CFD Study of the Numbering up of Membrane Microreactors for CO2 Capture
Abstract
:1. Introduction
2. Materials and Methods
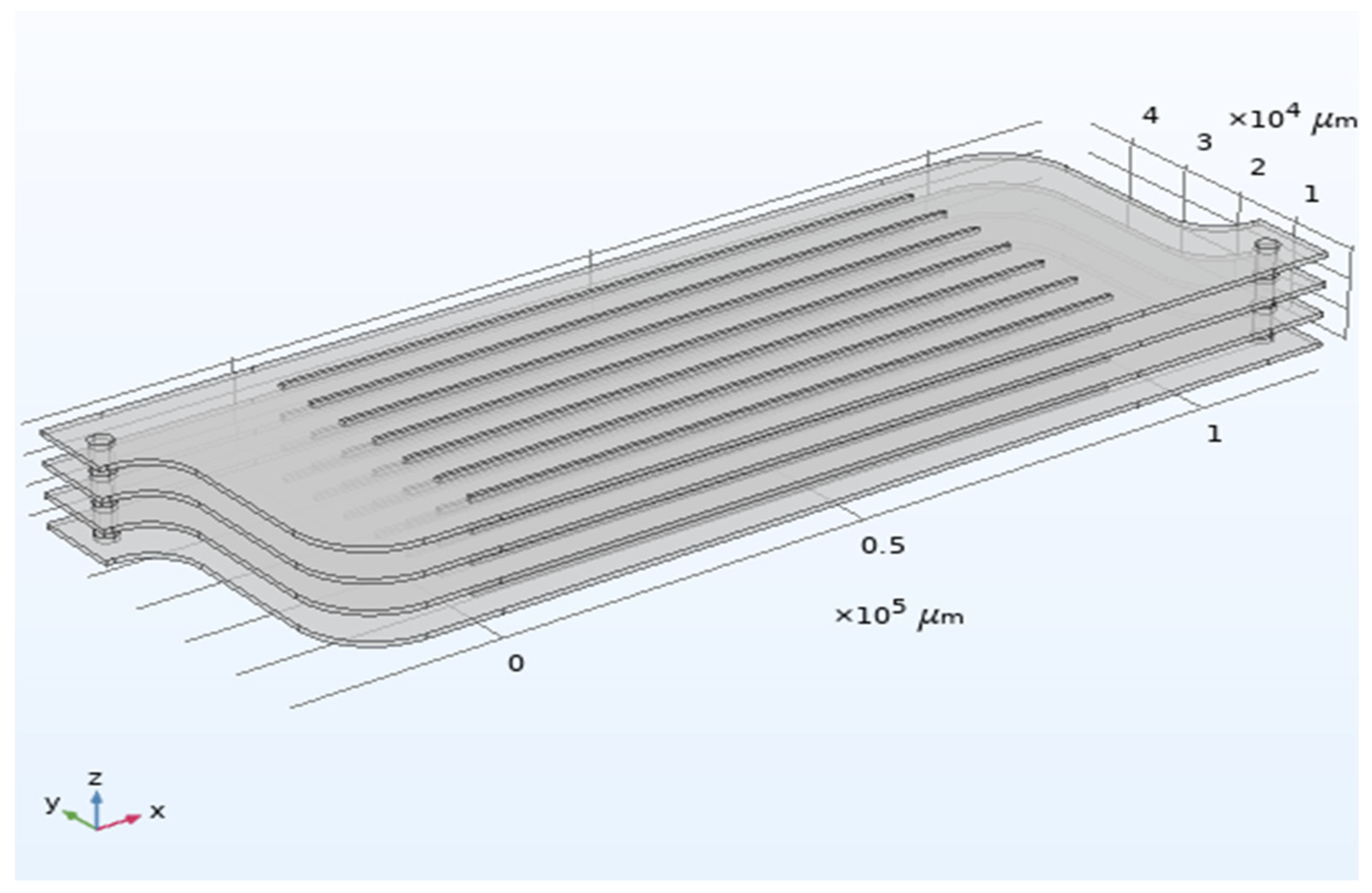
2.1. CFD Methodology
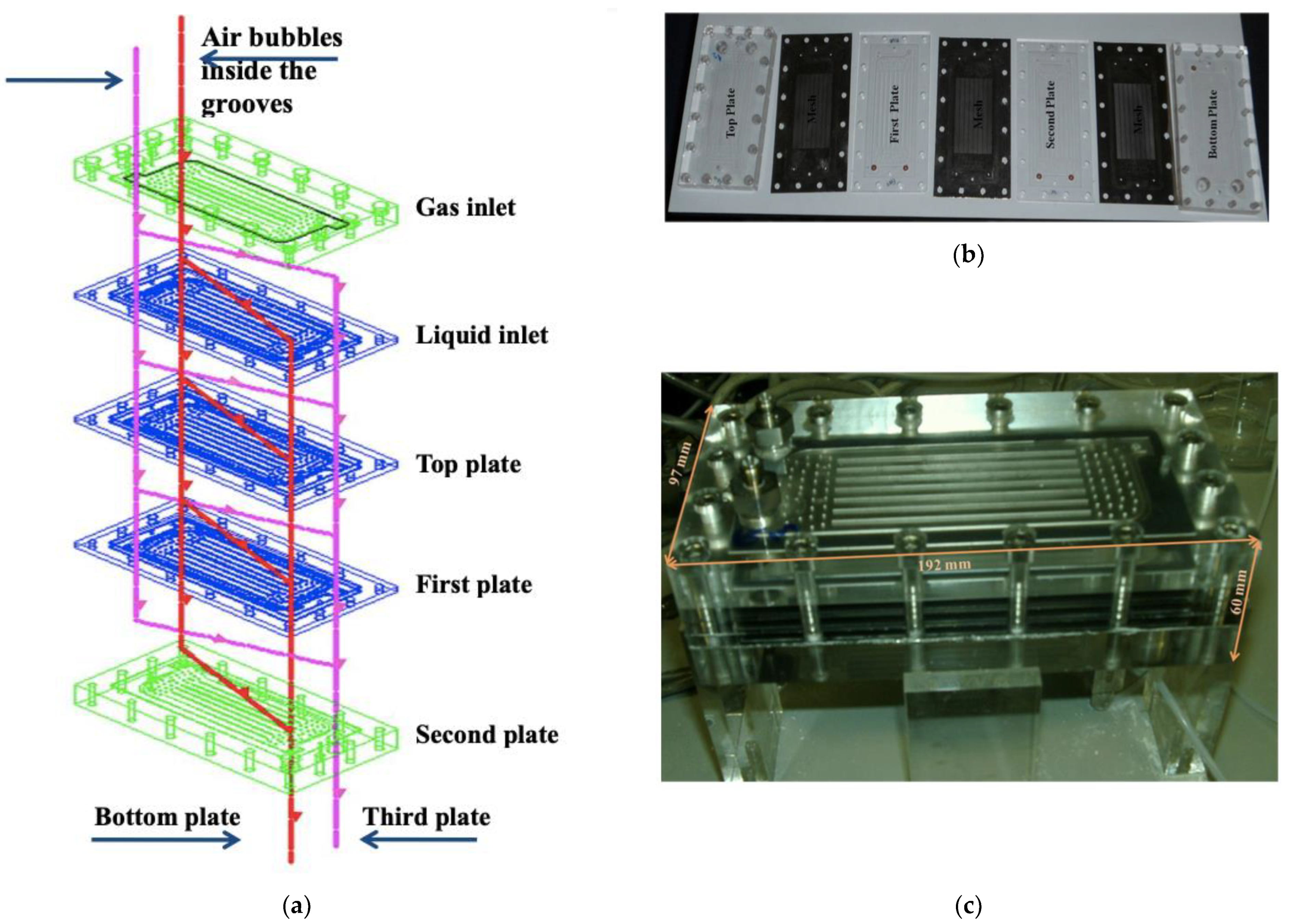
2.2. Experimental Methodology
3. Results and Discussion
3.1. CFD Results
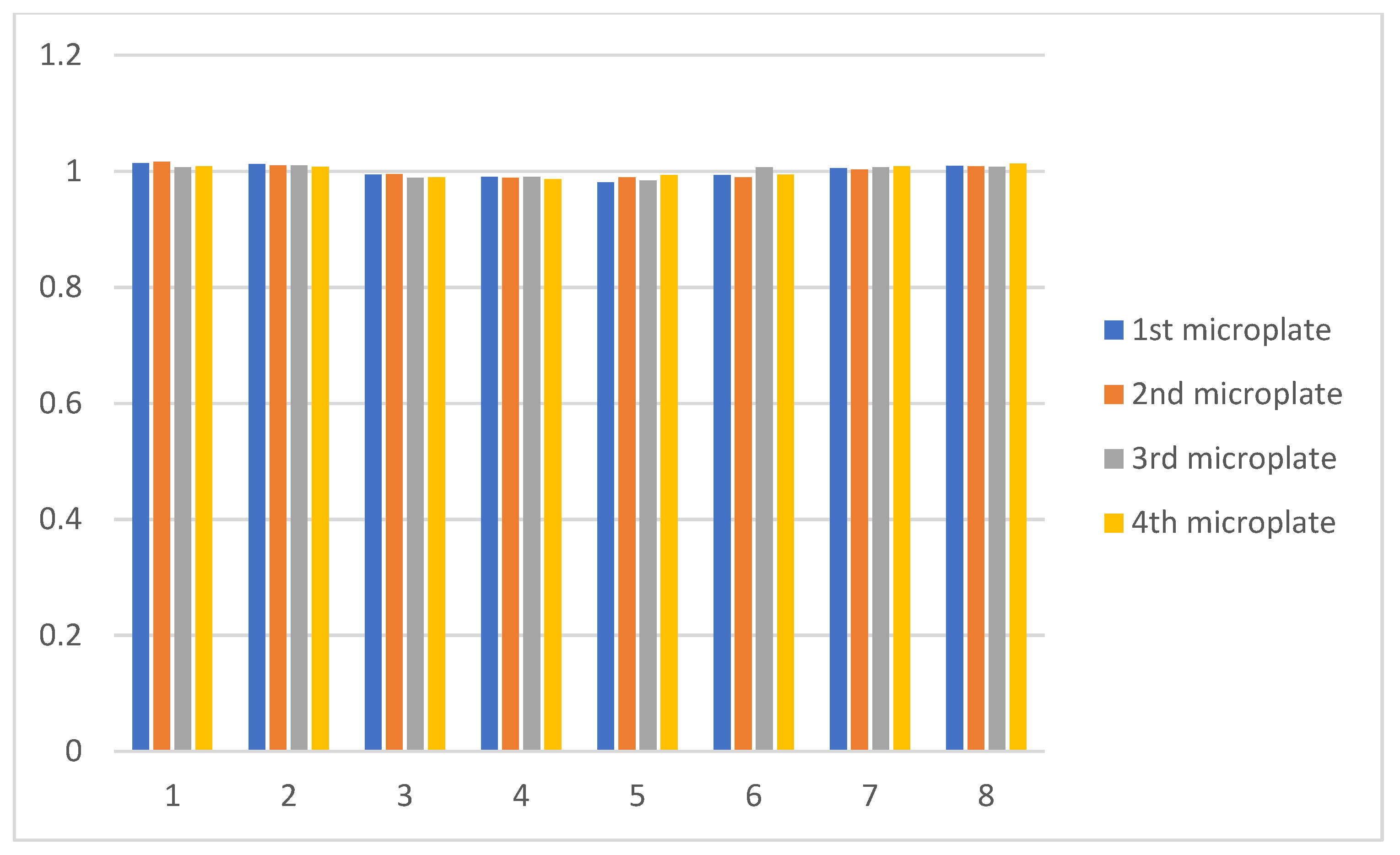
3.1.1. Flow Distribution in the Numbering-Up Microreactor
3.1.2. Pressure Profiles
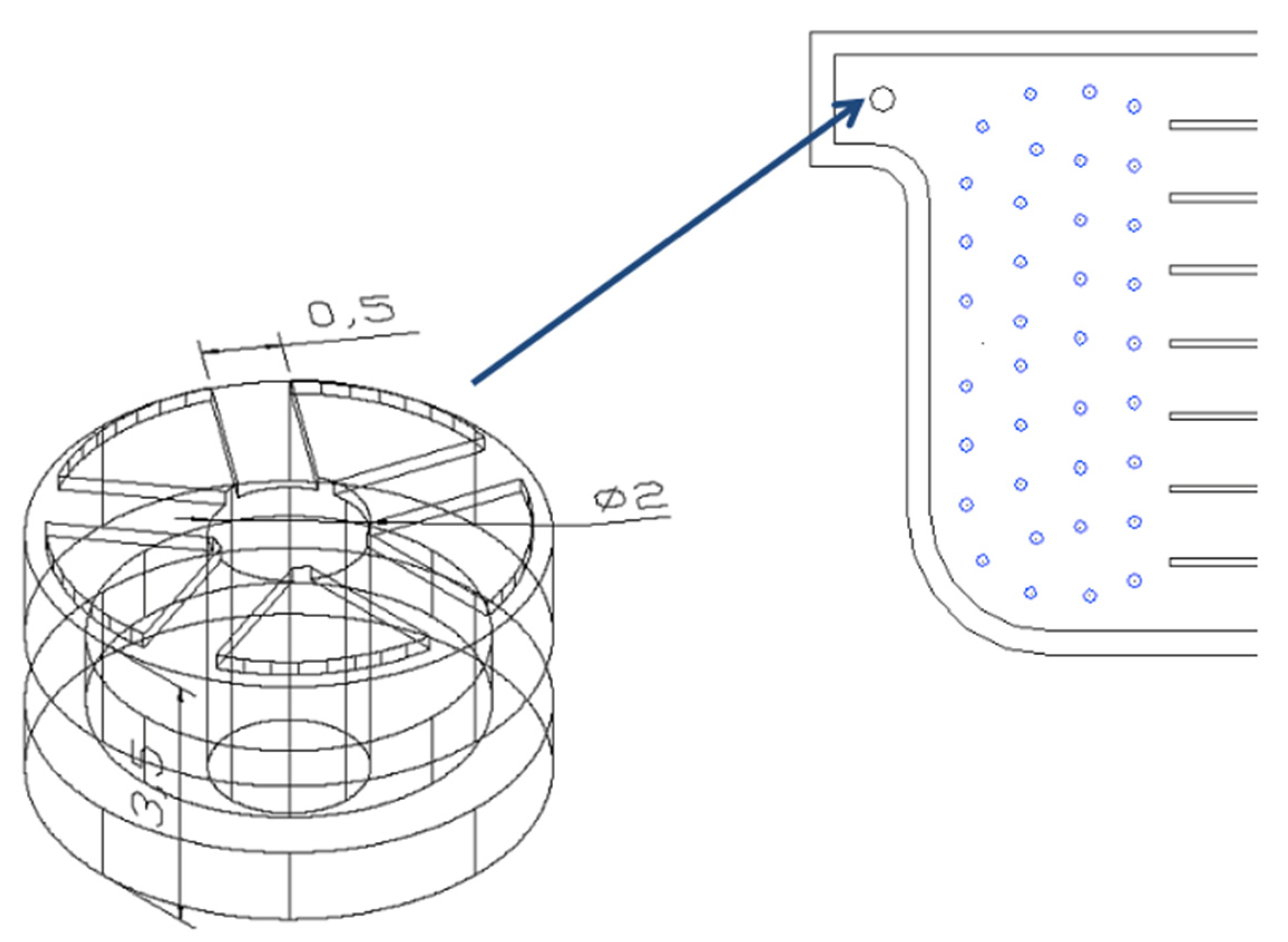
3.1.3. Effect of the Radius of Inlet and Outlet Tubes on the Flow Distribution
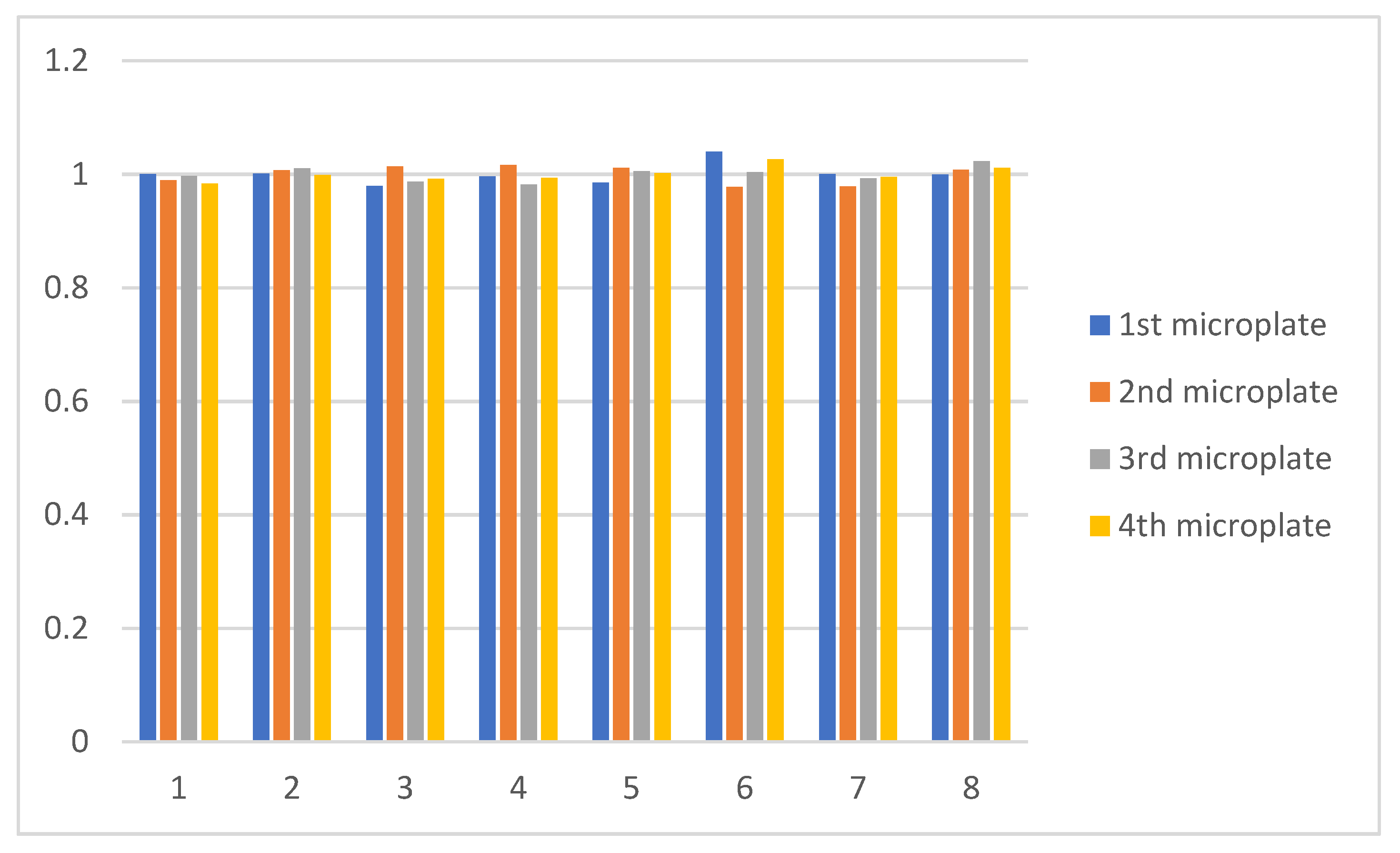
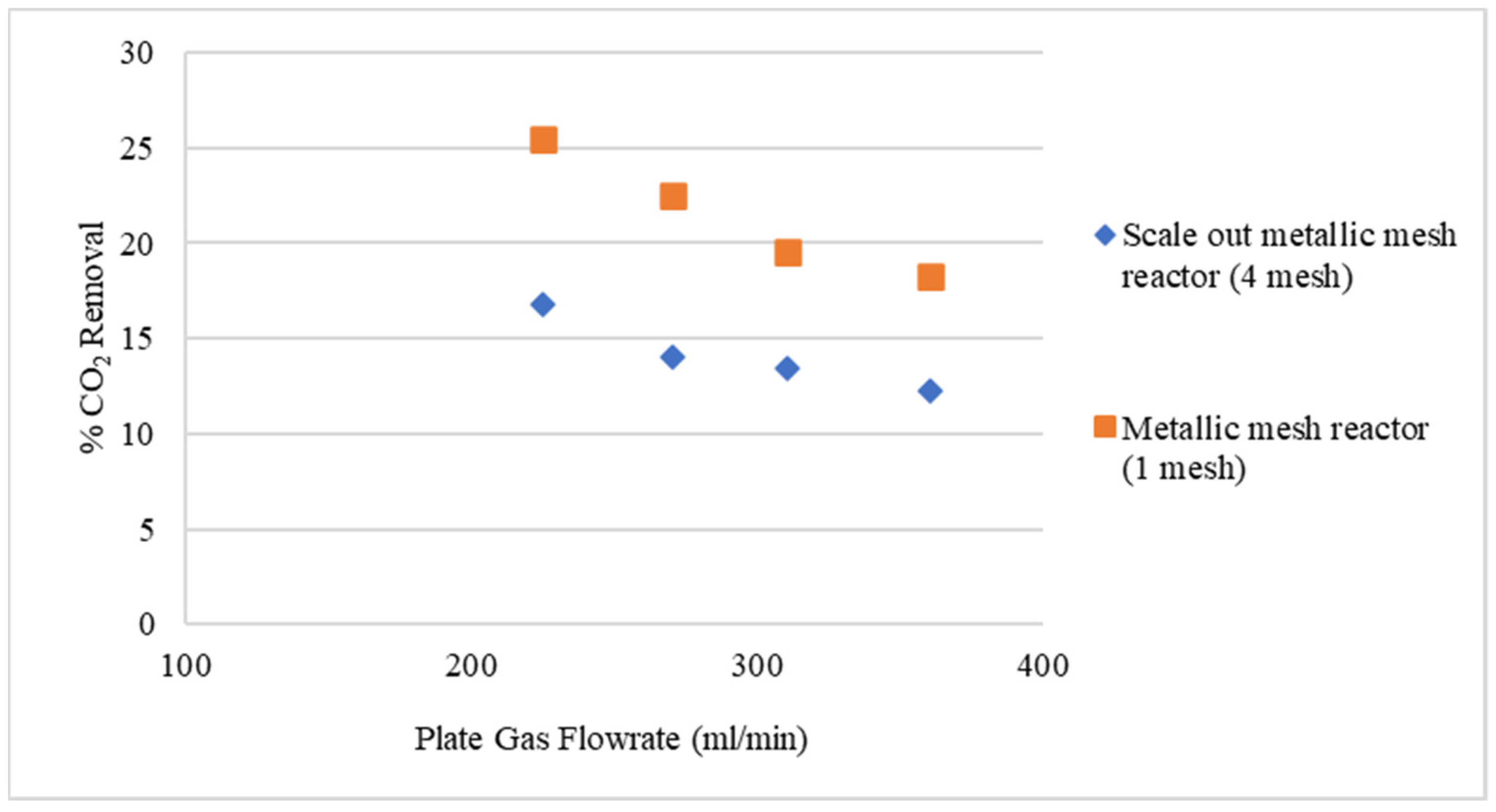
3.2. Experimental Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ma, D.; Zhu, C.; Fu, T.; Yuan, X.; Ma, Y. An effective hybrid solvent of MEA/DEEA for CO2 absorption and its mass transfer performance in microreactor. Sep. Purif. Technol. 2020, 242, 116795. [Google Scholar] [CrossRef]
- Abolhasani, M.; Günther, A.; Kumacheva, E. Microfluidic Studies of Carbon Dioxide. Angew. Chem. Int. Ed. 2014, 53, 7992–8002. [Google Scholar] [CrossRef]
- Meisen, A.; Shuai, X. Research and development issues in CO2 capture. Energy Convers. Manag. 1997, 38, S37–S42. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Zhu, K.; Yao, C.; Liu, Y.; Chen, G. Theoretical approach to CO2 absorption in microreactors and reactor volume prediction. Chem. Eng. Process. Process. Intensif. 2020, 150, 107904. [Google Scholar] [CrossRef]
- Constantinou, A.; Ghiotto, F.; Lam, K.F.; Gavriilidis, A. Stripping of acetone from water with microfabricated and membrane gas–liquid contactors. Analyst 2014, 139, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Constantinou, A.; Gavriilidis, A. Stripping of acetone from isopropanol solution with membrane and mesh gas–liquid contactors. Chem. Eng. Process. Process. Intensif. 2011, 50, 991–997. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; El-Naas, M.H.; Zhang, Z.; Van der Bruggen, B. CO2 Capture Using Hollow Fiber Membranes: A Review of Membrane Wetting. Energy Fuels 2018, 32, 963–978. [Google Scholar] [CrossRef]
- Hafeez, S.; Safdar, T.; Pallari, E.; Manos, G.; Aristodemou, E.; Zhang, Z.; Al-Salem, S.M.; Constantinou, A. CO2 capture using membrane contactors: A systematic literature review. Front. Chem. Sci. Eng. 2020, 15, 720–754. [Google Scholar] [CrossRef]
- Constantinou, A.; Barrass, S.; Pronk, F.; Bril, T.; Wenn, D.A.; Shaw, J.E.A.; Gavriilidis, A. CO2 absorption in a high efficiency silicon nitride mesh contactor. Chem. Eng. J. 2012, 207–208, 766–771. [Google Scholar] [CrossRef]
- Constantinou, A.; Barrass, S.; Gavriilidis, A. CO2 Absorption in Polytetrafluoroethylene Membrane Microstructured Contactor Using Aqueous Solutions of Amines. Ind. Eng. Chem. Res. 2014, 53, 9236–9242. [Google Scholar] [CrossRef]
- Constantinou, A.; Gavriilidis, A. CO2 Absorption in a Microstructured Mesh Reactor. Ind. Eng. Chem. Res. 2010, 49, 1041–1049. [Google Scholar] [CrossRef]
- Constantinou, A.; Barrass, S.; Gavriilidis, A. CO2 absorption in flat membrane microstructured contactors of different wettability using aqueous solution of NaOH. Green Process. Synth. 2018, 7, 471–476. [Google Scholar] [CrossRef]
- Rostami, S.; Keshavarz, P.; Raeissi, S. Experimental study on the effects of an ionic liquid for CO2 capture using hollow fiber membrane contactors. Int. J. Greenh. Gas. Control. 2018, 69, 1–7. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Darabi, M.; Ghaleh, V.R.; Bakhtiari, O. CFD Modeling of CO2 Absorption in Membrane Contactors Using Aqueous Solutions of Monoethanolamine–Ionic Liquids. Ind. Eng. Chem. Res. 2020, 59, 18629–18639. [Google Scholar] [CrossRef]
- Magnone, E. A performance comparison study of five single and sixteen blended amine absorbents for CO2 capture using ceramic hollow fiber membrane contactors. J. Ind. Eng. Chem. 2021, 100, 174–185. [Google Scholar] [CrossRef]
- Sohaib, Q. Rigorous non-isothermal modeling approach for mass and energy transport during CO2 absorption into aqueous solution of amino acid ionic liquids in hollow fiber membrane contactors. Sep. Purif. Technol. 2021, 254, 117644. [Google Scholar] [CrossRef]
- Griffini, G.; Gavriilidis, A. Effect of Microchannel Plate Design on Fluid Flow Uniformity at Low Flow Rates. Chem. Eng. Technol. 2007, 30, 395–406. [Google Scholar] [CrossRef]
- Mohammadi, M.; Jovanovic, G.N.; Sharp, K.V. Numerical study of flow uniformity and pressure characteristics within a microchannel array with triangular manifolds. Comput. Chem. Eng. 2013, 52, 134–144. [Google Scholar] [CrossRef]
- Tonomura, O.; Tanaka, S.; Noda, M.; Kano, M.; Hasebe, S.; Hashimoto, I. CFD-based optimal design of manifold in plate-fin microdevices. Chem. Eng. J. 2004, 101, 397–402. [Google Scholar] [CrossRef]
- Huang, C.-H.; Wang, C.-H.; Kim, S. A manifold design problem for a plate-fin microdevice to maximize the flow uniformity of system. Int. J. Heat Mass Transf. 2016, 95, 22–34. [Google Scholar] [CrossRef]
- Castedo, A.; Uriz, I.; Soler, L.; Gandía, L.M.; Llorca, J. Kinetic analysis and CFD simulations of the photocatalytic production of hydrogen in silicone microreactors from water-ethanol mixtures. Appl. Catal. B Environ. 2017, 203, 210–217. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Kuijpers, K.; Hessel, V.; Noël, T. A convenient numbering-up strategy for the scale-up of gas–liquid photoredox catalysis in flow. React. Chem. Eng. 2016, 1, 73–81. [Google Scholar] [CrossRef]
- Togashi, S.; Miyamoto, T.; Sano, T.; Suzuki, M. Microreactor System Using the Concept of Numbering-Up. In New Trends in Fluid Mechanics Research; Zhuang, F.G., Li, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 678–681. ISBN 978-3-540-75994-2. [Google Scholar]
- Kikutani, Y.; Hibara, A.; Uchiyama, K.; Hisamoto, H.; Tokeshi, M.; Kitamori, T. Pile-up glass microreactor. Lab. Chip 2002, 2, 193. [Google Scholar] [CrossRef]
- Su, Y.; Lautenschleger, A.; Chen, G.; Kenig, E.Y. A Numerical Study on Liquid Mixing in Multichannel Micromixers. Ind. Eng. Chem. Res. 2014, 53, 390–401. [Google Scholar] [CrossRef]
- Zhao, F.; Cambié, D.; Janse, J.; Wieland, E.W.; Kuijpers, K.P.L.; Hessel, V.; Debije, M.G.; Noël, T. Scale-up of a Luminescent Solar Concentrator-Based Photomicroreactor via Numbering-up. ACS Sustain. Chem. Eng. 2018, 6, 422–429. [Google Scholar] [CrossRef]
- Tonomura, O.; Taniguchi, S.; Nishi, K.; Nagaki, A.; Yoshida, J.; Hirose, K.; Ishizuka, N.; Hasebe, S. Blockage Detection and Diagnosis of Externally Parallelized Monolithic Microreactors. Catalysts 2019, 9, 308. [Google Scholar] [CrossRef] [Green Version]
- Cussler, E.L. Diffusion Mass Transfer in Fluid Systems; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Versteeg, G.F.; van Swaaij, W.P.M. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions—I. Primary and secondary amines. Chem. Eng. Sci. 1988, 43, 573–585. [Google Scholar] [CrossRef] [Green Version]
- Nijsing, R.A.T.O.; Hendriksz, R.H.; Kramers, H. Absorption of CO2 in jets and falling films of electrolyte solutions, with and without chemical reaction. Chem. Eng. Sci. 1959, 10, 88–104. [Google Scholar] [CrossRef]
- Pohorecki, R.; Moniuk, W. Kinetics of reaction between carbon dioxide and hydroxyl ions in aqueous electrolyte solutions. Chem. Eng. Sci. 1988, 43, 1677–1684. [Google Scholar] [CrossRef]
- Yue, J.; Boichot, R.; Luo, L.; Gonthier, Y.; Chen, G.; Yuan, Q. Flow distribution and mass transfer in a parallel microchannel contactor integrated with constructal distributors. Aiche J. 2009, 56, 298–317. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harkou, E.; Hafeez, S.; Manos, G.; Constantinou, A. CFD Study of the Numbering up of Membrane Microreactors for CO2 Capture. Processes 2021, 9, 1515. https://doi.org/10.3390/pr9091515
Harkou E, Hafeez S, Manos G, Constantinou A. CFD Study of the Numbering up of Membrane Microreactors for CO2 Capture. Processes. 2021; 9(9):1515. https://doi.org/10.3390/pr9091515
Chicago/Turabian StyleHarkou, Eleana, Sanaa Hafeez, George Manos, and Achilleas Constantinou. 2021. "CFD Study of the Numbering up of Membrane Microreactors for CO2 Capture" Processes 9, no. 9: 1515. https://doi.org/10.3390/pr9091515
APA StyleHarkou, E., Hafeez, S., Manos, G., & Constantinou, A. (2021). CFD Study of the Numbering up of Membrane Microreactors for CO2 Capture. Processes, 9(9), 1515. https://doi.org/10.3390/pr9091515







