Hierarchical Design of Co(OH)2/Ni3S2 Heterostructure on Nickel Foam for Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrodeposition of Co(OH)2/Ni3S2 Heterostructure on NF
2.3. Characterization
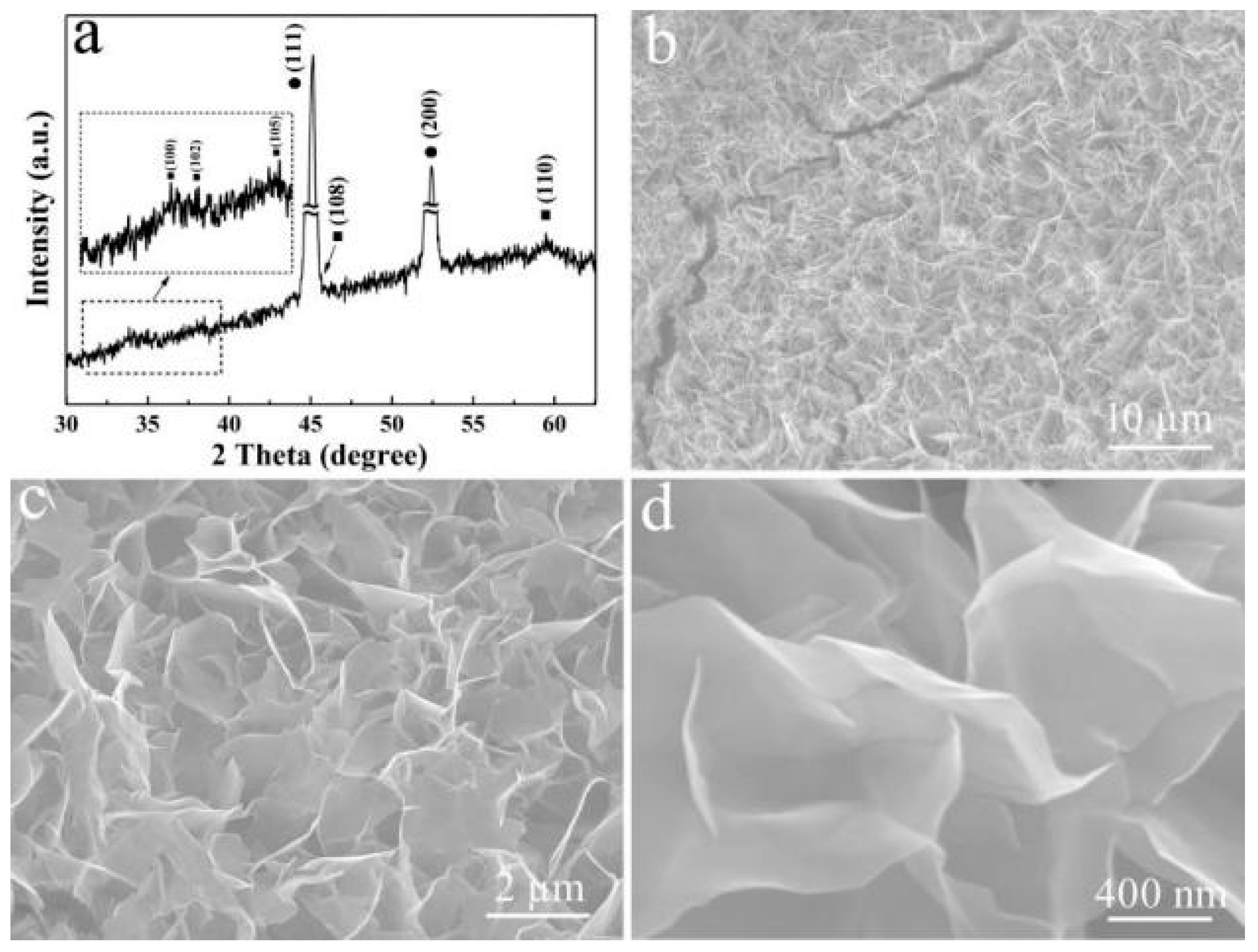
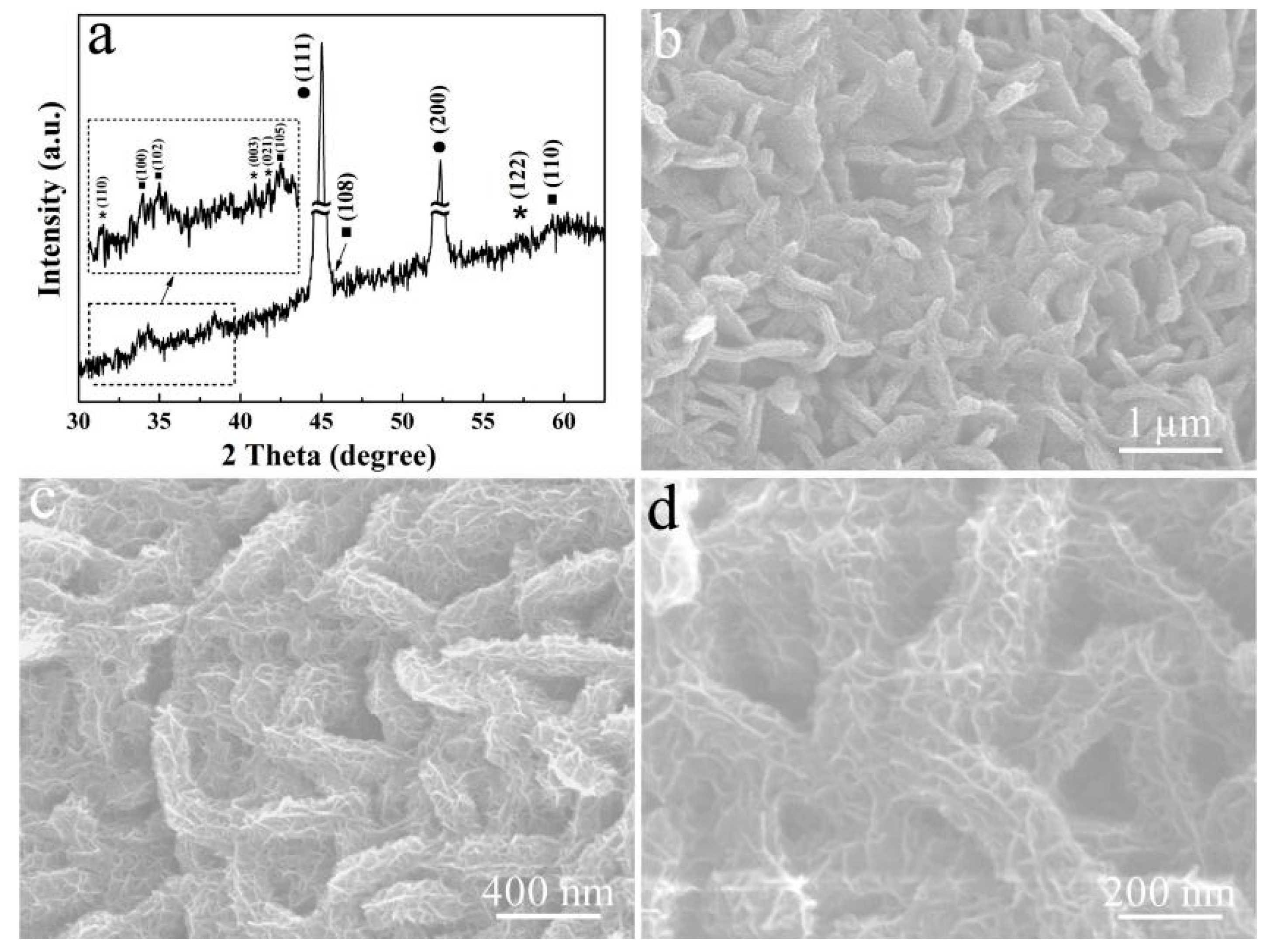
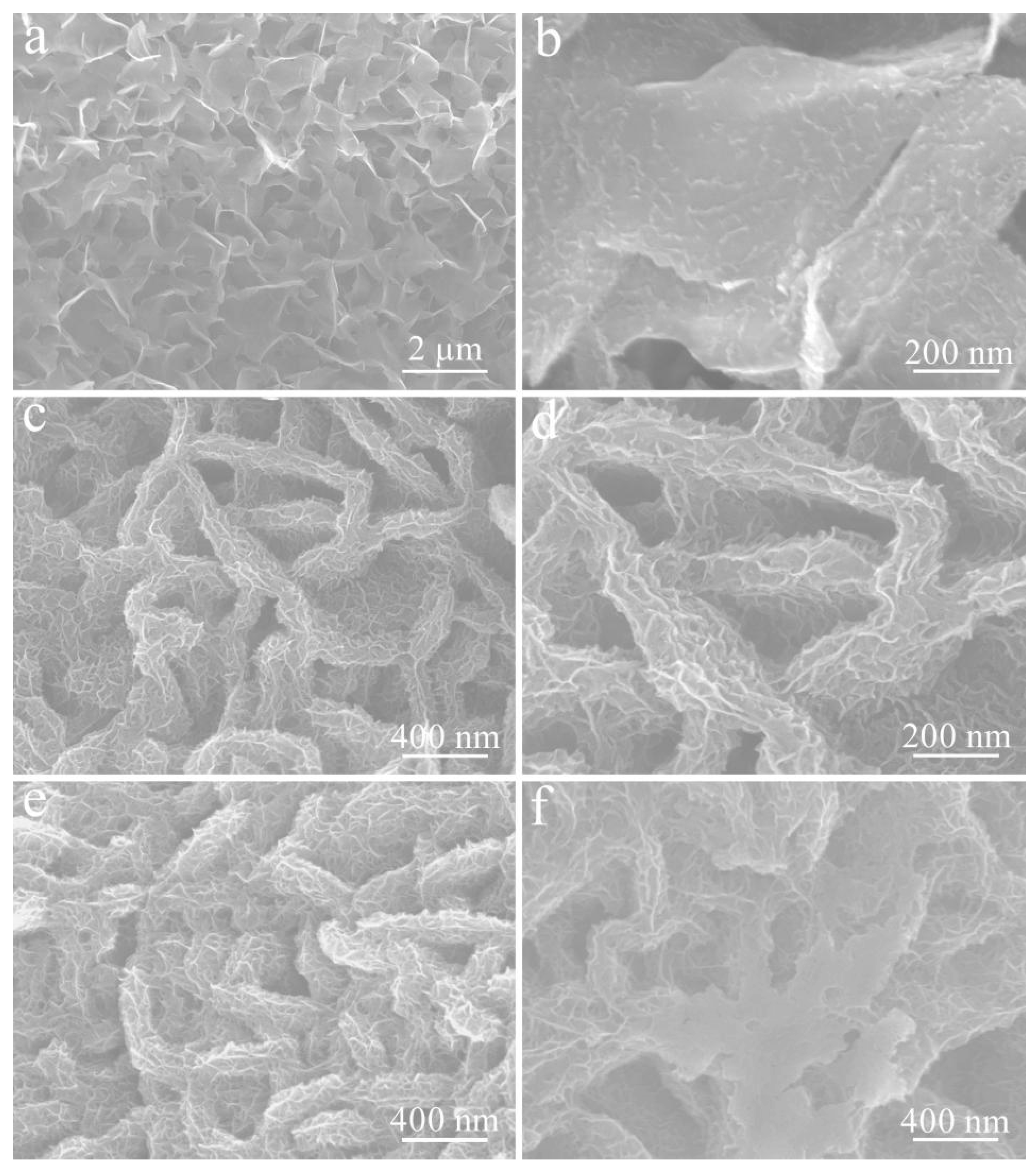
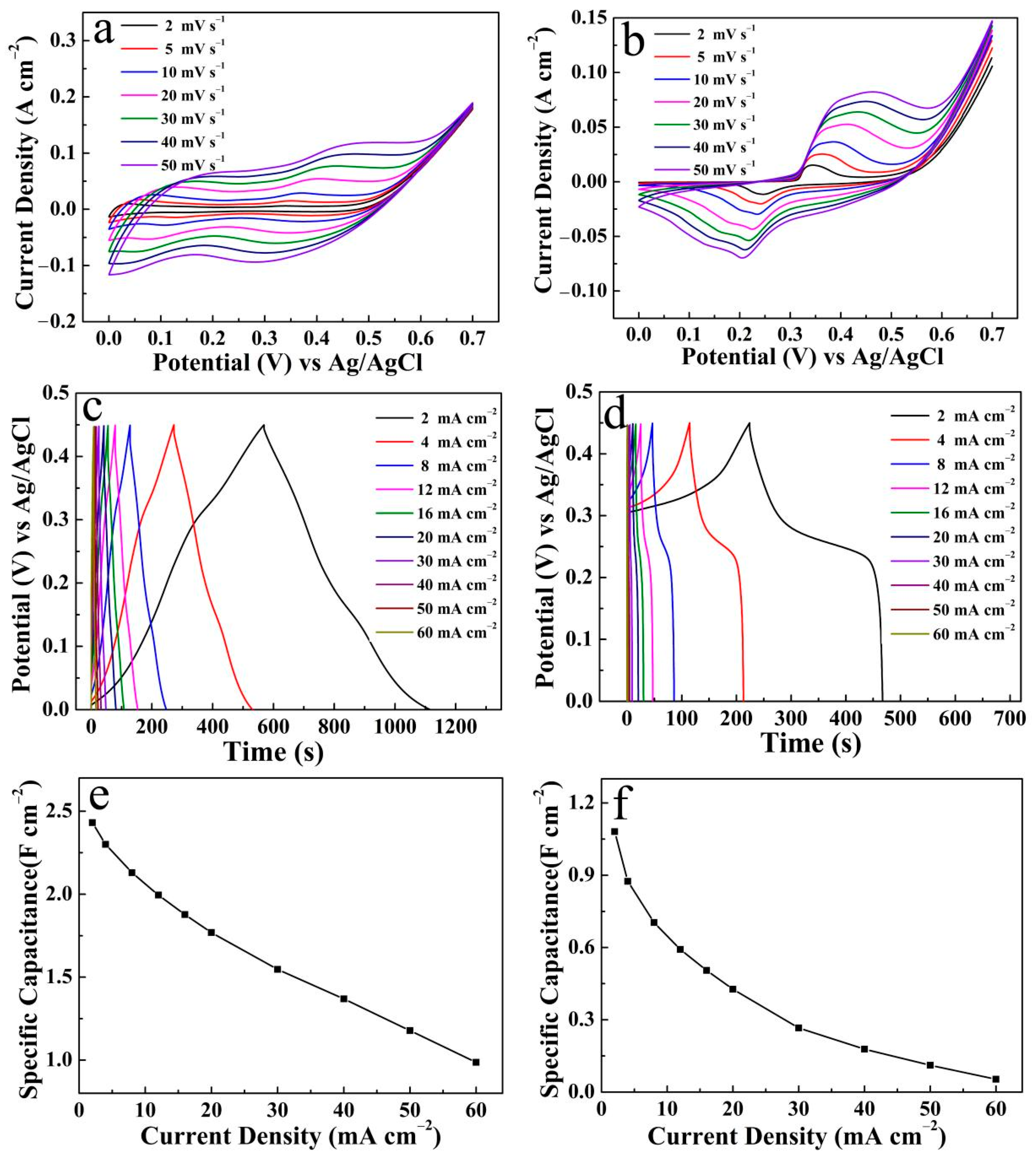
3. Results
| Electrode Materials | Electrode Substrate | Electrolyte | Current Density (mA cm−2) | Cs (F cm−2) | Literature |
|---|---|---|---|---|---|
| Co(OH)2/CoOOH/ Co3O4/Cu(OH)2 | Cu foam | 1 M KOH | 1 | 1.94 | [32] |
| Co(OH)2/Co9S8 | NiFe skeleton | 3 M KOH | 10 | 5.28 | [33] |
| Co9S8@Co(OH)2 | Ni foam | 3 M KOH | 1.5 | 5.62 | [13] |
| Co3S4-Ni3S2 | Ni foam | 6 M KOH | 2 | 2.83 | [34] |
| Ni3S2/rGO | Ni foam | 2 M KOH | 2 | 1.96 | [27] |
| Ni3S2/CoFeLDH | Ni foam | 1 M KOH | 2 | 5.08 | [35] |
| Cu(OH)2/Ni3S2 | Cu foam | 2 M NaOH | 2 | 4.85 | [10] |
| NiCoLDH@Ni3S2 | Ni foam | 3 M KOH | 3 | 4.95 | [36] |
| Co(OH)2/Ni3S2 | Ni foam | 2 M NaOH | 2 | 5.73 | Present work |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Tan, C.; Ou, T.; Zhang, S.R.; Li, L.; Ji, X.H. Constructing advanced electrode materials for low-temperature lithium-ion batteries: A review. Energy Rep. 2022, 8, 4525–4534. [Google Scholar]
- Tomy, M.; Rajappan, A.A.; Vimuna, V.; Suryabai, X.T. Emergence of Novel 2D Materials for High-Performance Supercapacitor Electrode Applications: A Brief Review. Energy Fuel 2021, 35, 19881–19900. [Google Scholar] [CrossRef]
- Mone, P.; Deore, S.; Balgude, S.; Pandit, V. Fabrication, design and performance evaluation of supercapacitors review. Mater. Today Proc. 2022, 53, 130–133. [Google Scholar] [CrossRef]
- Patel, K.K.; Singhal, T.; Pandey, V.; Sumangala, T.P.; Sreekanth, M.S. Evolution and recent developments of high performance electrode material for supercapacitors: A review. J. Energy Storage 2021, 44, 103366. [Google Scholar] [CrossRef]
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Namini, A.S.; Le, Q.V.; Shokouhimehr, M.; Asl, M.S.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, L.J. Review on recent advances in nanostructured transition-metal-sulfide-based electrode materials for cathode materials of asymmetric supercapacitors. Chem. Eng. J. 2022, 430, 132745. [Google Scholar] [CrossRef]
- Miller, E.E.; Hua, Y.; Tezel, F.H. Materials for energy storage: Review of electrode materials and methods of increasing capacitance for supercapacitors. J. Energy Storage 2018, 20, 30–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.X.; Cao, Y.; Yan, X.H.; Yan, J.; Gao, H.L.; Luo, H.W.; Wang, S.W.; Jia, X.D.; Kachalova, L.; et al. Recent advances and challenges of electrode materials for flexible supercapacitors. Coordin. Chem. Rev. 2021, 438, 213910. [Google Scholar] [CrossRef]
- Satpathy, B.K.; Patnaik, S.; Pradhan, D. Room-Temperature Growth of Co(OH)2 Nanosheets on Nanobelt-like Cu(OH)2 Arrays for a Binder-Free High-Performance All-Solid-State Supercapacitor. ACS Appl. Energy Mater. 2022, 1, 77–87. [Google Scholar] [CrossRef]
- Lv, S.; Shang, W.S.; Wang, H.; Chu, X.F.; Chi, Y.D.; Wang, C.; Yang, J.; Geng, P.Y.; Yang, X.T. Design and Construction of Cu(OH)2/Ni3S2 Composite Electrode on Cu Foam by Two-Step Electrodeposition. Micromachines 2022, 13, 237. [Google Scholar] [CrossRef]
- Huang, J.; Wei, J.C.; Xiao, Y.B.; Xu, Y.Z.; Xiao, Y.J.; Wang, Y.; Tan, L.C.; Yuan, K.; Chen, Y.W. When Al-Doped Cobalt Sulfide Nanosheets Meet Nickel Nanotube Arrays: A Highly Efficient and Stable Cathode for Asymmetric Supercapacitors. ACS Nano 2018, 12, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Hu, L.B.; Zhou, X.Y.; Zhang, S.; Qiao, Q.S.; Xu, L.; Tang, S.C. Three-Dimensional Porous Network Electrodes with Cu(OH)2 Nanosheet/Ni3S2 Nanowire 2D/1D Heterostructures for Remarkably Cycle-Stable Supercapacitors. ACS Omega 2021, 6, 34276–34285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Ye, W.Q.; Yang, P.J.; Song, J.L.; Shi, X.W.; Zheng, H.J. Manganese doping to boost the capacitance performance of hierarchical Co9S8@Co(OH)2 nanosheet arrays. Green Energy Environ. 2021; in press. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Cheng, Z.X.; Wang, X.L.; Dou, S.X.; Kong, X.Y. High area-specific capacitance of Co(OH)2/hierarchical nickel/nickel foam supercapacitors and its increase with cycling. J. Mater. Chem. A 2017, 5, 7968–7978. [Google Scholar] [CrossRef]
- Maile, N.C.; Shinde, S.K.; Koli, R.R.; Fulari, A.V.; Kim, D.Y.; Fulari, V.J. Effffect of difffferent electrolytes and deposition time on the supercapacitor properties of nanoflflake-like Co(OH)2 electrodes. Ultrason. Sonochem. 2019, 51, 49–57. [Google Scholar] [PubMed]
- Li, R.; Wang, S.L.; Huang, Z.C.; Lu, F.X.; He, T.B. NiCo2S4@Co(OH)2 core-shell nanotube arrays in situ grown on Ni foam for high performances asymmetric supercapcitors. J. Power Sources 2016, 312, 156–164. [Google Scholar] [CrossRef]
- Dileep, N.P.; Vineesh, T.V.; Sarma, P.V.; Chalil, M.V.; Prasad, C.S.; Shaijumon, M.M. Electrochemically Exfoliated β-Co(OH)2 Nanostructures for Enhanced Oxygen Evolution Electrocatalysis. ACS Appl. Energy Mater. 2020, 3, 1461–1467. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Z.L.; Wang, Z.X.; Li, X.H.; Guo, H.J.; Jiexi Wang, J.X.; Zhang, D.C. Facile construction of Co(OH)2@Ni(OH)2 core-shell nanosheets on nickel foam as three dimensional free-standing electrode for supercapacitors. Electrochim. Acta 2019, 293, 40–46. [Google Scholar] [CrossRef]
- He, D.; Wan, J.N.; Liu, G.L.; Suo, H.; Zhao, C. Design and construction of hierarchical α-Co(OH)2-coated ultra-thin ZnO flower nanostructures on nickel foam for high performance supercapacitors. J. Alloys Compd. 2020, 838, 155556–155564. [Google Scholar] [CrossRef]
- Tang, S.S.; Li, X.G.; Courté, M.; Peng, J.J.; Fichou, D. Hierarchical Cu(OH)2@Co(OH)2 Nanotrees for Water Oxidation Electrolysis. ChemCatChem 2020, 12, 4038–4043. [Google Scholar] [CrossRef]
- Guo, W.W.; Kim, J.; Kim, H.; Han, G.H.; Jang, H.W.; Kim, S.Y.; Sang Hyun Ahn, S.H. Sandwich-like Co(OH)x/Ag/Co(OH)2 nanosheet composites for oxygen evolution reaction in anion exchange membrane water electrolyzer. J. Alloys Compd. 2021, 889, 161674. [Google Scholar] [CrossRef]
- Chen, F.S.; Wang, H.; Ji, S.; Linkov, V.; Wang, R.F. Core-shell structured Ni3S2@Co(OH)2 nano-wires grown on Ni foam as binder-free electrode for asymmetric supercapacitors. Chem. Eng. J. 2018, 345, 48–57. [Google Scholar] [CrossRef]
- Wang, X.L.; Jin, E.M.; Chen, J.S.; Bandyopadhyay, P.; Jin, B.; Jeong, S.M. Facile In Situ Synthesis of Co(OH)2-Ni3S2 Nanowires on Ni Foam for Use in High-Energy-Density Supercapacitors. Nanomaterials 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Yu, Y.; Mu, Z.C.; Wang, Y.H.; Usman, A.; Jing, S.Y.; Xing, S.X. Urea-assisted enhanced electrocatalytic activity of MoS2-Ni3S2 for overall water splitting. Inorg. Chem. Front. 2020, 7, 3588–3597. [Google Scholar] [CrossRef]
- Fu, S.Q.; Yang, X.C.; Zhao, P.D.; Yao, X.; Jiao, Z.; Cheng, L.L. Regulable Electron Transfer on ZnS/CoS2/CC Prepared by an MOF-on-MOF Strategy for Robust LIB Performance. ACS Appl. Energy Mater. 2022, 5, 5159–5169. [Google Scholar] [CrossRef]
- Ding, J.F.; Zhao, D.P.; Xia, T.; Xia, Q.; Li, G.L.; Qu, Y.D. Hierarchical Co3O4@Ni3S2 electrode materials for energy storage and conversion. Dalton Trans. 2022, 51, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.H.; Wu, D.J.; Gao, X.R.; Wang, W.; Wu, W.P.; Tao, S.; Fang, Y.; Han, Z.D.; Qian, B.; Jiang, X.F.; et al. Ni3S2/rGO nanoparticles ensemble by an in-situ microwave irradiation route for supercapacitors. J. Alloys Compd. 2021, 890, 161435. [Google Scholar] [CrossRef]
- Pan, X.B.; Zhao, L.J.; Liu, H.B.; Guo, M.Y.; Han, C.D.; Wang, W.Q. Hierarchical structure Ni3S2/Ni(OH)2 nanoarrays towards high-performance supercapacitors. J. Solid State Chem. 2022, 309, 122974. [Google Scholar] [CrossRef]
- Liu, G.L.; He, X.; He, D.; Cui, B.Y.; Zhu, L.; Suo, H. Construction of CuO@Ni-Fe layered double hydroxide hierarchical core-shell nanorods arrays on copper foam for high-performance Supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 2080–2088. [Google Scholar] [CrossRef]
- An, Q.; Sun, X.H.; Guo, J.Z.; Cai, S.; Zheng, C.M. Review-Key Strategies to Increase the Rate Capacity of Cathode Materials for High Power Lithium-Ion Batteries. J. Electrochem. Soc. 2020, 167, 140528. [Google Scholar] [CrossRef]
- Liu, G.L.; Zhao, C.; Liu, T.Y.; He, D.; Suo, H. Facile route to achieve book-like tricobalt tetraoxide microstructures on copper foam for high performance supercapacitor. Mater. Lett. 2018, 220, 78–81. [Google Scholar] [CrossRef]
- Yu, Y.W.; Wang, H.M.; Zhang, H.Z.; Tan, Y.R.; Wang, Y.L.; Song, K.F.; Yang, B.Q.; Yuan, L.F.; Shen, X.D.; Hu, X.L. Blanket-like Co(OH)2/CoOOH/Co3O4/Cu(OH)2 composites on Cu foam for hybrid supercapacitor. Electrochim. Acta 2020, 334, 135559. [Google Scholar] [CrossRef]
- Wu, B.X.; Zhang, F.F.; Nie, Z.W.; Qian, H.; Liu, P.; He, H.; Wu, J.H.; Chen, Z.Y.; Chen, S.G. A high-performance battery-like supercapacitor electrode with a continuous NiTe network skeleton running throughout Co(OH)2/Co9S8 nanohybrid. Electrochim. Acta 2021, 365, 137325. [Google Scholar] [CrossRef]
- Li, Z.L.; Ren, J.; Yang, C.M.; He, Y.X.; Liang, Y.; Liu, J.L.; Waterhouse, G.I.N.; Li, J.H.; Qian, D. Sodium 5-sulfosalicylate-assisted hydrothermal synthesis of a selfsupported Co3S4-Ni3S2@nickel foam electrode for all-solid-state asymmetric supercapacitors. J. Alloys Compd. 2021, 889, 161661. [Google Scholar]
- Wang, Y.X.; Zhang, W.J.; Guo, X.L.; Liu, Y.Y.; Zheng, Y.M.; Zhang, M.; Li, R.; Peng, Z.B.; Zhao, Y.H. Construction of high-performance asymmetric supercapacitor based on the hierarchical Ni3S2/CoFe LDH/Ni foam hybrid. Appl. Surf. Sci. 2021, 561, 150049. [Google Scholar] [CrossRef]
- Wen, J.; Li, S.Z.; Chen, T.; Yue, Y.; Liu, N.S.; Gao, Y.H.; Li, B.R.; Song, Z.C.; Xiong, L.B.; Chen, Z.; et al. Three-dimensional hierarchical NiCo hydroxide@Ni3S2 nanorod hybrid structure as high performance positive material for asymmetric supercapacitor. Electrochim. Acta 2016, 222, 965–975. [Google Scholar] [CrossRef]
- Dong, X.; Wang, M.T.; He, L.Q.; Hu, P.; Wang, H.; Zhang, Z.H.; Huang, Z.L.; Shang, C.Q. Ternary Co-Ni sulfides deposited on Co(OH)2 nanoflakes decorated carbon cloth as electrode for supercapacitor. Inorg. Chem. Commun. 2022, 140, 109443. [Google Scholar]
- Liang, H.Y.; Lin, T.S.; Wang, S.Y.; Jia, H.N.; Li, C.; Cao, J.; Feng, J.C.; Fei, W.D.; Qi, J.L. A free-standing manganese cobalt sulfifide@cobalt nickel layered double hydroxide core-shell heterostructure for an asymmetric supercapacitor. Dalton Trans. 2020, 49, 196. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, S.; Shang, W.; Chi, Y.; Wang, H.; Chu, X.; Geng, P.; Wang, C.; Yang, J.; Cheng, Z.; Yang, X. Hierarchical Design of Co(OH)2/Ni3S2 Heterostructure on Nickel Foam for Energy Storage. Processes 2022, 10, 1255. https://doi.org/10.3390/pr10071255
Lv S, Shang W, Chi Y, Wang H, Chu X, Geng P, Wang C, Yang J, Cheng Z, Yang X. Hierarchical Design of Co(OH)2/Ni3S2 Heterostructure on Nickel Foam for Energy Storage. Processes. 2022; 10(7):1255. https://doi.org/10.3390/pr10071255
Chicago/Turabian StyleLv, Sa, Wenshi Shang, Yaodan Chi, Huan Wang, Xuefeng Chu, Peiyu Geng, Chao Wang, Jia Yang, Zhifei Cheng, and Xiaotian Yang. 2022. "Hierarchical Design of Co(OH)2/Ni3S2 Heterostructure on Nickel Foam for Energy Storage" Processes 10, no. 7: 1255. https://doi.org/10.3390/pr10071255
APA StyleLv, S., Shang, W., Chi, Y., Wang, H., Chu, X., Geng, P., Wang, C., Yang, J., Cheng, Z., & Yang, X. (2022). Hierarchical Design of Co(OH)2/Ni3S2 Heterostructure on Nickel Foam for Energy Storage. Processes, 10(7), 1255. https://doi.org/10.3390/pr10071255





