Effect of Wettability on Vacuum-Driven Bubble Nucleation
Abstract
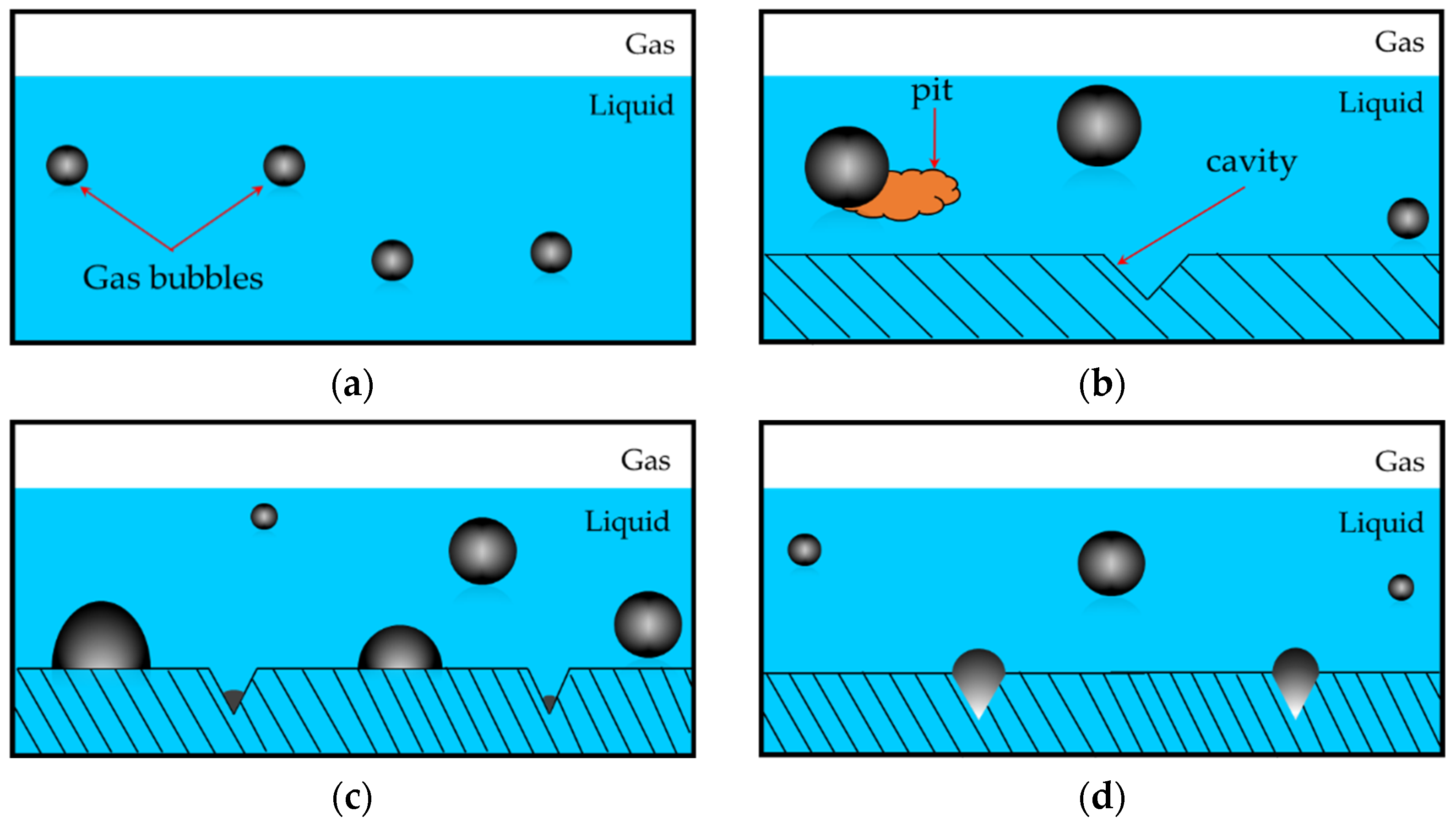
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Equipment Details
2.2. Chemicals and Equipment Details
2.3. Static Contact Angle and Roughness Measurements
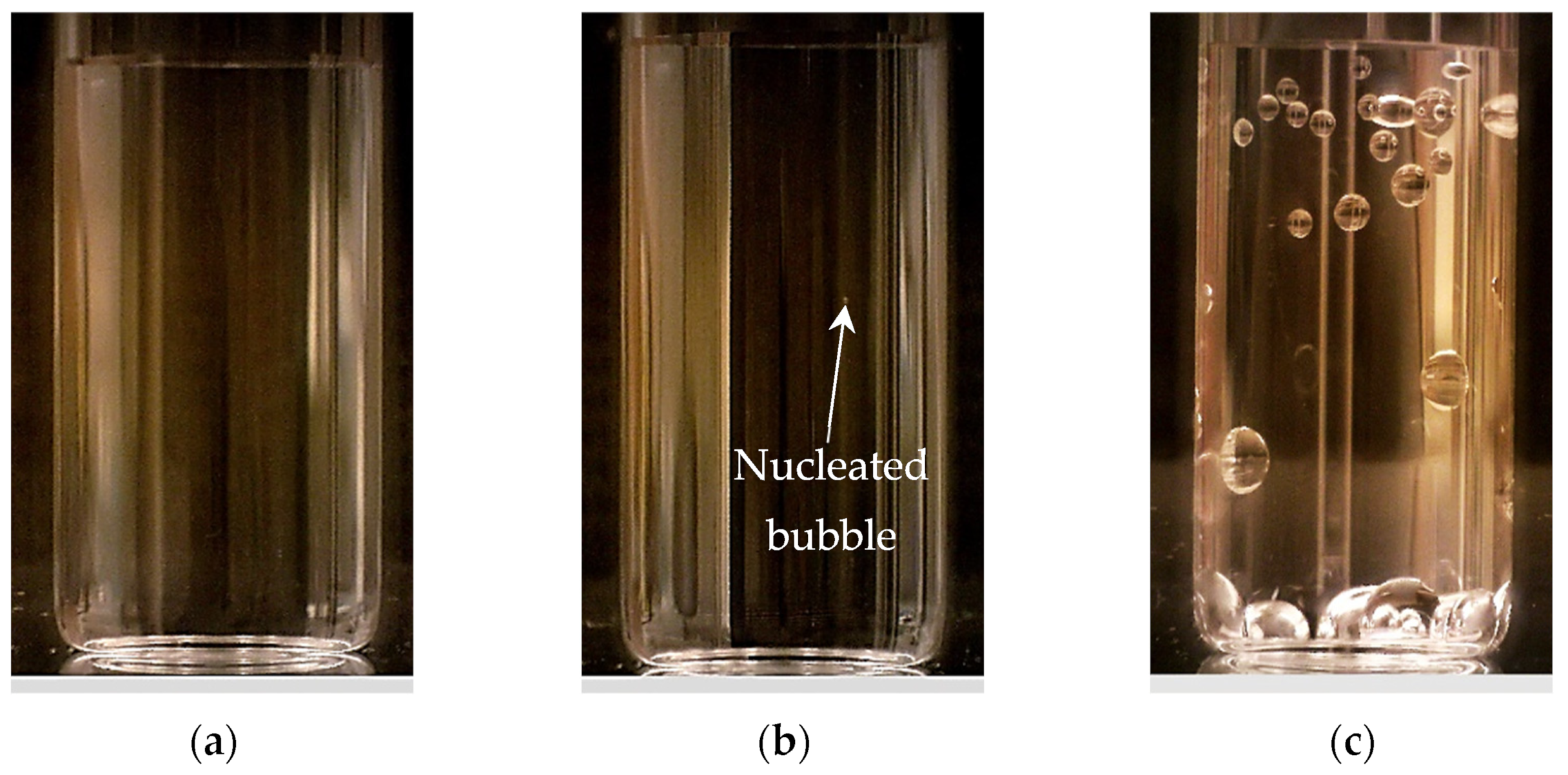
2.4. Bubble Nucleation Experiments in Vacuum Desiccator
3. Results and Discussion
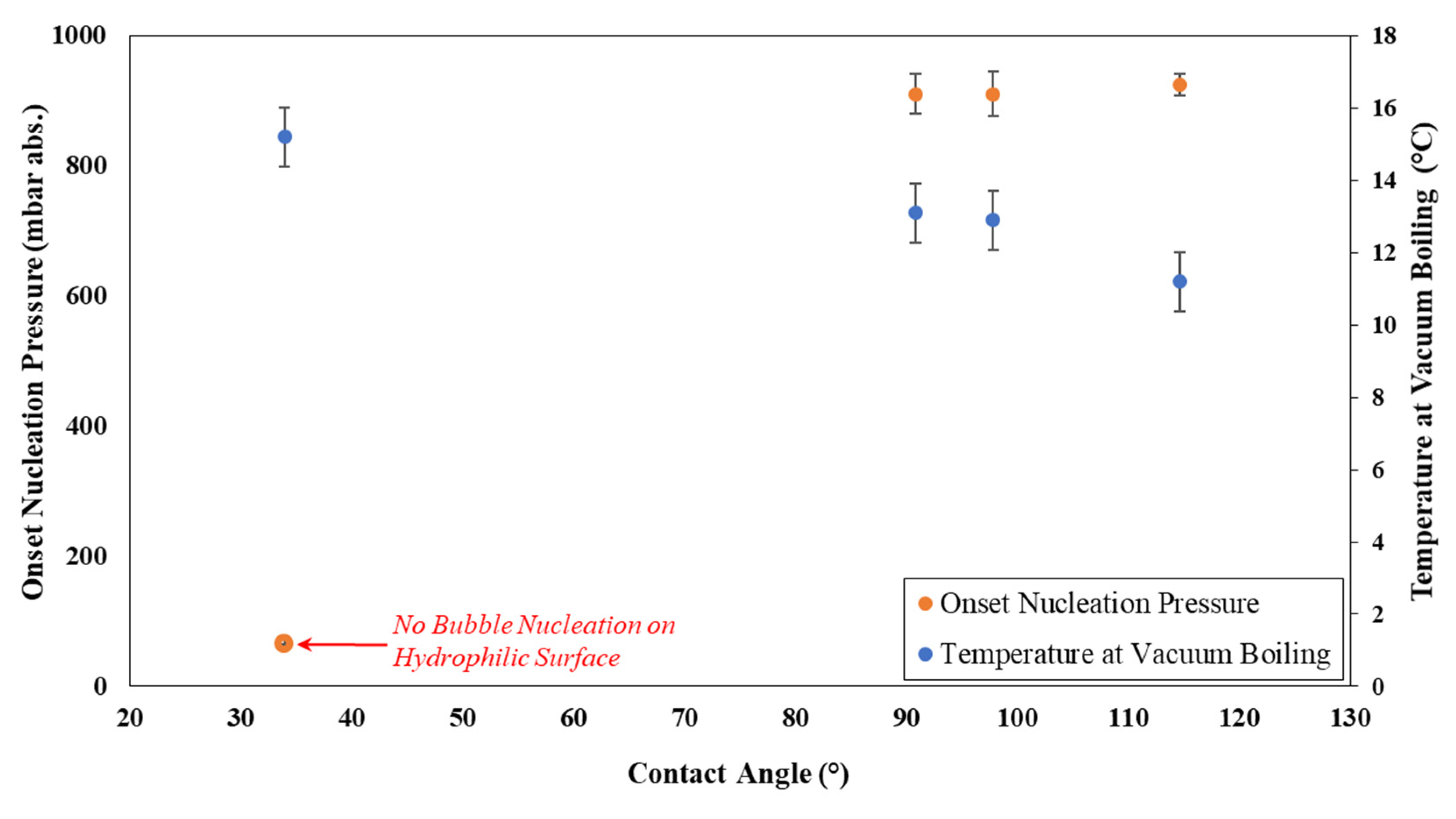
3.1. Contact Angle and Roughness Measurements
3.2. Vacuum-Driven Bubble Nucleation Results
3.2.1. Effect of Wettability on Onset Vacuum for Bubble Nucleation
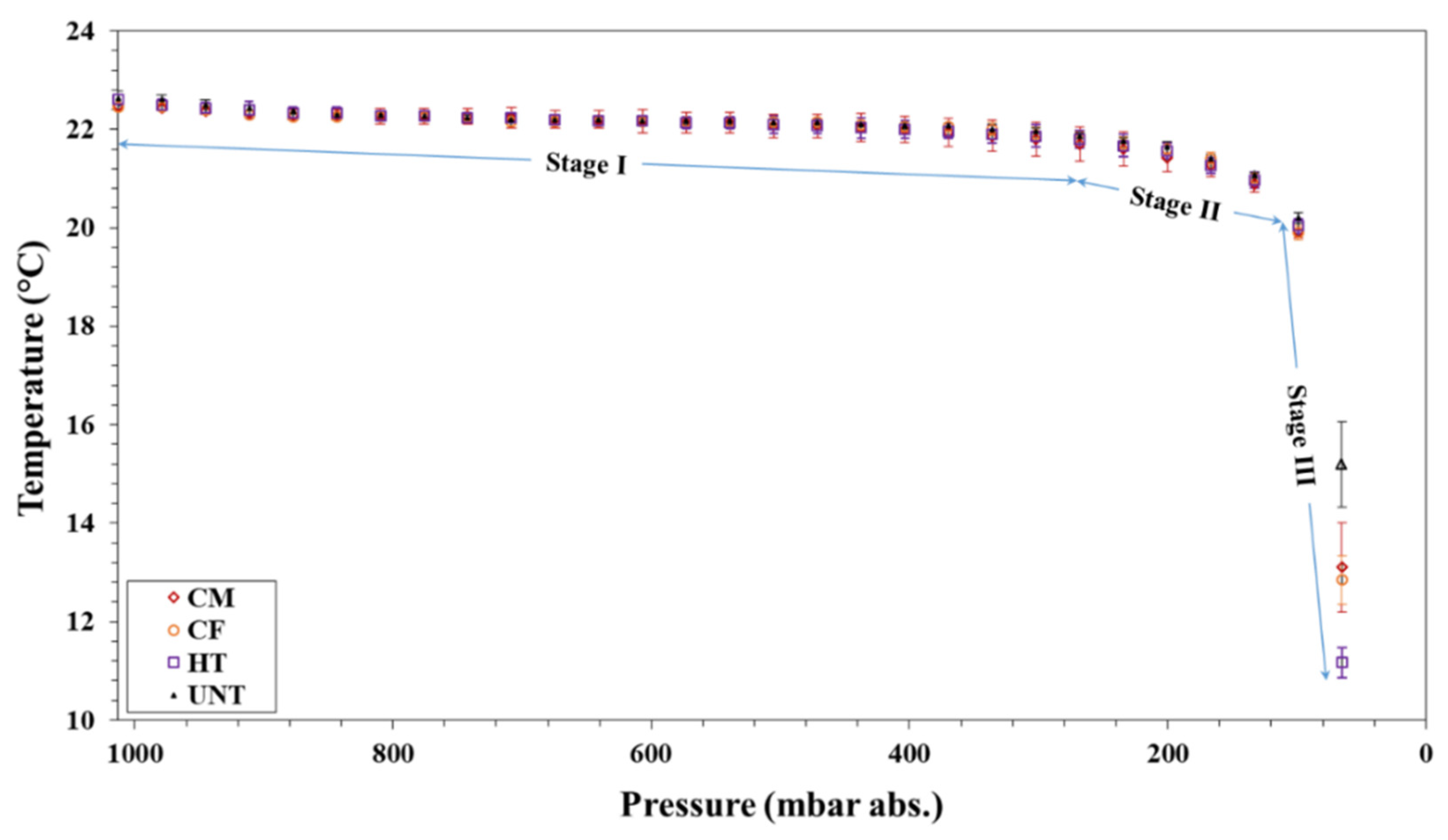
3.2.2. Effect of Wettability on Evaporative Cooling during Bubble Nucleation
4. Conclusions
- The wettability of the container surface has a strong influence on the onset vacuum for vapor/gas bubble nucleation, rate of vacuum boiling, and evaporative cooling.
- As the hydrophobicity increases the rate of vacuum boiling, and hence the rate of evaporative cooling increases.
- At higher vacuum levels (stage III in Figure 4), a relatively higher decrease in the temperature of the water was observed in the hydrophobic vials compared to the hydrophilic vials due to an increase in the rate of evaporative cooling.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions–A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Blander, M.; Katz, J.L. Bubble nucleation in liquids. AIChE J. 1975, 21, 833–848. [Google Scholar] [CrossRef]
- Jones, S.; Evans, G.; Galvin, K. Bubble nucleation from gas cavities—A review. Adv. Colloid Interface Sci. 1999, 80, 27–50. [Google Scholar] [CrossRef]
- Diemand, J.; Angélil, R.; Tanaka, K.K.; Tanaka, H. Direct simulations of homogeneous bubble nucleation: Agreement with classical nucleation theory and no local hot spots. Phys. Rev. E 2014, 90, 052407. [Google Scholar] [CrossRef]
- Vachaparambil, K.J.; Einarsrud, K.E. Explanation of bubble nucleation mechanisms: A gradient theory approach. J. Electrochem. Soc. 2018, 165, E504. [Google Scholar] [CrossRef]
- Pradhan, S.; Qader, R.J.; Sedai, B.R.; Bikkina, P.K. Influence of Wettability on Pressure-Driven Bubble Nucleation: A Potential Method for Dissolved Gas Separation. Sep. Purif. Technol. 2019, 217, 31–39. [Google Scholar] [CrossRef]
- Bikkina, P.K.; Pradhan, S. A potential solution for boiling crisis. In Proceedings of the 18th International Topical Meeting on Nuclear Reactor Thermal Hydraulics (NURETH 18), Portland, OR, USA, 18–23 August 2019; American Nuclear Society: La Grange Park, IL, USA, 2019; pp. 1383–1396. [Google Scholar]
- Eddington, R.; Kenning, D. The effect of contact angle on bubble nucleation. Int. J. Heat Mass Transf. 1979, 22, 1231–1236. [Google Scholar] [CrossRef]
- Tong, W.; Bar-Cohen, A.; Simon, T.; You, S. Contact angle effects on boiling incipience of highly-wetting liquids. Int. J. Heat Mass Transf. 1990, 33, 91–103. [Google Scholar] [CrossRef]
- Nam, Y.; Ju, Y.S. Bubble nucleation on hydrophobic islands provides evidence to anomalously high contact angles of nanobubbles. Appl. Phys. Lett. 2008, 93, 103115. [Google Scholar] [CrossRef]
- Sakuma, G.; Fukunaka, Y.; Matsushima, H. Nucleation and growth of electrolytic gas bubbles under microgravity. Int. J. Hydrogen Energy 2014, 39, 7638–7645. [Google Scholar] [CrossRef]
- Han, Y.; Agyeman, F.; Green, H.; Tao, W. Stable, high-rate anaerobic digestion through vacuum stripping of digestate. Bioresour. Technol. 2022, 343, 126133. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Shirakashi, R. Simulation of air/vacuum desiccation process for high-quality preservation of proteins. J. Food Process Eng 2022, e13962. [Google Scholar] [CrossRef]
- Ermolaev, A.; Krysanov, K.; Tekiev, M.; Silaev, V.; Zaseev, S. Vacuum-evaporative method of juice concentration. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Omsk, Russia, 29–30 March 2021; IOP Publishing: Tokyo, Japan, 2021. [Google Scholar]
- Kim, J.; Huh, C.; Kim, M.H. On the growth behavior of bubbles during saturated nucleate pool boiling at sub-atmospheric pressure. Int. J. Heat Mass Transf. 2007, 50, 3695–3699. [Google Scholar] [CrossRef]
- Wu. A molecular dynamics simulation of bubble nucleation in homogeneous liquid under heating with constant mean negative pressure. Microscale Thermophys. Eng. 2003, 7, 137–151. [Google Scholar] [CrossRef]
- Serdyukov, V.; Patrin, G.; Malakhov, I.; Surtaev, A. Biphilic surface to improve and stabilize pool boiling in vacuum. Appl. Therm. Eng. 2022, 209, 118298. [Google Scholar] [CrossRef]
- Može, M.; Vajc, V.; Zupančič, M.; Šulc, R.; Golobič, I. Pool boiling performance of water and self-rewetting fluids on hybrid functionalized aluminum surfaces. Processes 2021, 9, 1058. [Google Scholar] [CrossRef]
- Može, M.; Vajc, V.; Zupančič, M.; Golobič, I. Hydrophilic and Hydrophobic Nanostructured Copper Surfaces for Efficient Pool Boiling Heat Transfer with Water, Water/Butanol Mixtures and Novec 649. Nanomaterials 2021, 11, 3216. [Google Scholar] [CrossRef]
- Liao, M.-J.; Duan, L.-Q. Effect of the Hybrid Hydrophobic-Hydrophilic Nanostructured Surface on Explosive Boiling. Coatings 2021, 11, 212. [Google Scholar] [CrossRef]
- Regis, F.; Arsiccio, A.; Bourlès, E.; Scutellà, B.; Pisano, R. Surface Treatment of Glass Vials for Lyophilization: Implications for Vacuum-Induced Surface Freezing. Pharmaceutics 2021, 13, 1766. [Google Scholar] [CrossRef]
- Jones, S.; Galvin, K.; Evans, G.; Jameson, G.J. Carbonated water: The physics of the cycle of bubble production. Chem. Eng. Sci. 1998, 53, 169–173. [Google Scholar] [CrossRef]
- Jones, S.; Evans, G.; Galvin, K. The cycle of bubble production from a gas cavity in a supersaturated solution. Adv. Colloid Interface Sci. 1999, 80, 51–84. [Google Scholar] [CrossRef]
- Hanafizadeh, P.; Eshraghi, J.; Kosari, E.; Ahmed, W. The effect of gas properties on bubble formation, growth, and detachment. Part. Sci. Technol. 2015, 33, 645–651. [Google Scholar] [CrossRef]
- Yang, Z.; Dinh, T.-N.; Nourgaliev, R.; Sehgal, B. Numerical investigation of bubble growth and detachment by the lattice-Boltzmann method. Int. J. Heat Mass Transf. 2001, 44, 195–206. [Google Scholar] [CrossRef]
- Boubendir, L.; Chikh, S.; Tadrist, L. On the surface tension role in bubble growth and detachment in a micro-tube. Int. J. Multiph. Flow 2020, 124, 103196. [Google Scholar] [CrossRef]
- Georgoulas, A.; Koukouvinis, P.; Gavaises, M.; Marengo, M. Numerical investigation of quasi-static bubble growth and detachment from submerged orifices in isothermal liquid pools: The effect of varying fluid properties and gravity levels. Int. J. Multiph. Flow 2015, 74, 59–78. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Bikkina, P.K. An Analytical Method to Estimate Supersaturation in Gas–Liquid Systems as a Function of Pressure-Reduction Step and Waiting Time. Eng 2022, 3, 116–123. [Google Scholar] [CrossRef]
- Pradhan, S.; Bikkina, P. The Effect of Step-Down Pressure and Wettability on Pressure-Driven Bubble Nucleation for Dissolved Gas Separation. In Proceedings of the 2020 Virtual AIChE Annual Meeting, Virtual, 16–20 November 2020; AIChE: New York, NY, USA, 2020. [Google Scholar]
- Pradhan, S. Influence of Wettability on Dissolved Gas Separation, Nucleate Boiling, and Enhanced Oil Recovery. Ph.D. Dissertation, School of Chemical Engineering, Oklahoma State University, Stillwater, OK, USA, 2021. [Google Scholar]
- Fuchs, F. Ultrasonic cleaning and washing of surfaces. In Power Ultrasonics; Gallego-Juárez, J.A., Graff, K.F., Eds.; Elsevier: Cambridge, UK, 2015; pp. 577–609. [Google Scholar]
- Francis, M.J. Effect of degassing on the electrical conductivity of pure water and potassium chloride solutions. J. Phys. Chem. C 2008, 112, 14563–14569. [Google Scholar] [CrossRef]
- Degassing Water Methods. Available online: https://www.veoliawatertech.com/en/solutions/technologies/whittier-filtration-separation/degasification (accessed on 22 April 2022).
- CF, UNT and CM Vials Containing Degassed and DI Water at 65 mbar Absolute (28 inHg rel.) Pressure. Available online: https://youtu.be/QilmjSg0O5c (accessed on 21 December 2020).
- HT Vial Containing Degassed and DI Water at 65 mbar Absolute (28 inHg rel.) pressure. Available online: https://youtu.be/E07DaBB6Q_I (accessed on 21 December 2020).


| Average Contact Angle (°) | Average Roughness (nm) | ||
|---|---|---|---|
| Surface | Flat Surface | Flat Surface | Vial (Curved) Surface |
| Untreated | 33.9 ± 0.4 | 1.1 ± 0.1 | 3.6 ± 1.3 |
| CM | 90.8 ± 0.8 | 6.6 ± 0.7 | 6.4 ± 2.7 |
| CF | 97.8 ± 0.4 | 2.9 ± 0.2 | 4.4 ± 1.3 |
| HT | 114.6 ± 0.75 | 2.4 ± 0.2 | 7.4 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, S.; Counts, S.; Enget, C.; Bikkina, P.K. Effect of Wettability on Vacuum-Driven Bubble Nucleation. Processes 2022, 10, 1073. https://doi.org/10.3390/pr10061073
Pradhan S, Counts S, Enget C, Bikkina PK. Effect of Wettability on Vacuum-Driven Bubble Nucleation. Processes. 2022; 10(6):1073. https://doi.org/10.3390/pr10061073
Chicago/Turabian StylePradhan, Sushobhan, Sage Counts, Charissa Enget, and Prem Kumar Bikkina. 2022. "Effect of Wettability on Vacuum-Driven Bubble Nucleation" Processes 10, no. 6: 1073. https://doi.org/10.3390/pr10061073
APA StylePradhan, S., Counts, S., Enget, C., & Bikkina, P. K. (2022). Effect of Wettability on Vacuum-Driven Bubble Nucleation. Processes, 10(6), 1073. https://doi.org/10.3390/pr10061073








