Valorization of Spent Sugarcane Fermentation Broth as a Source of Phenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Recovery of Phenolic Compounds
2.2.1. Sample Preparation
2.2.2. Solid Phase Extraction (SPE)
2.3. Analytical Methods
2.3.1. Total Phenolic Compounds (TPC) Content
2.3.2. LC−ESI−UHR−QqTOF−MS Analysis
2.3.3. Phenolic Compound Quantification
2.3.4. Antioxidant Activity
Radical Scavenging Activity (ABTS Assay)
Oxygen Radical Absorbance Capacity (ORAC Assay)
3. Results and Discussion
3.1. Spent Broth’s Phenolic Compound Characterization
3.2. Recovery of Phenolic Compounds
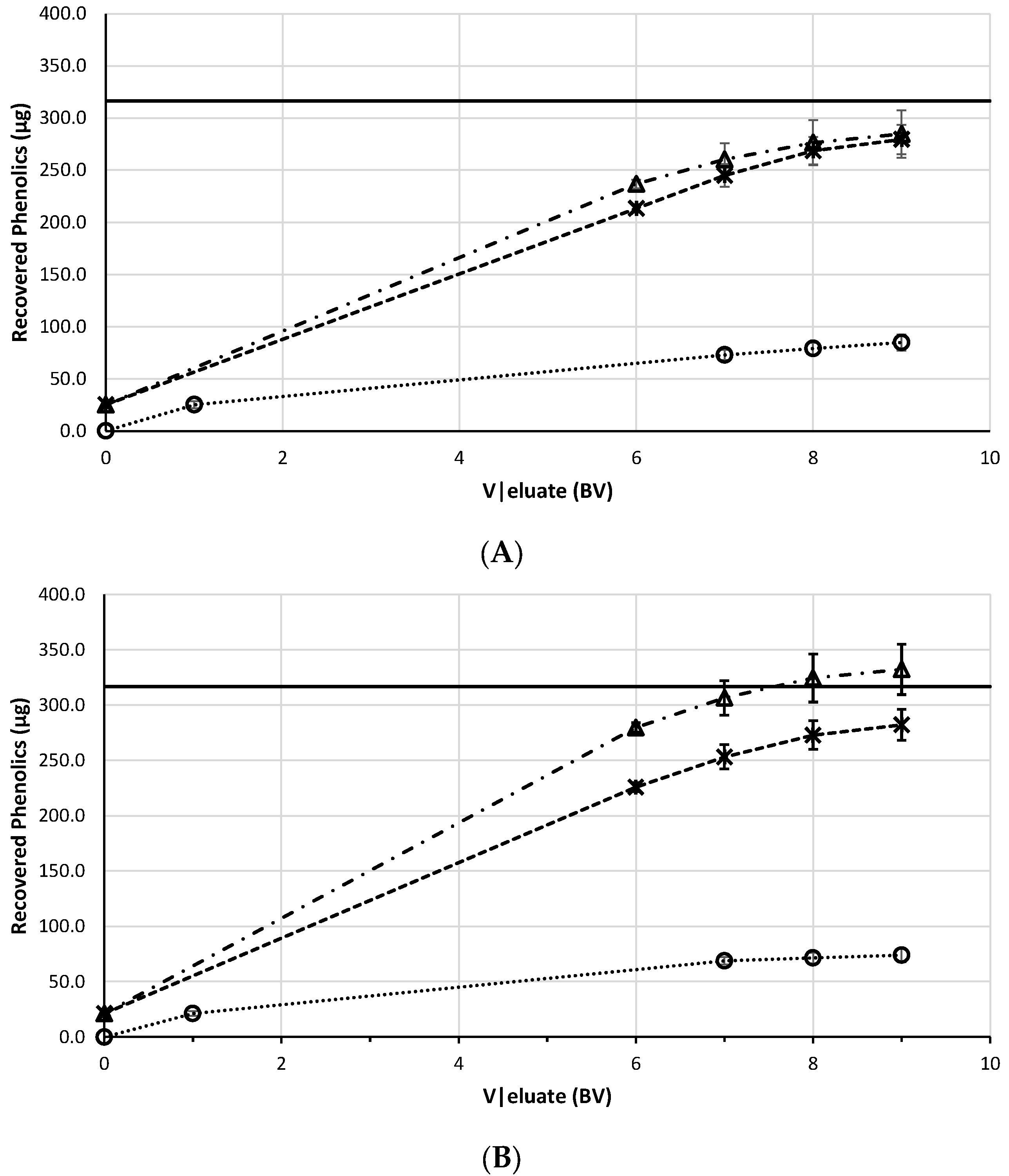
3.2.1. Selection of the Resin and Desorption Agent
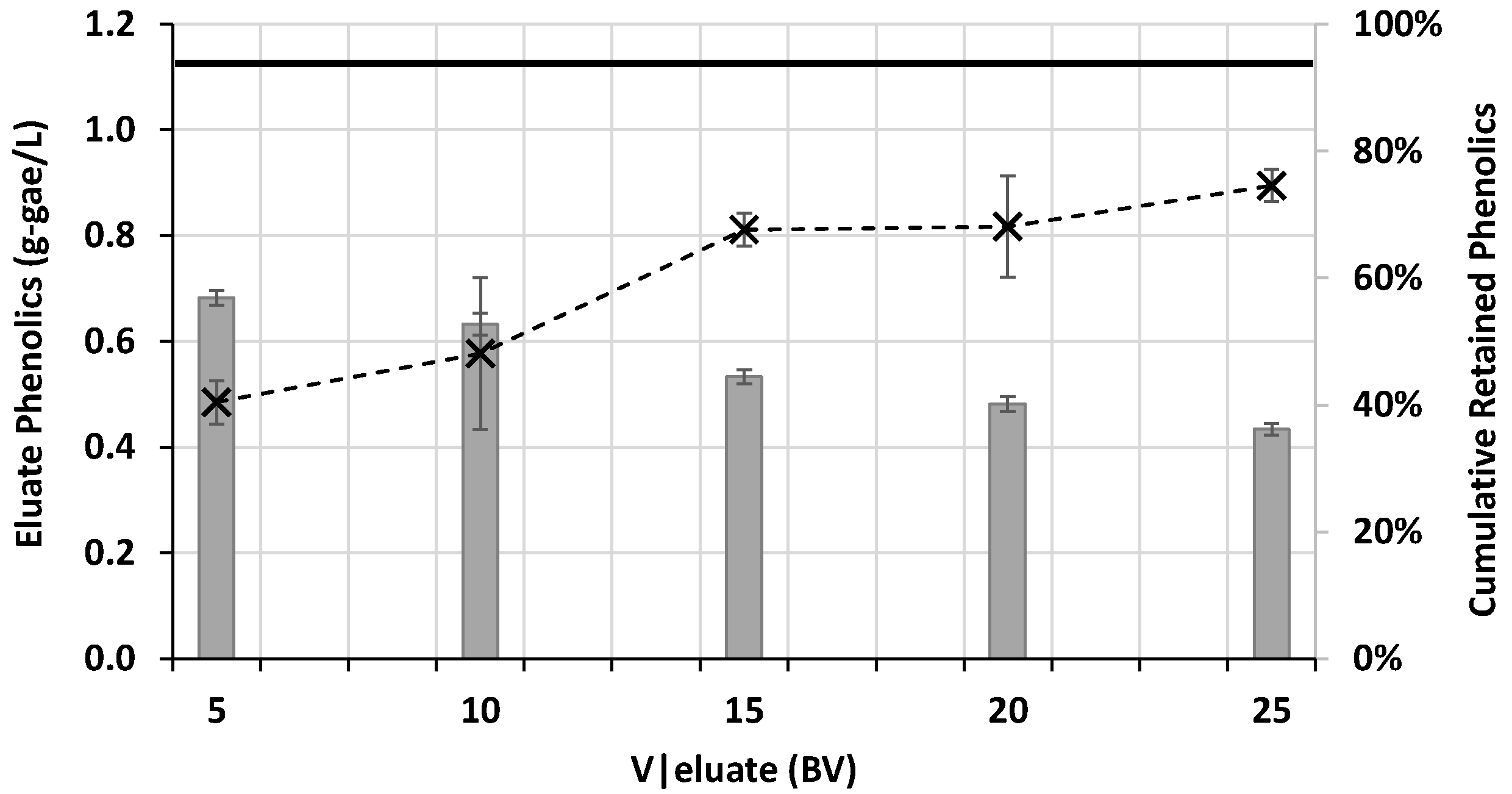
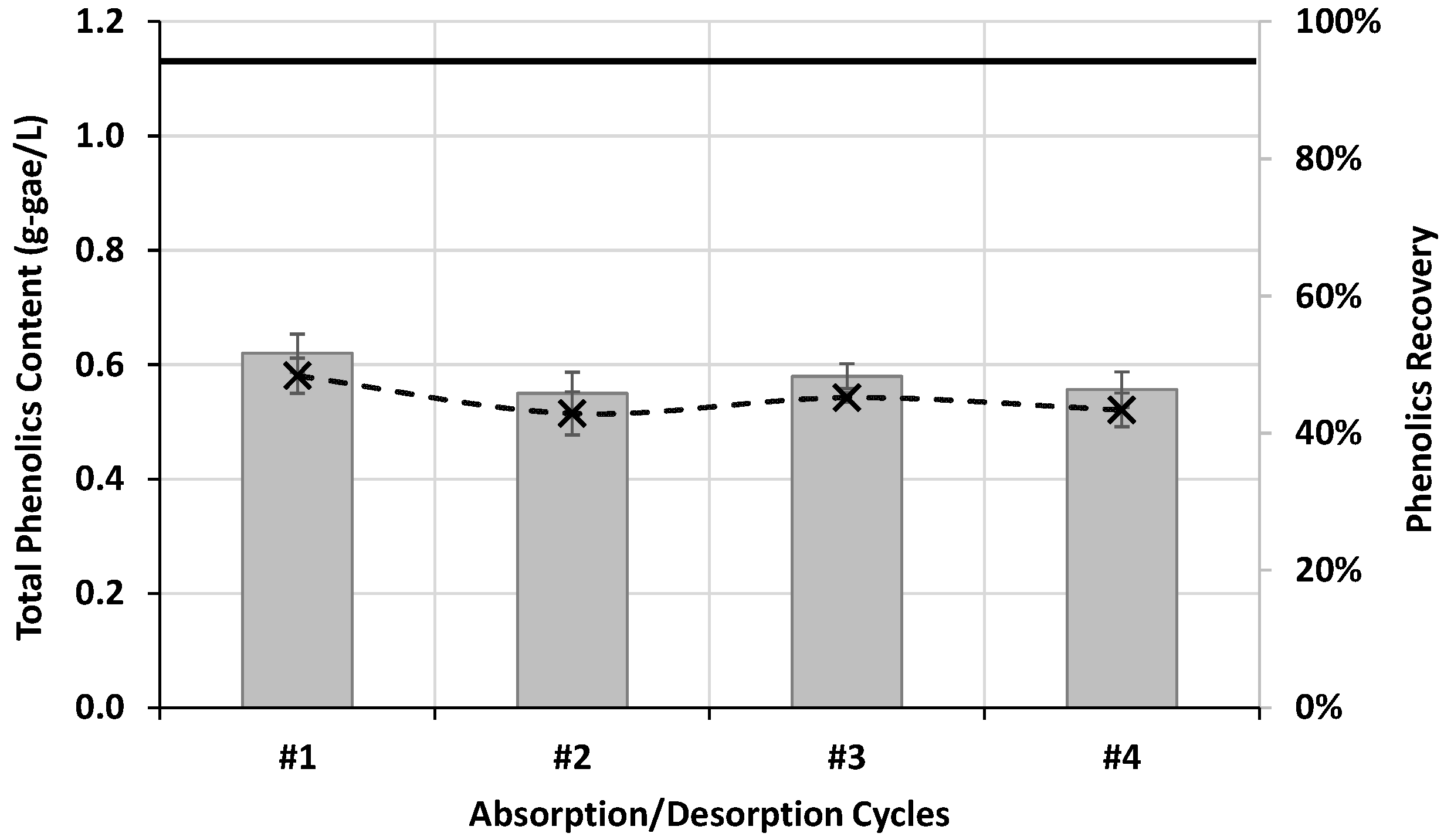
3.2.2. Process Assessment
3.3. Phenolic-Rich Extract Characterization
3.3.1. Phenolic-Rich Extract Characterization using LC−ESI−UHR−QqTOF−MS
3.3.2. Antioxidant Capacity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marques de Oliveira, C.M.D. Pathways of Oxidative Degradation of Wine. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2014. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry occurrence and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications. limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Caleja, C.; Ribeiro, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Nutr. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Shams, K.A.; Abdel-Azim, N.S.; Saleh, I.A.; Hegazy, M.-E.F.; El-Missiry, M.M.; Hammouda, F.M. Green technology: Economically and environmentally innovative methods for extraction of medicinal & aromatic plants (MAP) in Egypt. J. Chem. Pharm. Res. 2015, 7, 1050–1074. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet extraction of phenolic compounds from Vernonia cinerea leaves and its antioxidant activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Azhari, N.H. Vernonia cinerea leaves as the source of phenolic compounds. antioxidants and anti-diabetic activity using microwave-assisted extraction technique. Ind. Crops Prod. 2018, 122, 533–544. [Google Scholar] [CrossRef]
- O’Hara, I.M. The Sugarcane Industry, Biofuel, and Bioproduct Perspectives. In Sugarcane-Based Biofuels and Bioproducts, 1st ed.; O’Hara, I., Sagadevan, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; p. 4. [Google Scholar]
- Duarte-Almeida, J.M.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Genovese, M.I. Antioxidant Activity of Phenolics Compounds from Sugar Cane (Saccharum officinarum L.). Juice. Plant Foods Hum. Nutr. 2006, 61, 187–192. [Google Scholar] [CrossRef]
- Martini, C.; Verruma-Bernardi, M.R.; Borges, M.T.M.R.; Margarido, L.A.C.; Ceccato-Antonini, S.R. Yeast Composition of Sugar Cane Juice in Relation to Plant Varieties and Seasonality. Biosci, J. Uberlândia 2011, 27, 710–717. [Google Scholar]
- Thao, N.T.; Tuan, H.Q. Recovering Bioactive Compounds from Cane Sugar Wastes, 1st ed.; Nguyen, V.T., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Luz, D.A.; Gomes, A.C.C.; Simas, N.K.; Heringer, O.A.; Romão, W.; Lovatti, B.P.O.; Scherer, R.; Filgueiras, P.R.; Kuster, R.M. Sugarcane waste products as source of phytotoxic compounds for agriculture. Int. J. Recycl. Org. Waste Agric. 2020, 9, 385–397. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Brochier, B.; de Medeiros, J.K.; Marczak, L.D.F.; Mercali, G.D. Phenolic profile of sugarcane juice: Effects of harvest season and processing by ohmic heating and ultrasound. Food Chem. 2021, 347, 129058. [Google Scholar] [CrossRef] [PubMed]
- Da Gama, R.; Petrides, D. Production of Farnesene (a Terpene) via Fermentation-Process Modeling and Techno-Economic Assessment (TEA) using SuperPro Designe; Intelligen Inc.: Scotch Plains, NJ, USA, 2020. [Google Scholar]
- Jimenez, A.M.; Borja, R.; Martín, A.; Raposo, F. Kinetic analysis of the anaerobic digestion of untreated vinasses and vinasses previously treated with Penicillium decumbens. J. Environ. Manage. 2006, 80, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Siles, J.A.; García-García, I.; Martín, A.; Martín, M.A. Integrated ozonation and biomethanization treatments of vinasse derived from ethanol manufacturing. J. Hazard. Mater. 2011, 188, 247–253. [Google Scholar] [CrossRef]
- García-García, I.; Bonilla-Venceslada, J.L.; Jiménez-Peña, P.R.; Ramos-Gómez, E. Biodegradation of phenol compounds in vinasse using Aspergillus terreus and Geotrichum candidum. Water Res. 1997, 31, 2005–2011. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Pramanik, A.; Maji, S.K.; Haldar, S.; Mukhopadhyay, U.K.; Mukherjee, J. Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express 2012, 2, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.M.; Barros, A.S.; Silva Ferreira, A.C.; Silva, A.M.S. Influence of the temperature and oxygen exposure in red Port wine: A kinetic approach. Food Res. Int. 2015, 75, 337–347. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
- An, C.Y.; Hossain, M.; Alam, F.; Islam, A.; Khalil, I.; Alam, N.; Gan, S.H. Efficiency of Polyphenol Extraction from Artificial Honey Using C18 Cartridges and Amberlite® XAD-2 Resin: A Comparative Study. J. Chem. 2016, 2016, 8356739. [Google Scholar] [CrossRef] [Green Version]
- Weston, R.J.; Brocklebank, L.K.; Lu, Y. Identification and quantitative levels of antibacterial components of some New Zealand honeys. Food Chem. 2000, 70, 427–435. [Google Scholar] [CrossRef]
- Soto, M.L.; Conde, E.; González-López, N.; Conde, M.J.; Moure, A.; Sineiro, J.; Falqué, E.; Domínguez, H.; Núñez, M.J.; Parajó, J.C. Recovery and Concentration of Antioxidants from Winery Wastes. Molecules 2012, 17, 3008–3024. [Google Scholar] [CrossRef]
- Sakaki, K.; Habe, H.; Ikegami, T.; Yanagishita, T. Separation and Functional Evaluation of Dark Brown Colorants in Distillery Wastewater from a Sugarcane-Molasses-Derived Bioethanol Production Process. J. Water Environ. Technol. 2014, 12, 407–420. [Google Scholar] [CrossRef] [Green Version]
- Câmara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef] [PubMed]
- Keçili, R.; Büyüktiryaki, S.; Dolak, İ.; Hussain, C.M. The Use of Magnetic Nanoparticles in Sample Preparation Devices and Tools. In Handbook of Nanomaterials in Analytical Chemistry: Modern Trends in Analysis; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–95. [Google Scholar] [CrossRef]
- Brewer, A.; Flokrek, J.; Kleitz, F. A perspective on developing solid-phase extraction technologies for industrial-scale critical materials recovery. Green Chem. 2022, 24, 2753–2765. [Google Scholar] [CrossRef]
- Sunder, G.S.S.; Adhikari, S.; Rohanifar, A.; Poudel, A.; Kirchhoff, J.R. Evolution of environmentally friendly strategies for metal extraction. Separations 2020, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Scoma, A.; Pintucci, C.; Bertin, L.; Carlozzi, P.; Fava, F. Increasing the large-scale feasibility of a solid phase extraction procedure for the recovery of natural antioxidants from olive mill wastewaters. Chem. Eng. J. 2012, 198–199, 103–109. [Google Scholar] [CrossRef]
- Pavel, H. Comparison of batch and fixed bed column adsorption: A critical review. Int. J. Environ. Sci. Technol. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Type of Green Solvents Used in Separation and Preconcentration Methods. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Soylak, M., Yilmaz, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–266. [Google Scholar] [CrossRef]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic Acid: A green compound for food. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Pahalagedara, A.S.N.W.; Flint, S.; Palmer, J.; Brightwell, G.; Gupta, T.B. Antibacterial efficacy and possible mechanism of action of 2-hydroxyisocaproic acid (HICA). PLoS ONE 2022, 17, e0266406. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Bhagwat, S. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2; US Department of Agriculture: Washington, DC, USA, 2010; pp. 10–41. [Google Scholar]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Kalasariya, H.S.; Patel, N.B.; Yadav, A.; Perveen, K.; Yadav, V.K.; Munshi, F.M.; Yadav, K.K.; Alam, S.; Jung, Y.-K.; Jeon, B.-H. Characterization of Fatty Acids, Polysaccharides, Amino Acids, and Minerals in Marine Macroalga Chaetomorpha crassa and Evaluation of Their Potentials in Skin Cosmetics. Molecules 2021, 26, 7515. [Google Scholar] [CrossRef] [PubMed]

| Resin ID | XAD-2 | HP-20 |
|---|---|---|
| Brand (Grade name) | Amberlite® XAD®-2 | Diaion® HP-20 |
| Matrix | Styrene-divinylbenzene | Polystyrene/divinylbenzene |
| Surface area (m2/g) | 330 | 600 |
| Pore radius (Å) | 90 | 260 |
| Particle size (mm) | 0.25–0.84 | 0.25–0.60 |
| Density (g/mL) | 1.02 | 1.01 |
| Time (min) | Phase A 1 (%) Ionization Mode | |
|---|---|---|
| Negative | Positive | |
| 0.0 | 100 | 95 |
| 10.0 | 79 | - |
| 14.0 | 73 | 5 |
| 18.3 | 42 | 5 |
| 20.0 | 0 | 5 |
| 22.0 | 0 | 95 |
| 24.0 | 0 | 95 |
| 24.1 | 100 | 95 |
| 26.0 | 100 | 95 |
| Ionization Mode | ||
|---|---|---|
| Negative | Positive | |
| End plate offset voltage (V) | 500 | 500 |
| Capillary voltage (V) | 3000 | 4500 |
| Drying gas temperature (°C) | 200 | 220 |
| Drying gas flow (L/min) | 8.0 | 9.0 |
| Nebulizing gas pressure (bar) | 2.0 | 0.6 |
| Phenolic Compound | RT (min) | [M-H]− | Slope | Intercept | Linear Range (mg/L) |
|---|---|---|---|---|---|
| Gallic acid | 4.40 | 169.013 | 2563438 | 111233 | 0.02–1.00 |
| Protocatechuic acid | 6.03 | 153.017 | 2665367 | 90988 | 0.02–1.00 |
| Neochlorogenic acid | 6.73 | 353.088 | 3056952 | 101395 | 0.02–1.00 |
| Xanthurenic acid | 7.04 | 204.029 | 2728392 | 141330 | 0.02–1.00 |
| Kynurenic acid | 7.67 | 188.034 | 2467655 | 167725 | 0.02–1.00 |
| Gentisic acid | 7.78 | 153.017 | 2818874 | 176264 | 0.02–1.00 |
| Chlorogenic acid | 8.21 | 353.084 | 3299726 | 44199 | 0.02–1.00 |
| Dihydrocaffeic acid | 8.42 | 181.049 | 3224983 | 178143 | 0.02–1.00 |
| Cryptochlorogenic acid | 8.46 | 353.084 | 2951195 | 45491 | 0.02–1.00 |
| Vanillic acid | 8.81 | 167.033 | 226048 | 10686 | 0.02–1.00 |
| Caffeic acid | 8.95 | 179.033 | 4475963 | 201012 | 0.02–1.00 |
| Syringic acid | 9.28 | 197.043 | 412367 | 23715 | 0.02–1.00 |
| Vanillin | 10.52 | 151.038 | 332462 | 1281 | 0.02–1.00 |
| Schaftoside | 10.56 | 563.140 | 3318783 | 57986 | 0.02–1.00 |
| Isoorientin | 10.77 | 447.093 | 1664575 | 189245 | 0.02–1.00 |
| Isoschaftoside | 10.80 | 563.141 | 3141630 | 57687 | 0.02–1.00 |
| p-Coumaric acid | 10.85 | 163.038 | 3884267 | 179952 | 0.02–1.00 |
| Orientin | 10.86 | 447.093 | 2954171 | 137752 | 0.02–1.00 |
| Vitexin | 11.68 | 431.098 | 3187049 | 55612 | 0.02–1.00 |
| Vitexin-2″-O-rhamnoside | 11.71 | 577.156 | 1836598 | 23619 | 0.02–1.00 |
| Ferulic acid | 11.73 | 193.049 | 1769345 | 119285 | 0.02–1.00 |
| Isoferulic acid | 12.10 | 193.049 | 916475 | 63247 | 0.02–1.00 |
| Luteolin-4′-O-glucoside | 13.65 | 447.093 | 4435319 | 29097 | 0.02–1.00 |
| Prunin | 14.20 | 433.114 | 2131750 | 26077 | 0.02–1.00 |
| Luteolin | 16.74 | 285.040 | 13011525 | 158518 | 0.02–1.00 |
| Naringenin | 17.82 | 271.070 | 6506256 | 162853 | 0.02–1.00 |
| Apigenin | 17.91 | 269.045 | 8655494 | 235913 | 0.02–1.00 |
| Diosmetin | 18.13 | 299.055 | 9390446 | 124375 | 0.02–1.00 |
| Phenolic Compound Classes | Concentration (mg/L) | Phenolic Compounds |
|---|---|---|
| Hydroxybenzoic acids | 17.29 ± 0.38 | Gallic acid; Protocatechuic acid; Syringic acid; Gentisic acid; Dihydrobenzoic acid derivative; and Vanillic acid |
| Flavone glycosides | 2.45 ± 0.10 | Schaftoside; Isoschaftoside; Orientin; Isoorientin; Vitexin; and Vitexin-2″-O-rhamnoside |
| Hydroxycinnamic acids | 1.88 ± 0.06 | Caffeic acid; Caffeic acid derivative; p-Coumaric acid; p-Coumaroylmalic acid isomer I; p-Coumaroylmalic acid isomer II; Ferulic acid; Isoferulic acid; Feruloylquinic acid isomer I;ewº+’« Feruloylquinic acid isomer I; and Dihydrocaffeic acid |
| Chlorogenic acids | 1.35 ± 0.02 | Feruloylquinic acid isomer I; Feruloylquinic acid isomer II; cis-5-O-p-Coumaroylquinic acid; Neochlorogenic acid; Criptochlorogenic acid; and Chlorogenic acid |
| Flavones | 0.03 ± 0.00 | Luteolin; Apigenin; Tricin; and Diosmetin |
| Flavanones | 0.01 ± 0.00 | Naringenin |
| Rt (min) | m/z | Negative Mode Ions | Name | Molecular Formula | mSigma | MS/MS Score | Class |
|---|---|---|---|---|---|---|---|
| 7.04 | 204.029 | [M-H]− | Xanthurenic acid | C10H7NO4 | 4.5 | 986.2 | Organic acid |
| 7.67 | 188.034 | [M-H]− | Kynurenic acid | C10H7NO3 | 4.1 | 992.8 | Organic acid |
| 11.13 | 165.054 | [M-H]−. [M+Cl]− | Phenyllactic acid | C9H10O3 | 0.5 | 984.6 | Organic acid |
| 9.75 | 131.070 | [M-H]− | Leucinic acid | C6H12O3 | 0.6 | 908.6 | Organic acid |
| 6.33 | 117.054 | [M-H]− | 2-Hydroxy-2-methylbutyric acid | C5H10O3 | 1.0 | 982.5 | Organic acid |
| 11.14 | 195.065 | [M-H]− | Homoveratric acid | C10H12O4 | 0.7 | 920.1 | Organic acid |
| 10.56 | 563.140 | [M-H]−. [M-H-H2O]− | Schaftoside | C26H28O14 | 3.7 | 910.2 | Phenolic |
| 8.42 | 181.049 | [M-H]− | 3,4-Dihydroxyhydrocinnamic acid (Dihydrocaffeic acid) | C9H10O4 | 0.7 | 937.1 | Phenolic |
| 7.76 | 137.022 | [M-H]− | 4-Hydroxibenzoic acid | C7H6O3 | 0.2 | 999.7 | Phenolic |
| 7.82 | 175.060 | [M-H]− | 2-Isopropylmalic acid | C7H12O5 | 0.8 | 901.6 | Organic acid |
| 6.96 | 181.049 | [M-H]− | Hydroxyphenyllactic acid isomer I | C9H10O4 | 0.6 | 857.1 | Organic acid |
| 11.56 | 206.081 | [M-H]− | N-Acetyl-D-phenylalanine | C11H13NO3 | 3.1 | 842.9 | AA |
| 12.40 | 245.092 | [M-H]− | N-Acetyl-DL-tryptophan | C13H14N2O3 | 2.5 | 920.0 | AA |
| 1.94 | 117.018 | [M-H]− | Succinic acid | C4H6O4 | 4.1 | 942.3 | Organic acid |
| 9.01 | 153.018 | [M-H]− | Dihydroxybenzoic acid derivative | C7H6O4 | 0.5 | 737.5 | Phenolic |
| 1.74 | 89.023 | [M-H]− | Lactic acid | C3H6O3 | 6.3 | 979.4 | Organic acid |
| 7.18 | 109.028 | [M-H]− | Pyrocatechol | C6H6O2 | 7.0 | 939.6 | Phenolic |
| 9.28 | 197.043 | [M-H]− | Syringic acid | C9H10O5 | 4.7 | 972.6 | Phenolic |
| 1.57 | 191.054 | [M-H]− | Quinic acid isomer I | C7H12O6 | 8.7 | 884.2 | Organic acid |
| 4.68 | 195.065 | [M-H]− | Acetosyringone | C10H12O4 | 2.2 | 636.8 | Phenolic |
| 1.53 | 179.054 | [M-H]− | β-D-Galactopyranose | C6H12O6 | 8.5 | 913.4 | Saccharide |
| 8.53 | 179.034 | [M-H]− | Acetylsalicylic acid | C9H8O4 | 7.6 | 870.5 | Organic acid |
| 3.84 | 147.065 | [M-H]− | Mevalonic acid isomer I | C6H12O4 | 2.4 | 992.8 | Organic acid |
| 7.78 | 153.017 | [M-H]− | Gentisic acid | C7H6O4 | 1.0 | 996.8 | Phenolic |
| 8.81 | 167.033 | [M-H]− | Vanillic acid | C8H8O4 | 6.8 | 963.9 | Phenolic |
| 9.15 | 137.023 | [M-H]− | p-Salicylic acid isomer I | C7H6O3 | 7.3 | 982.6 | Organic acid |
| 13.76 | 137.023 | [M-H]− | Salicylic acid | C7H6O3 | 4.8 | 998.6 | Organic acid |
| 1.78 | 179.052 | [M-H]− | D-Tagatose | C6H12O6 | 13.3 | 727.3 | Saccharide |
| 7.30 | 222.076 | [M-H]− | Acetyl-L-tyrosine isomer I | C11H13NO4 | 2.0 | 793.5 | AA |
| 6.03 | 153.017 | [M-H]− | Protocatechuic acid | C7H6O4 | 0.4 | 979.6 | Phenolic |
| 11.80 | 613.214 | [M-H]− | Galα1-4Galβ1-4GlcNacβ-Sp | C22H38N4O16 | 16.5 | 673.0 | Saccharide |
| 11.71 | 577.156 | [M-H]− | Vitexin-2″-O-rhamnoside | C27H30O14 | 3.4 | 980.2 | Phenolic |
| 8.78 | 159.065 | [M-H]− | Pimelic acid | C7H12O4 | 8.4 | 842.0 | Organic acid |
| 13.90 | 187.096 | [M-H]− | Nonanedioic acid | C9H16O4 | 1.0 | 818.5 | Organic acid |
| 12.14 | 204.065 | [M-H]− | Indolelactic acid | C11H11NO3 | 7.8 | 793.8 | Organic acid |
| 11.68 | 431.098 | [M-H]− | Vitexin | C21H20O10 | 15.1 | 947.4 | Phenolic |
| 7.62 | 194.045 | [M-H]− | N-Acetyl-5-aminosalicylic acid | C9H9NO4 | 7.9 | 875.2 | Organic acid |
| 10.43 | 172.097 | [M-H]− | Acetyl-DL-Leucine isomer I | C8H15NO3 | 4.4 | 922.8 | AA |
| 8.75 | 177.018 | [M-H]−. [M+Cl]− | Esculetin | C9H6O4 | 7.3 | 802.5 | Phenolic |
| 17.82 | 271.060 | [M-H]− | Naringenin | C15H12O5 | 6.4 | 899.8 | Phenolic |
| 17.94 | 357.133 | [M-H]− | Matairesinol | C20H22O6 | 10.0 | 934.9 | Lignan |
| 1.82 | 103.002 | [M-H]−. [M+Cl]− | β-Hydroxypyruvic acid | C3H4O4 | 5.0 | 901.5 | Organic acid |
| 8.95 | 179.033 | [M-H]− | Caffeic acid | C9H8O4 | 2.0 | 964.2 | Phenolic |
| 18.13 | 299.055 | [M-H]− | Diosmetin | C16H12O6 | 19.2 | 923.2 | Phenolic |
| 5.66 | 212.001 | [M-H]− | Indoxylsulfuric acid | C8H7NO4S | 1.6 | 938.0 | Organic acid |
| 3.95 | 161.044 | [M-H]− | 3-Hydroxymethylglutaric acid | C6H10O5 | 9.2 | 964.1 | Organic acid |
| 5.74 | 218.102 | [M-H]− | Pantothenic acid | C9H17NO5 | 1.5 | 880.1 | Vitamin |
| 1.69 | 341.106 | [M-H]−. [M-H-H2O]− | Sucrose | C12H22O11 | 5.4 | 981.6 | Saccharide |
| 9.30 | 121.028 | [M-H]- | 4-hydroxybenzaldehyde | C7H6O2 | 14.5 | 968.9 | Phenolic |
| 7.47 | 137.023 | [M-H]- | 3,4-Dihydrobenzaldehyde | C7H6O3 | 0.3 | 676.8 | Phenolic |
| 1.77 | 133.012 | [M-H]- | L-Malic acid | C4H6O5 | 6.4 | 948.6 | Organic acid |
| 10.31 | 367.103 | [M-H]- | Feruloylquinic acid isomer II | C17H20O9 | 15.4 | 956.4 | Phenolic |
| 10.86 | 447.093 | [M-H]-. [M+Cl]- | Orientin | C21H20O11 | 9.4 | 970.2 | Phenolic |
| 9.67 | 375.130 | [M-H]- | Riboflavin | C17H20N4O6 | 9.7 | 748.7 | Vitamin |
| 9.90 | 172.097 | [M-H]- | Acetyl-DL-Leucine isomer II | C8H15NO3 | 5.8 | 852.0 | AA |
| 1.71 | 177.039 | [M-H]- | L-Gulonic γ-lactone | C6H10O6 | 9.8 | 727.0 | Lactone |
| 8.10 | 223.061 | [M-H]- | Sinapic acid | C11H12O5 | 9.9 | 615.6 | Phenolic |
| 8.94 | 181.050 | [M-H]- | Hydroxyphenyllactic acid isomer II | C9H10O4 | 7.6 | 718.0 | Organic acid |
| 10.77 | 447.093 | [M-H]- | Isoorientin | C21H20O11 | 10.2 | 981.2 | Phenolic |
| 16.74 | 285.040 | [M-H]- | Luteolin | C15H10O6 | 10.3 | 831.0 | Phenolic |
| 10.85 | 163.038 | [M-H]-. [M+Cl]- | p-Coumaric acid | C9H8O3 | 6.1 | 994.5 | Phenolic |
| 8.52 | 367.103 | [M-H]-. [M-H-H2O]- | Feruloylquinic acid isomer I | C17H20O9 | 3.3 | 850.5 | Phenolic |
| 16.71 | 201.111 | [M-H]- | Sebacic acid | C10H18O4 | 8.0 | 709.0 | Organic acid |
| 11.73 | 193.049 | [M-H]- | Ferulic acid | C10H10O4 | 8.0 | 946.7 | Phenolic |
| 5.54 | 167.034 | [M-H]- | Homogentisic acid | C8H8O4 | 7.2 | 734.7 | Phenolic |
| 19.30 | 211.133 | [M-H]-. [M+Cl]- | Dihydrojasmonic acid | C12H20O3 | 12.4 | 961.9 | Lipid |
| 5.19 | 129.018 | [M-H]- | Citraconic acid | C5H6O4 | 5.8 | 625.4 | Organic acid |
| 17.91 | 269.045 | [M-H]- | Apigenin | C15H10O5 | 13.3 | 751.6 | Phenolic |
| 13.98 | 174.055 | [M-H]− | 3-Indoleacetic acid | C10H9NO2 | 17.6 | 888.6 | Organic acid |
| 1.75 | 503.159 | [M-H]− | 1-Kestose | C18H32O16 | 10.0 | 627.8 | Saccharide |
| 12.82 | 144.044 | [M-H]− | Indole-4-carboxaldehyde | C9H7NO | 22.2 | 648.8 | Alkaloid |
| 7.56 | 152.034 | [M-H]− | 3-Hydroxyanthranilic acid | C7H7NO3 | 12.0 | 839.1 | Organic acid |
| 9.65 | 337.093 | [M-H]− | cis-5-O-p-Coumaroylquinic acid | C16H18O8 | 11.5 | 845.2 | Phenolic |
| 8.46 | 353.088 | [M-H]− | Cryptochlorogenic acid | C16H18O9 | 7.2 | 860.4 | Phenolic |
| 1.80 | 191.017 | [M-H]− | Isocitric acid | C6H8O7 | 10.6 | 987.8 | Organic acid |
| 16.88 | 359.149 | [M-H]− | Lariciresinol | C20H24O6 | 19.7 | 638.8 | Lignan |
| 4.40 | 169.013 | [M-H]− | Gallic acid | C7H6O5 | 14.0 | 982.1 | Phenolic |
| 6.73 | 353.088 | [M-H]− | Neochlorogenic | C16H18O9 | 30.7 | 768.5 | Phenolic |
| 5.58 | 222.076 | [M-H]− | Acetyl-L-tyrosine isomer II | C11H13NO4 | 12.2 | 734.2 | AA |
| 2.39 | 173.007 | [M-H]− | Trans-Aconitic acid | C6H6O6 | 0.9 | 997.1 | Organic acid |
| 16.16 | 181.069 | [M-H]− | Sorbitol isomer I | C6H14O6 | 18.4 | 847.7 | Saccharide |
| 3.22 | 147.028 | [M-H]−. [M+Cl]− | 2-Hydroxy-2-methylbutanedioic acid | C5H8O5 | 7.9 | 613.6 | Organic acid |
| 16.47 | 181.069 | [M-H]− | Sorbitol isomer II | C6H14O6 | 13.0 | 837.1 | Saccharide |
| 8.21 | 191.054 | [M-H]− | Quinic acid isomer II | C7H12O6 | 6.6 | 601.6 | Organic acid |
| Rt (min) | m/z | Negative Mode Ions | Name | Molecular Formula | mSigma | MS/MS Score | Class |
|---|---|---|---|---|---|---|---|
| 5.25 | 653.164 | [M+H]+ | Tricin 7-rhamnosyl-(1->2)-galacturonide | C29H32O17 | 8.8 | 733.3 | Phenolic |
| 3.97 | 377.142 | [M+H]+, [M+Na]+ | Riboflavin | C17H20N4O6 | 6.9 | 976.6 | Vitamin |
| 5.37 | 639.184 | [M+H]+ | 6-Hydroxyluteolin 6,4′-dimethyl ether 7-rutinoside isomer II | C29H34O16 | 9.8 | 891.3 | Phenolic |
| 5.24 | 639.185 | [M+H]+ | 6-Hydroxyluteolin 6,4′-dimethyl ether 7-rutinoside isomer I | C29H34O16 | 19.9 | 931.2 | Phenolic |
| 14.42 | 282.277 | [M+H]+ | Elaidamide | C18H35NO | 2.5 | 909.4 | Lipid |
| 7.15 | 331.077 | [M+H]+ | Malvidin | C17H14O7 | 15.2 | 695.3 | Phenolic |
| 1.22 | 527.152 | [M+Na]+, [M+K]+ | Levan | C18H32O16 | 18.3 | 725.4 | Saccharide |
| 5.31 | 403.098 | [M+H]+ | 4-O-Demethyl-13-dihydroadriamycinone | C20H18O9 | 19.7 | 878.0 | Phenolic |
| 5.22 | 376.219 | [M+H]+ | Ile Pro Phe | C20H29N3O4 | 17.2 | 693.9 | Peptide |
| 1.21 | 365.101 | [M+Na]+, [M+K]+ | Lactulose | C12H22O11 | 13.9 | 838.6 | Saccharide |
| 4.22 | 355.113 | [M+H]+ | Asp-Leu-OH | C15H18N2O8 | 19.7 | 890.8 | Peptide |
| 3.60 | 371.130 | [M+H]+ | Perilloside E | C17H22O9 | 20.6 | 635.2 | Phenolic |
| 6.54 | 309.084 | [M+H]+ | Flazine | C17H12N2O4 | 15.0 | 632.0 | Alkaloid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, C.M.; Horta, B.; Leal, T.; Pintado, M.; Oliveira, C.S.S. Valorization of Spent Sugarcane Fermentation Broth as a Source of Phenolic Compounds. Processes 2022, 10, 1339. https://doi.org/10.3390/pr10071339
Oliveira CM, Horta B, Leal T, Pintado M, Oliveira CSS. Valorization of Spent Sugarcane Fermentation Broth as a Source of Phenolic Compounds. Processes. 2022; 10(7):1339. https://doi.org/10.3390/pr10071339
Chicago/Turabian StyleOliveira, Carla Maria, Bruno Horta, Tânia Leal, Manuela Pintado, and Catarina S. S. Oliveira. 2022. "Valorization of Spent Sugarcane Fermentation Broth as a Source of Phenolic Compounds" Processes 10, no. 7: 1339. https://doi.org/10.3390/pr10071339
APA StyleOliveira, C. M., Horta, B., Leal, T., Pintado, M., & Oliveira, C. S. S. (2022). Valorization of Spent Sugarcane Fermentation Broth as a Source of Phenolic Compounds. Processes, 10(7), 1339. https://doi.org/10.3390/pr10071339








