; Lisboa, H.D.M.; Meier, H.F.; Vieira, M.V.; Hartmann, E.M. Avaliação das emissões de CO, NO, NOX e SO2 provenientes da combustão, em motor monocilíndrico, de misturas de diesel e biodiesel de óleo de fritura. Rev. Ciências Ambient. 2013, 7, 33–43. [Google Scholar]
- Völz, M.; Pozzebon, A.; Oliveira, G.; D’oca, M.G.M.; Villararreyes, J. Comportamento da esterificação de ácidos graxos livres óleo de soja. In Proceedings of the Congresso Latinoamericano de Óleos e Gorduras, Florianópolis, Brazil, 12–14 November 2000. [Google Scholar]
- Elhalwagy, M.; Zhang, C. Uma Cinética de Combustão de Biodiesel Proposta com base na dinâmica do fluido computacional resulta em um testador de qualidade de ignição. J. Energy Resour. Technol. Trans. ASME 2019, 141, 082204. [Google Scholar] [CrossRef]
- Mahalingam, A.; Deverajan, Y. Radhakrishnan, S.; Vellaiyan, S.; Nagappan, B. Análise de emissões em biodiesel de óleo de mahua e misturas mais altas de álcool no motor diesel. Alex. Univ.-Alex. Eng. J. 2018, 57, 2627–2631. [Google Scholar] [CrossRef]
- Oliveira, K.G.D. Obtenção de Antioxidantes Naturais e Aplicação em Misturas de Diesel e Biodiesel Sintetizado a Partir das Oleaginosas de Soja e Algodão. Master’s Thesis, Postgraduate Program in Chemistry. Universidade Federal do rio Grande do Norte Instituto de Química, Natal, Brazil, 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).