Parametric Sensitivity Analysis and Performance Evaluation of High-Temperature Anion-Exchange Membrane Fuel Cell
Abstract
:1. Introduction
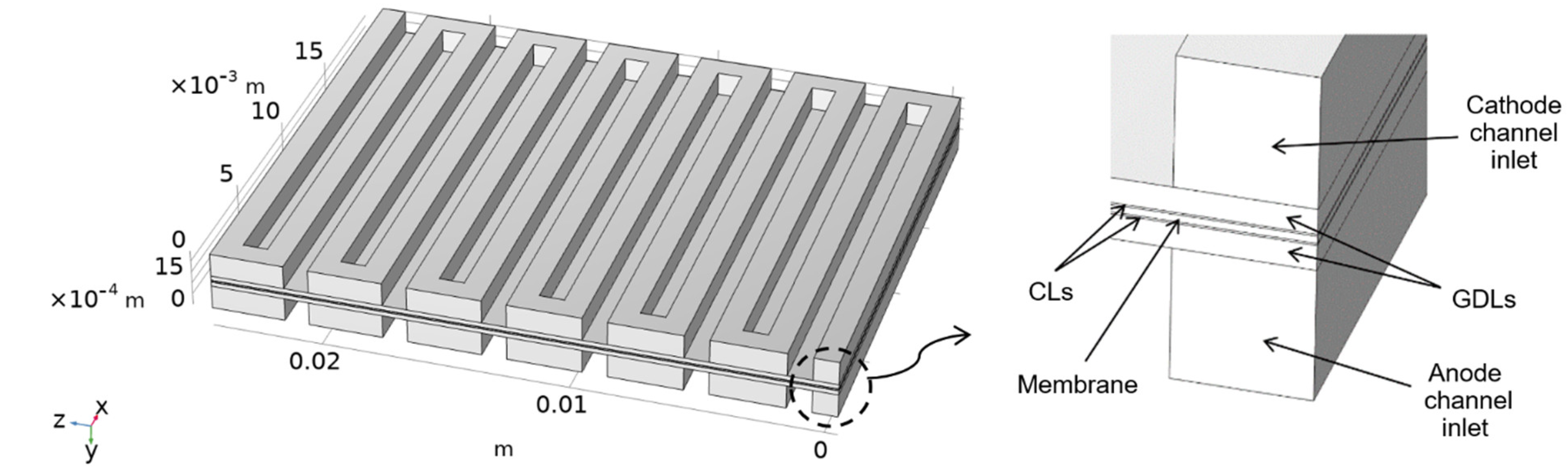
2. Model Description
2.1. Assumptions
- The cell operates in a steady-state mode;
- The ideal gas assumption is considered for both individual gases and gas mixtures;
- The flow is described as laminar due to the usually low fluid speed;
- The isothermal model is chosen because temperature variations are typically negligible in fuel cells with small active areas;
- In HT-AEMFC, water is only expected to exist in the vapor phase; hence, a single-phase flow is assumed;
- GDLs, CLs, and membranes are considered isotropic materials with uniformly distributed porosities;
- There is no cross-over of reactants across the membrane; and
- The current model ignores water that dissolves in the membrane as well as its impact on ionic conductivity because the processes underlying these phenomena are yet unclear for the LDPE-BTMA AEM. Nevertheless, in the current model, the relative humidity in both the anode and the cathode is at the highest level, deterring extensive water concentration gradient across the membrane. In addition, according to the observed data [28,29], the water penetration via the membrane–gas interface is the limiting transport mechanism because water is carried more easily across the membrane when it is exposed to liquid water, and not vapor, on its sides.
2.2. Governing Equations
2.2.1. Conservation of Mass
2.2.2. Conservation of Momentum
2.2.3. Conservation of Species
2.2.4. Conservation of Charge
2.3. Reaction Kinetics
2.4. Boundary Conditions
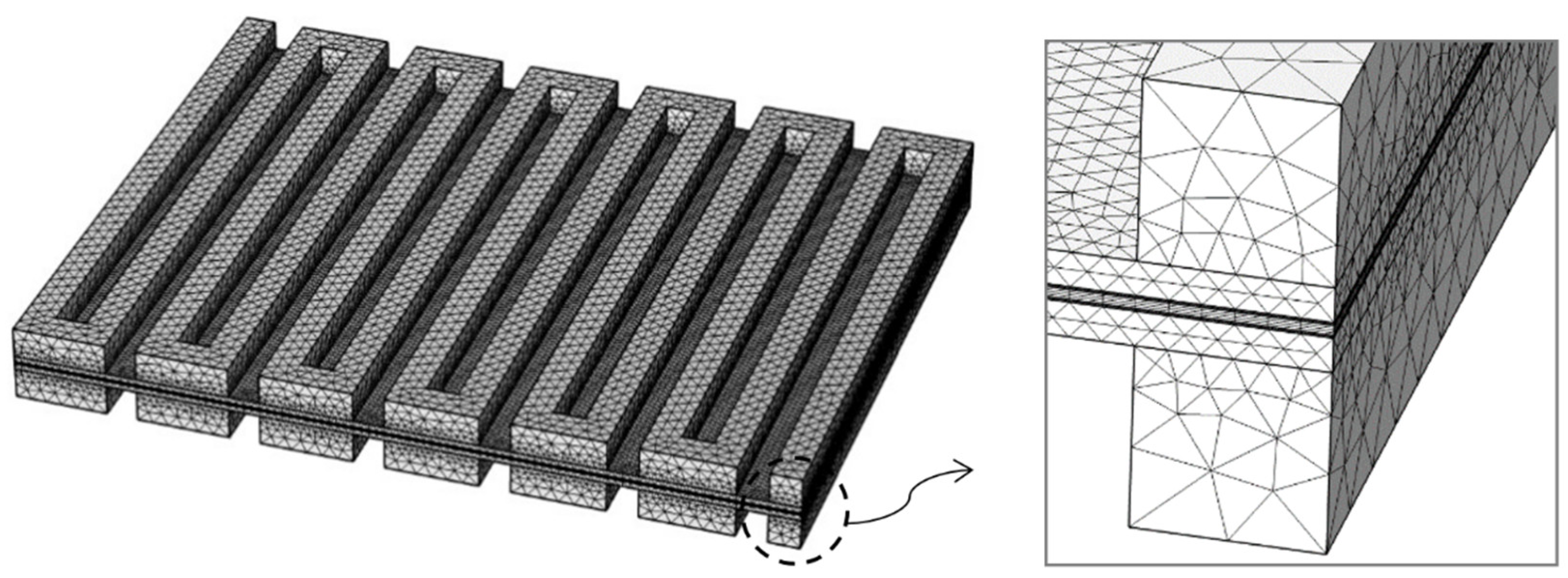
2.5. Numerical Approach
3. Results and Discussion
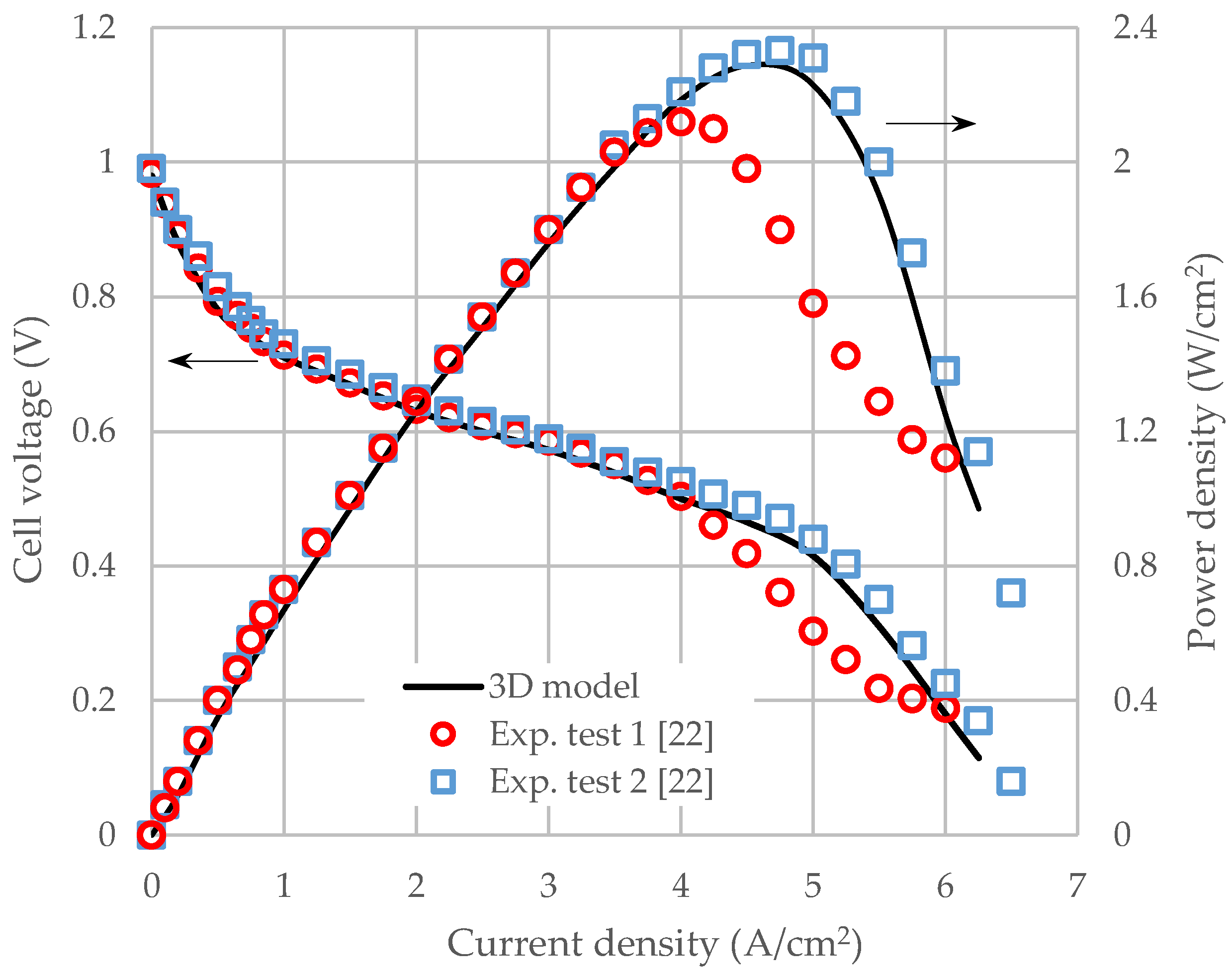
3.1. Model Validation
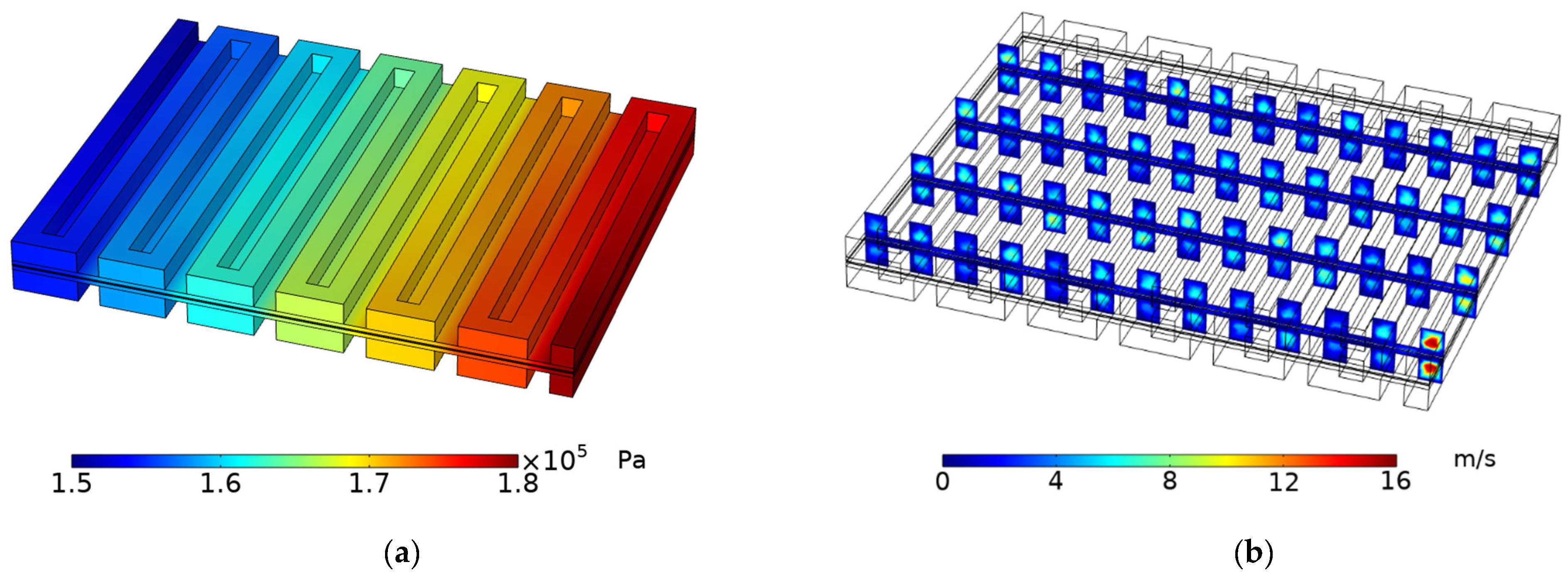
3.2. Pressure and Velocity Distribution
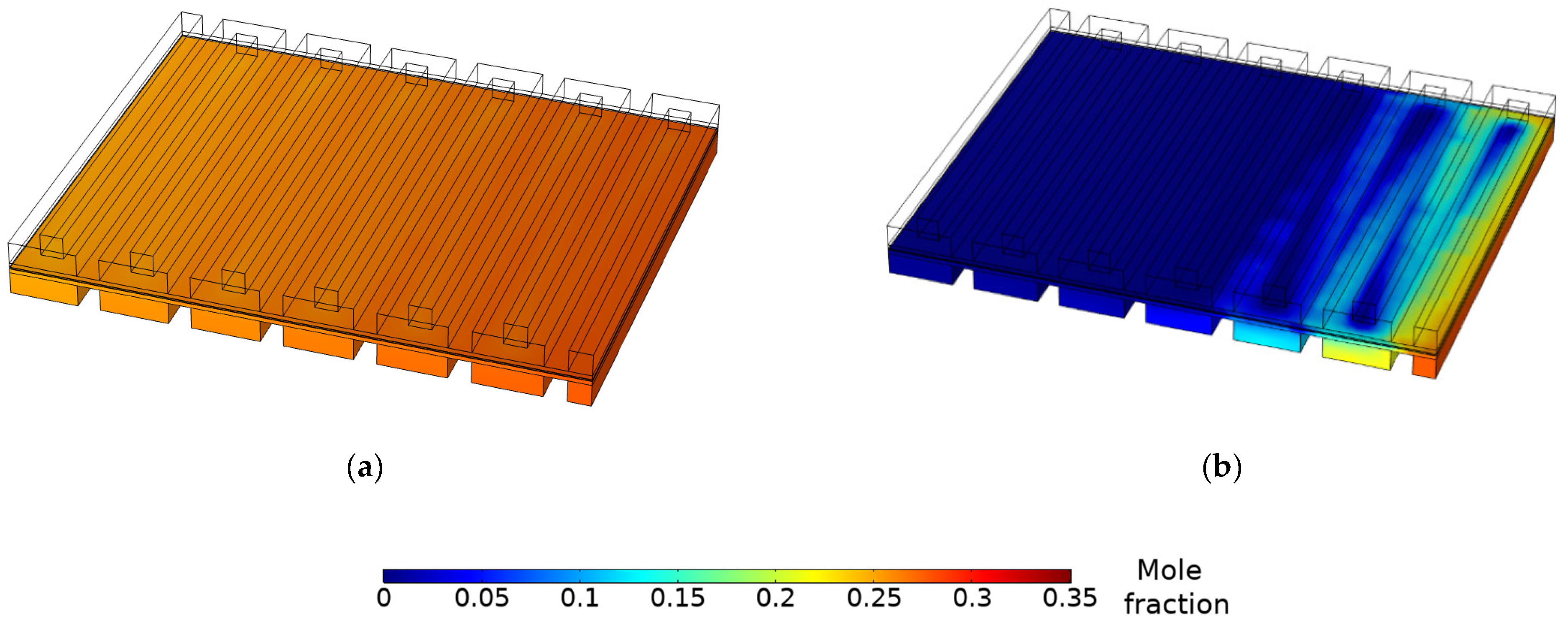
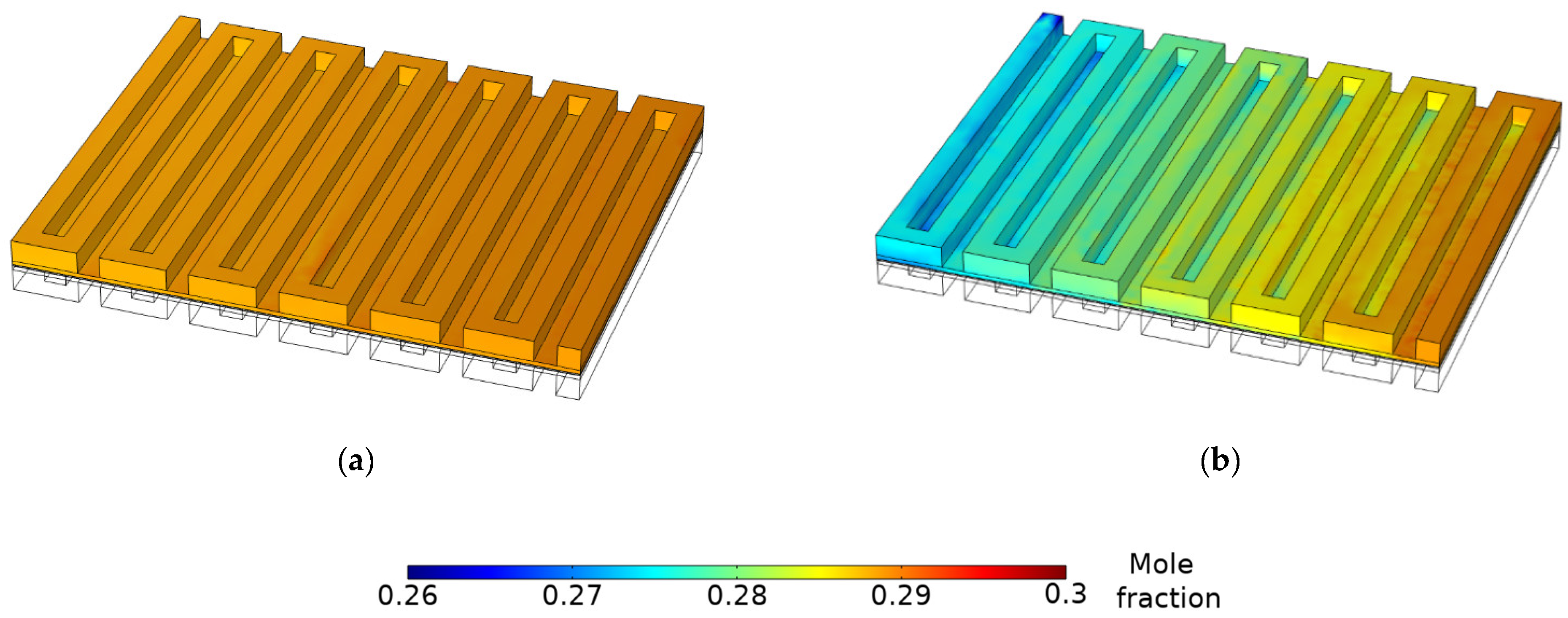
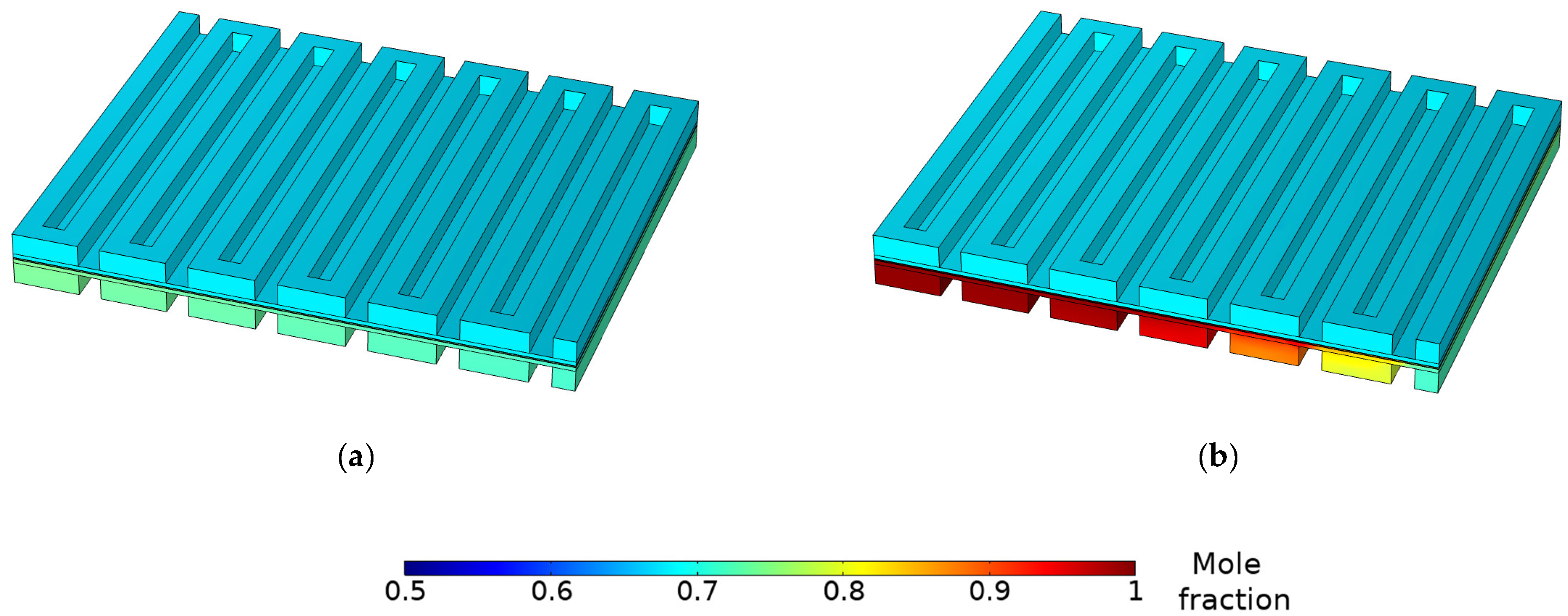
3.3. Species Concentration Distribution
3.4. Current Density Distribution
3.5. Effect of Operating Temperature
3.6. Effect of Flow Rate
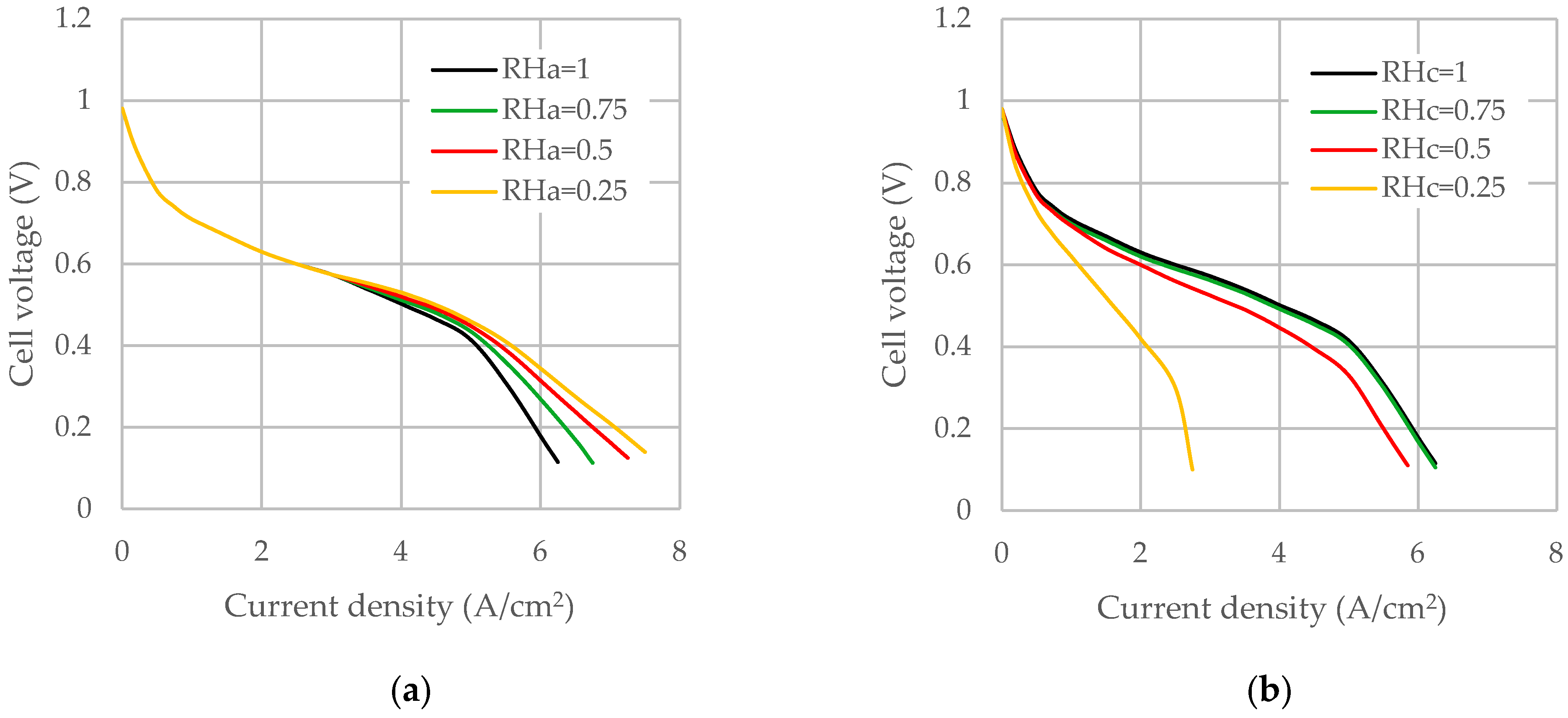
3.7. Effect of Inlet Humidification
4. Conclusions
- Due to the relatively long reactant flow path created by the single serpentine flow field design, there is a large pressure drop from the inlet to the exit. Moreover, the water generated at the anode is challenging to remove, which results in poor cell performance.
- Through its impact on species transport, membrane conductivity, and electrochemical kinetics, the temperature is seen to have a substantial impact on the fuel cell performance.
- The gas and water concentration has a major effect on the magnitude of local current density. Due to reactant depletion and water formation along the channel, the region near the inlet demonstrates the most current, whereas the region near the outlet shows low current values. Limiting current occurs because of mass transport restrictions on the anode side.
- High inlet flow rates increase oxygen and water consumption in the cathode while reducing water accumulation at the anode, with the latter being more crucial.
- The inlet RH of gases plays an important role in cell performance. It was found that the increase in the humidity of cathode inlet gas has a favorable effect on fuel cell performance while the increase in the humidity of the anode inlet has a detrimental effect on the cell performance. At high current densities, the sensitivity of cell performance to this parameter increases substantially. Additional humidification of the cathode improves overall performance, although the benefit becomes less pronounced at higher humidification levels. By lowering the RHa while maintaining high RHc, the cell water can be efficiently regulated.
Funding
Conflicts of Interest
Nomenclature
| A | Surface area, |
| AEMFC | anion exchange membrane fuel cell |
| CL | catalyst layer |
| D | diffusivity, m2 s−1 |
| F | Faraday’s constant, C mol−1 |
| GDL | gas diffusion layer |
| h | height, m |
| i | volumetric reaction rate, A cm−3 |
| K | permeability, m2 |
| M | molecular weight, kg mol−1 |
| P | pressure, Pa |
| PEMFC | proton exchange membrane fuel cell |
| R | universal gas constant, 8.314 J mol−1 K−1 |
| RH | relative humidity |
| S | source term |
| T | temperature, K |
| u | velocity, m s−1 |
| V | Voltage, V |
| w | width, m |
| molar fraction | |
| Greek symbols | |
| α | charge transfer coefficient |
| δ | thickness, m |
| ε | porosity |
| σ | electric conductivity, S m−1 |
| μ | dynamic viscosity, kg m−1 s−1 |
| η | activation overpotential, V |
| electric potential, V | |
| molar volume of species, m3 mol−1 | |
| mass fraction | |
| ρ | density, kg m−3 |
| Subscripts and superscripts | |
| a | anode |
| c | cathode |
| ch | channel |
| dp | dew point |
| i | species index |
| in | inlet |
| j | species index |
| m | membrane phase |
| mix | mixture |
| ref | reference value |
| rev | reversible |
| s | solid phase |
| sat | saturation |
| 0 | standard value |
References
- Dekel, D.R.; Rasin, I.G.; Page, M.; Brandon, S. Steady State and Transient Simulation of Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 191–204. [Google Scholar] [CrossRef]
- Lee, K.S.; Maurya, S.; Kim, Y.S.; Kreller, C.R.; Wilson, M.S.; Larsen, D.; Elangovan, S.E.; Mukundan, R. Intermediate Temperature Fuel Cells: Via an Ion-Pair Coordinated Polymer Electrolyte. Energy Environ. Sci. 2018, 11, 979–987. [Google Scholar] [CrossRef]
- Mehrtash, M.; Tari, I.; Yesilyurt, S. Impacts of Inhomogeneous Clamping Force on Local Performance and Liquid Water Formation in Polymer Electrolyte Fuel Cells. Int. J. Hydrogen Energy 2017, 42, 19227–19245. [Google Scholar] [CrossRef]
- Zhou, Z.; Zholobko, O.; Wu, X.F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2021, 14, 135. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-Exchange Membranes in Electrochemical Energy Systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef] [Green Version]
- Tang, D.P.; Pan, J.; Lu, S.F.; Zhuang, L.; Lu, J.T. Alkaline Polymer Electrolyte Fuel Cells: Principle, Challenges, and Recent Progress. Sci. China Chem. 2010, 53, 357–364. [Google Scholar] [CrossRef]
- Truong, V.M.; Tolchard, J.R.; Svendby, J.; Manikandan, M.; Miller, H.A.; Sunde, S.; Yang, H.; Dekel, D.R.; Barnett, A.O. Platinum and Platinum Group Metal-Free Catalysts for Anion Exchange Membrane Fuel Cells. Energies 2020, 13, 582. [Google Scholar] [CrossRef] [Green Version]
- Miller, H.A.; Lavacchi, A.; Vizza, F.; Marelli, M.; di Benedetto, F.; D’Acapito, F.; Paska, Y.; Page, M.; Dekel, D.R. A Pd/C-CeO2Anode Catalyst for High-Performance Platinum-Free Anion Exchange Membrane Fuel Cells. Angew. Chem. Int. Ed. 2016, 55, 6004–6007. [Google Scholar] [CrossRef]
- Dekel, D.R. Review of Cell Performance in Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 158–169. [Google Scholar] [CrossRef]
- Gottesfeld, S.; Dekel, D.R.; Page, M.; Bae, C.; Yan, Y.; Zelenay, P.; Kim, Y.S. Anion Exchange Membrane Fuel Cells: Current Status and Remaining Challenges. J. Power Sources 2018, 375, 170–184. [Google Scholar] [CrossRef]
- Yu, S.; Ma, X.; Liu, H.; Hao, J. Highly Stable Double Crosslinked Membrane Based on Poly(Vinylbenzyl Chloride) for Anion Exchange Membrane Fuel Cell. Polym. Bull. 2018, 75, 5163–5177. [Google Scholar] [CrossRef]
- Peng, H.; Li, Q.; Hu, M.; Xiao, L.; Lu, J.; Zhuang, L. Alkaline Polymer Electrolyte Fuel Cells Stably Working at 80 °C. J. Power Sources 2018, 390, 165–167. [Google Scholar] [CrossRef]
- Wang, L.; Bellini, M.; Miller, H.A.; Varcoe, J.R. A High Conductivity Ultrathin Anion-Exchange Membrane with 500+ h Alkali Stability for Use in Alkaline Membrane Fuel Cells That Can Achieve 2 W Cm-2 at 80 °c. J. Mater. Chem. A 2018, 6, 15404–15412. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Mandal, M.; Peng, X.; Yang-Neyerlin, A.C.; Pivovar, B.S.; Mustain, W.E.; Kohl, P.A. Composite Poly(Norbornene) Anion Conducting Membranes for Achieving Durability, Water Management and High Power (3.4 W/Cm 2) in Hydrogen/Oxygen Alkaline Fuel Cells. J. Electrochem. Soc. 2019, 166, F637–F644. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Peng, X.; Mustain, W.E.; Varcoe, J.R. Radiation-Grafted Anion-Exchange Membranes: The Switch from Low-to High-Density Polyethylene Leads to Remarkably Enhanced Fuel Cell Performance. Energy Environ. Sci 2019, 12, 1575–1579. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.Y.; Yang, Q.; Gao, X.L.; Zhu, Z.Y.; Sun, Q.H.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Novel Crosslinked Aliphatic Anion Exchange Membranes with Pendant Pentafluorophenyl Groups. Electrochim. Acta 2019, 321, 134634. [Google Scholar] [CrossRef]
- Ouadah, A.; Xu, H.; Luo, T.; Gao, S.; Zhang, Z.; Li, Z.; Zhu, C. Synthesis of Novel Copolymers Based on: P -Methylstyrene, N, N -Butylvinylimidazolium and Polybenzimidazole as Highly Conductive Anion Exchange Membranes for Fuel Cell Application. RSC Adv. 2017, 7, 47806–47817. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, Y.; Setzler, B.P.; Rojas-Carbonell, S.; ben Yehuda, C.; Amel, A.; Page, M.; Wang, L.; Hu, K.; Shi, L.; et al. Poly (Aryl Piperidinium) Membranes and Ionomers for Hydroxide Exchange Membrane Fuel Cells. Nat. Energy 2019, 4, 392–398. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, T.; Li, L.; Zhou, S.; Fang, H.; Li, X.; Wei, H.; Ding, Y. Chemo-Stable Poly (Quinquephenylene-Co-Diphenylene Piperidinium) Ionomers for Anion Exchange Membrane Fuel Cells. J. Power Sources 2021, 506, 230184. [Google Scholar] [CrossRef]
- Lu, C.; Long, C.; Li, Y.; Li, Z.; Zhu, H. Chemically Stable Poly (Meta-Terphenyl Piperidinium) with Highly Conductive Side Chain for Alkaline Fuel Cell Membranes. J. Membr. Sci. 2020, 598, 117797. [Google Scholar] [CrossRef]
- Wang, L.; Brink, J.J.; Liu, Y.; Herring, A.M.; Ponce-González, J.; Whelligan, D.K.; Varcoe, J.R. Non-Fluorinated Pre-Irradiation-Grafted (Peroxidated) LDPE-Based Anion-Exchange Membranes with High Performance and Stability. Energy Environ. Sci. 2017, 10, 2154–2167. [Google Scholar] [CrossRef] [Green Version]
- Douglin, J.C.; Varcoe, J.R.; Dekel, D.R. A High-Temperature Anion-Exchange Membrane Fuel Cell. J. Power Sources Adv. 2020, 5, 100023. [Google Scholar] [CrossRef]
- Huo, S.; Deng, H.; Chang, Y.; Jiao, K. Water Management in Alkaline Anion Exchange Membrane Fuel Cell Anode. Int. J. Hydrogen Energy 2012, 37, 18389–18402. [Google Scholar] [CrossRef]
- Deng, H.; Huo, S.; Chang, Y.; Zhou, Y.; Jiao, K. Transient Analysis of Alkaline Anion Exchange Membrane Fuel Cell Anode. Int. J. Hydrogen Energy 2013, 38, 6509–6525. [Google Scholar] [CrossRef]
- Jiao, K.; He, P.; Du, Q.; Yin, Y. Three-Dimensional Multiphase Modeling of Alkaline Anion Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2014, 39, 5981–5995. [Google Scholar] [CrossRef]
- Machado, B.S.; Chakraborty, N.; Das, P.K. Influences of Flow Direction, Temperature and Relative Humidity on the Performance of a Representative Anion Exchange Membrane Fuel Cell: A Computational Analysis. Int. J. Hydrogen Energy 2017, 42, 6310–6323. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, M.R.; Pant, L.M.; Weber, A.Z. Along-the-Channel Impacts of Water Management and Carbon-Dioxide Contamination in Hydroxide-Exchange-Membrane Fuel Cells: A Modeling Study. J. Electrochem. Soc. 2019, 166, F3180–F3192. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, B.; Grimler, H.; Carlson, A.; Ekström, H.; Wreland Lindström, R.; Lindbergh, G.; Lagergren, C. Quantifying Water Transport in Anion Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 4930–4939. [Google Scholar] [CrossRef]
- Adachi, M.; Navessin, T.; Xie, Z.; Frisken, B.; Holdcroft, S. Correlation of In Situ and Ex Situ Measurements of Water Permeation Through Nafion NRE211 Proton Exchange Membranes. J. Electrochem. Soc. 2009, 156, B782. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, H.C. A Calculation of the Viscous Force Exerted by a Flowing Fluid on a Dense Swarm of Particles. Flow Turbul. Combust. 1949, 1, 27–34. [Google Scholar] [CrossRef]
- Bird, R.; Stewart, W.; Lightfoot, E. Transport Phenomena; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Omasta, T.J.; Wang, L.; Peng, X.; Lewis, C.A.; Varcoe, J.R.; Mustain, W.E. Importance of Balancing Membrane and Electrode Water in Anion Exchange Membrane Fuel Cells. J. Power Sources 2018, 375, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Omasta, T.J.; Park, A.M.; Lamanna, J.M.; Zhang, Y.; Peng, X.; Wang, L.; Jacobson, D.L.; Varcoe, J.R.; Hussey, D.S.; Pivovar, B.S.; et al. Beyond Catalysis and Membranes: Visualizing and Solving the Challenge of Electrode Water Accumulation and Flooding in AEMFCs. Energy Env. Sci. 2018, 11, 551–558. [Google Scholar] [CrossRef] [Green Version]
- van Truong, M.; Duong, N.B.; Wang, C.L.; Yang, H. Effects of Cell Temperature and Reactant Humidification on Anion Exchange Membrane Fuel Cells. Materials 2019, 12, 2048. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Ohashi, H.; Tamaki, T.; Yamaguchi, T. Direction and Management of Water Movement in Solid-State Alkaline Fuel Cells. J. Phys. Chem. C 2012, 116, 7650–7657. [Google Scholar] [CrossRef]
- Zhegur-Khais, A.; Kubannek, F.; Krewer, U.; Dekel, D.R. Measuring the True Hydroxide Conductivity of Anion Exchange Membranes. J. Membr. Sci. 2020, 612, 118461. [Google Scholar] [CrossRef]
- Oshiba, Y.; Hiura, J.; Suzuki, Y.; Yamaguchi, T. Improvement in the Solid-State Alkaline Fuel Cell Performance through Efficient Water Management Strategies. J. Power Sources 2017, 345, 221–226. [Google Scholar] [CrossRef]




| Volumetric Source Terms | Units |
|---|---|
| Kg m−3 s−1 | |
| Kg m−3 s−1 | |
| A m−3 | |
| A m−3 |
| Property, Symbol | Value, Unit |
|---|---|
| 102, 10−3 | |
| 0.5, 1.5, 3.5, 0.5 | |
| 5 × 10−4 m2 | |
| Channel height, hch | 1 × 10−3 m |
| Channel width, wch | 1 × 10−3 m |
| 190 × 10−6 m | |
| 10 × 10−6 m | |
| 50 × 10−6 m | |
| Faraday’s constant, F | 96,487 C mol−1 |
| Universal gas constant, R | 8.31 J mol−1 K−1 |
| 16.6 × 10−6 m3 mol−1 | |
| 6 × 10−6 m3 mol−1 | |
| 12.7 × 10−6 m3 mol−1 | |
| 0.032 kg mol−1 | |
| 0.002 kg mol−1 | |
| 0.018 kg mol−1 | |
| 0.78 | |
| 0.3 | |
| 1000 S m−1 | |
| 290 S m−1 | |
| 290 S m−1 | |
| 1 × 10−11 m2 | |
| 1 × 10−12 m2 | |
| Operating temperature, T | 383.15 K |
| Dew point temperature, Tdp | 383.15 K |
| 2 atm | |
| 1.42 atm | |
| Relative humidity for anode and cathode, RHa, RHc | 1, 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrtash, M. Parametric Sensitivity Analysis and Performance Evaluation of High-Temperature Anion-Exchange Membrane Fuel Cell. Processes 2022, 10, 1315. https://doi.org/10.3390/pr10071315
Mehrtash M. Parametric Sensitivity Analysis and Performance Evaluation of High-Temperature Anion-Exchange Membrane Fuel Cell. Processes. 2022; 10(7):1315. https://doi.org/10.3390/pr10071315
Chicago/Turabian StyleMehrtash, Mehdi. 2022. "Parametric Sensitivity Analysis and Performance Evaluation of High-Temperature Anion-Exchange Membrane Fuel Cell" Processes 10, no. 7: 1315. https://doi.org/10.3390/pr10071315
APA StyleMehrtash, M. (2022). Parametric Sensitivity Analysis and Performance Evaluation of High-Temperature Anion-Exchange Membrane Fuel Cell. Processes, 10(7), 1315. https://doi.org/10.3390/pr10071315






