Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Brachychiton Populneus Leaves
2.2. Preparation of Leaves Extracts
2.3. Formation of Silver Nitrate (AgNO3) Dilutions
2.4. Leaf Extract-Mediated BP-AgNPs Synthesis
2.5. Characterization of Silver Nanoparticles
2.5.1. Ultraviolet-Visible Spectrophotometer
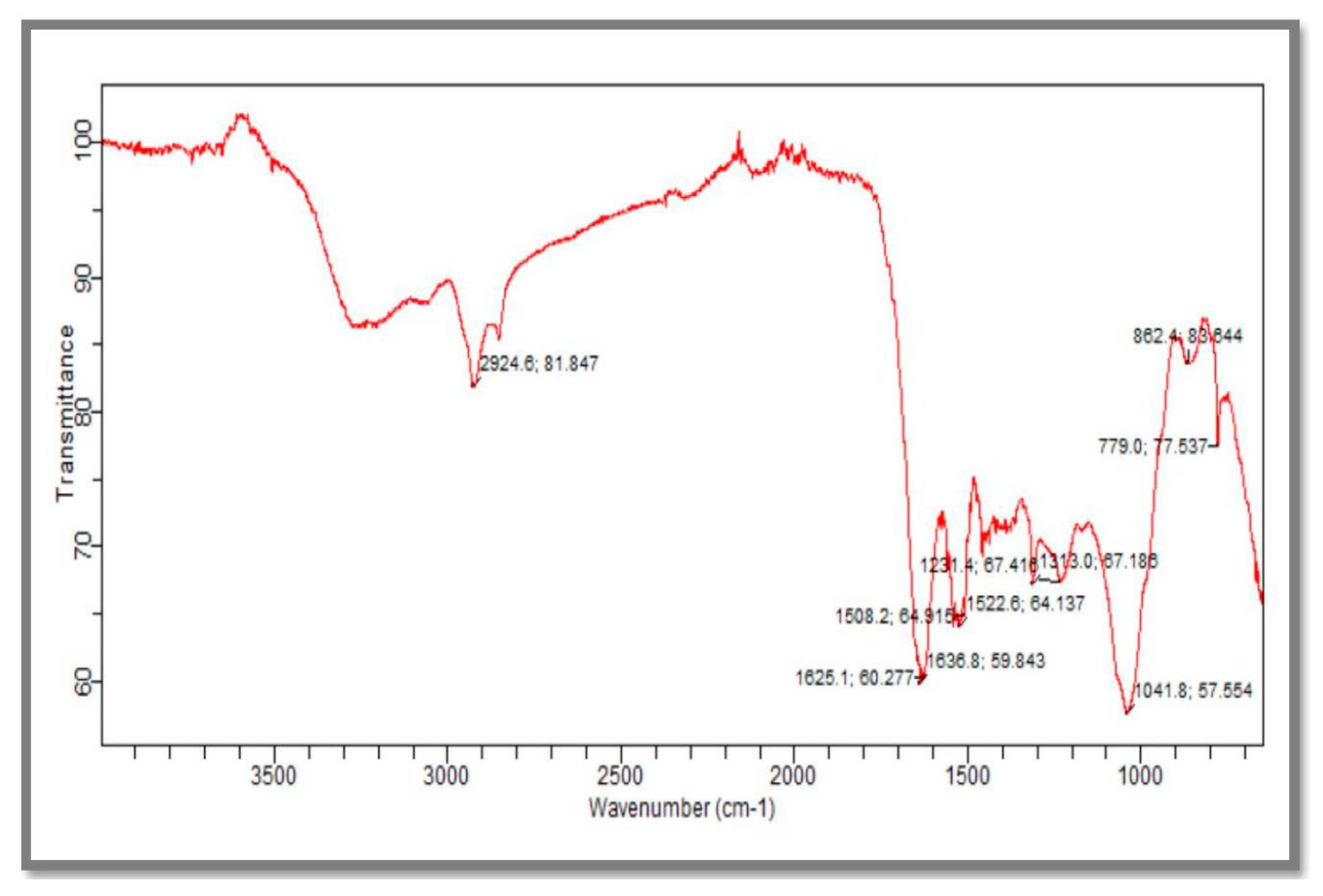
2.5.2. Fourier-Transform Infrared (FTIR) Spectroscopy
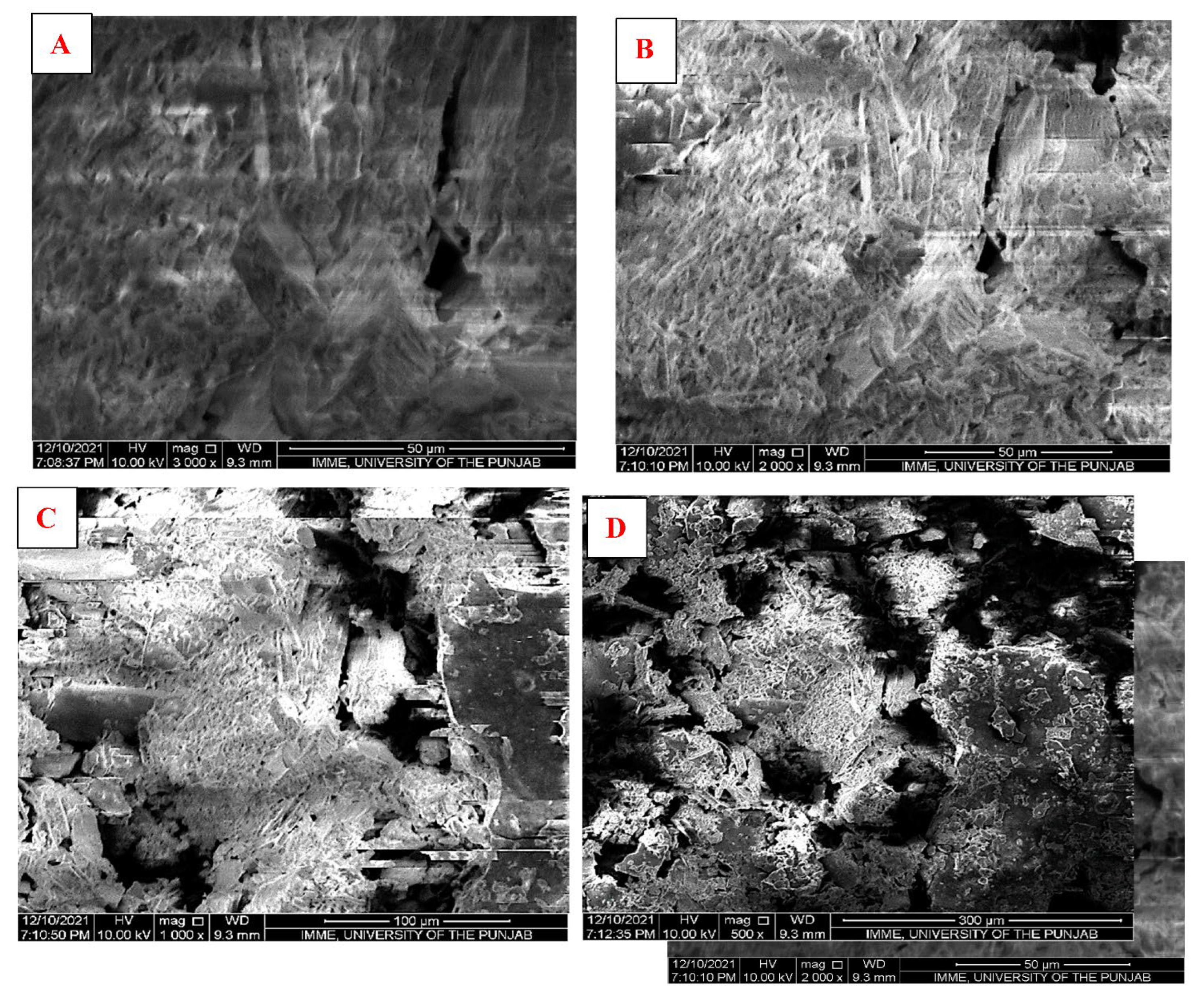
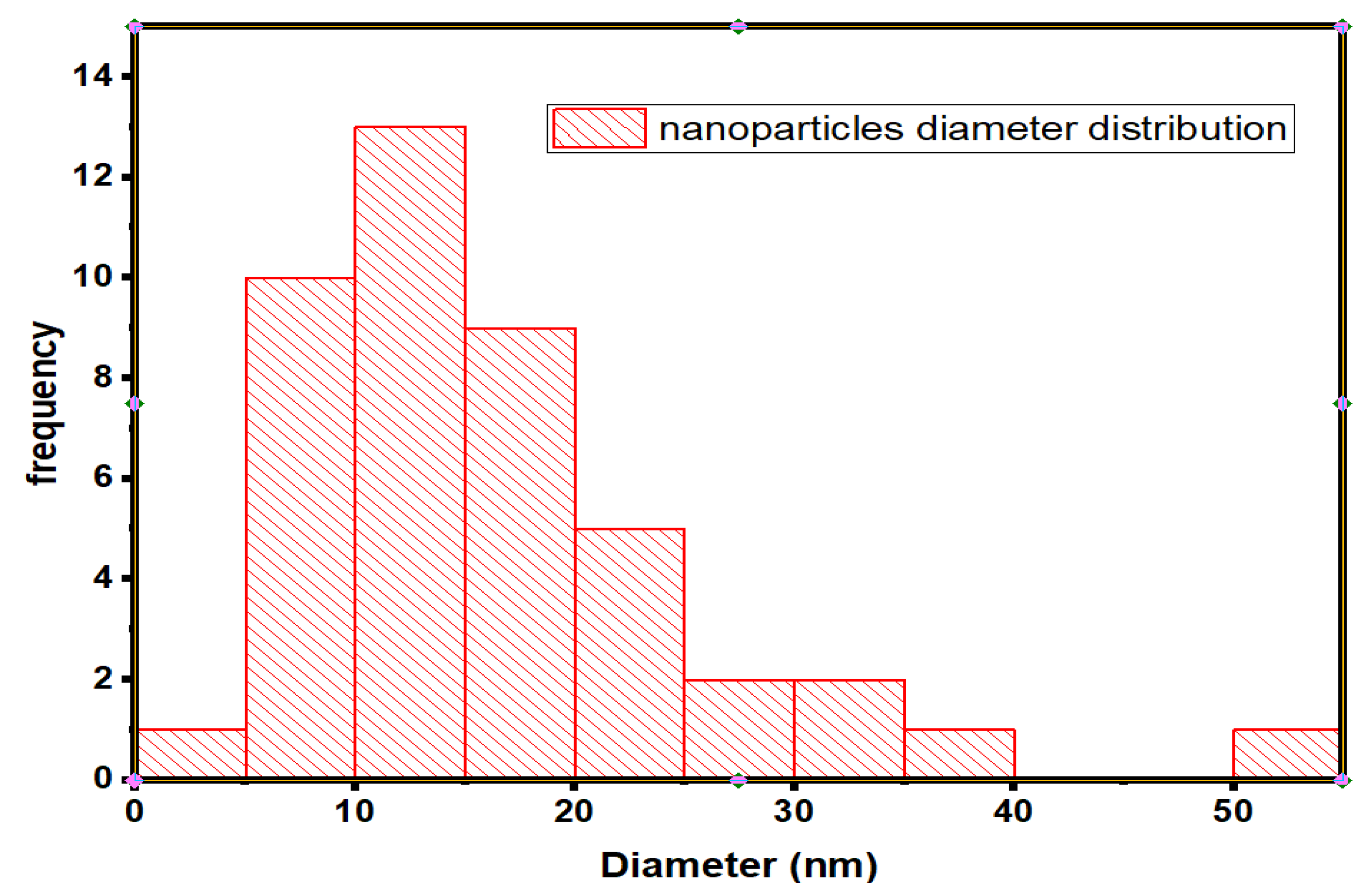
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. Energy Dispersive X-ray Analysis (EDX)
2.6. In Vitro Biological Screening of Silver Nanoparticles
2.6.1. Antioxidant Activity of Silver Nanoparticles (DPPH ASSAY)
2.6.2. Inhibition of Enzymatic Activity of α-Amylase by BP-AgNPs
2.6.3. Inhibition of Protein Denaturation by BP-AgNPs
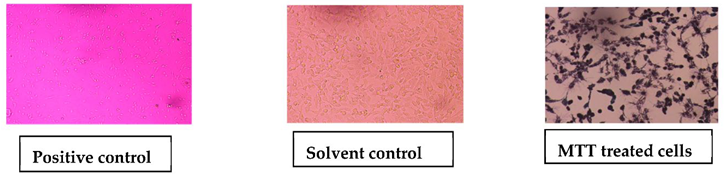
2.6.4. Cytotoxic Activity of AgNPs on Cell Lines (MTT Assay)
Culturing of Cell Lines
Assay for % Cell Viability
2.7. Statistical Analysis
3. Results
3.1. Phyto-Mediated Reduction of Ag+ Ions to Agº
3.2. Characterization of Silver Nanoparticles
3.2.1. UV-Visible Spectrophotometric Analysis
3.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.2.3. Scanning Electron Microscopy (SEM)
3.2.4. Energy Dispersive X-ray Analysis (EDX)
3.3. In vitro Biological Screening of Brachychiton Populneus Mediated AgNPs
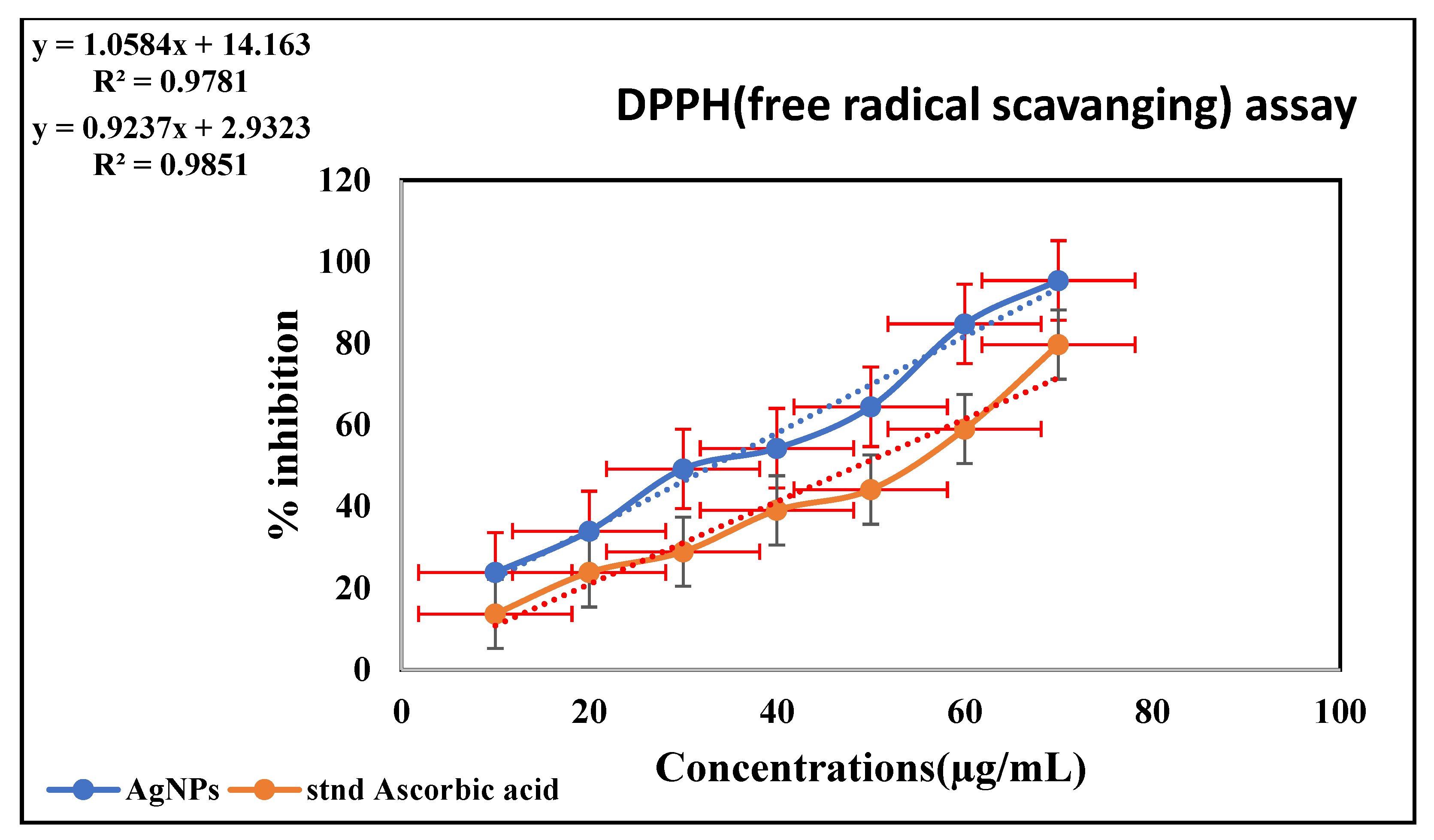
3.3.1. Antioxidant Activity Silver Nanoparticles (DPPH Assay)
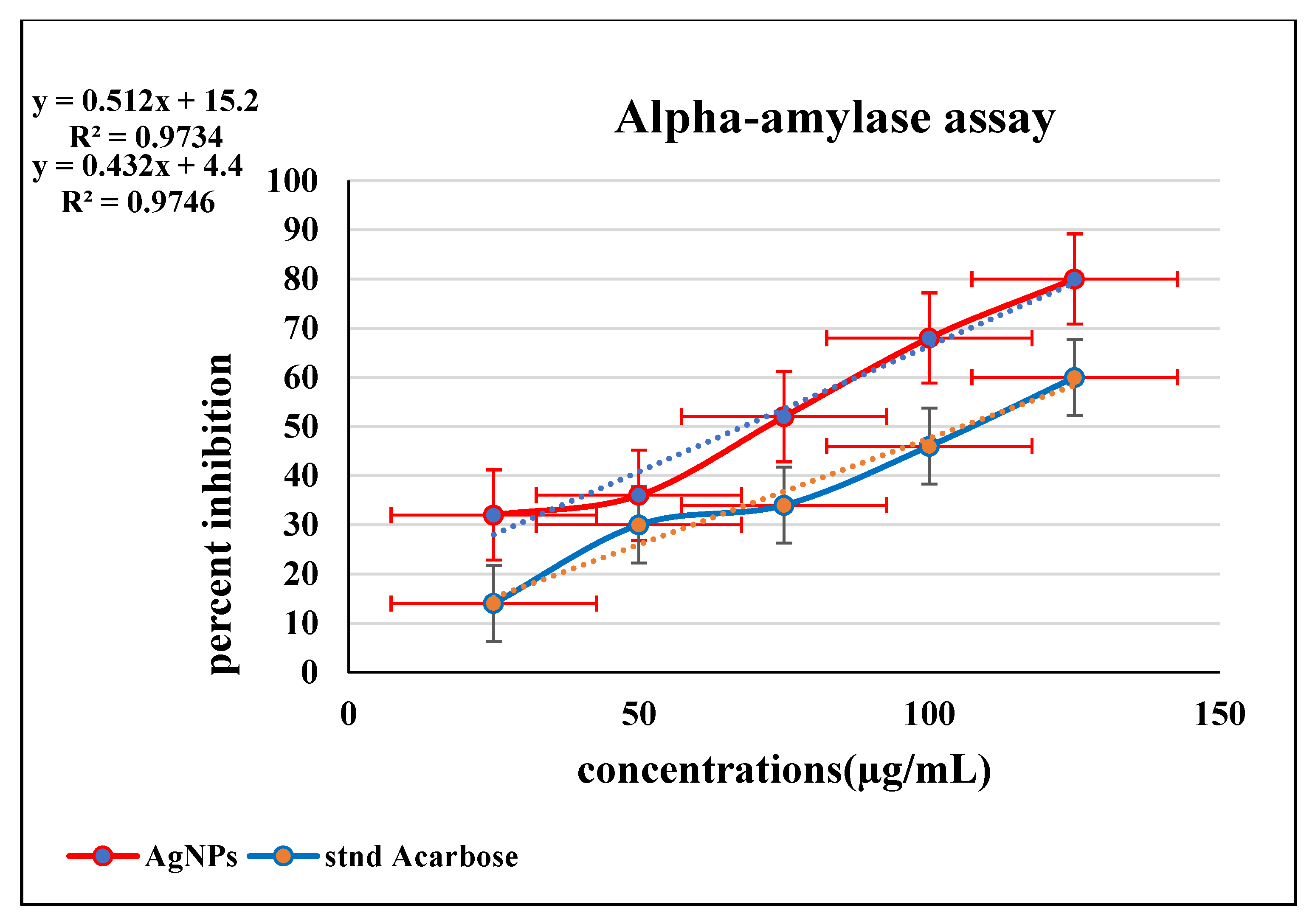
3.3.2. Inhibition of Enzymatic Activity of α-Amylase by BP-AgNPs
3.3.3. Inhibition of Protein Denaturation by BP-AgNPs
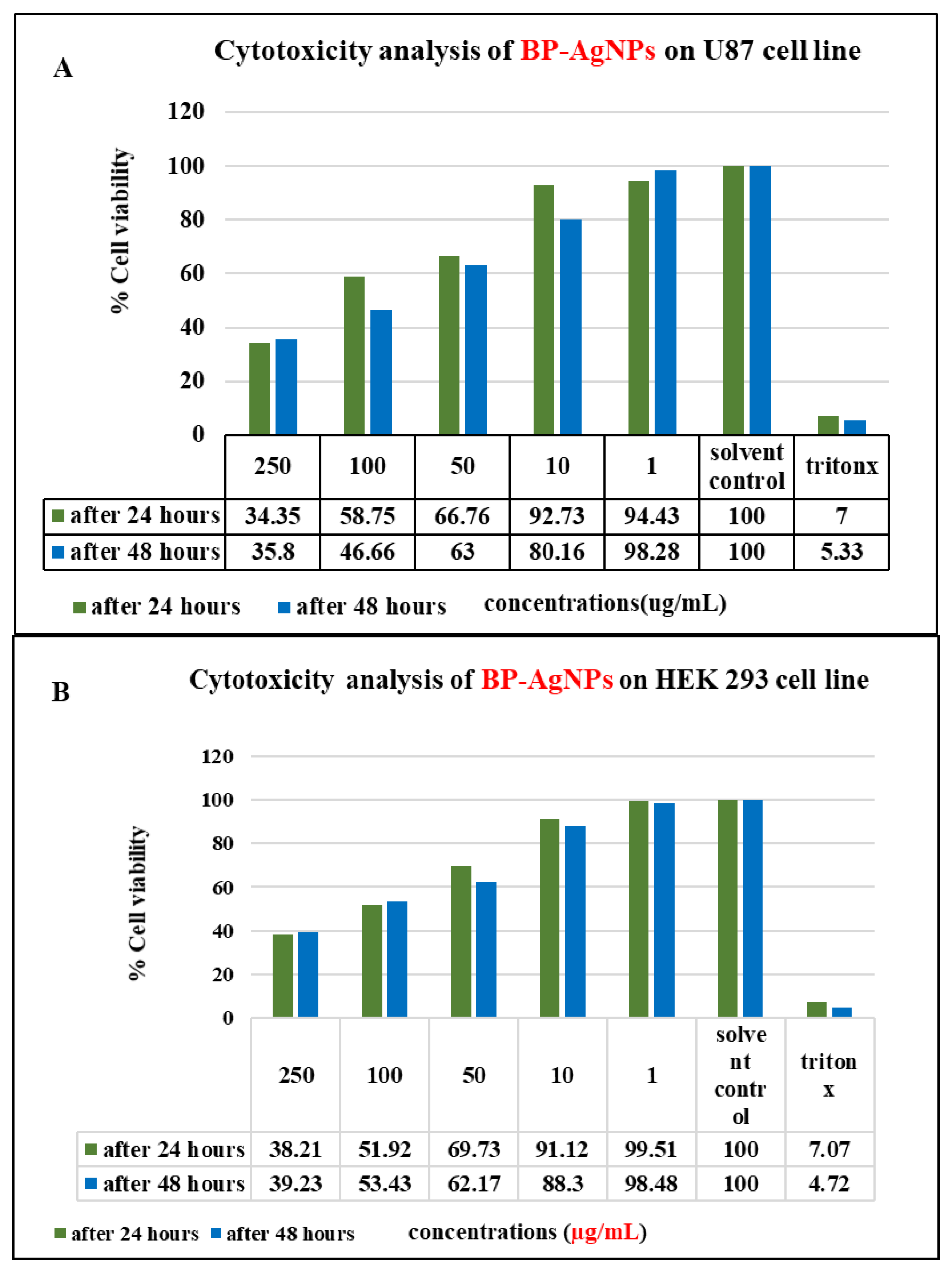
3.3.4. Cytotoxic Activity of AgNPs (MTT Assay)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 27, 112. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. Supramol. Chem. Mol. Nanomaterials. 2012, 2, 1–14. [Google Scholar]
- Sriramulu, M.; Shanmugam, S.; Ponnusamy, V.K. Agaricus bisporus mediated biosynthesis of copper nanoparticles and its biological effects: An in-vitro study. Colloids Interface Sci. Commun. 2020, 35, 100254. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications–An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Arif, R.; Uddin, R. A review on recent developments in the biosynthesis of silver nanoparticles and its biomedical applications. Med. Devices Sens. 2021, 4, e10158. [Google Scholar] [CrossRef]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of Silver Nanoparticles, and their Biomedical Applications–A Comprehensive Review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef]
- Jouyban, A.; Rahimpour, E. Optical sensors based on silver nanoparticles for determination of pharmaceuticals: An overview of advances in the last decade. Talanta 2020, 217, 121071. [Google Scholar] [CrossRef]
- Zeid, A.H.A.; Farag, M.A.; Hamed, M.A.A.; Kandil, Z.A.A.; El-Akad, R.H.; El-Rafie, H.M. Flavonoid chemical composition and antidiabetic potential of Brachychiton acerifolius leaves extract. Asian Pac. J. Trop. Biomed. 2017, 7, 389–396. [Google Scholar] [CrossRef]
- Ragheb, A.Y.; Kassem, E.S.; El-Sherei, M.; Marzouk, M.; Saleh, A.M.; Saleh, N.A.M. Morphological, phytochemical, and anti-hyperglycemic evaluation of Brachychiton populneus. Rev. Bras. Farmacogn. 2019, 29, 559–569. [Google Scholar] [CrossRef]
- Agyare, C.; Koffuor, G.A.; Boamah, V.E.; Adu, F.; Mensah, K.B.; Adu-Amoah, L. Antimicrobial and Anti-Inflammatory Activities of Pterygota macrocarpa and Cola gigantea (Sterculiaceae). Evid Based Complement. Altern. Med. 2012, 2012, 902394. [Google Scholar] [CrossRef] [Green Version]
- Aadil, K.R.; Pandey, N.; Mussatto, S.I.; Jha, H. Green synthesis of silver nanoparticles using acacia lignin, their cytotoxicity, catalytic, metal ion sensing capability and antibacterial activity. J. Environ. Chem. Eng. 2019, 7, 103296. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-inflammatory mechanism of various metal and metal oxide nanoparticles synthesized using plant extracts: A review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Koroth, J.; Srinivasan, P.P.; Singh, A.; Mukundan, S.; Karki, S.S.; Choudhary, B.; Gupta, C.M. Effects of green synthesised silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. Int. J. Nanomed. 2019, 16, 5257–5270. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed. Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [Green Version]
- Darvish, S.; Kahrizi, M.S.; Özbolat, G.; Khaleghi, F.; Mortezania, Z.; Sakhaei, D. Silver nanoparticles: Biosynthesis and cytotoxic performance against breast cancer MCF-7 and MDA-MB-231 cell lines. Nanomed. Res. J. 2022, 7, 83–92. [Google Scholar]
- Bhardwaj, M.; Yadav, P.; Dalal, S.; Kataria, S.K. A review on ameliorative green nanotechnological approaches in diabetes management. Biomed. Pharm. 2020, 127, 110198. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders. Biochim. Biophys Acta Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef]
- Mohammad, G.; Mishra, V.K.; Pandey, H.P. Antioxidant properties of some nanoparticles may enhance wound healing in T2DM patient. Dig. J. Nanomater. Biostructures 2008, 3, 159–162. [Google Scholar]
- Lushchak, O.; Zayachkivska, A.; Vaiserman, A. Metallic Nanoantioxidants as Potential Therapeutics for Type 2 Diabetes: A Hypothetical Background and Translational Perspectives. Oxid Med. Cell Longev. 2018, 2018, 27. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Chen, L.; Xiong, Y.; Panayi, A.C.; Abududilibaier, A.; Hu, Y.; Yu, C.; Zhou, W.; Sun, Y.; Liu, M.; et al. Antioxidant Therapy and Antioxidant-Related Bionanomaterials in Diabetic Wound Healing. Front. Bioeng. Biotechnol. 2021, 24, 707479. [Google Scholar] [CrossRef]
- Bawazeer, S.; Rauf, A.; Shah, S.U.A.; Shawky, A.M.; Al-Awthan, Y.S.; Bahattab, O.S.; Uddin, G.; Sabir, J.; El-Esawi, M.A. Green synthesis of silver nanoparticles using Tropaeolum majus: Phytochemical screening and antibacterial studies. Green Proc. Synth. 2021, 10, 85–94. [Google Scholar] [CrossRef]
- Braca, A.; de Tommasi, N.; di Bari, L.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant Principles from Bauhinia tarapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Ng, K.; Zhang, P.; Warner, R.D.; Shen, S.; Tang, H.Y.; Liang, Z.; Fang, Z. In Vitro α-Glucosidase and α-Amylase Inhibitory Activities of Free and Bound Phenolic Extracts from the Bran and Kernel Fractions of Five Sorghum Grain Genotypes. Foods 2020, 15, 1301. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Nguyen, N.H.A.; Padil, V.V.T.; Slaveykova, V.I.; Černík, M.; Ševců, A. Green Synthesis of Metal and Metal Oxide Nanoparticles and Their Effect on the Unicellular Alga Chlamydomonas reinhardtii. Nanoscale Res. Lett. 2018, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Chandrasekaran, R. Biogenic nanoparticles: A comprehensive perspective in synthesis, characterization, application, and its challenges. J. Genet. Eng. Biotechnol. 2020, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 17, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 12, e0220950. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, E.H.; Kilany, M.; Ghramh, H.A.; Khan, K.A.; Ul Islam, S. Cellular proliferation/cytotoxicity, and antimicrobial potentials of green synthesized silver nanoparticles (AgNPs) using Juniperus procera. Saudi J. Biol. Sci. 2019, 26, 1689–1694. [Google Scholar] [CrossRef]
- Aslam, M.; Fozia, F.; Gul, A.; Ahmad, I.; Ullah, R.; Bari, A.; Mothana, R.A.; Hussain, H. Phyto-Extract-Mediated Synthesis of Silver Nanoparticles Using Aqueous Extract of Sanvitalia procumbens and Characterization, Optimization and Photocatalytic Degradation of Azo Dyes Orange G and Direct Blue-15. Molecules 2021, 12, 6144. [Google Scholar] [CrossRef] [PubMed]
- Krithiga, N.; Rajalakshmi, A.; Jayachitra, A. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria ternatea and Solanum nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 2015, 928204. [Google Scholar] [CrossRef] [Green Version]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [Green Version]
- Abdellatif, A.A.H.; Alhathloul, S.S.; Aljohani, A.S.M.; Maswadeh, H.; Abdallah, E.M.; Musa, K.H.; el Hamd, M.A. Green Synthesis of Silver Nanoparticles Incorporated Aromatherapies Utilized for Their Antioxidant and Antimicrobial Activities against Some Clinical Bacterial Isolates. Bioinorg. Chem. Appl. 2022, 2022, 2432758. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A.; Afolayan, A.J. Chemical Composition, Antibacterial and in vitro Anti-Inflammatory Potentials of Essential Oils from Different Plant Parts of Moringa oleifera Lam. Am. J. Biochem. Biotechnol. 2018, 14, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Mofolo, M.J.; Kadhila, P.; Chinsembu, K.C.; Mashele, S.; Sekhoacha, M. Green synthesis of silver nanoparticles from extracts of Pechuel-loeschea leubnitziae: Their anti-proliferative activity against the U87 cell line. Inorg. Nano-Met. Chem. 2020, 50, 949–955. [Google Scholar] [CrossRef]
- Algebaly, A.S.; Mohammed, A.E.; Abutaha, N.; Elobeid, M.M. Biogenic synthesis of silver nanoparticles: Antibacterial and cytotoxic potential. Saudi J. Biol. Sci. 2020, 27, 1340–1351. [Google Scholar] [CrossRef]
- Mittal, A.K.; Thanki, K.; Jain, S.; Banerjee, U.C. Comparative studies of anticancer and antimicrobial potential of bioinspired silver and silver-selenium nanoparticles. J. Mater. Nanosci. 2016, 3, 22–27. [Google Scholar]


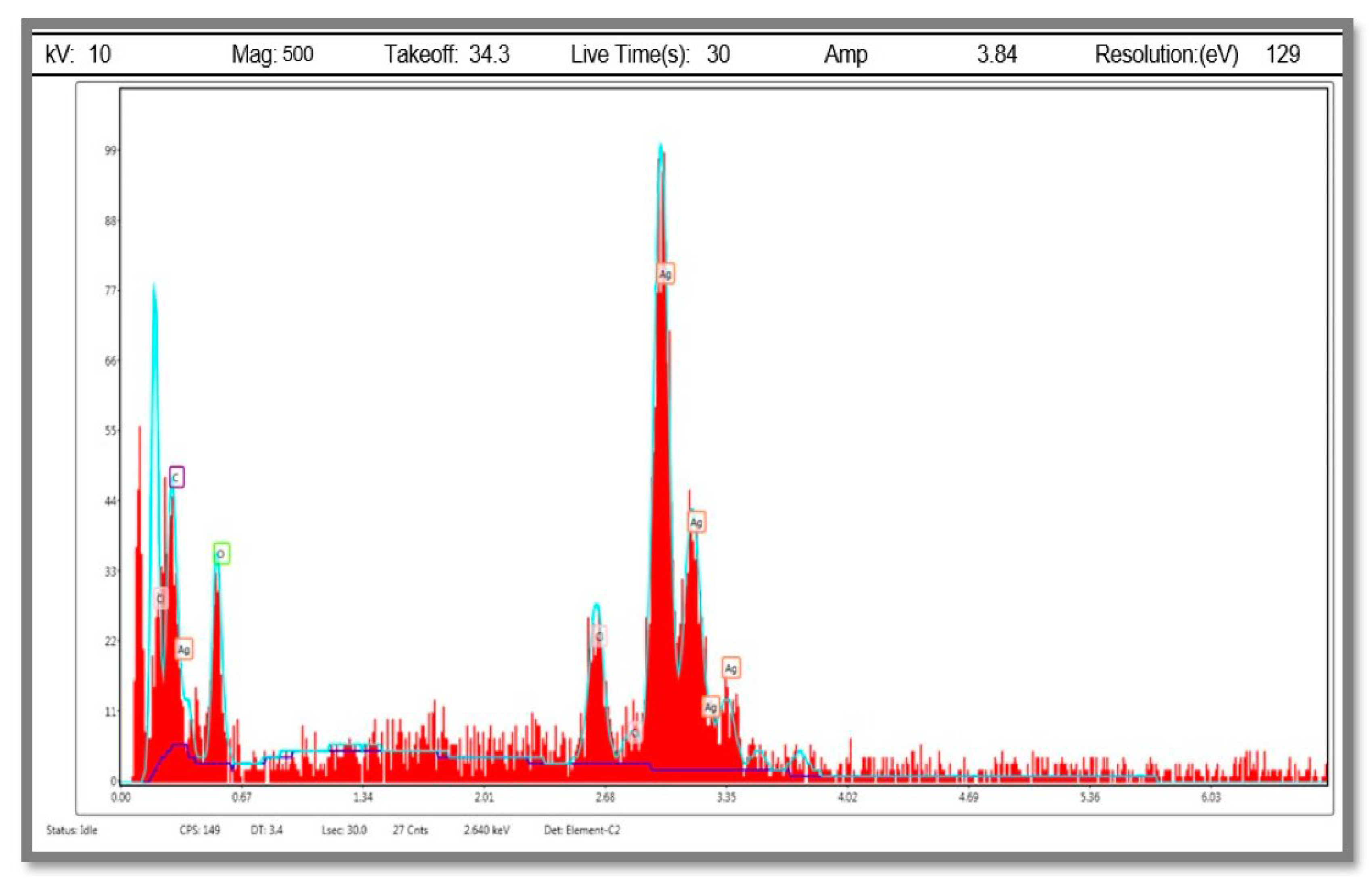

| Element | Weight % | Atomic % | Net Int. |
|---|---|---|---|
| C K | 4.56 | 20.13 | 6.02 |
| O K | 9.13 | 30.3 | 6.11 |
| ClK | 7.05 | 10.56 | 7.44 |
| AgL | 79.26 | 39.01 | 33.36 |
| Concentrations (µg/mL) | Mean ± SD (AgNPs) | Mean ± SD (Ascorbic Acid) |
|---|---|---|
| 10 | 0.15 ± 0.01 | 0.17 ± 0.02 |
| 20 | 0.13 ± 0.01 | 0.15 ± 0.01 |
| 30 | 0.10 ± 0.02 | 0.14 ± 0.03 |
| 40 | 0.09 ± 0.01 | 0.12 ± 0.02 |
| 50 | 0.07 ± 0.02 | 0.11 ± 0.01 |
| 60 | 0.03 ± 0.01 | 0.08 ± 0.03 |
| 70 | 0.009 ± 0.00 | 0.08 ± 0.01 |
| Concentrations (µg/mL) | Mean ± SD (AgNPs) | Mean ± SD (Acarbose) |
|---|---|---|
| 25 | 0.34 ± 0.034 | 0.43 ± 0.01 |
| 50 | 0.32 ± 0.036 | 0.35 ± 0.01 |
| 75 | 0.32 ± 0.03 | 0.32 ± 0.032 |
| 100 | 0.27 ± 0.01 | 0.22 ± 0.05 |
| 125 | 0.20 ± 0.10 | 0.14 ± 0.01 |
| Concentrations (µg/mL) | (Mean ± SD) (AgNPs) | (Mean ± SD) (Diclofenc Sodium) |
|---|---|---|
| 100 | 0.39 ± 0.00 | 0.45 ± 0.15 |
| 200 | 0.33 ± 0.005 | 0.40 ± 0.10 |
| 300 | 0.24 ± 0.017 | 0.32 ± 0.032 |
| 400 | 17 ± 0.010 | 0.22 ± 0.05 |
| 500 | 0.1 ± 0.00 | 0.14 ± 0.01 |
| Cell Lines | Concentrations (µg/mL) of (BP-AgNPs) | |||||
|---|---|---|---|---|---|---|
| 250 | 100 | 50 | 10 | 1 | ||
| HEK Cell Line | After 24 h |  |  |  |  |  |
| U87-MG Cell Line |  |  | 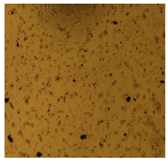 |  | 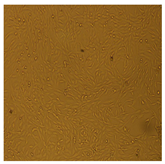 | |
 | ||||||
| DPPH Assay IC50 | Alpha-Amylase Assay IC50 | MTT Assay IC50 | |||
|---|---|---|---|---|---|
| BP-AgNPs | Ascorbic acid | BP-AgNPs | Acarbose | U87 after 24 and 48 h) | HEK293 (after 24 and 48 h) |
| 33.85 µg/mL | 50 µg/mL | 67 µg/mL | 110 µg/mL | 164.85 µg/mL, 150.38 µg/mL | 168.97 µg/mL, 167.79 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naveed, M.; Batool, H.; Rehman, S.u.; Javed, A.; Makhdoom, S.I.; Aziz, T.; Mohamed, A.A.; Sameeh, M.Y.; Alruways, M.W.; Dablool, A.S.; et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes 2022, 10, 1521. https://doi.org/10.3390/pr10081521
Naveed M, Batool H, Rehman Su, Javed A, Makhdoom SI, Aziz T, Mohamed AA, Sameeh MY, Alruways MW, Dablool AS, et al. Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes. 2022; 10(8):1521. https://doi.org/10.3390/pr10081521
Chicago/Turabian StyleNaveed, Muhammad, Hira Batool, Shafiq ur Rehman, Aneela Javed, Syeda Izma Makhdoom, Tariq Aziz, Amal A. Mohamed, Manal Y. Sameeh, Mashael W. Alruways, Anas S. Dablool, and et al. 2022. "Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract" Processes 10, no. 8: 1521. https://doi.org/10.3390/pr10081521
APA StyleNaveed, M., Batool, H., Rehman, S. u., Javed, A., Makhdoom, S. I., Aziz, T., Mohamed, A. A., Sameeh, M. Y., Alruways, M. W., Dablool, A. S., Almalki, A. A., Alamri, A. S., & Alhomrani, M. (2022). Characterization and Evaluation of the Antioxidant, Antidiabetic, Anti-Inflammatory, and Cytotoxic Activities of Silver Nanoparticles Synthesized Using Brachychiton populneus Leaf Extract. Processes, 10(8), 1521. https://doi.org/10.3390/pr10081521








