Repeated Transient Transfection: An Alternative for the Recombinant Production of Difficult-to-Express Proteins Like BMP2
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cells and Maintenance
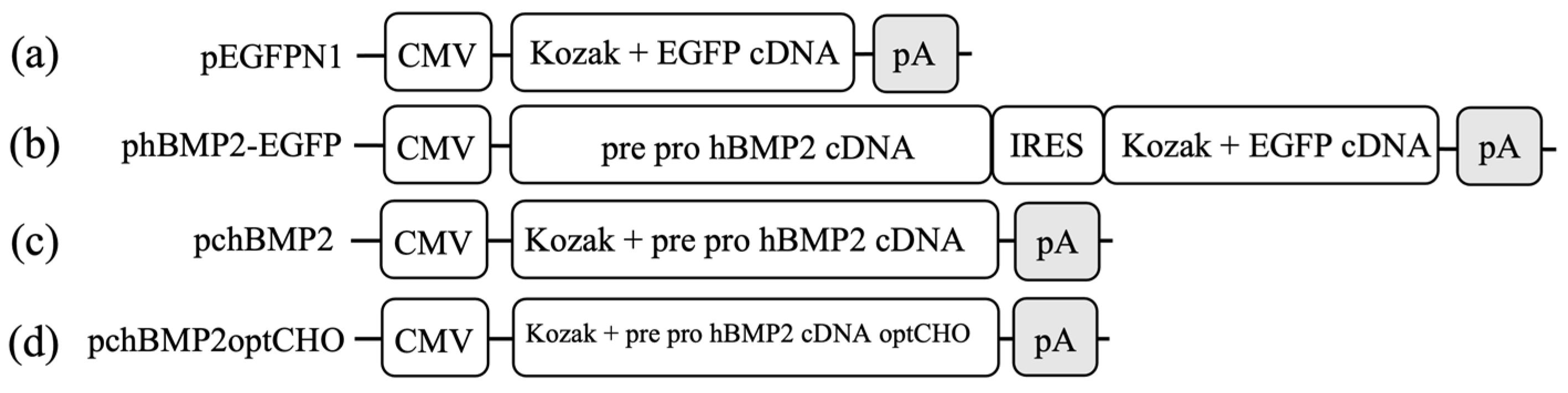
2.3. Plasmids
2.4. Transfection Agent
2.5. High Cell Density Transfection Protocol for HEKsus and CHOsus Cells
2.6. RTT of HEKsus Cells for EGFP and BMP2 Production
2.7. Scale-Up of RTT
2.8. Flow Cytometry Analysis of EGFP Expression
2.9. Quantification of BMP2
2.10. Statistical Analysis
3. Results and Discussion
3.1. Transient BMP2 Production in CHOsus Cells at the Micro-Scale
3.2. Transient BMP2 Production in HEKsus Cells at the Micro-Scale
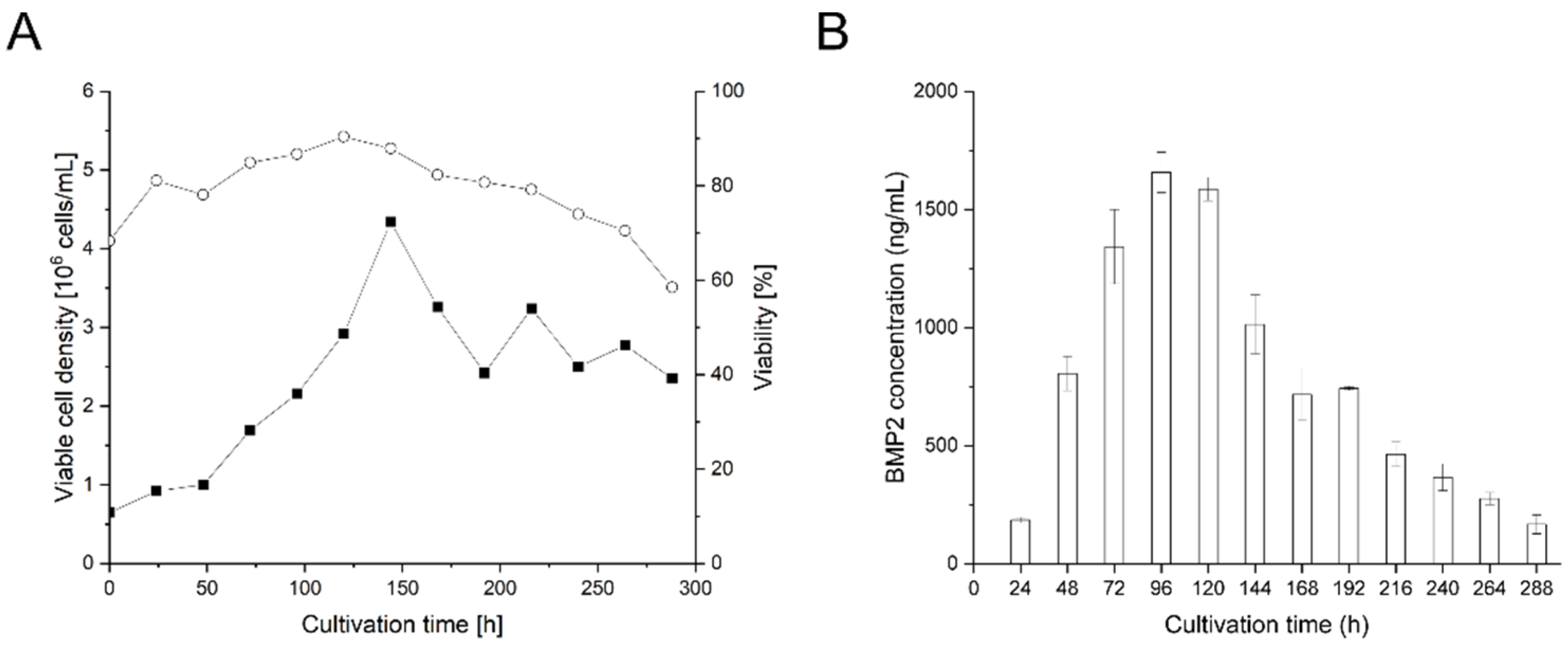
3.3. Small-Scale Transient BMP2 Production in HEKsus Cells
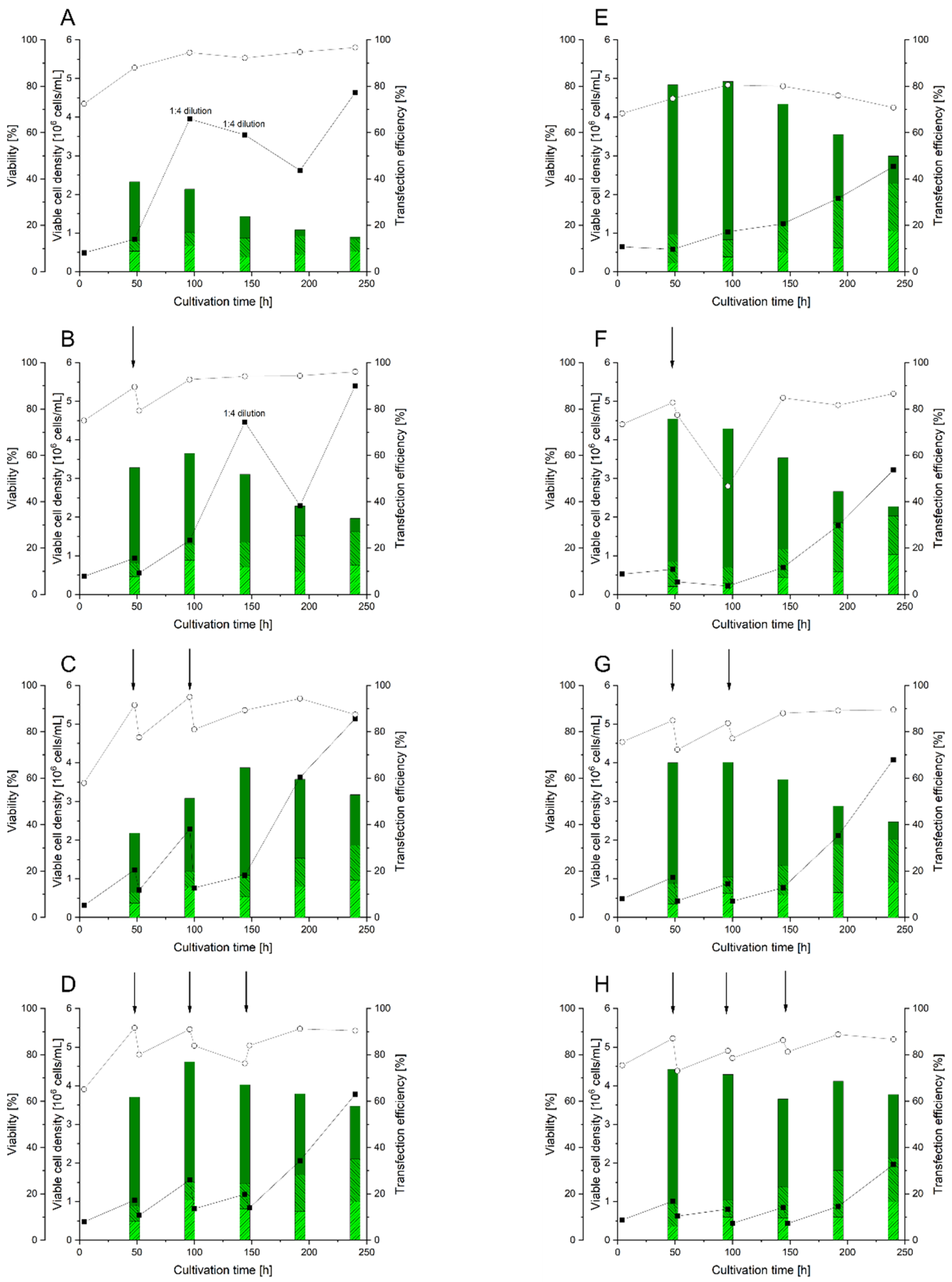
3.4. Implementation of the RTT Procedure
3.5. Use of RTT for BMP2 Production in HEKsus Cells at the Micro-Scale
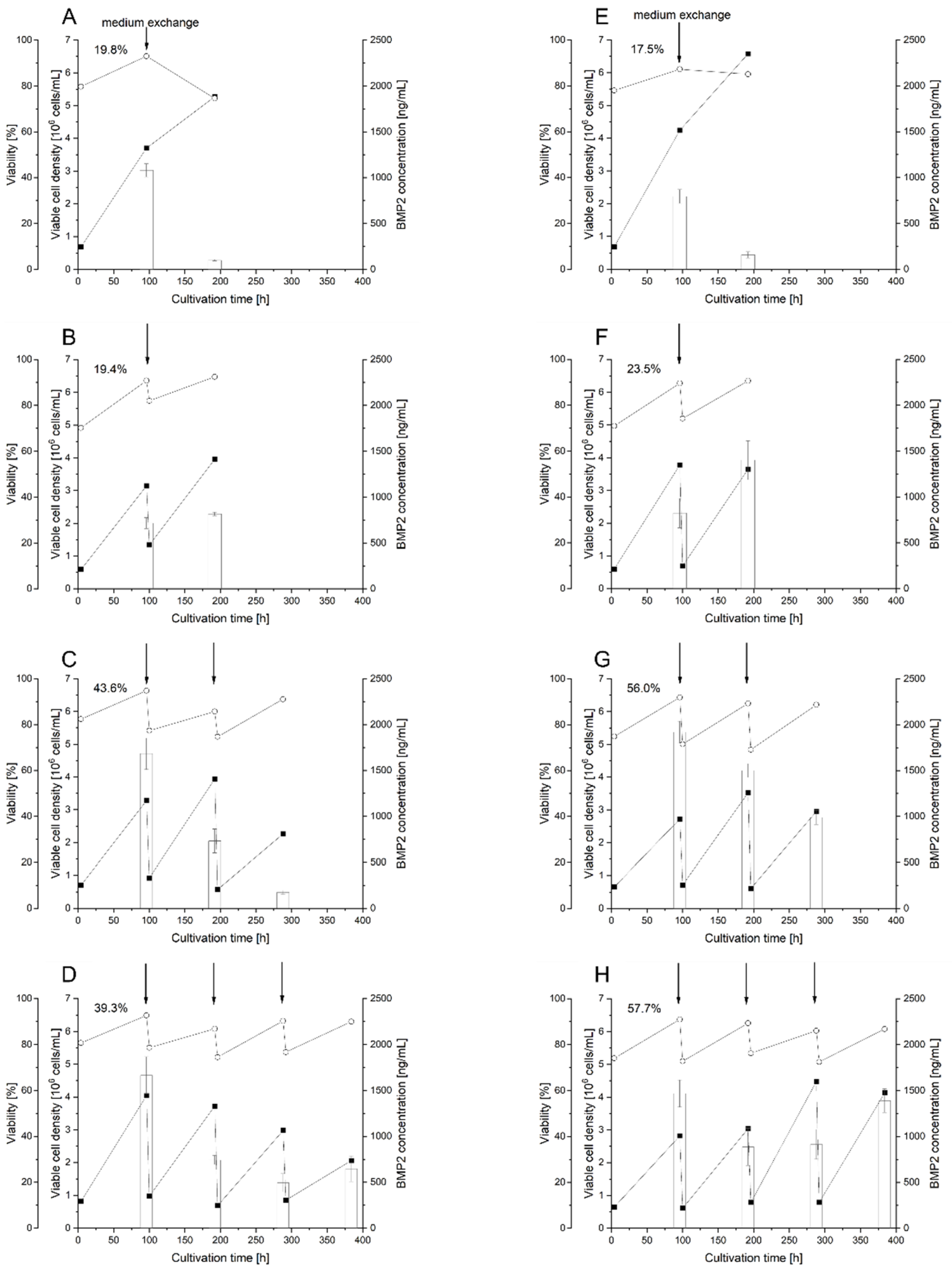
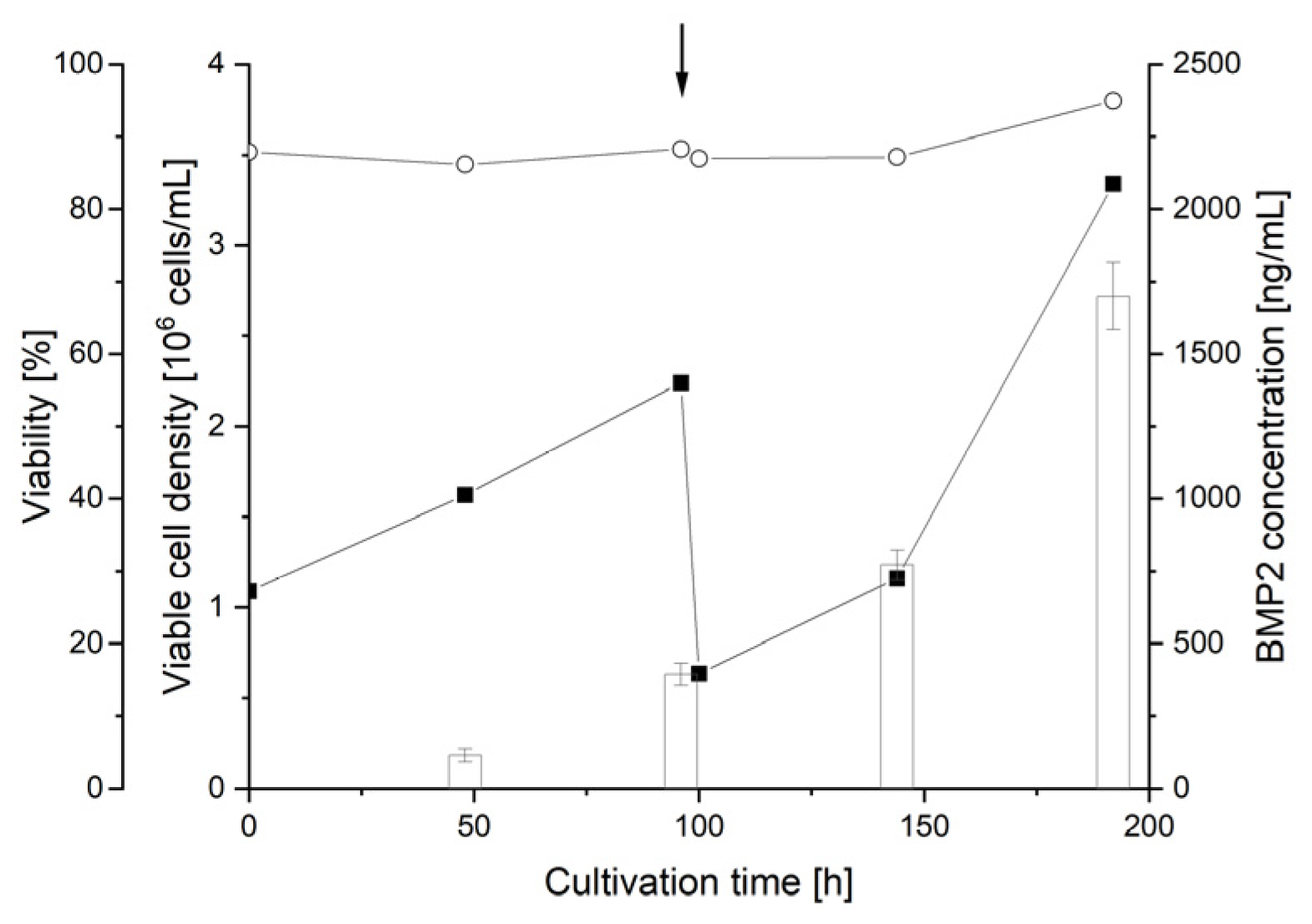
3.6. Scale-Up of BMP2 Production by RTT
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carreira, A.C.; Lojudice, F.H.; Halcsik, E.; Navarro, R.D.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Facts, Challenges, and Future Perspectives. J. Dent. Res. 2014, 93, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Zambuzzi, W.F.; Rossi, M.C.; Astorino Filho, R.; Sogayar, M.C.; Granjeiro, J.M. Bone morphogenetic proteins: Promising molecules for bone healing, bioengineering, and regenerative medicine. Vitam. Horm. 2015, 99, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [PubMed]
- Lissenberg-Thunnissen, S.N.; de Gorter, D.J.J.; Sier, C.F.M.; Schipper, I.B. Use and efficacy of bone morphogenetic proteins in fracture healing. Int. Orthop. 2011, 35, 1271–1280. [Google Scholar] [CrossRef]
- Nguyen, V.; Meyers, C.A.; Yan, N.; Agarwal, S.; Levi, B.; James, A.W. BMP-2-induced bone formation and neural inflammation. J. Orthop. 2017, 14, 252–256. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, K.-H.; Kim, S.; Lee, Y.-M.; Seol, Y.-J. BMP-2 gene delivery-based bone regeneration in dentistry. Pharmaceutics 2019, 11, 393. [Google Scholar] [CrossRef]
- Schmidt-Bleek, K.; Willie, B.M.; Schwabe, P.; Seemann, P.; Duda, G.N. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2016, 27, 141–148. [Google Scholar] [CrossRef]
- Hang, Q.; Zhou, Y.; Hou, S.; Zhang, D.; Yang, X.; Chen, J.; Ben, Z.; Cheng, C.; Shen, A. Asparagine-linked glycosylation of bone morphogenetic protein-2 is required for secretion and osteoblast differentiation. Glycobiology 2014, 24, 292–304. [Google Scholar] [CrossRef]
- Kisiel, M.; Ventura, M.; Oommen, O.P.; George, A.; Walboomers, X.F.; Hilborn, J.; Varghese, O.P. Critical assessment of rhBMP-2 mediated bone induction: An in vitro and in vivo evaluation. J. Control Release 2012, 162, 646–653. [Google Scholar] [CrossRef]
- Jérôme, V.; Thoring, L.; Salzig, D.; Kubick, S.; Freitag, R. Comparison of cell-based versus cell-free mammalian systems for the production of a recombinant human bone morphogenic growth factor. Eng. Life Sci. 2017, 17, 1097–1107. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, C.L.; Kim, Y.S.; Jang, J.W.; Lee, G.M. Selective endocytosis of recombinant human BMPs through cell surface heparan sulfate proteoglycans in CHO cells: BMP-2 and BMP-7. Sci. Rep. 2021, 11, 3378. [Google Scholar] [CrossRef]
- Zhou, A.J.; Clokie, C.M.L.; Peel, S.A.F. Polyarginine peptide IND-1 enhances recombinant human bone morphogenetic protein-2 yield in mammalian cells. Biotechnol. Lett. 2012, 34, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.L.; Wu, X.; Maceren, J.P.; Mao, Y.; Kohn, J. In vitro evaluation of recombinant bone morphogenetic protein-2 bioactivity for regenerative medicine. Tissue Eng. Part C Methods 2019, 25, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.G.; Kim, N.; Heo, W.D.; Lee, G.M. Heparan sulfate proteoglycan synthesis in CHO DG44 and HEK293 cells. Biotechnol. Bioprocess. Eng. 2016, 21, 439–445. [Google Scholar] [CrossRef]
- Kudo, T.; Kanetaka, H.; Watanabe, A.; Okumoto, A.; Asano, M.; Zhang, Y.; Zhao, F.; Kano, M.R.; Shimizu, Y.; Tamura, S.; et al. Investigating bone morphogenetic protein (BMP) signaling in a newly established human cell line expressing BMP receptor type II. Tohoku J. Exp. Med. 2010, 222, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Israel, D.I.; Nove, J.; Kerns, K.M.; Moutsatsos, I.K.; Kaufman, R.J. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors 1992, 7, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lalonde, M.E.; Durocher, Y. Therapeutic glycoprotein production in mammalian cells. J. Biotechnol. 2017, 251, 128–140. [Google Scholar] [CrossRef]
- Puetz, J.; Wurm, F.M. Recombinant proteins for industrial versus pharmaceutical purposes: A review of process and pricing. Processes 2019, 7, 476. [Google Scholar] [CrossRef]
- Wurm, F.; Wurm, M. Cloning of CHO cells, productivity and genetic stability—A discussion. Processes 2017, 5, 20. [Google Scholar] [CrossRef]
- Gilleskie, G.; Rutter, C.; McCuen, B. Chapter 3. Good Manufacturing Practice (GMP) for biopharmaceutical production. In Biopharmaceutical Manufacturing: Principles, Processes, and Practices; De Gruyter: Berlin, Germany, 2021; pp. 64–91. [Google Scholar]
- Baldi, L.; Hacker, D.L.; Adam, M.; Wurm, F.M. Recombinant protein production by large-scale transient gene expression in mammalian cells: State of the art and future perspectives. Biotechnol. Lett. 2007, 29, 677–684. [Google Scholar] [CrossRef]
- Geisse, S. Reflections on more than 10 years of TGE approaches. Protein Expr. Purif. 2009, 64, 99–107. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, R.; Bergin, A.; Flampouri, E.; Mota, L.M.; Obaidi, I.; Quigley, A.; Xie, Y.; Butler, M. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Biotechnol. Adv. 2020, 43, 107552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ge, Y.; Sun, L.; Cao, J.; Wu, Q.; Guo, L.; Wang, Z. Effect of bone morphogenetic protein-2 on proliferation and apoptosis of gastric cancer cells. Int. J. Med. Sci. 2012, 9, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Dai, T.; Wei, Y.; Zhang, L.; Zheng, M.; Zhou, F. A systematic review of SARS-CoV-2 vaccine candidates. Signal Transduc. Target Ther. 2020, 5, 237. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Gutiérrez-Granados, S.; Cervera, L.; Kamen, A.A.; Gòdia, F. Advancements in mammalian cell transient gene expression (TGE) technology for accelerated production of biologics. Crit. Rev. Biotechnol. 2018, 38, 918–940. [Google Scholar] [CrossRef]
- Cervera, L.; Gutierrez-Granados, S.; Berrow, N.S.; Segura, M.M.; Godia, F. Extended gene expression by medium exchange and repeated transient transfection for recombinant protein production enhancement. Biotechnol. Bioeng. 2015, 112, 934–946. [Google Scholar] [CrossRef]
- Ladd, B.; Bowes, K.; Lundgren, M.; Gräslund, T.; Chotteau, V. Proof-of-concept of continuous transfection for adeno-associated virus production in microcarrier-based culture. Processes 2022, 10, 515. [Google Scholar] [CrossRef]
- Backliwal, G.; Hildinger, M.; Hasija, V.; Wurm, F.M. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. Bioeng. 2008, 99, 721–727. [Google Scholar] [CrossRef]
- Rajendra, Y.; Kiseljak, D.; Baldi, L.; Hacker, D.L.; Wurm, F.M. A simple high-yielding process for transient gene expression in CHO cells. J. Biotechnol. 2011, 153, 22–26. [Google Scholar] [CrossRef]
- Elshereef, A.A.; Jochums, A.; Lavrentieva, A.; Stuckenberg, L.; Scheper, T.; Solle, D. High cell density transient transfection of CHO cells for TGF-β1 expression. Eng. Life Sci. 2019, 19, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Backliwal, G.; Hildinger, M.; Chenuet, S.; Wulhfard, S.; Jesus, M.; Wurm, F.M. Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res. 2008, 36, e96. [Google Scholar] [CrossRef] [PubMed]
- Hacker, D.L.; Kiseljak, D.; Rajendra, Y.; Thurnheer, S.; Baldi, L.; Wurm, F.M. Polyethyleneimine-based transient gene expression processes for suspension-adapted HEK-293E and CHO-DG44 cells. Protein Expr. Purif. 2013, 92, 67–76. [Google Scholar] [CrossRef]
- Raymond, C.; Tom, R.; Perret, S.; Moussouami, P.; L’Abbé, D.; St-Laurent, G.; Durocher, Y. A simplified polyethylenimine-mediated transfection process for large-scale and high-throughput applications. Methods 2011, 55, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Tennant, J.R. Evaluation of the trypan blue technique for determination of cell viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Rajendra, Y.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Comparison of three transposons for the generation of highly productive recombinant CHO cell pools and cell lines. Biotechnol. Bioeng. 2016, 113, 1234–1243. [Google Scholar] [CrossRef]
- Baldi, L.; Muller, N.; Picasso, S.; Jacquet, R.; Girard, P.; Thanh, H.P.; Derow, E.; Wurm, F.M. Transient Gene Expression in Suspension HEK-293 Cells: Application to Large-Scale Protein Production. Biotechnol. Prog. 2005, 21, 148–153. [Google Scholar] [CrossRef]
- Rajendra, Y.; Hougland, M.D.; Alam, R.; Morehead, T.A.; Barnard, G.C. A high cell density transient transfection system for therapeutic protein expression based on a CHO GS-knockout cell line: Process development and product quality assessment. Biotechnol. Bioeng. 2015, 112, 977–986. [Google Scholar] [CrossRef]
- Rajendra, Y.; Kiseljak, D.; Manoli, S.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Role of non-specific DNA in reducing coding DNA requirement for transient gene expression with CHO and HEK-293E cells. Biotechnol. Bioeng. 2012, 109, 2271–2278. [Google Scholar] [CrossRef]
- Al-Allaf, F.A.; Abduljaleel, Z.; Athar, M.; Taher, M.M.; Khan, W.; Mehmet, H.; Colakogullari, M.; Apostolidou, S.; Bigger, B.; Waddington, S.; et al. Modifying inter-cistronic sequence significantly enhances IRES dependent second gene expression in bicistronic vector: Construction of optimised cassette for gene therapy of familial hypercholesterolemia. Noncoding RNA Res. 2019, 4, 1–14. [Google Scholar] [CrossRef]
- Dalton, A.C.; Barton, W.A. Overexpression of secreted proteins from mammalian cell lines. Protein Sci. 2014, 23, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Raup, A.; Jérôme, V.; Freitag, R.; Synatschke, C.V.; Müller, A.H.E. Promoter, transgene, and cell line effects in the transfection of mammalian cells using PDMAEMA-based nano-stars. Biotechnol. Rep. 2016, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pick, H.M.; Meissner, P.; Preuss, A.K.; Tromba, P.; Vogel, H.; Wurm, F.M. Balancing GFP reporter plasmid quantity in large-scale transient transfections for recombinant anti-human Rhesus-D IgG1 synthesis. Biotechnol. Bioeng. 2002, 79, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.L.; Bang, Y.L.; Kim, Y.S.; Jang, J.W.; Lee, G.M. Alleviation of proteolytic degradation of recombinant human bone morphogenetic protein-4 by repeated batch culture of Chinese hamster ovary cells. Process. Biochem. 2016, 51, 1078–1084. [Google Scholar] [CrossRef]
- Kim, C.L.; Jung, M.Y.; Kim, Y.S.; Jang, J.W.; Lee, G.M. Improving the production of recombinant human bone morphogenetic protein-4 in Chinese hamster ovary cell cultures by inhibition of undesirable endocytosis. Biotechnol. Bioeng. 2018, 115, 2565–2575. [Google Scholar] [CrossRef]
- Kim, C.L.; Lee, G.M. Improving recombinant bone morphogenetic protein-4 (BMP-4) production by autoregulatory feedback loop removal using BMP receptor-knockout CHO cell lines. Metab. Eng. 2019, 52, 57–67. [Google Scholar] [CrossRef]
- Alborzinia, H.; Schmidt-Glenewinkel, H.; Ilkavets, I.; Breitkopf-Heinlein, K.; Cheng, X.; Hortschansky, P.; Dooley, S.; Wölfl, S. Quantitative kinetics analysis of BMP2 uptake into cells and its modulation by BMP antagonists. J. Cell Sci. 2013, 126, 117–127. [Google Scholar] [CrossRef][Green Version]
- Khattar, V.; Lee, J.H.; Wang, H.; Bastola, S.; Ponnazhagan, S. Structural determinants and genetic modifications enhance BMP2 stability and extracellular secretion. FASEB Bioadv. 2019, 1, 180–190. [Google Scholar] [CrossRef]
| Micro-Scale | Small-Scale | Medium-Scale | Large-Scale | |
|---|---|---|---|---|
| Cell number | 5 × 106 cells | 100 × 106 cells | 500 × 106 cells | 1000 × 106 cells |
| Transfection volume | 0.25 mL | 5 mL | 25 mL | 50 mL |
| Transfection vessel | 2 mL reaction tube | 50 mL conical tube | 50 mL conical tube | 250 mL spinner |
| Rotational speed | 20 rpm | 20 rpm | 20 rpm | 75 rpm |
| HEKsus | ||||
| pDNA | 12.5 µg | 250 µg | 1250 µg | 2500 µg |
| l-PEI | 25 µg | 500 µg | 2500 µg | 5000 µg |
| CHOsus | ||||
| pDNA | 7.5 µg | 150 µg | 750 µg | 1500 µg |
| l-PEI | 12.5 µg | 250 µg | 1250 µg | 2500 µg |
| Cultivation Post Transfection | ||||
| Cultivation vessel | Cell culture plate (10 mL) a 6-well plate (2 mL) b | 250 mL spinner flask | 1 L spinner flask | 250 mL spinner flask c |
| Cultivation volume post transfection | 5 a/2 b mL | 100 mL | 500 mL | 50 c mL |
| TE (%) | MFIEGFP (a.u.) | BMP2 (ng/mL) | YieldBMP2 (ng/106 Cells) | ||
|---|---|---|---|---|---|
| pEGFP-N1 (4.7 kb) | Exp. 1 | 36.6 | 20.7 | n.a. | n.a. |
| Exp. 2 | 38.9 | 18.5 | n.a. | n.a. | |
| Exp. 3 | 44.0 | 25.4 | n.a. | n.a. | |
| phBMP2-EGFP (5.9 kb) | Exp. 1 | 9.5 | 8.4 | 1.5 ± 0.7 | 0.8 |
| Exp. 2 | 9.6 | 7.3 | 1.4 ± 0.2 | 0.5 | |
| Exp. 3 | 11.6 | 8.1 | 1.8 ± 0.7 | 1.0 | |
| pchBMP2 (6.5 kb) | Exp. 1 | n.a. | n.a. | 5.1 ± 0.6 | 2.5 |
| Exp. 2 | n.a. | n.a. | 6.7 ± 1.0 | 2.2 | |
| Exp. 3 | n.a. | n.a. | 11.4 ± 1.0 | 6.0 | |
| pchBMP2optCHO (6.5 kb) | Exp. 1 | n.a. | n.a. | 11.9 ± 0.8 | 6.3 |
| Exp. 2 | n.a. | n.a. | 9.7 ± 0.3 | 3.7 | |
| Exp. 3 | n.a. | n.a. | 13.4 ± 2.3 | 6.8 |
| TE (%) | MFIEGFP (a.u.) | BMP2 (ng/mL) | YieldBMP2 (ng/106 Cells) | ||
|---|---|---|---|---|---|
| pEGFP-N1 | Exp. 1 | 52.4 | 105.0 | n.a. | n.a. |
| Exp. 2 | 65.7 | 132.0 | n.a. | n.a. | |
| phBMP2-EGFP | Exp. 1 | 8.0 | 2.7 | 38 ± 7 | 27.5 |
| Exp. 2 | 18.7 | 2.7 | 132 ± 16 | 86.4 | |
| pchBMP2 | Exp. 1 | n.a. | n.a. | 794 ± 65 | 470.0 |
| Exp. 2 | n.a. | n.a. | 820 ± 81 | 411.8 |
| Cultivation Time Post Transfection (hours) | |||||
|---|---|---|---|---|---|
| 96 | 192 | 288 | 384 | ||
| Initial Tf | Exp. 1 | 291.6 | 18.7 | - | - |
| Exp. 2 | 187 | 24 | - | - | |
| 2nd Tf | Exp. 1 | 228 | 206 | - | - |
| Exp. 2 | 218 | 384 | - | - | |
| 3rd Tf | Exp. 1 | 513 | 186 | 77 | - |
| Exp. 2 | 705 | 426 | 335 | - | |
| 4th Tf | Exp. 1 | 412 | 200 | 166 | 313 |
| Exp. 2 | 520 | 292 | 205 | 336 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedl, S.A.B.; Jérôme, V.; Freitag, R. Repeated Transient Transfection: An Alternative for the Recombinant Production of Difficult-to-Express Proteins Like BMP2. Processes 2022, 10, 1064. https://doi.org/10.3390/pr10061064
Riedl SAB, Jérôme V, Freitag R. Repeated Transient Transfection: An Alternative for the Recombinant Production of Difficult-to-Express Proteins Like BMP2. Processes. 2022; 10(6):1064. https://doi.org/10.3390/pr10061064
Chicago/Turabian StyleRiedl, Simon A. B., Valérie Jérôme, and Ruth Freitag. 2022. "Repeated Transient Transfection: An Alternative for the Recombinant Production of Difficult-to-Express Proteins Like BMP2" Processes 10, no. 6: 1064. https://doi.org/10.3390/pr10061064
APA StyleRiedl, S. A. B., Jérôme, V., & Freitag, R. (2022). Repeated Transient Transfection: An Alternative for the Recombinant Production of Difficult-to-Express Proteins Like BMP2. Processes, 10(6), 1064. https://doi.org/10.3390/pr10061064








