A Review of the Preparation, Machining Performance, and Application of Fe-Based Amorphous Alloys
Abstract
1. Introduction
2. Preparation and Properties of Amorphous Alloys
2.1. Amorphous Alloys versus Crystalline Materials
2.2. Preparation of Amorphous Alloys
2.3. Properties of Amorphous Alloys
2.4. Machining Performance of Amorphous Alloys
3. Preparation, Machining, and Application of Fe-Based Amorphous Alloys
3.1. Preparation and Application of Fe-Based Amorphous Alloys
3.2. Machining of Fe-Based Amorphous Alloys
3.3. Difficult-to-Machine Property of Fe-Based Amorphous Alloys
4. Assisted Machining Methods
4.1. Tool-Assisted Machining
4.1.1. Ultrasonic Vibration-Assisted Machining
4.1.2. Tool Servo-Assisted Machining
4.2. Low-Temperature Lubrication Assisted Machining
4.2.1. Fe-Based Amorphous Alloys at Low Temperature
4.2.2. Advantages of Low-Temperature Lubrication Assisted Machining
4.2.3. Device for Low-Temperature Lubrication Assisted Machining
4.3. Magnetic Field-Assisted Machining
4.3.1. Application of Magnetic Field on SPDT
4.3.2. Application of Magnetic Field on Fe-Based Amorphous Alloys
4.4. Other Assisted Machining Methods
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, G.D.; Li, J.P.; Zhou, C.W.; Yang, F. Fe-based Amorphous Soft Magnetic Alloy and Its Crystallization. J. Nanjing Univ. Sci. Technol. 1998, 22, 544–547. (In Chinese) [Google Scholar] [CrossRef]
- Pei, Y.; Zhou, G.; Luan, N.; Zong, B.; Qiao, M.; Tao, F.F. Synthesis and catalysis of chemically reduced metal–metalloid amorphous alloys. Chem. Soc. Rev. 2012, 41, 8140–8162. [Google Scholar] [CrossRef] [PubMed]
- Miracle, D.B.; Lord, E.A.; Ranganathan, S. Candidate atomic cluster configurations in metallic glass structures. Mater. Trans. 2006, 47, 1737–1742. [Google Scholar] [CrossRef][Green Version]
- Cheng, Y.; Ma, E. Atomic-level structure and structure–property relationship in metallic glasses. Prog. Mater. Sci. 2011, 56, 379–473. [Google Scholar] [CrossRef]
- Greer, A.L. Confusion by design. Nature 1993, 366, 303–304. [Google Scholar] [CrossRef]
- Souza, C.; Ribeiro, D.; Kiminami, C. Corrosion resistance of Fe-Cr-based amorphous alloys: An overview. J. Non-Cryst. Solids 2016, 442, 56–66. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Jain, V.K. Nanofinishing Science and Technology: Basic and Advanced Finishing and Polishing Processes; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Li, R.T. Fundamental Study on the Ultra-Precision Machining of Brittle Material and Construction of Micro-Cutting System; Tianjin University: Tianjin, China, 2018; (In Chinese). [Google Scholar] [CrossRef]
- Hatefi, S.; Abou-El-Hossein, K. Review of non-conventional technologies for assisting ultra-precision single-point diamond turning. Int. J. Adv. Manuf. Technol. 2020, 111, 2667–2685. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, C.; Shek, C. Bulk metallic glasses. Mater. Sci. Eng. R Rep. 2004, 44, 45–89. [Google Scholar] [CrossRef]
- Jalali, A.; Malekan, M.; Park, E.S.; Rashidi, R.; Bahmani, A.; Yoo, G.H. Thermal behavior of newly developed Zr33Hf8Ti6Cu32Ni10Co5Al6 high-entropy bulk metallic glass. J. Alloys Compd. 2022, 892, 162220. [Google Scholar] [CrossRef]
- Wang, L. The Research on Preparation and Properties of Fe-Based Amorphous Alloys; Northeastern University: Shenyang, China, 2014. (In Chinese) [Google Scholar]
- Liu, Y.; Wang, D.; Nakajima, K.; Zhang, W.; Hirata, A.; Nishi, T.; Inoue, A.; Chen, M. Characterization of nanoscale mechanical heterogeneity in a metallic glass by dynamic force microscopy. Phys. Rev. Lett. 2011, 106, 125504. [Google Scholar] [CrossRef] [PubMed]
- Ichitsubo, T.; Matsubara, E.; Yamamoto, T.; Chen, H.; Nishiyama, N.; Saida, J.; Anazawa, K. Microstructure of fragile metallic glasses inferred from ultrasound-accelerated crystallization in Pd-based metallic glasses. Phys. Rev. Lett. 2005, 95, 245501. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bedorf, D.; Kuechemann, S.; Schwabe, M.; Zhang, B.; Arnold, W.; Samwer, K. Local elastic properties of a metallic glass. Nat. Mater. 2011, 10, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H. The nature and properties of amorphous matter. Prog. Phys. 2013, 33, 177–351. (In Chinese) [Google Scholar]
- Park, E.S. Understanding of the shear bands in amorphous metals. Appl. Microsc. 2015, 45, 63–73. [Google Scholar] [CrossRef]
- Lewandowski, J.; Shazly, M.; Nouri, A.S. Intrinsic and extrinsic toughening of metallic glasses. Scr. Mater. 2006, 54, 337–341. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super plastic bulk metallic glasses at room temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef]
- Conner, R.; Li, Y.; Nix, W.; Johnson, W. Shear band spacing under bending of Zr-based metallic glass plates. Acta Mater. 2004, 52, 2429–2434. [Google Scholar] [CrossRef]
- Wang, L.; Bei, H.; Gao, Y.; Lu, Z.P.; Nieh, T. Effect of residual stresses on the hardness of bulk metallic glasses. Acta Mater. 2011, 59, 2858–2864. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Bei, H.; Nieh, T.-G. On the shear-band direction in metallic glasses. Acta Mater. 2011, 59, 4159–4167. [Google Scholar] [CrossRef]
- Şopu, D.; Stukowski, A.; Stoica, M.; Scudino, S. Atomic-level processes of shear band nucleation in metallic glasses. Phys. Rev. Lett. 2017, 119, 195503. [Google Scholar] [CrossRef] [PubMed]
- Klement, W.; Willens, R.; Duwez, P. Non-crystalline structure in solidified gold–silicon alloys. Nature 1960, 187, 869–870. [Google Scholar] [CrossRef]
- Turnbull, D. Under what conditions can a glass be formed? Contemp. Phys. 1969, 10, 473–488. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Zr–Al–Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 177–183. [Google Scholar] [CrossRef]
- Axinte, E.; Bofu, A.; Wang, Y.; Abdul-Rani, A.M.; Aliyu, A.A.A. An overview on the conventional and nonconventional methods for manufacturing the metallic glasses. MATEC Web Conf. 2017, 112, 03003. [Google Scholar] [CrossRef]
- Chen, M. A brief overview of bulk metallic glasses. NPG Asia Mater. 2011, 3, 82–90. [Google Scholar] [CrossRef]
- Ruhl, R.C. Cooling rates in splat cooling. Mater. Sci. Eng. 1967, 1, 313–320. [Google Scholar] [CrossRef]
- Chen, H.S.; Turnbull, D. Formation, stability and structure of palladium-silicon based alloy glasses. Acta Metall. 1969, 17, 1021–1031. [Google Scholar] [CrossRef]
- Spaepen, F. A microscopic mechanism for steady state inhomogeneous flow in metallic glasses. Acta Metall. 1977, 25, 407–415. [Google Scholar] [CrossRef]
- Argon, A.S. Plastic deformation in metallic glasses. Acta Metall. 1979, 27, 47–58. [Google Scholar] [CrossRef]
- Huang, R.; Suo, Z.; Prevost, J.H.; Nix, W.D. Inhomogeneous deformation in metallic glasses. J. Mech. Phys. Solids 2002, 50, 1011–1027. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, Y.; Ma, D.; Feng, Y.; Li, Y. Optimum glass formation at off-eutectic composition and its relation to skewed eutectic coupled zone in the La based La–Al–(Cu, Ni) pseudo ternary system. Acta Mater. 2003, 51, 4551–4561. [Google Scholar] [CrossRef]
- Ekambaram, R.; Thamburaja, P.; Nikabdullah, N. On the evolution of free volume during the deformation of metallic glasses at high homologous temperatures. Mech. Mater. 2008, 40, 487–506. [Google Scholar] [CrossRef]
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Xingchao, Z.; Yong, Z.; Hao, T.; Yong, L.; Xiaohua, C.; Guoliang, C. Micro-electro-discharge machining of bulk metallic glasses. In Proceedings of the 2007 International Symposium on High Density packaging and Microsystem Integration, Shanghai, China, 26–28 June 2007; pp. 1–4. [Google Scholar] [CrossRef]
- Rizzi, P.; Habib, A.; Castellero, A.; Battezzati, L. Ductility and toughness of cold-rolled metallic glasses. Intermetallics 2013, 33, 38–43. [Google Scholar] [CrossRef]
- Li, J.B.; Lin, H.C.; Jang, J.S.C.; Kuo, C.N.; Huang, J.C. Novel open-cell bulk metallic glass foams with promising characteristics. Mater. Lett. 2013, 105, 140–143. [Google Scholar] [CrossRef]
- Li, X.; Kang, C.; Huang, H.; Sercombe, T. The role of a low-energy–density re-scan in fabricating crack-free Al85Ni5Y6Co2Fe2 bulk metallic glass composites via selective laser melting. Mater. Des. 2014, 63, 407–411. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; Carstensen, J.; Ketkaew, J.; Ojeda Mota, R.M.; Guest, J.K.; Schroers, J. 3D metallic glass cellular structures. Acta Mater. 2016, 105, 35–43. [Google Scholar] [CrossRef]
- Perim, E.; Lee, D.; Liu, Y.; Toher, C.; Gong, P.; Li, Y.; Simmons, W.N.; Levy, O.; Vlassak, J.J.; Schroers, J. Spectral descriptors for bulk metallic glasses based on the thermodynamics of competing crystalline phases. Nat. Commun. 2016, 7, 12315. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, C.; Xing, W.; Liu, L. 3D printing of Zr-based bulk metallic glasses with complex geometries and enhanced catalytic properties. Intermetallics 2018, 94, 22–28. [Google Scholar] [CrossRef]
- Mohr, M.; Wunderlich, R.K.; Zweiacker, K.; Prades-Rödel, S.; Sauget, R.; Blatter, A.; Logé, R.; Dommann, A.; Neels, A.; Johnson, W.L. Surface tension and viscosity of liquid Pd43Cu27Ni10P20 measured in a levitation device under microgravity. Npj Microgravity 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Johnson, W. Formation of Ti–Zr–Cu–Ni bulk metallic glasses. J. Appl. Phys. 1995, 78, 6514–6519. [Google Scholar] [CrossRef]
- Li, H.; Lu, Z.; Wang, S.; Wu, Y.; Lu, Z. Fe-based bulk metallic glasses: Glass formation, fabrication, properties and applications. Prog. Mater. Sci. 2019, 103, 235–318. [Google Scholar] [CrossRef]
- Zhu, P.-Z.; Qiu, C.; Fang, F.-Z.; Yuan, D.-D.; Shen, X.-C. Molecular dynamics simulations of nanometric cutting mechanisms of amorphous alloy. Appl. Surf. Sci. 2014, 317, 432–442. [Google Scholar] [CrossRef]
- Cao, T.F. Studies of Relationship between Melting Entropy and Melting Point Viscosity of Zr2ni-Ti2ni Alloys; Yanshan University: Qinhuangdao, China, 2019. (In Chinese) [Google Scholar] [CrossRef]
- Johnson, W.L.; Kaltenboeck, G.; Demetriou, M.D.; Schramm, J.P.; Liu, X.; Samwer, K.; Kim, C.P.; Hofmann, D.C. Beating crystallization in glass-forming metals by millisecond heating and processing. Science 2011, 332, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Kaltenboeck, G.; Harris, T.; Sun, K.; Tran, T.; Chang, G.; Schramm, J.P.; Demetriou, M.D.; Johnson, W.L. Accessing thermoplastic processing windows in metallic glasses using rapid capacitive discharge. Sci. Rep. 2014, 4, 6441. [Google Scholar] [CrossRef]
- Inoue, A.; Nakamura, T.; Nishiyama, N.; Masumoto, T. Mg–Cu–Y bulk amorphous alloys with high tensile strength produced by a high-pressure die casting method. Mater. Trans. JIM 1992, 33, 937–945. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Hua, N.; Zhang, T. Co-based ternary bulk metallic glasses with ultrahigh strength and plasticity. J. Mater. Res. 2011, 26, 2072–2079. [Google Scholar] [CrossRef]
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Makino, A.; Inoue, A.; Masumoto, T. Nanocrystalline soft magnetic Fe–M–B (M = Zr, Hf, Nb) alloys produced by crystallization of amorphous phase (overview). Mater. Trans. JIM 1995, 36, 924–938. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Launey, M.E.; Garrett, G.; Schramm, J.P.; Hofmann, D.C.; Johnson, W.L.; Ritchie, R.O. A damage-tolerant glass. Nat. Mater. 2011, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Bian, X.; Wang, P.; Luo, G. Application of Fe-based metallic glasses in wastewater treatment. Mater. Sci. Eng. B 2012, 177, 92–95. [Google Scholar] [CrossRef]
- Pratap, A.; Kasyap, S.; Prajapati, S.; Upadhyay, D. Bio-corrosion studies of Fe-based metallic glasses. Mater. Today Proc. 2021, 42, 1669–1672. [Google Scholar] [CrossRef]
- Yi, H.Q. Research of the Fe76al4p12b4si4 Fe-Based Amorphous Alloy; Shanghai Jiao Tong University: Shanghai, China, 2009. (In Chinese) [Google Scholar]
- Gong, P.; Deng, L.; Jin, J.; Wang, S.; Wang, X.; Yao, K. Review on the research and development of Ti-based bulk metallic glasses. Metals 2016, 6, 264. [Google Scholar] [CrossRef]
- Li, F.; Zhang, T.; Guan, S.; Shen, N. A novel dual-amorphous-phased bulk metallic glass with soft magnetic properties. Mater. Lett. 2005, 59, 1453–1457. [Google Scholar] [CrossRef]
- Schroers, J. Bulk metallic glasses. Phys. Today 2013, 66, 32. [Google Scholar] [CrossRef]
- Parisi, G.; Sciortino, F. Flying to the bottom. Nat. Mater. 2013, 12, 94–95. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical behavior of emerging materials. Mater. Today 2012, 15, 486–498. [Google Scholar] [CrossRef]
- Wang, W. Bulk metallic glasses with functional physical properties. Adv. Mater. 2009, 21, 4524–4544. [Google Scholar] [CrossRef]
- Highmore, R.; Greer, A. Eutectics and the formation of amorphous alloys. Nature 1989, 339, 363–365. [Google Scholar] [CrossRef]
- Shen, J.; Chen, Q.; Sun, J.; Fan, H.; Wang, G. Exceptionally high glass-forming ability of an FeCoCrMoCBY alloy. Appl. Phys. Lett. 2005, 86, 151907. [Google Scholar] [CrossRef]
- Inoue, A.; Shinohara, Y.; Gook, J.S. Thermal and magnetic properties of bulk Fe-based glassy alloys prepared by copper mold casting. Mater. Trans. JIM 1995, 36, 1427–1433. [Google Scholar] [CrossRef]
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates (overview). Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, N.; Kimura, H. Preparation and thermal stability of bulk amorphous Pd40Cu30Ni10P20 alloy cylinder of 72 mm in diameter. Mater. Trans. JIM 1997, 38, 179–183. [Google Scholar] [CrossRef]
- Chen, M. Mechanical behavior of metallic glasses: Microscopic understanding of strength and ductility. Annu. Rev. Mater. Res. 2008, 38, 445–469. [Google Scholar] [CrossRef]
- Maroju, N.K.; Jin, X. Mechanism of chip segmentation in orthogonal cutting of Zr-based bulk metallic glass. J. Manuf. Sci. Eng. 2019, 141, 081003. [Google Scholar] [CrossRef]
- Komanduri, R.; Schroeder, T.; Hazra, J.; Von Turkovich, B.; Flom, D. On the catastrophic shear instability in high-speed machining of an AISI 4340 steel. J. Eng. Ind. May 1982, 102, 121–131. [Google Scholar] [CrossRef]
- Molinari, A.; Musquar, C.; Sutter, G. Adiabatic shear banding in high speed machining of Ti–6Al–4V: Experiments and modeling. Int. J. Plast. 2002, 18, 443–459. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, H. Micro machining of bulk metallic glasses: A review. Int. J. Adv. Manuf. Tech. 2019, 100, 637–661. [Google Scholar] [CrossRef]
- Zhu, P.; Fang, F. On the mechanism of material removal in nanometric cutting of metallic glass. Appl. Phys. A 2014, 116, 605–610. [Google Scholar] [CrossRef]
- Chong, F.; To, S.; Chan, K.C. Cutting characteristics of lanthanum base metallic glass in single point diamond turning. In Key Engineering Materials; Trans Tech Publ.: Zurich, Switzerland, 2012. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, H.; Zhang, G.; Chen, Y.; Ma, J.; Mo, R. Machinability and surface generation of Pd40Ni10Cu30P20 bulk metallic glass in single-point diamond turning. Micromachines 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Nagato, K.; Liu, S.; Sugita, N.; Nakao, M. Investigation into surface integrity and magnetic property of FeSiB metallic glass in two-dimensional cutting. J. Manuf. Process 2021, 64, 1098–1104. [Google Scholar] [CrossRef]
- Bakkal, M.; Liu, C.T.; Watkins, T.R.; Scattergood, R.O.; Shih, A.J. Oxidation and crystallization of Zr-based bulk metallic glass due to machining. Intermetallics 2004, 12, 195–204. [Google Scholar] [CrossRef]
- Fu, E.; Carter, J.; Martin, M.; Xie, G.; Zhang, X.; Wang, Y.; Littleton, R.; McDeavitt, S.; Shao, L. Ar-ion-milling-induced structural changes of Cu50Zr45Ti5 metallic glass. Nucl. Instrum. Meth. B 2010, 268, 545–549. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, M.; Song, A.; Liang, S.; Hao, Q.; Tan, C.; Jing, Q.; Liu, R. Temperature rise and flow of Zr-based bulk metallic glasses under high shearing stress. Sci. China Phys. Mech. 2011, 54, 1972–1976. [Google Scholar] [CrossRef]
- Basak, A.; Zhang, L. Deformation of Ti-Based bulk metallic glass under a cutting tip. Tribol. Lett. 2018, 66, 1–8. [Google Scholar] [CrossRef]
- Wang, J.Q.; Liu, Y.H.; Chen, M.W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A.; Perepezko, J.H. Rapid degradation of azo dye by Fe-based metallic glass powder. Adv. Funct. Mater. 2012, 22, 2567–2570. [Google Scholar] [CrossRef]
- Sun, H.; Zheng, H.; Yang, X. Efficient degradation of orange II dye using Fe-based metallic glass powders prepared by commercial raw materials. Intermetallics 2021, 129, 107030. [Google Scholar] [CrossRef]
- Zhou, J.; Di, S.-Y.; Sun, B.-A.; Zhao, R.; Zeng, Q.-S.; Wang, J.-G.; Sun, Z.-Z.; Wang, W.-H.; Shen, B.-L. Pronounced β-relaxation in plastic FeNi-based bulk metallic glasses and its structural origin. Intermetallics 2021, 136, 107234. [Google Scholar] [CrossRef]
- Cheng, Y.; Hao, Q.; Pelletier, J.; Pineda, E.; Qiao, J. Modelling and physical analysis of the high-temperature rheological behavior of a metallic glass. Int. J. Plast. 2021, 146, 103107. [Google Scholar] [CrossRef]
- Yang, W.; Liu, H.; Xue, L.; Li, J.; Dun, C.; Zhang, J.; Zhao, Y.; Shen, B. Magnetic properties of (Fe1− xNix) 72B20Si4Nb4 (x= 0.0–0.5) bulk metallic glasses. J. Magn. Magn. Mater. 2013, 335, 172–176. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Z.; Du, Q.; Li, H.; Wu, Y.; Wang, H.; Liu, X.; Lu, Z. Fe-based bulk metallic glass composites without any metalloid elements. Acta Mater. 2013, 61, 3214–3223. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, C.; Thompson, J.; Porter, W. Structural amorphous steels. Phys. Rev. Lett. 2004, 92, 245503. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.a.; Oguma, S.; Yamauchi, K. New Fe-based soft magnetic alloys composed of ultrafine grain structure. J. Appl. Phys. 1988, 64, 6044–6046. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, N.; Amiya, K.; Zhang, T.; Masumoto, T. Ti-based amorphous alloys with a wide supercooled liquid region. Mater. Lett. 1994, 19, 131–135. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Inoue, A. Iron-based bulk metallic glasses. Int. Mater. Rev. 2013, 58, 131–166. [Google Scholar] [CrossRef]
- Zhao, Y.Y. Molecular Dynamic Simulation of Fe-Based Amorphous Alloys; Shijiazhuang Tiedao University: Shijiazhuang, China, 2014. (In Chinese) [Google Scholar]
- Wang, W.M.; Gebert, A.; Roth, S.; Kuehn, U.; Schultz, L. Effect of Si on the glass-forming ability, thermal stability and magnetic properties of Fe–Co–Zr–Mo–W–B alloys. J. Alloys Compd. 2008, 459, 203–208. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, Y.; Fan, H.; Shen, J. Synthesis of Fe–Cr–Mo–C–B amorphous coating with high corrosion resistance. Mater. Lett. 2012, 89, 229–232. [Google Scholar] [CrossRef]
- Lenain, A.; Blandin, J.; Kapelski, G.; Volpi, F.; Gravier, S. Hf-rich bulk metallic glasses as potential insulating structural material. Mater. Des. 2018, 139, 467–472. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Lv, M.; Hu, Z. Decolorization of azo dye solution by Fe–Mo–Si–B amorphous alloy. J. Non-Cryst Solids 2010, 356, 1703–1706. [Google Scholar] [CrossRef]
- Ou, C.-H.; Lin, Y.-C.; Keikoin, Y.; Ono, T.; Esashi, M.; Tsai, Y.-C. Two-dimensional MEMS Fe-based metallic glass micromirror driven by an electromagnetic actuator. Jpn. J. Appl. Phys. 2019, 58, SDDL01. [Google Scholar] [CrossRef]
- Wang, M. Experimental Study on Machineability of Fe-based Amorphous Alloy. Mach. Tool Hydraul. 2019, 47, 112–115. [Google Scholar] [CrossRef]
- Fan, G.; Quan, M.; Hu, Z. Induced magnetic anisotropy in Fe80B20 metallic glass by mechanical milling. Appl. Phys. Lett. 1996, 68, 1159–1161. [Google Scholar] [CrossRef]
- Fang, Z.; Nakao, M. Local magnetic deterioration on work-hardening layer of FeSiB metallic glass by milling. CIRP Ann. 2020, 69, 501–504. [Google Scholar] [CrossRef]
- Tsui, H.-P.; Lee, P.-H.; Yeh, C.-C.; Hung, J.-C. Ultrasonic vibration-assisted electrical discharge machining on Fe-based metallic glass by adding conductive powder. Procedia CIRP 2020, 95, 425–430. [Google Scholar] [CrossRef]
- Quintana, I.; Dobrev, T.; Aranzabe, A.; Lalev, G. Laser micromachining of metallic glasses: Investigation of the material response to machining with micro-second and pico-second lasers. In Laser Applications in Microelectronic and Optoelectronic Manufacturing XV; International Society for Optics and Photonics: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- He, Y.; Shiflet, G.; Poon, S. Ball milling-induced nanocrystal formation in aluminum-based metallic glasses. Acta Metal. Mater. 1995, 43, 83–91. [Google Scholar] [CrossRef]
- Ramasamy, P.; Shahid, R.N.; Scudino, S.; Eckert, J.; Stoica, M. Influencing the crystallization of Fe80Nb10B10 metallic glass by ball milling. J. Alloys Compd. 2017, 725, 227–236. [Google Scholar] [CrossRef]
- Guo, F.; Lu, K. Ball-milling-induced crystallization and ball-milling effect on thermal crystallization kinetics in an amorphous FeMoSiB alloy. Metall. Mater. Trans. A 1997, 28, 1123–1131. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, Q. Internal friction behavior and thermal conductivity of Fe-based bulk metallic glasses with different crystallization. Thermochim. Acta 2018, 666, 36–40. [Google Scholar] [CrossRef]
- Han, D.; Wang, G.; Li, J.; Chan, K.C.; To, S.; Wu, F.; Gao, Y.; Zhai, Q. Cutting characteristics of Zr-based bulk metallic glass. J. Mater. Sci. Technol. 2015, 31, 153–158. [Google Scholar] [CrossRef]
- Chen, X.; Xiao, J.; Zhu, Y.; Tian, R.; Shu, X.; Xu, J. Micro-machinability of bulk metallic glass in ultra-precision cutting. Mater. Des. 2017, 136, 1–12. [Google Scholar] [CrossRef]
- Wang, D.D. Experomental and Simulation Study on Ultrasonic Torsional Vibration Assisted Milling of Amorphous Alloys; Yanshan University: Qinhuangdao, China, 2019. (In Chinese) [Google Scholar] [CrossRef]
- Zhou, S.X.; Lu, Z.C.; Chen, J.C. Amorphous state physics and the manufacture of soft magnetic materials. Physical 2002, 31, 430–436. (In Chinese) [Google Scholar]
- Wang, M.; Xu, B.; Dong, S.; Zhang, J.; Wei, S. Experimental investigations of cutting parameters influence on cutting forces in turning of Fe-based amorphous overlay for remanufacture. Int. J. Adv. Manuf. Technol. 2013, 65, 735–743. [Google Scholar] [CrossRef]
- Ding, F.; Wang, C.; Zhang, T.; Zheng, L.; Zhu, X. High performance cutting of Zr-based bulk metallic glass: A review of chip formation. Procedia Cirp. 2018, 77, 421–424. [Google Scholar] [CrossRef]
- Komanduri, R.; Lucca, D.; Tani, Y. Technological advances in fine abrasive processes. CIRP Ann. 1997, 46, 545–596. [Google Scholar] [CrossRef]
- Venkatesh, V.; Inasaki, I.; Toenshof, H.; Nakagawa, T.; Marinescu, I. Observations on polishing and ultraprecision machining of semiconductor substrate materials. CIRP Ann. 1995, 44, 611–618. [Google Scholar] [CrossRef]
- Gee, A.E.; Spragg, R.; Puttick, K.E.; Rudman, M. Single-point diamond form-finishing of glasses and other macroscopically brittle materials. Commer. Appl. Precis. Manuf. Sub-Micron Level 1992, 1573, 39–48. [Google Scholar] [CrossRef]
- Fang, F.; Chen, L. Ultra-precision cutting for ZKN7 glass. CIRP Ann. 2000, 49, 17–20. [Google Scholar] [CrossRef]
- Fang, F.; Venkatesh, V. Diamond cutting of silicon with nanometric finish. CIRP Ann. 1998, 47, 45–49. [Google Scholar] [CrossRef]
- Shimada, S.; Ikawa, N.; Inamura, T.; Takezawa, N.; Ohmori, H.; Sata, T. Brittle-ductile transition phenomena in microindentation and micromachining. CIRP Ann. 1995, 44, 523–526. [Google Scholar] [CrossRef]
- Lucca, D.; Brinksmeier, E.; Goch, G. Progress in assessing surface and subsurface integrity. CIRP Ann. 1998, 47, 669–693. [Google Scholar] [CrossRef]
- Ayomoh, M.; Abou-El-Hossein, K. Surface finish in ultra-precision diamond turning of single-crystal silicon. In Optifab 2015; International Society for Optics and Photonics: Washington, DC, USA, 2015. [Google Scholar] [CrossRef]
- Guo, X.; Zhai, C.; Jin, Z.; Guo, D. The study of diamond graphitization under the action of iron-based catalyst. Chin. J. Mech. Eng. 2015, 51, 162–168. [Google Scholar] [CrossRef]
- Zou, L.; Yin, J.; Huang, Y.; Zhou, M. Essential causes for tool wear of single crystal diamond in ultra-precision cutting of ferrous metals. Diamond Relat. Mater. 2018, 86, 29–40. [Google Scholar] [CrossRef]
- Biček, M.; Dumont, F.; Courbon, C.; Pušavec, F.; Rech, J.; Kopač, J. Cryogenic machining as an alternative turning process of normalized and hardened AISI 52100 bearing steel. J. Mater. Process. Technol. 2012, 212, 2609–2618. [Google Scholar] [CrossRef]
- Hatefi, S.; Abou-El-Hossein, K. Review of single-point diamond turning process in terms of ultra-precision optical surface roughness. Int. J. Adv. Manuf. Tech. 2020, 106, 2167–2187. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Chen, F.; Zheng, H.; Yang, X.; Wu, L.; Song, J.; Xu, W. Diamond-cutting ferrous metals assisted by cold plasma and ultrasonic elliptical vibration. Int. J. Adv. Manuf. Tech. 2016, 85, 673–681. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Z.; Wang, J.; Li, X. Diamond tool wear in precision turning of titanium alloy. Mater. Manuf. Process. 2013, 28, 1061–1064. [Google Scholar] [CrossRef]
- Wang, Y.; Song, L.-X.; Liu, J.-G.; Wang, R.; Zhao, B.-C. Investigation on the sawing temperature in ultrasonic vibration assisted diamond wire sawing monocrystalline silicon. Mater. Sci. Semicond Process. 2021, 135, 106070. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Y.; Li, C.; Yang, C.; Xue, C. Ductile-brittle coupled cutting of a single-crystal silicon by ultrasonic assisted diamond turning. Opt. Express 2021, 29, 23847–23863. [Google Scholar] [CrossRef]
- Shen, J.; Wang, J.; Jiang, B.; Xu, X. Study on wear of diamond wheel in ultrasonic vibration-assisted grinding ceramic. Wear 2015, 332, 788–793. [Google Scholar] [CrossRef]
- Zhong, Z.; Lin, G. Diamond turning of a metal matrix composite with ultrasonic vibrations. Mater. Manuf. Process. 2005, 20, 727–735. [Google Scholar] [CrossRef]
- Zhong, Z.; Lin, G. Ultrasonic assisted turning of an aluminium-based metal matrix composite reinforced with SiC particles. Int. J. Adv. Manuf. Technol. 2006, 27, 1077–1081. [Google Scholar] [CrossRef]
- Zhong, Z.; Hung, N.P. Diamond turning and grinding of aluminum-based metal matrix composites. Mater. Manuf. Process. 2000, 15, 853–865. [Google Scholar] [CrossRef]
- Kumar, J. Ultrasonic machining—A comprehensive review. Mach. Sci. Technol. 2013, 17, 325–379. [Google Scholar] [CrossRef]
- Singh, R.; Khamba, J. Ultrasonic machining of titanium and its alloys: A review. J. Mater. Process Technol. 2006, 173, 125–135. [Google Scholar] [CrossRef]
- Koshimizu, S. Ultrasonic vibration-assisted cutting of titanium alloy. In Key Engineering Materials; Trans Tech Publ.: Zurich, Switzerland, 2009. [Google Scholar] [CrossRef]
- Zhu, Z.; To, S.; Xiao, G.; Ehmann, K.F.; Zhang, G. Rotary spatial vibration-assisted diamond cutting of brittle materials. Precis. Eng. 2016, 44, 211–219. [Google Scholar] [CrossRef]
- Tian, F.; Yin, Z.; Li, S. A novel long range fast tool servo for diamond turning. Int. J. Adv. Manuf. Technol. 2016, 86, 1227–1234. [Google Scholar] [CrossRef]
- Feng, H.; Xia, R.; Li, Y.; Chen, J.; Yuan, Y.; Zhu, D.; Chen, S.; Chen, H. Fabrication of freeform progressive addition lenses using a self-developed long stroke fast tool servo. Int. J. Adv. Manuf. Technol. 2017, 91, 3799–3806. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.; Weckenmann, A.; Zhang, G.; Evans, C. Manufacturing and measurement of freeform optics. CIRP Ann. 2013, 62, 823–846. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Fang, F.; Huang, S.; Zhang, X. Review on fast tool servo machining of optical freeform surfaces. Int. J. Adv. Manuf. Technol. 2018, 95, 2071–2092. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, X.; Xu, P. A new tool path for optical freeform surface fast tool servo diamond turning. Proc. Inst. Mech. Eng. Pt. B J. Eng. Manuf. 2014, 228, 1721–1726. [Google Scholar] [CrossRef]
- Yuan, J.; Lyu, B.; Hang, W.; Deng, Q. Review on the progress of ultra-precision machining technologies. Front. Mech. Eng. 2017, 12, 158–180. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Cai, Y.; Tohyama, K.; Shimizu, Y.; Ito, S.; Gao, W. Auto-tracking single point diamond cutting on non-planar brittle material substrates by a high-rigidity force controlled fast tool servo. Precis. Eng. 2017, 49, 253–261. [Google Scholar] [CrossRef]
- Yu, D.P.; Wong, Y.S.; Hong, G.S. Ductile-regime machining for fast tool servo diamond turning of micro-structured surfaces on brittle materials. In Advanced Materials Research; Trans Tech Publ.: Zurich, Switzerland, 2012. [Google Scholar] [CrossRef]
- Yin, Z.; Dai, Y.; Li, S.; Guan, C.; Tie, G. Fabrication of off-axis aspheric surfaces using a slow tool servo. Int. J. Mach. Tool Manuf. 2011, 51, 404–410. [Google Scholar] [CrossRef]
- Nagayama, K.; Yan, J. Deterministic error compensation for slow tool servo-driven diamond turning of freeform surface with nanometric form accuracy. J. Manuf. Process. 2021, 64, 45–57. [Google Scholar] [CrossRef]
- Singh, R.; Dureja, J.; Dogra, M.; Gupta, M.K.; Jamil, M.; Mia, M. Evaluating the sustainability pillars of energy and environment considering carbon emissions under machining ofTi-3Al-2.5 V. Sustain. Energy Technol. 2020, 42, 100806. [Google Scholar] [CrossRef]
- Maaß, R.; Klaumünzer, D.; Preiß, E.; Derlet, P.; Löffler, J.F. Single shear-band plasticity in a bulk metallic glass at cryogenic temperatures. Scr. Mater. 2012, 66, 231–234. [Google Scholar] [CrossRef]
- Daniil, M.; Osofsky, M.S.; Gubser, D.U.; Willard, M.A. (Fe, Si, Al)-based nanocrystalline soft magnetic alloys for cryogenic applications. Appl. Phys. Lett. 2010, 96, 162504. [Google Scholar] [CrossRef]
- Jin, X.J.; Zhu, X.B.; Xue, W.C.; Wang, G. Effect of Cryogenic Treatment on Microstructure and Properties of Fe -Based Amorphous Alloy Coating. Mater. Prot. 2019, 52, 90–93. [Google Scholar] [CrossRef]
- Fan, C.; Liu, C.; Yan, H. Mechanical properties of bulk metallic glasses at cryogenic temperatures. Mod. Phys. Lett. B 2009, 23, 2703–2722. [Google Scholar] [CrossRef]
- Salahinejad, E.; Amini, R.; Marasi, M.; Sritharan, T.; Hadianfard, M. The effect of nitrogen on the glass-forming ability and micro-hardness of Fe–Cr–Mn–N amorphous alloys prepared by mechanical alloying. Mater. Chem. Phys. 2009, 118, 71–75. [Google Scholar] [CrossRef]
- Evans, C.; Bryan, J. Cryogenic diamond turning of stainless steel. CIRP Ann. 1991, 40, 571–575. [Google Scholar] [CrossRef]
- An, Q.L. Cryogenic Mist Jet Impinging Cooling and Its Application in Machining of Titanium Alloy; Nanjing University of Aeronautics Astronautics: Nanjing, China, 2006. (In Chinese) [Google Scholar]
- Aramcharoen, A. Influence of cryogenic cooling on tool wear and chip formation in turning of titanium alloy. Procedia CIRP. 2016, 46, 83–86. [Google Scholar] [CrossRef]
- Bordin, A.; Sartori, S.; Bruschi, S.; Ghiotti, A. Experimental investigation on the feasibility of dry and cryogenic machining as sustainable strategies when turning Ti6Al4V produced by Additive Manufacturing. J. Clean. Prod. 2017, 142, 4142–4151. [Google Scholar] [CrossRef]
- Khanna, N.; Agrawal, C.; Gupta, M.K.; Song, Q. Tool wear and hole quality evaluation in cryogenic Drilling of Inconel 718 superalloy. Tribol. Int. 2020, 143, 106084. [Google Scholar] [CrossRef]
- Pereira, O.; Celaya, A.; Urbikaín, G.; Rodríguez, A.; Fernández-Valdivielso, A.; de Lacalle, L.N.L. CO2 cryogenic milling of Inconel 718: Cutting forces and tool wear. J. Mater. Res. Technol. 2020, 9, 8459–8468. [Google Scholar] [CrossRef]
- Gupta, M.K.; Song, Q.; Liu, Z.; Sarikaya, M.; Mia, M.; Jamil, M.; Singla, A.K.; Bansal, A.; Pimenov, D.Y.; Kuntoğlu, M. Tribological performance based machinability investigations in cryogenic cooling assisted turning of α-β titanium alloy. Tribol. Int. 2021, 160, 107032. [Google Scholar] [CrossRef]
- Hong, S.Y.; Ding, Y. Cooling approaches and cutting temperatures in cryogenic machining of Ti-6Al-4V. Int. J. Mach. Tool Manuf. 2001, 41, 1417–1437. [Google Scholar] [CrossRef]
- Huang, P.; Li, H.; Zhu, W.-L.; Wang, H.; Zhang, G.; Wu, X.; To, S.; Zhu, Z. Effects of eco-friendly cooling strategy on machining performance in micro-scale diamond turning of Ti–6Al–4V. J. Clean. Prod. 2020, 243, 118526. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, Y.; Liu, K.; Wang, S.; Yu, Q.; Che, C.; Liu, H. The development and experimental research of a cryogenic internal cooling turning tool. J. Clean. Prod. 2021, 319, 128787. [Google Scholar] [CrossRef]
- Sharma, A.K.; Tiwari, A.K.; Dixit, A.R. Effects of Minimum Quantity Lubrication (MQL) in machining processes using conventional and nanofluid based cutting fluids: A comprehensive review. J. Clean. Prod. 2016, 127, 1–18. [Google Scholar] [CrossRef]
- Sharma, V.S.; Singh, G.; Sørby, K. A Review on Minimum Quantity Lubrication for Machining Processes. Mater. Manuf. Process. 2015, 30, 935–953. [Google Scholar] [CrossRef]
- Kamata, Y.; Obikawa, T. High speed MQL finish-turning of Inconel 718 with different coated tools. J. Mater. Process. Technol. 2007, 192, 281–286. [Google Scholar] [CrossRef]
- Hadad, M.; Sadeghi, B. Minimum quantity lubrication-MQL turning of AISI 4140 steel alloy. J. Clean. Prod. 2013, 54, 332–343. [Google Scholar] [CrossRef]
- Kishawy, H.; Dumitrescu, M.; Ng, E.-G.; Elbestawi, M. Effect of coolant strategy on tool performance, chip morphology and surface quality during high-speed machining of A356 aluminum alloy. Int. J. Mach. Tool Manu. 2005, 45, 219–227. [Google Scholar] [CrossRef]
- Zhou, J.W. Effect of Cryogenic and Magnetic Pulse Treatment on Fe-Based Amorphous Microstructure and Magnetic Properties; Jiangsu University: Zhenjiang, China, 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, D.M.; Guo, L.J.; Liu, K.; Ren, F.; Liu, H.B.; Jiang, S.W.; Wang, S.Q. Research Status and Development Trend of Cryogenic Machining Technology. Aerosp. Shanghai (Chin. Engl.) 2020, 37, 11–21. (In Chinese) [Google Scholar] [CrossRef]
- Dhar, N.; Kishore, N.S.; Paul, S.; Chattopadhyay, A. The effects of cryogenic cooling on chips and cutting forces in turning AISI 1040 and AISI 4320 steels. Proc. Inst. Mech. Eng. Pt. B J Eng. Manuf. 2002, 216, 713–724. [Google Scholar] [CrossRef]
- Khan, A.; Ali, M.Y.; Haque, M. A New Approach of Applying Cryogenic Coolant in Turning AISI 304 Stainless Steel. Int. J. Mech. Mater. Eng. 2010, 5, 171–174. [Google Scholar]
- Sen, B.; Mia, M.; Krolczyk, G.; Mandal, U.K.; Mondal, S.P. Eco-friendly cutting fluids in minimum quantity lubrication assisted machining: A review on the perception of sustainable manufacturing. Int. J. Precis. Eng. Manuf.-Green Technol. 2021, 8, 249–280. [Google Scholar] [CrossRef]
- Gavili, A.; Zabihi, F.; Isfahani, T.D.; Sabbaghzadeh, J. The thermal conductivity of water base ferrofluids under magnetic field. Exp. Therm. Fluid Sci. 2012, 41, 94–98. [Google Scholar] [CrossRef]
- Altan, C.L.; Elkatmis, A.; Yüksel, M.; Aslan, N.; Bucak, S. Enhancement of thermal conductivity upon application of magnetic field to Fe3O4 nanofluids. J. Appl. Phys. 2011, 110, 093917. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.; Raj, B. Enhancement of thermal conductivity in magnetite based nanofluid due to chainlike structures. Appl. Phys. Lett. 2007, 91, 203108. [Google Scholar] [CrossRef]
- Younes, H.; Christensen, G.; Luan, X.; Hong, H.; Smith, P. Effects of alignment, p H, surfactant, and solvent on heat transfer nanofluids containing Fe2O3 and CuO nanoparticles. J. Appl. Phys. 2012, 111, 064308. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C. Investigation of thermal conductivity and viscosity of Fe3O4 nanofluid for heat transfer applications. Int. Commun. Heat Mass 2013, 44, 7–14. [Google Scholar] [CrossRef]
- Gonnet, P.; Liang, Z.; Choi, E.S.; Kadambala, R.S.; Zhang, C.; Brooks, J.S.; Wang, B.; Kramer, L. Thermal conductivity of magnetically aligned carbon nanotube buckypapers and nanocomposites. Curr. Appl. Phys. 2006, 6, 119–122. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Horton, M.; Hong, H.; Li, C.; Shi, B.; Peterson, G.; Jin, S. Magnetic alignment of Ni-coated single wall carbon nanotubes in heat transfer nanofluids. J. Appl. Phys. 2010, 107, 104320. [Google Scholar] [CrossRef]
- Yip, W.; To, S. Reduction of material swelling and recovery of titanium alloys in diamond cutting by magnetic field assistance. J. Alloys Compd. 2017, 722, 525–531. [Google Scholar] [CrossRef]
- Philip, J.; Shima, P.; Raj, B. Evidence for enhanced thermal conduction through percolating structures in nanofluids. Nanotechnology 2008, 19, 305706. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Pan, Z. Effect of chain-like magnetite nanoparticle aggregates on thermal conductivity of magnetic nanofluid in magnetic field. Exp. Therm. Fluid. Sci. 2013, 44, 607–612. [Google Scholar] [CrossRef]
- Yip, W.; To, S. Tool life enhancement in dry diamond turning of titanium alloys using an eddy current damping and a magnetic field for sustainable manufacturing. J. Clean. Prod. 2017, 168, 929–939. [Google Scholar] [CrossRef]
- Sodano, H.A.; Bae, J.-S. Eddy current damping in structures. Shock. Vib. Dig. 2004, 36, 469. [Google Scholar] [CrossRef]
- Sodano, H.A.; Bae, J.-S.; Inman, D.J.; Belvin, W.K. Improved concept and model of eddy current damper. J. Vib. Acoust. Jun 2006, 128, 294–302. [Google Scholar] [CrossRef]
- Bae, J.S.; Kwak, M.K.; Inman, D.J. Vibration suppression of a cantilever beam using eddy current damper. J. Sound Vib. 2005, 284, 805–824. [Google Scholar] [CrossRef]
- Khalil, A.K.; Yip, W.; To, S. Theoretical and experimental investigations of magnetic field assisted ultra-precision machining of titanium alloys. J. Mater. Process Technol. 2022, 300, 117429. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chao, Y.S. Nano-Crystallization of Amorphous Alloy Fe78Si9B13 by Treatment of Low-Frequency Pulsating Magnetic Field. J. Northeast. Univ. (Nat. Sci.) 2003, 24, 1018–1020. (In Chinese) [Google Scholar]
- Jin, Y.; Chao, Y.; Liu, F.; Wang, J.; Sun, M. Nanocrystallization and magnetostriction coefficient of Fe52Co34Hf7B6Cu1 amorphous alloy treated by medium-frequency magnetic pulse. J. Magn. Magn. Mater. 2018, 468, 181–184. [Google Scholar] [CrossRef]
- Guo, H.; Chao, Y.S.; Zhang, l. Nano-Crystallization of Amorphous Alloy by Magnetic Pulsing and Optimization of Soft Magnetic Properties. Rare Met. Mater. Eng. 2013, 42, 1236–1240. (In Chinese) [Google Scholar]
- Suzuki, K.; Makino, A.; Kataoka, N.; Inoue, A.; Masumoto, T. High saturation magnetization and soft magnetic properties of bcc Fe–Zr–B and Fe–Zr–B–M (M= transition metal) alloys with nanoscale grain size. Mater. Trans. JIM 1991, 32, 93–102. [Google Scholar] [CrossRef]
- Souza, C.; May, J.; Carlos, I.; de Oliveira, M.; Kuri, S.; Kiminami, C. Influence of the corrosion on the saturation magnetic density of amorphous and nanocrystalline Fe73Nb3Si15. 5B7. 5Cu1 and Fe80Zr3. 5Nb3. 5B12Cu1 alloys. J. Non-Cryst. Solids 2002, 304, 210–216. [Google Scholar] [CrossRef]
- Gostin, P.; Gebert, A.; Schultz, L. Comparison of the corrosion of bulk amorphous steel with conventional steel. Corros Sci. 2010, 52, 273–281. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, C.; Chen, Q.e.; Yang, Y.; Li, N.; Liu, L. Study of structure and corrosion resistance of Fe-based amorphous coatings prepared by HVAF and HVOF. Corros Sci 2011, 53, 2351–2356. [Google Scholar] [CrossRef]
- Klocke, F.; Krieg, T. Coated tools for metal cutting–features and applications. CIRP Ann. 1999, 48, 515–525. [Google Scholar] [CrossRef]
- Brinksmeier, E.; Gläbe, R. Advances in precision machining of steel. CIRP Ann. 2001, 50, 385–388. [Google Scholar] [CrossRef]
- Xiao, C. Properties of nano-SiC/Ni composite coating on diamond surfaces. Surf. Eng. 2018, 34, 832–837. [Google Scholar] [CrossRef]
- Stock, H.-R.; Schlett, V.; Kohlscheen, J.; Mayr, P. Characterization and mechanical properties of ion-implanted diamond surfaces. Surf. Coat. Technol. 2001, 146, 425–429. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hao, L.; Lüder, J.; Chaudhari, A.; Wang, S.; Manzhos, S.; Wang, H. Micromachining of ferrous metal with an ion implanted diamond cutting tool. Carbon 2019, 152, 598–608. [Google Scholar] [CrossRef]
- Prawer, S. Ion implantation of diamond and diamond films. Diamond Relat. Mater. 1995, 4, 862–872. [Google Scholar] [CrossRef]
- Zhang, G. Method for Extending Diamond Tool Life in Diamond Machining of Materials that Chemically React with Diamond. U.S. Patent 7198043B1, 3 April 2007. [Google Scholar]
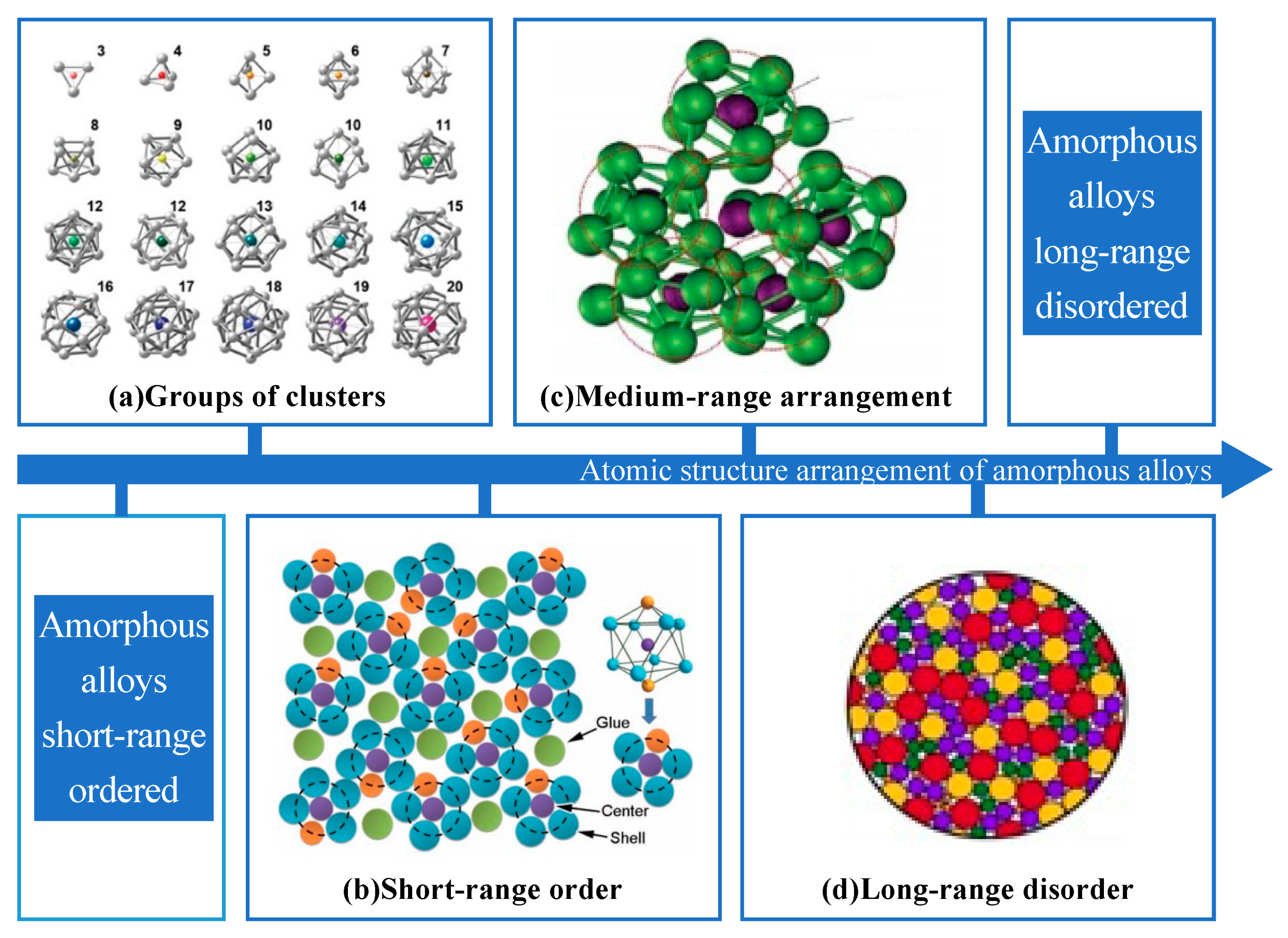
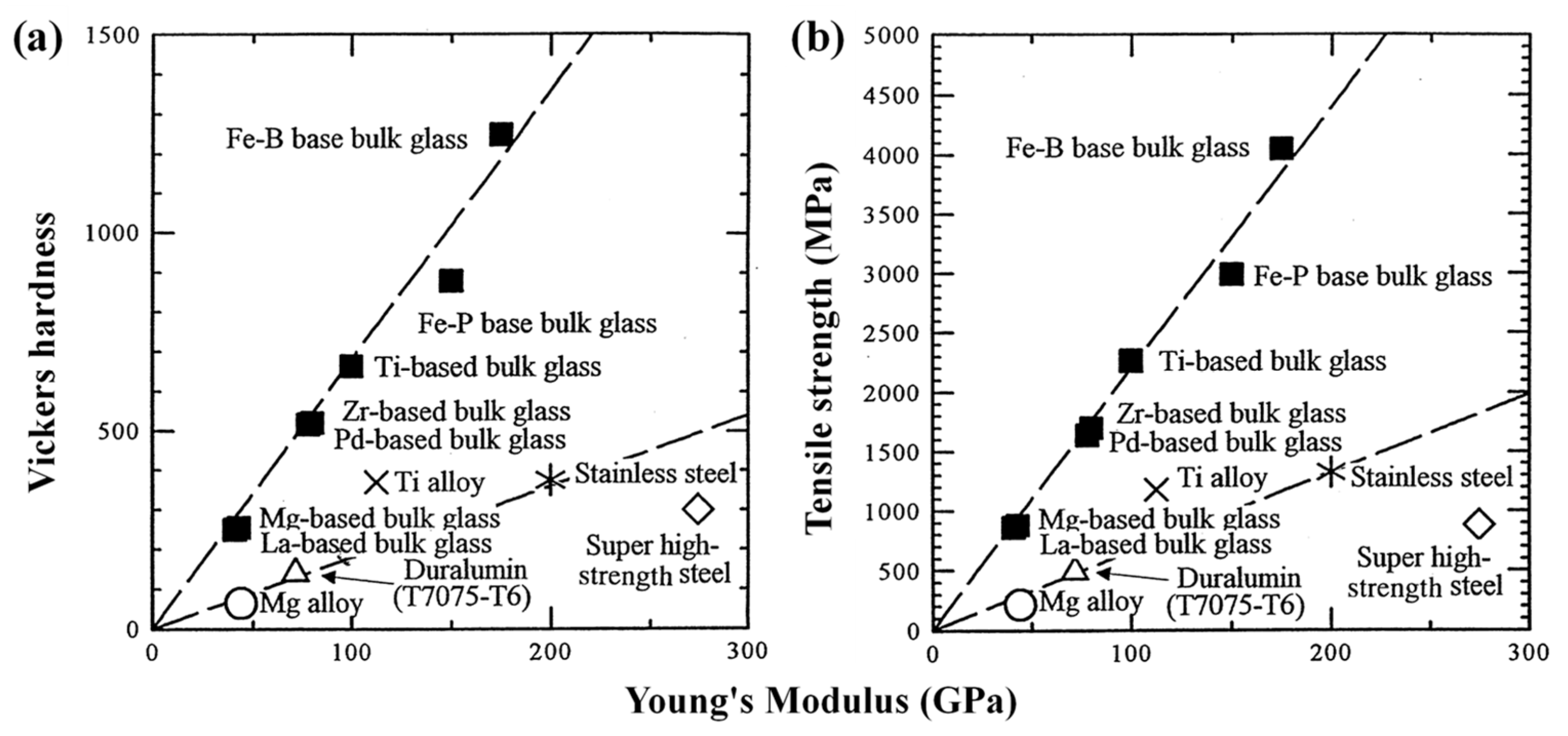
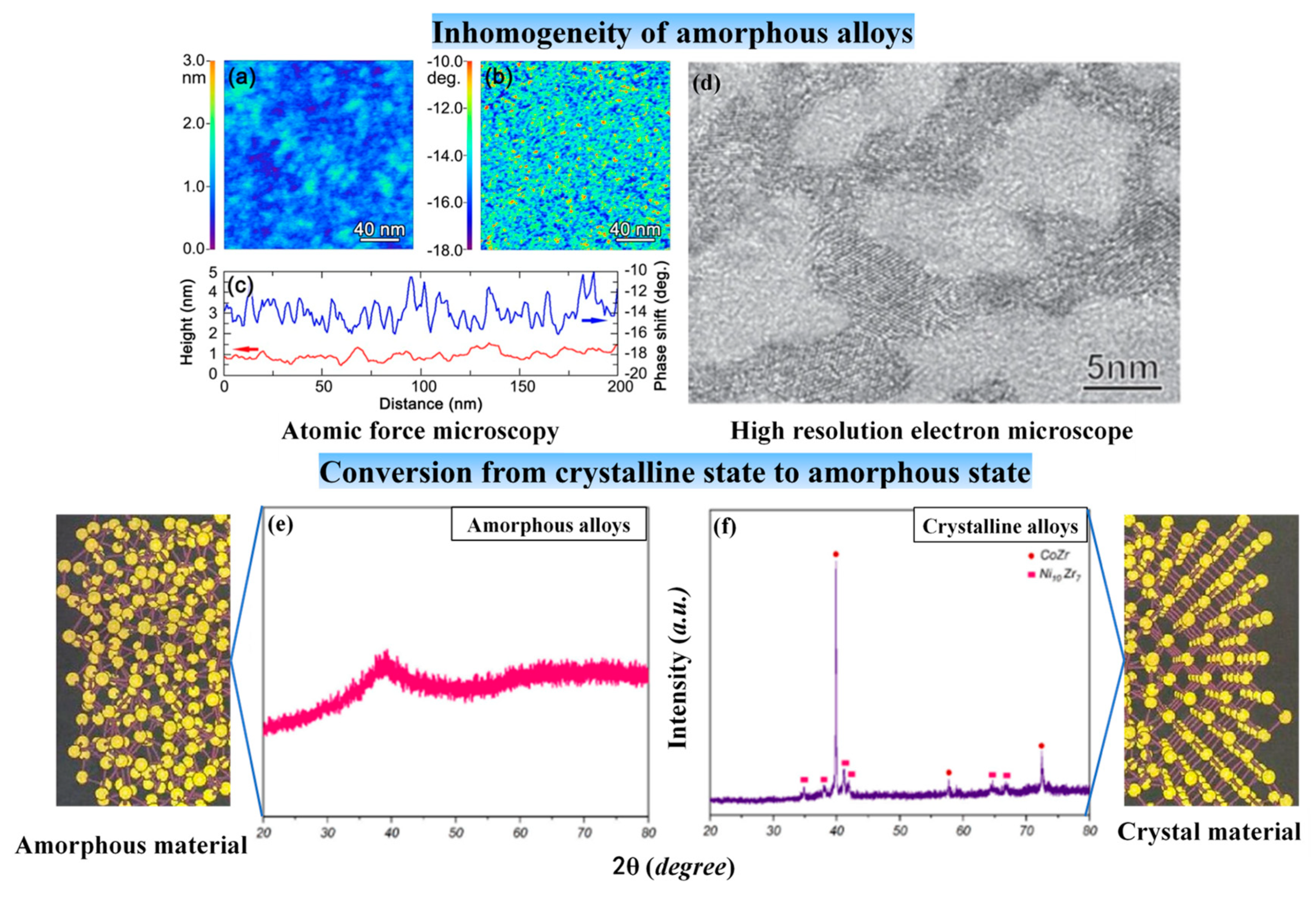


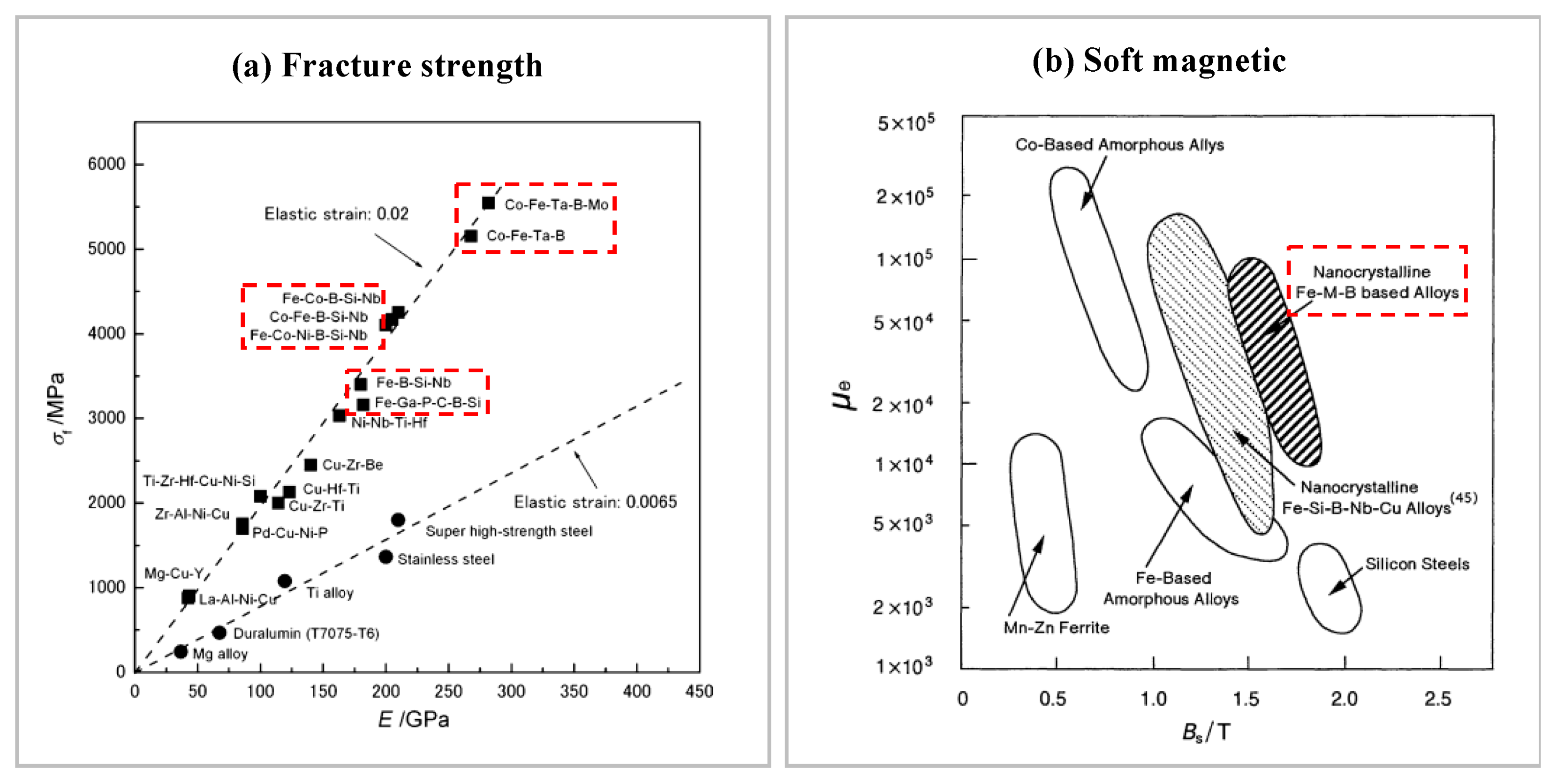
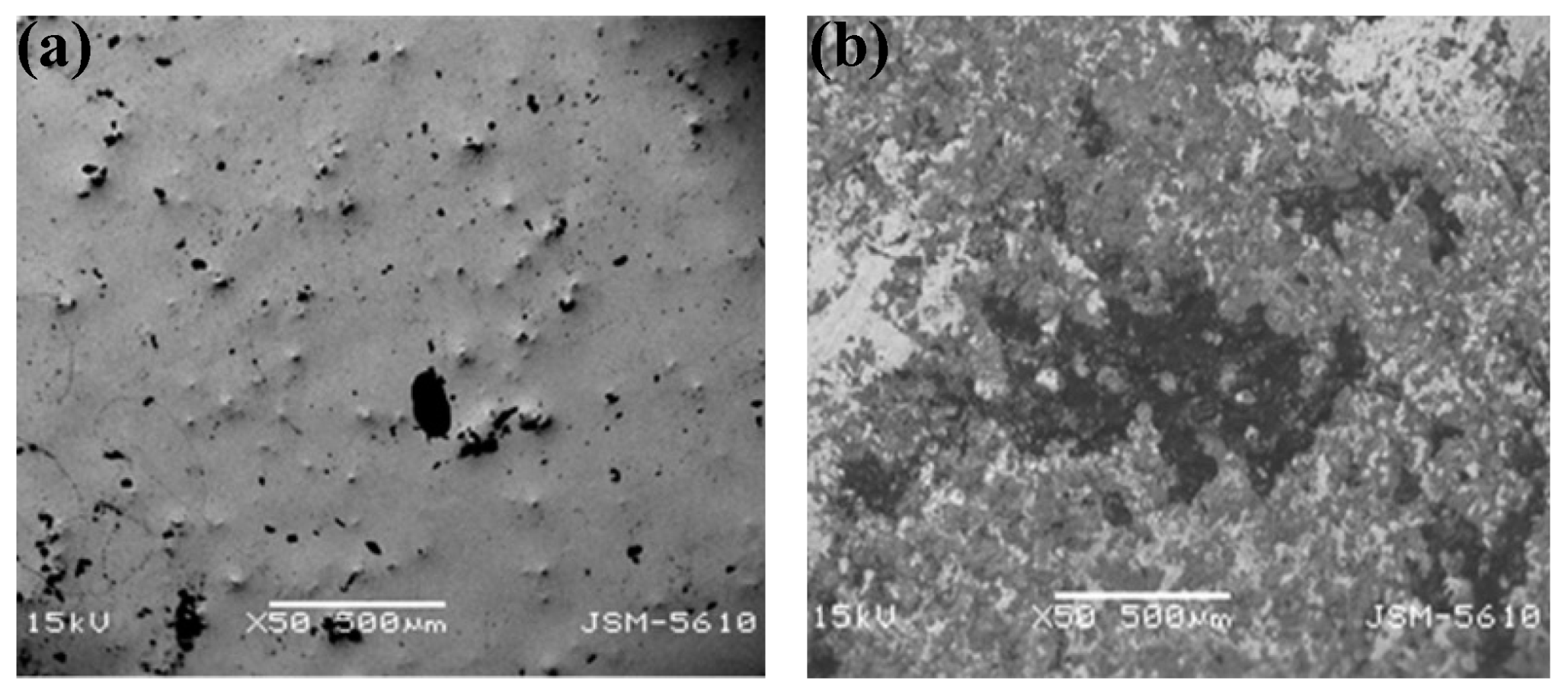

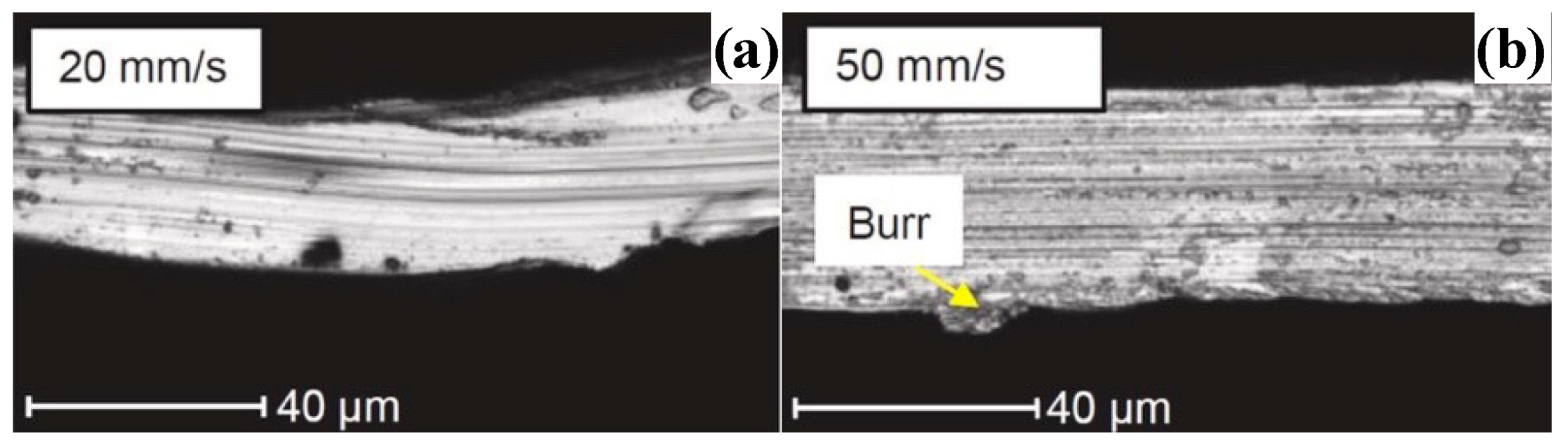
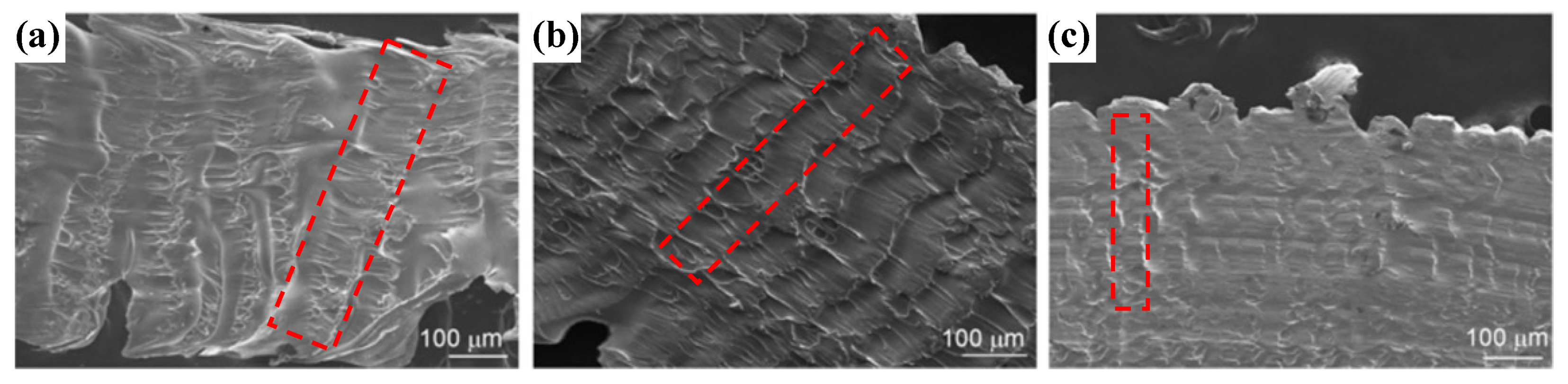

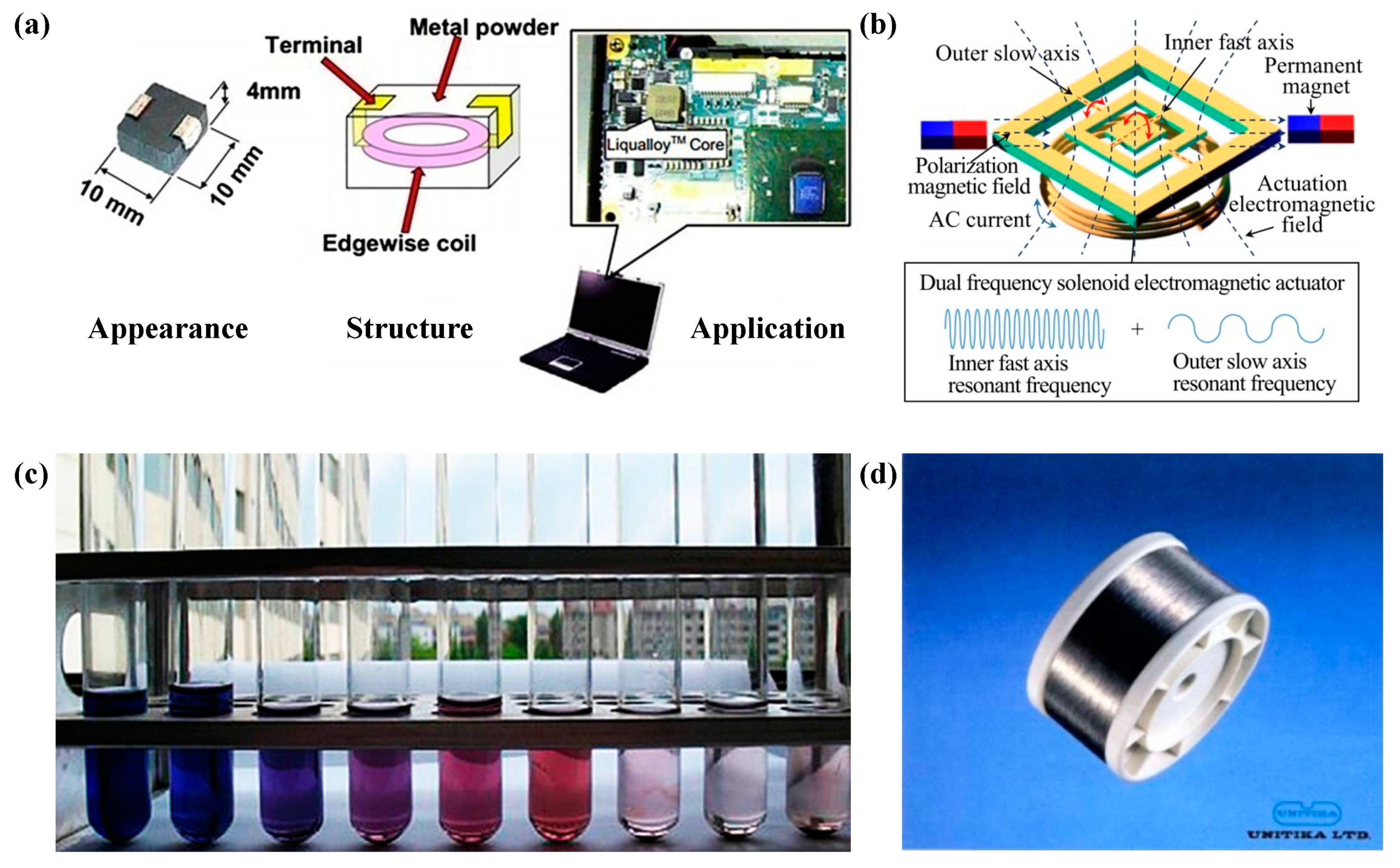
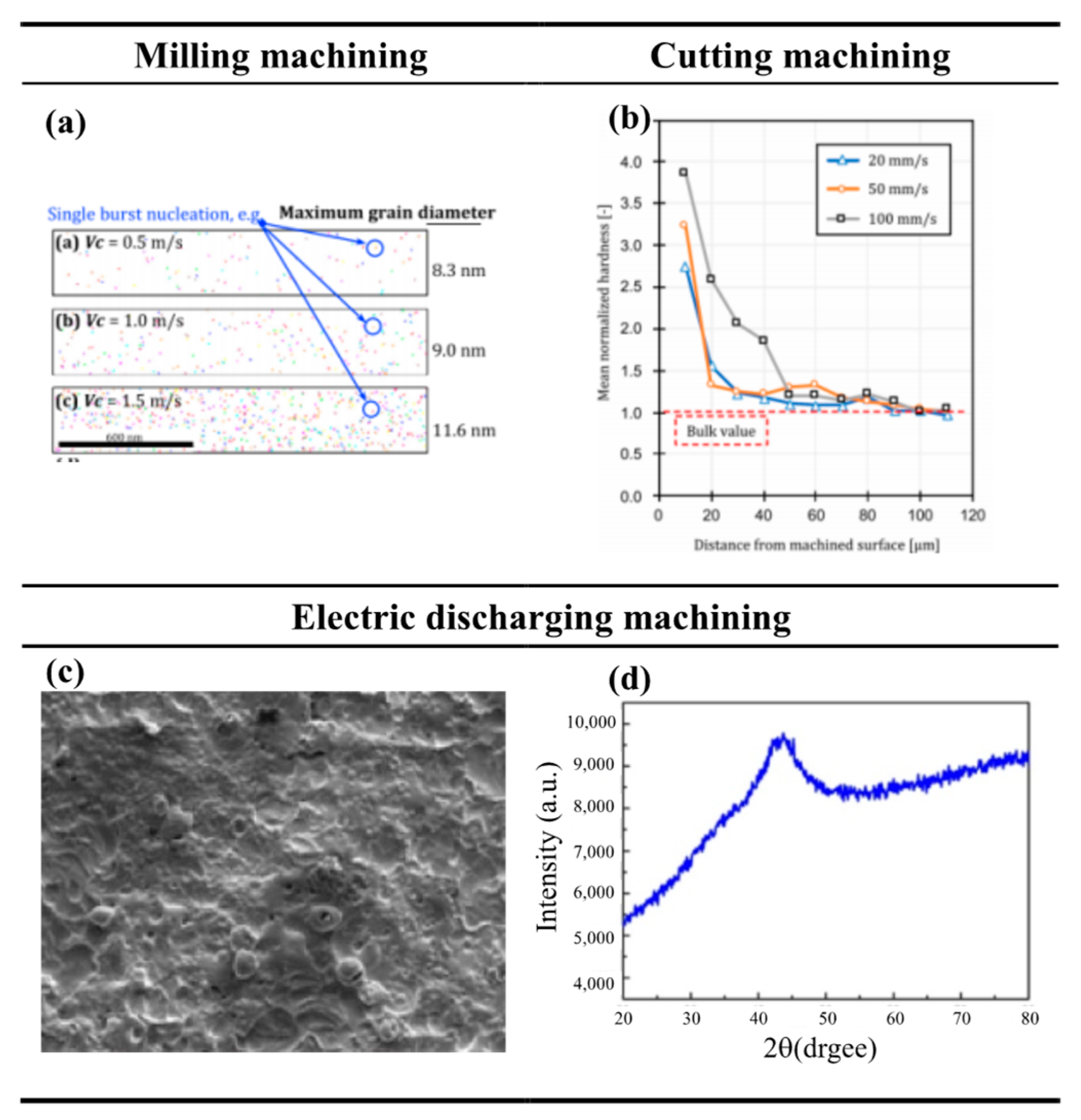
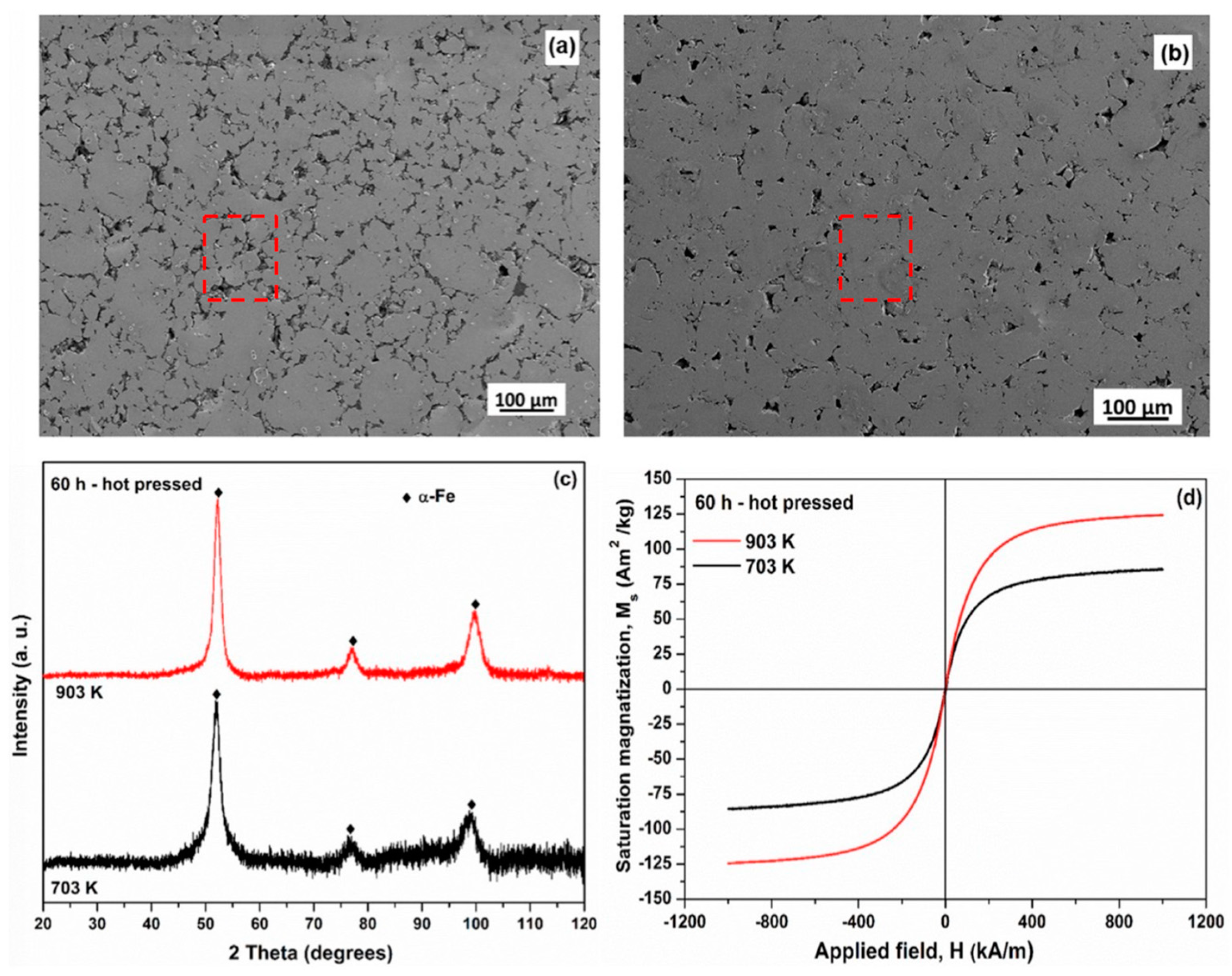
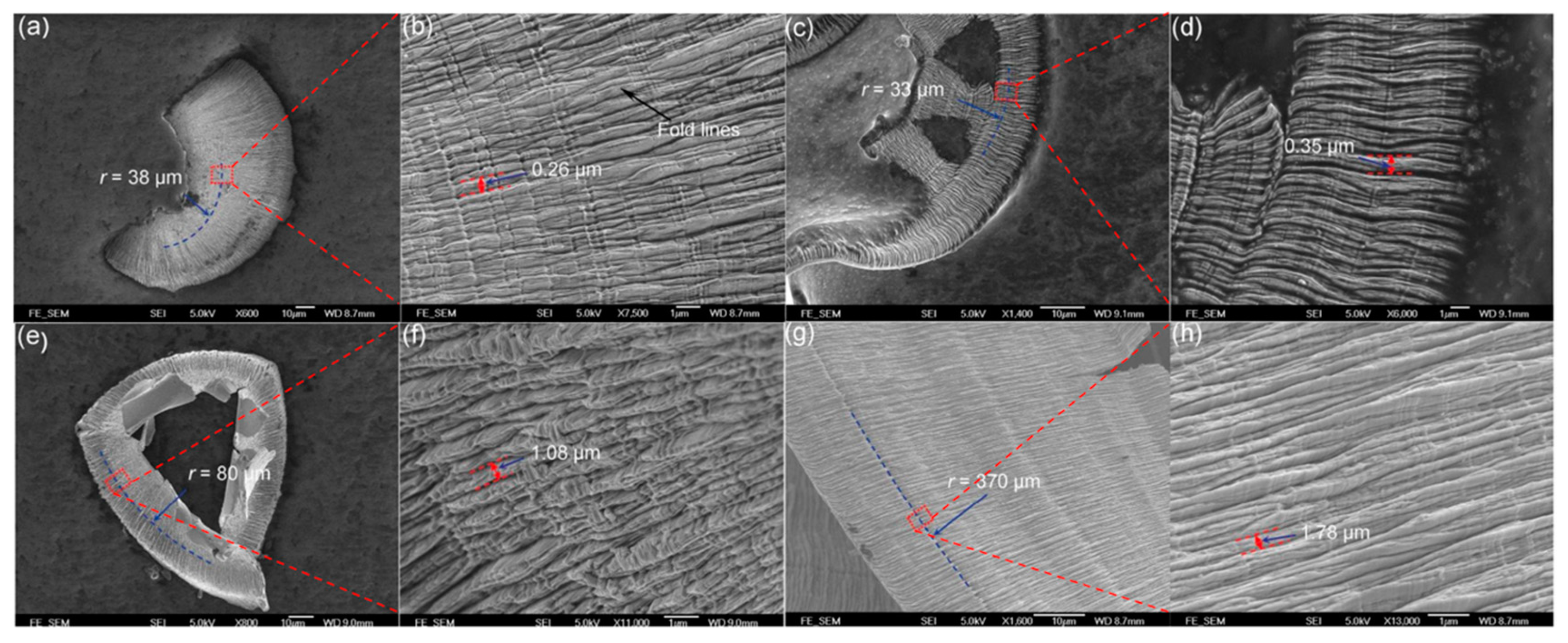

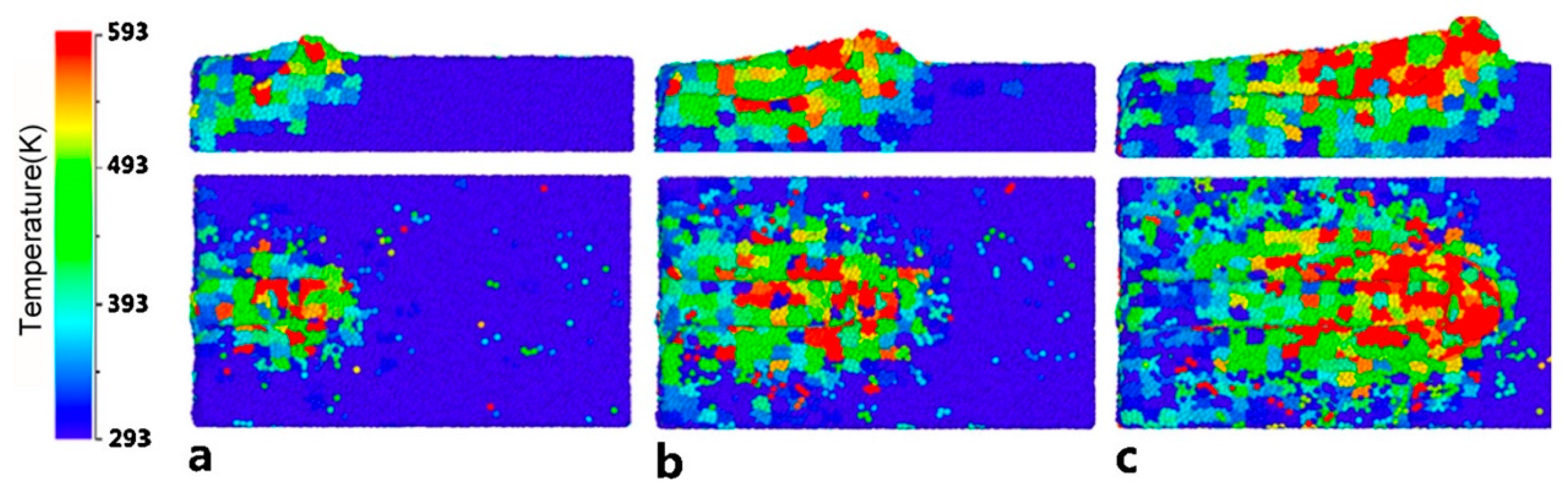
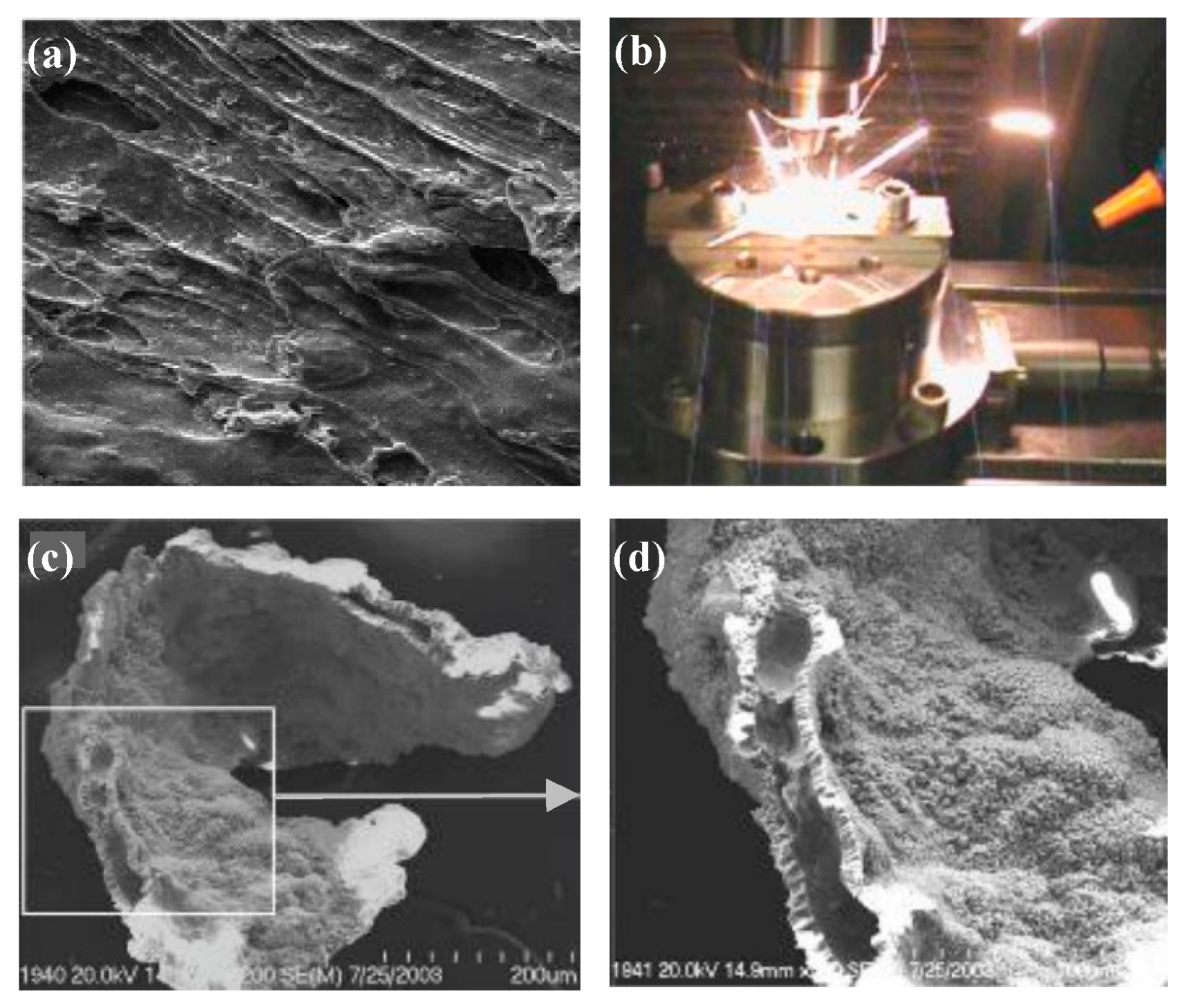
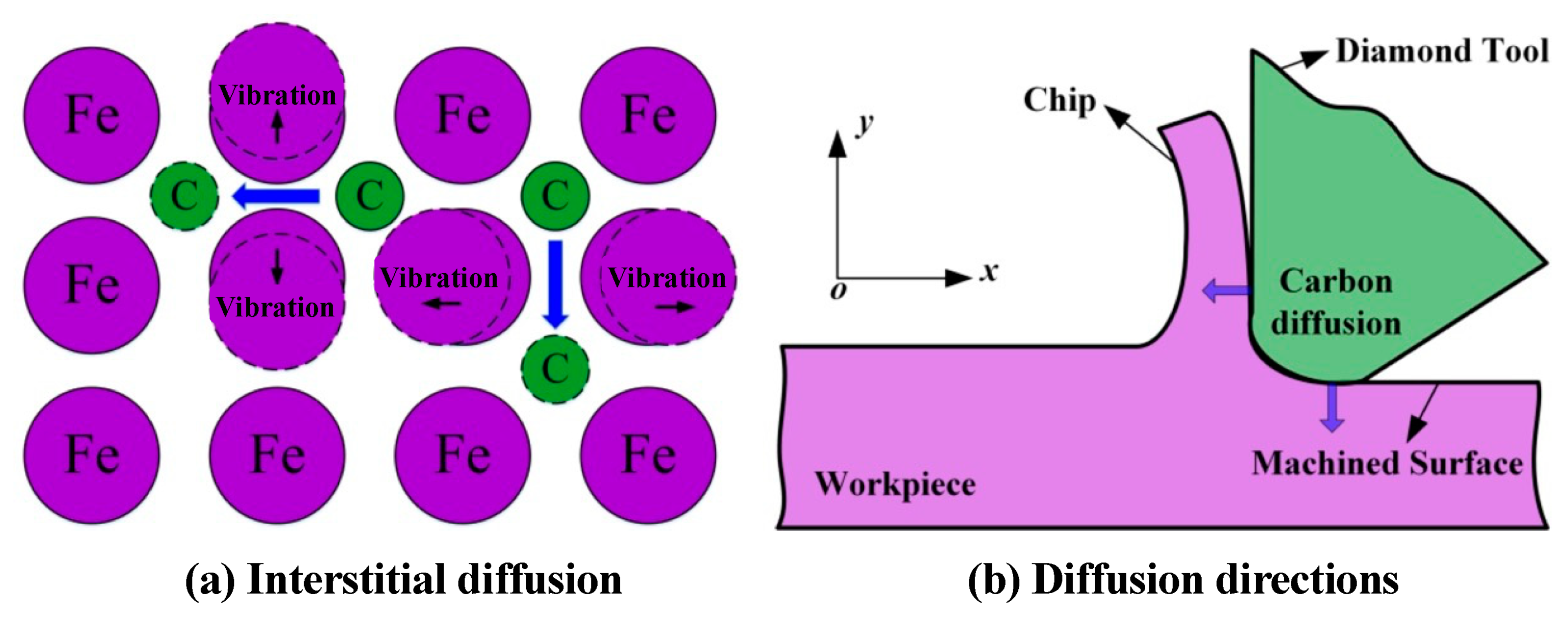
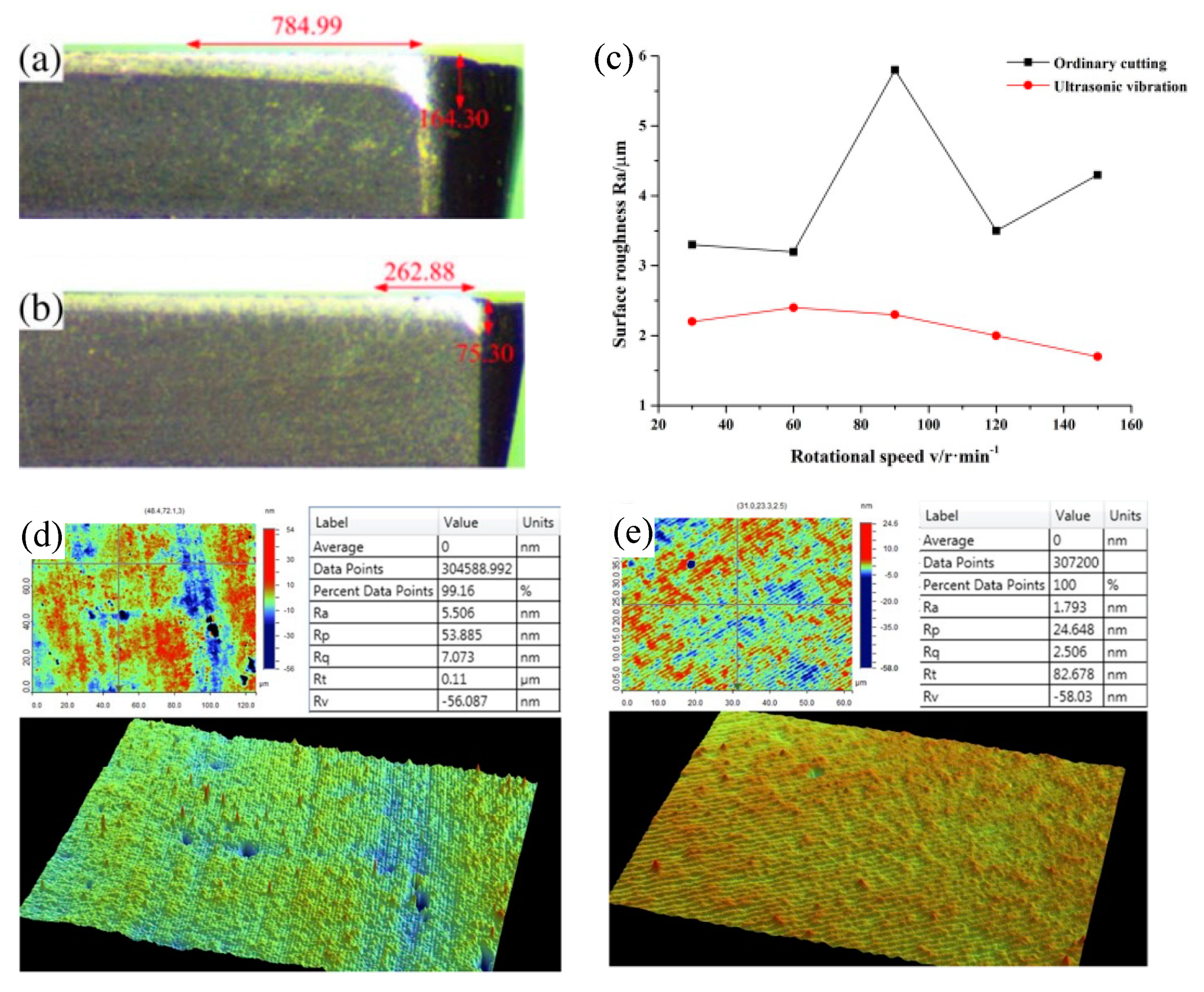

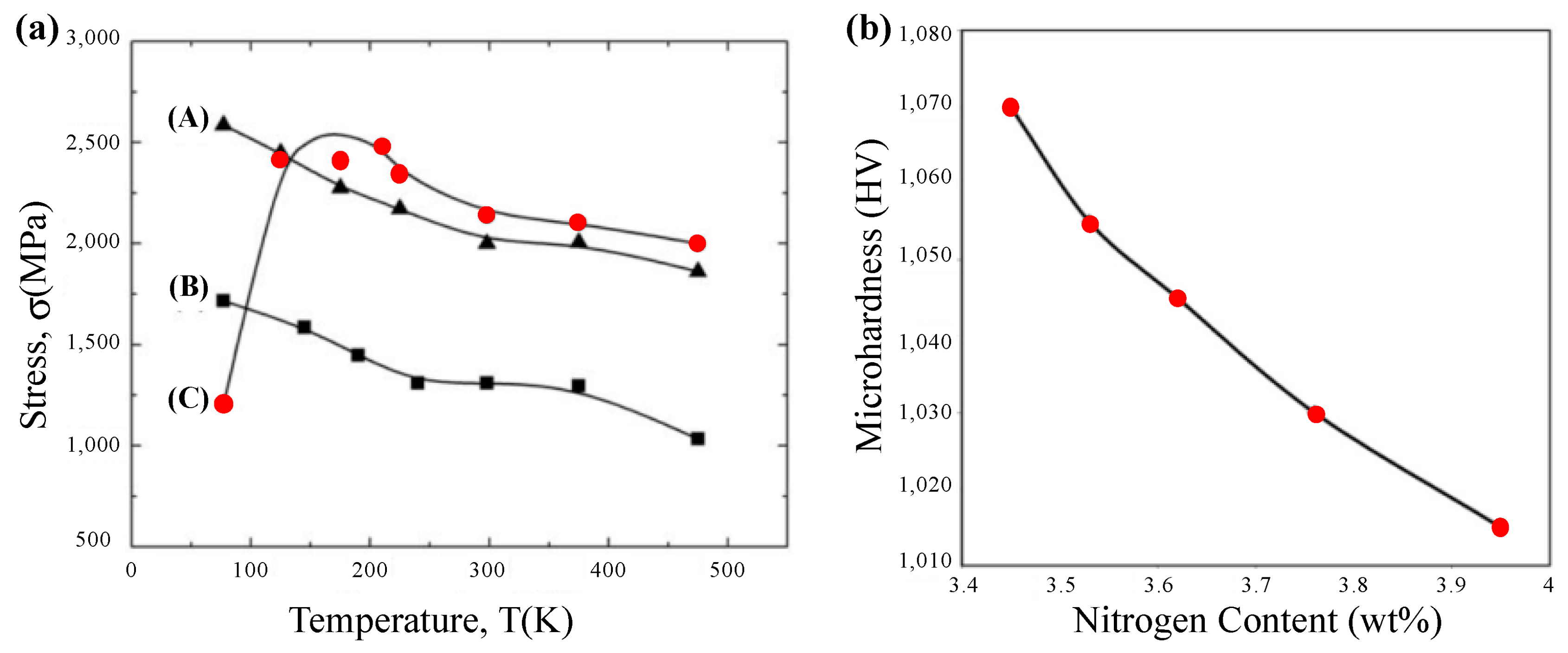
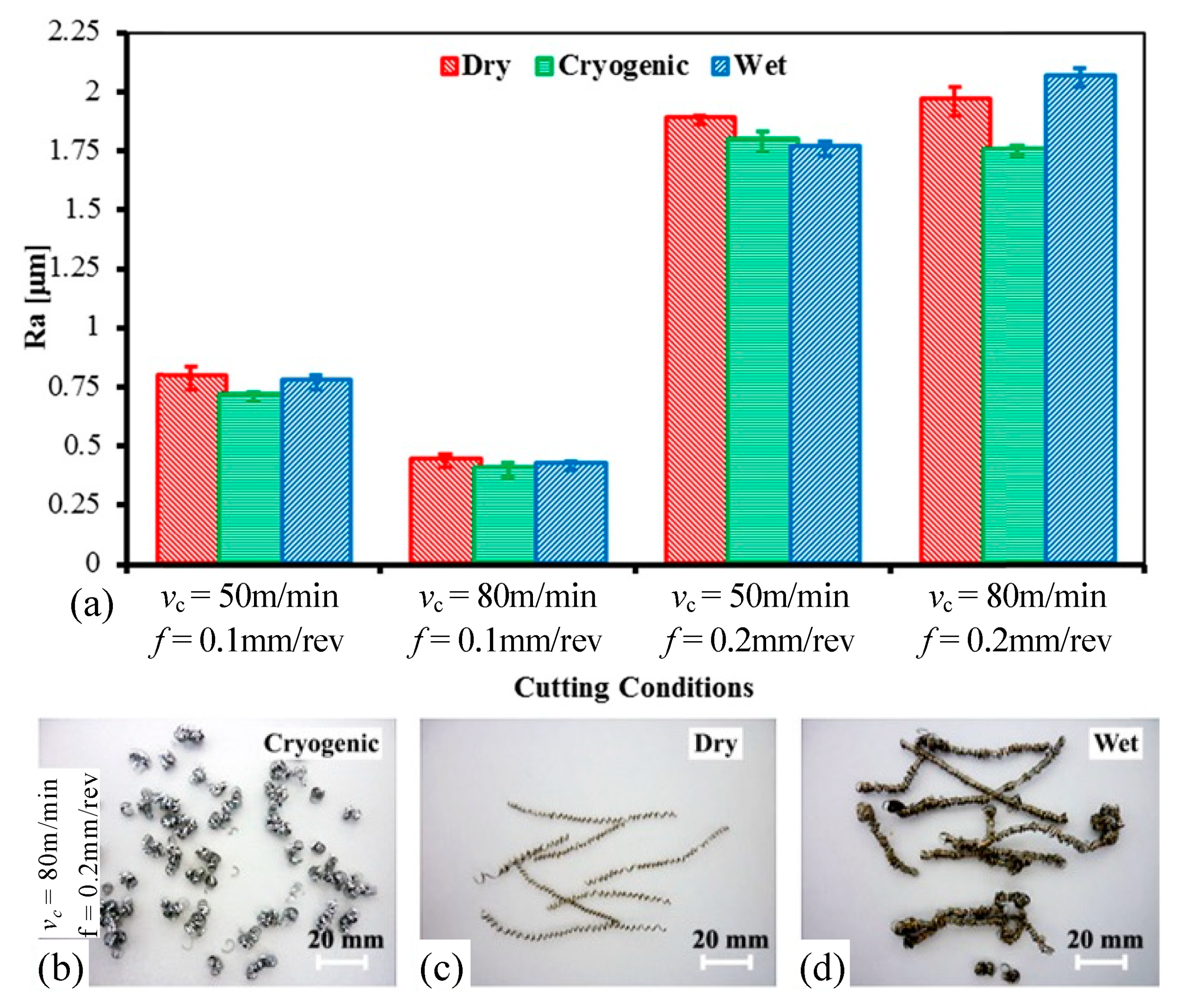
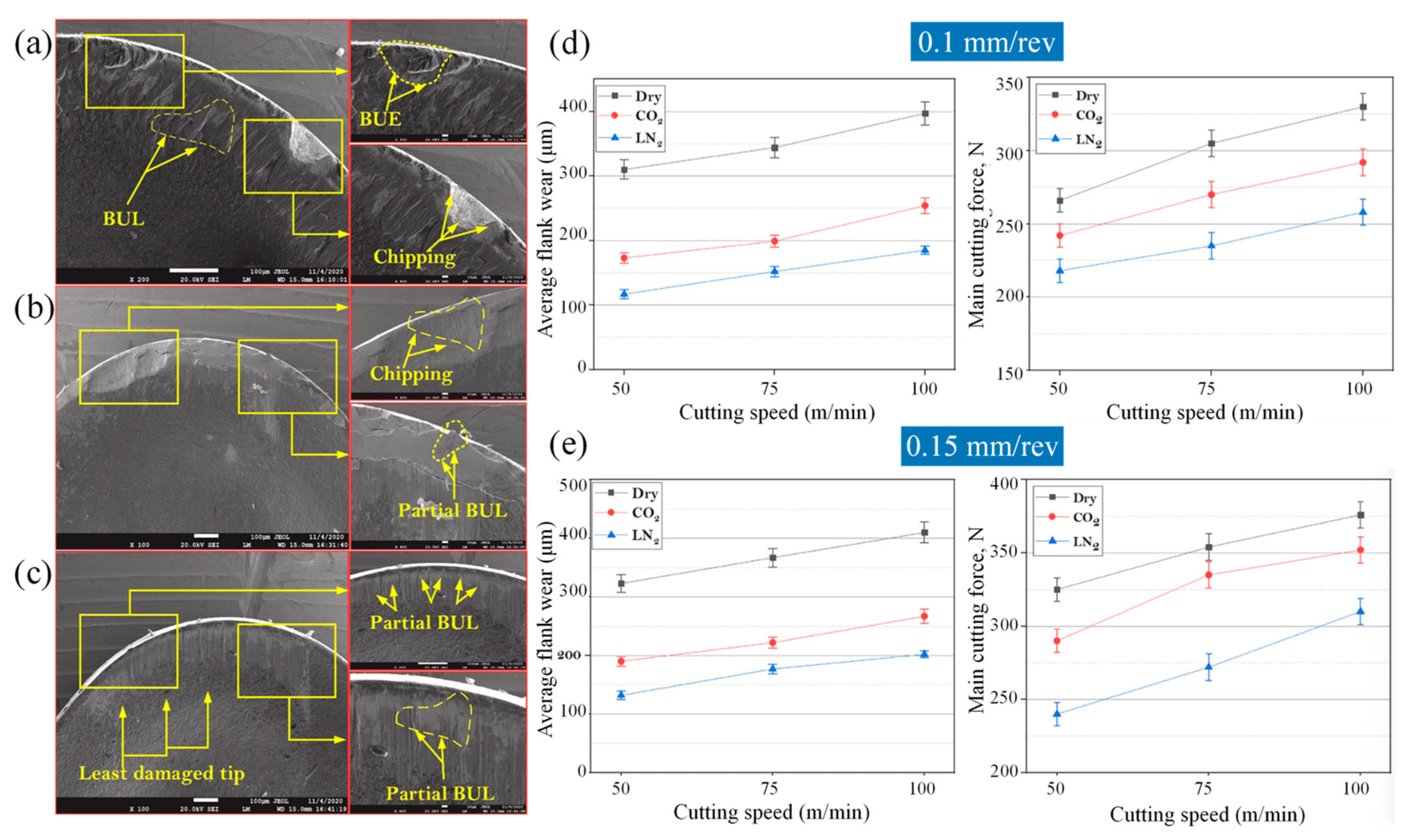

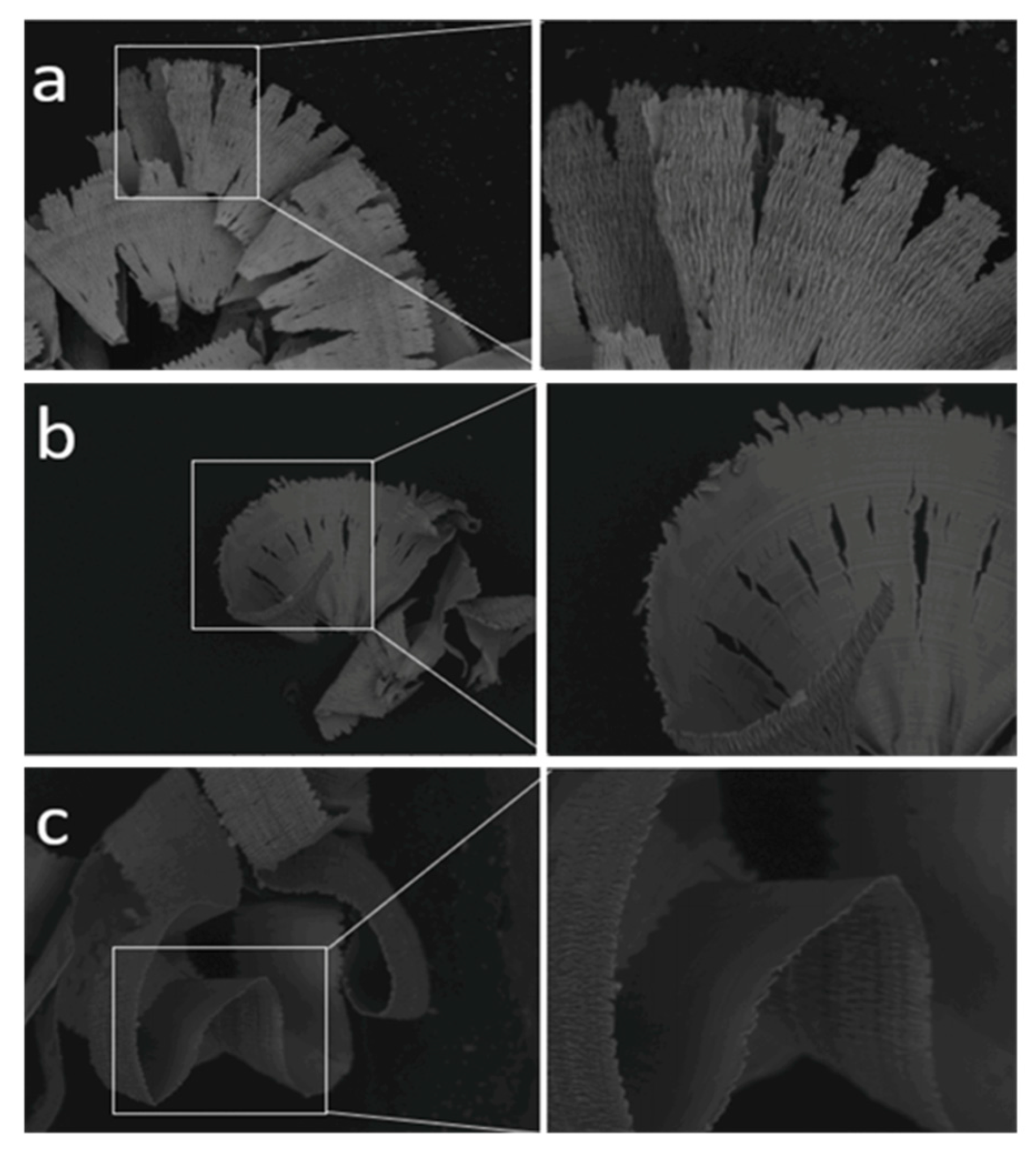
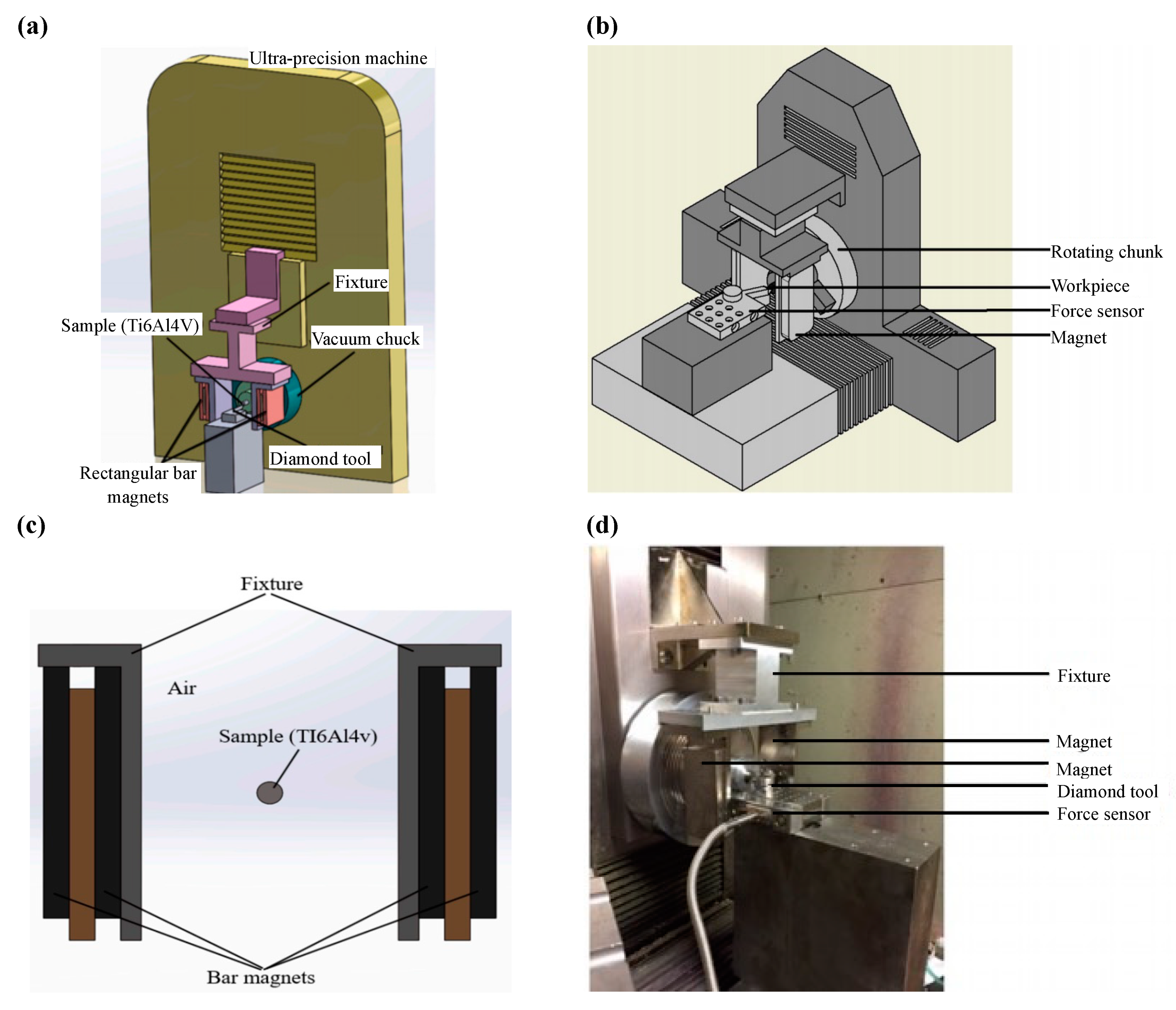


| Time | Events | References |
|---|---|---|
| 1959 | First MG from Cal. Tech. using splat quenching (Au-Si) | [29] |
| 1966 | Splat cooling additional findings (MIT) | [30] |
| 1969 | MG formation, stability, structure (Pd-Si) (Harvard) | [31] |
| 1977 | MG constitutive model | [32,33] |
| 1990 | Multi component MG formers using Copper cast (Tohoku University) | [29] |
| 2002 | MG tension, compression studies | [34] |
| 2003 | MG research with La-Al-Cu-Ni (GFA) | [35] |
| 2004 | Application in MEMS, biomedical, sporting goods, and electronics | [36] |
| 2005 | MG corrosion wear resistance | [37] |
| 2007 | Drilling, machining studies on MG | [38] |
| 2008 | MG model into ABAQUS FEA program | [36] |
| 2012 | MG cold rolling studies | [39] |
| 2013 | MG foam reduce osteopenia in biomedical | [40] |
| 2013 | MG by 3D SLM started | [41] |
| 2015 | Honeycomb MG | [42] |
| 2016 | MG descriptor on GFA best element combination (AFLOW framework) | [43] |
| 2017 | MG using 3D SLM with crack-free, complex geometry | [44] |
| 2019 | MG measured in a levitation device under microgravity | [45] |
| Sample ID | Feed Rate (mm/min) | DOC (μm) | Spindle Rate (r/min) | Groove Depth (nm) | Distance between Fold Lines (μm) | Tool Moving Speed (μm/s) |
|---|---|---|---|---|---|---|
| A | 10 | 1 | 2000 | 7 | 0.35 | 7 |
| B | 20 | 3 | 2000 | 14 | 1.08 | 16 |
| C | 10 | 3 | 2000 | 20 | 0.26 | 8 |
| D | 10 | 3 | 500 | 200 | 1.78 | 18 |
| Composition | MS (300 K) (A m2/kg) | MS (77 K) (A m2/kg) | HC (300 K) (A/m) | HC (300 K) (A/m) |
|---|---|---|---|---|
| Fe73.5Si13.5Nb3B9Cu1 | 139.5 | 151.9 | 0.52 | 0.81 |
| Fe68Si15.5Al3.5Nb3B9Cu1 | 113.3 | 127.6 | 0.35 | 1.26 |
| Fe65.5Si16.5Al5Nb3B9Cu1 | 98.3 | 98.3 | 0.50 | 0.58 |
| Fe63Si17.5Al6Nb3B9Cu1 | 80.4 | 80.4 | 1.12 | 0.45 |
| Fe62Si18Al7Nb3B9Cu1 | 64.1 | 64.1 | 1.48 | 1.45 |
| Cooling Conditions | MQL | CG | MQL + CG |
|---|---|---|---|
| Tool wear | 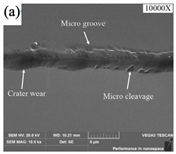 | 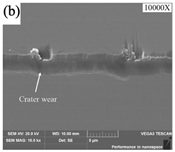 | 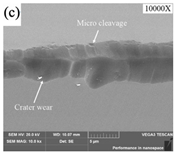 |
| Chip adhesion | 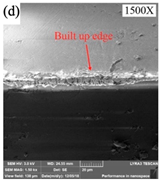 | 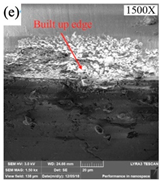 | 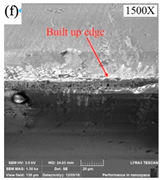 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Z.; Zhang, G.; Han, J.; Wang, J.; Ma, S.; Wang, H. A Review of the Preparation, Machining Performance, and Application of Fe-Based Amorphous Alloys. Processes 2022, 10, 1203. https://doi.org/10.3390/pr10061203
Huo Z, Zhang G, Han J, Wang J, Ma S, Wang H. A Review of the Preparation, Machining Performance, and Application of Fe-Based Amorphous Alloys. Processes. 2022; 10(6):1203. https://doi.org/10.3390/pr10061203
Chicago/Turabian StyleHuo, Zexuan, Guoqing Zhang, Junhong Han, Jianpeng Wang, Shuai Ma, and Haitao Wang. 2022. "A Review of the Preparation, Machining Performance, and Application of Fe-Based Amorphous Alloys" Processes 10, no. 6: 1203. https://doi.org/10.3390/pr10061203
APA StyleHuo, Z., Zhang, G., Han, J., Wang, J., Ma, S., & Wang, H. (2022). A Review of the Preparation, Machining Performance, and Application of Fe-Based Amorphous Alloys. Processes, 10(6), 1203. https://doi.org/10.3390/pr10061203






