Green Extraction of Date Palm Fruits via Ultrasonic-Assisted Approach: Optimizations and Antioxidant Enrichments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Date Palm Fruits Materials
2.2. Preparation of Date Palm Fruit Extracts via Conventional Extraction
2.3. Determination of Total Phenolic Content (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Ferric Reducing Antioxidant Power Assay (FRAP)
2.6. Radical Scavenging Antioxidant Capacity by ABTS
2.7. Green Extraction of Date Palm Fruits Using UAE Techniques
2.7.1. The Experimental Design
2.7.2. Date Palm Fruit Sample Preparation for UAE
2.7.3. UAE Method
3. Results
3.1. Analysis of Different Cultivars of Date Palm Fruits’ Ethanol and Water Conventional Extract
3.2. Production of Date Palm Fruits’ Extract Applying the UAE Approach
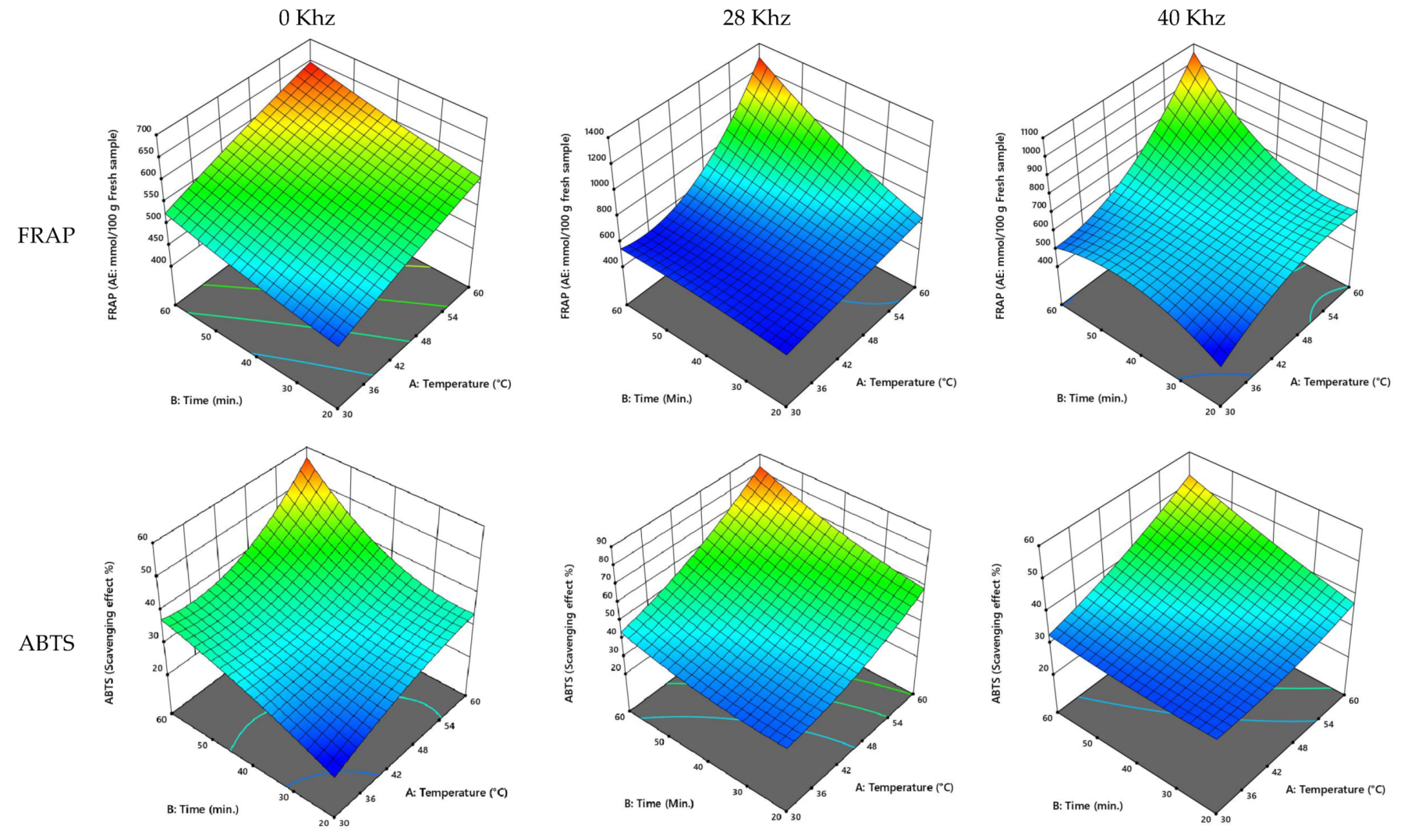
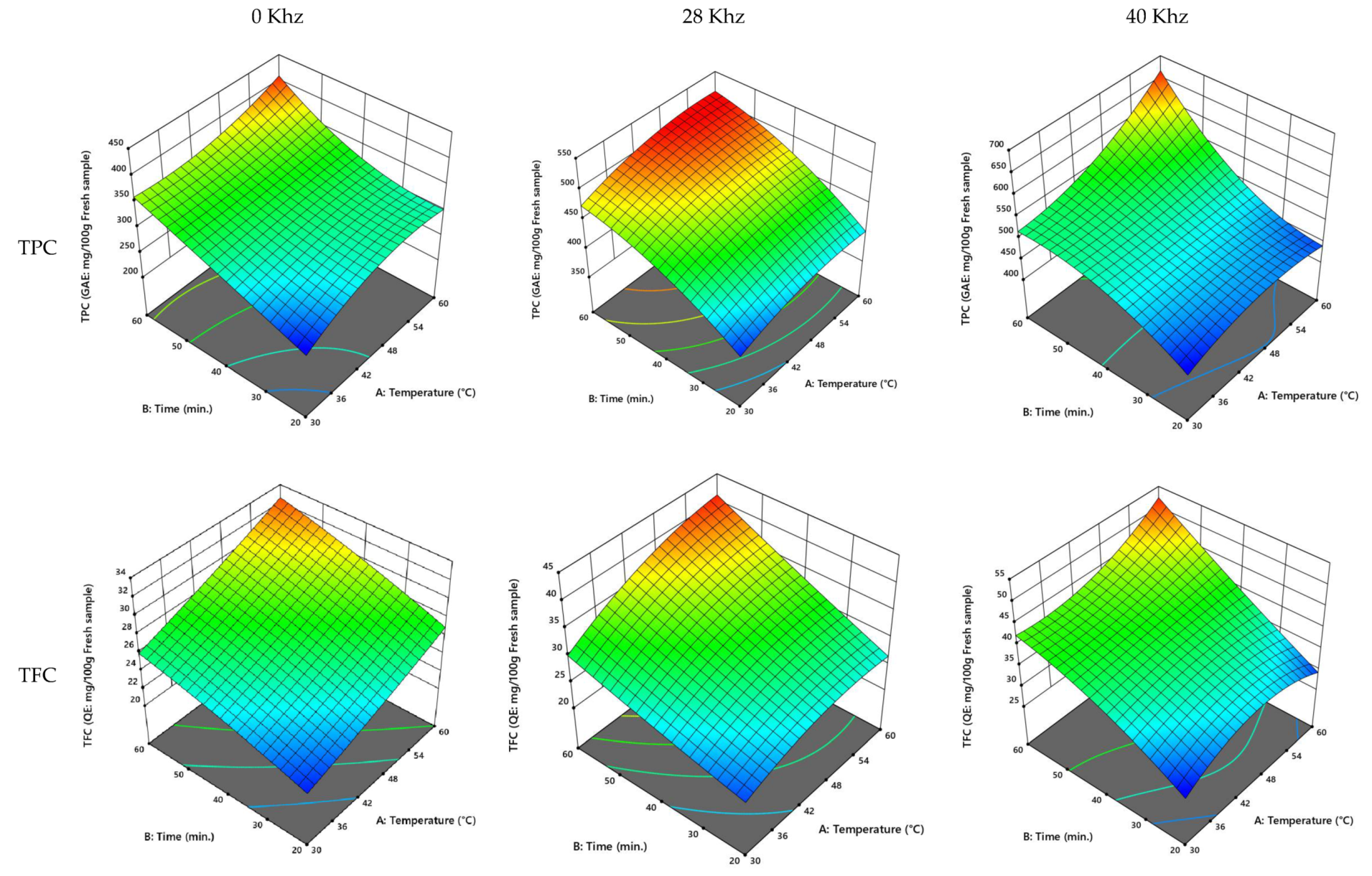
3.2.1. UAE of Agwa Cultivar of Date Palm Fruit
3.2.2. UAE of the Anbarah Cultivar of Date Palm Fruit
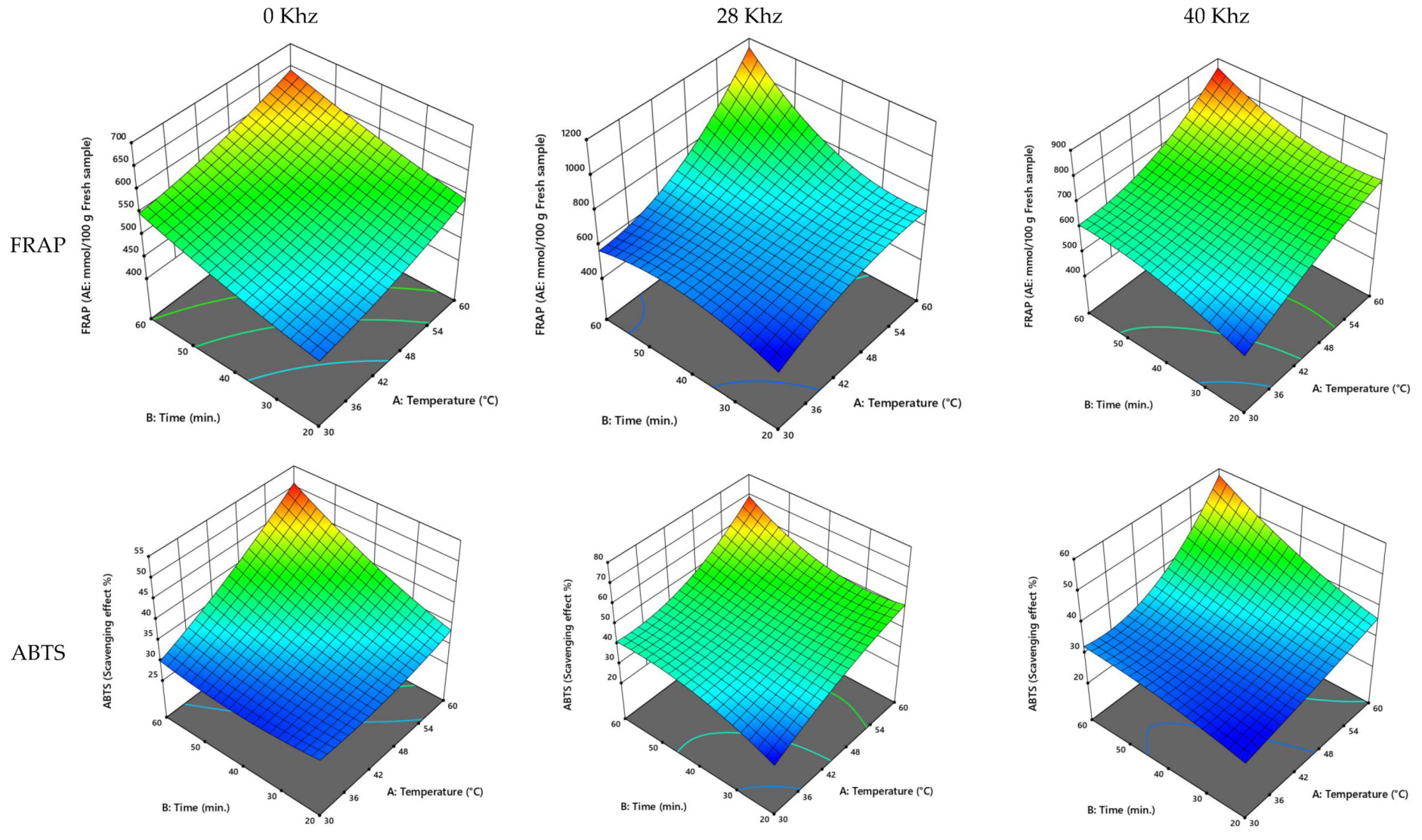
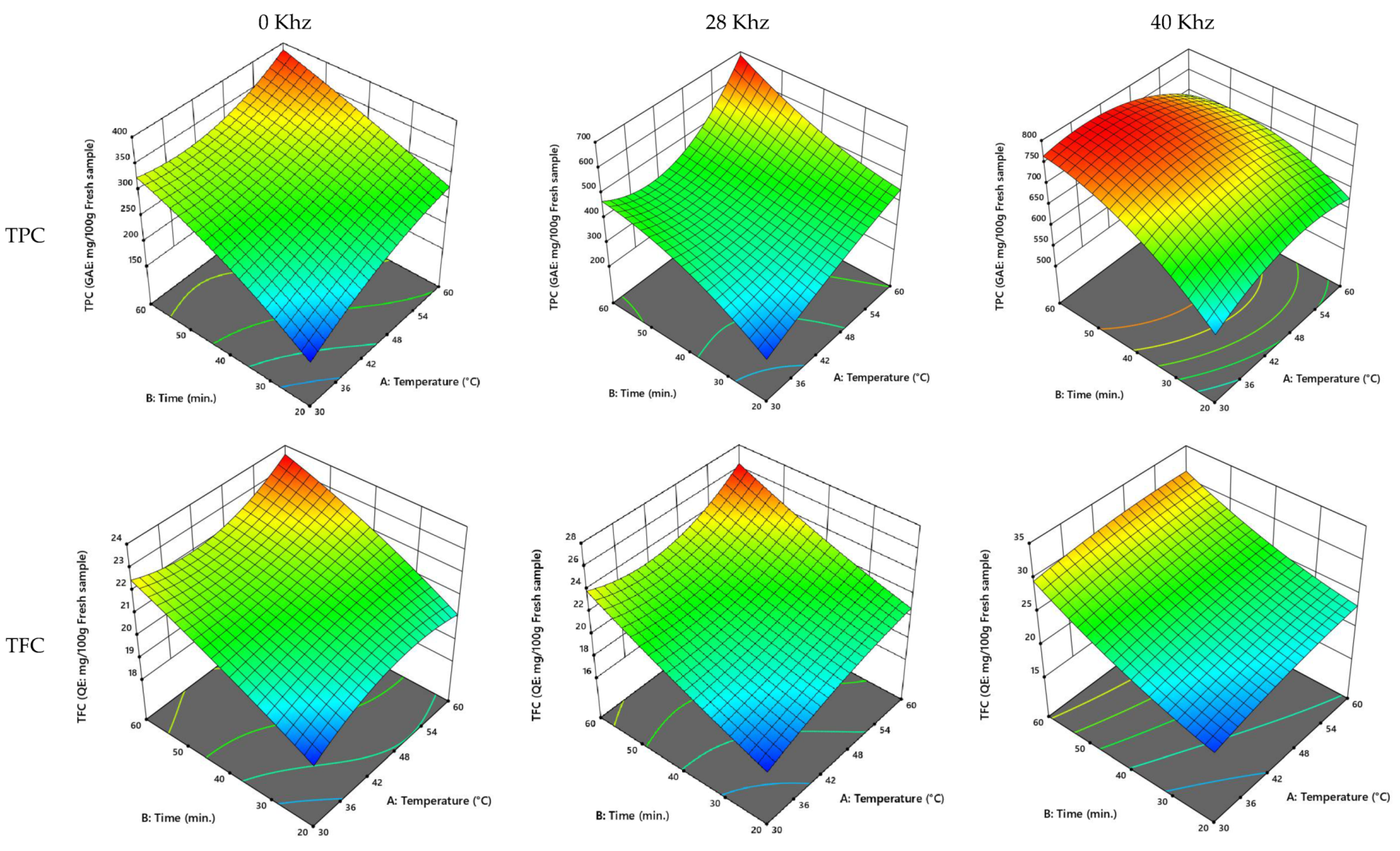
3.2.3. UAE of the Khalase Cultivar of Date Palm Fruit
3.2.4. UAE of the Reziz Cultivar of Date Palm Fruit
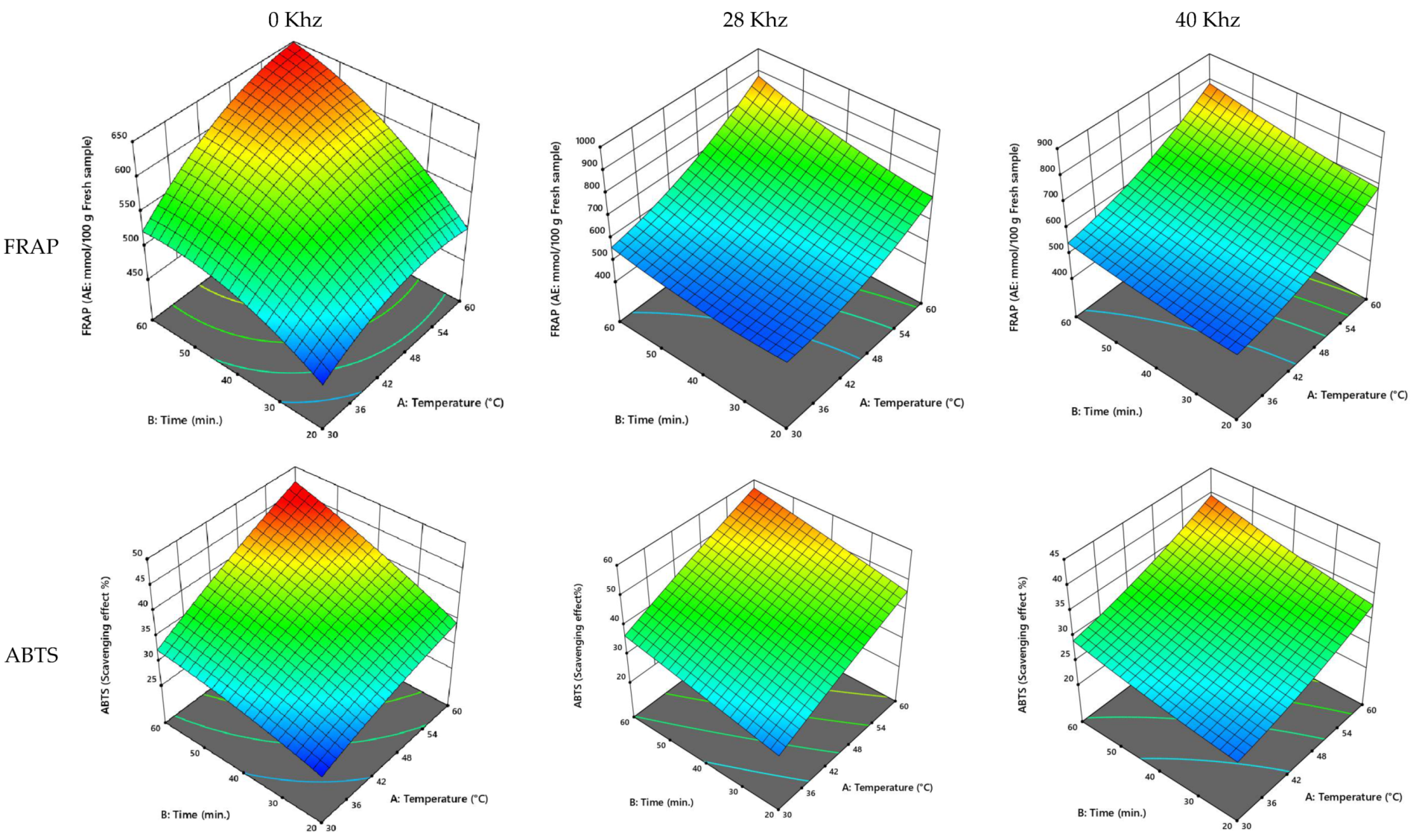
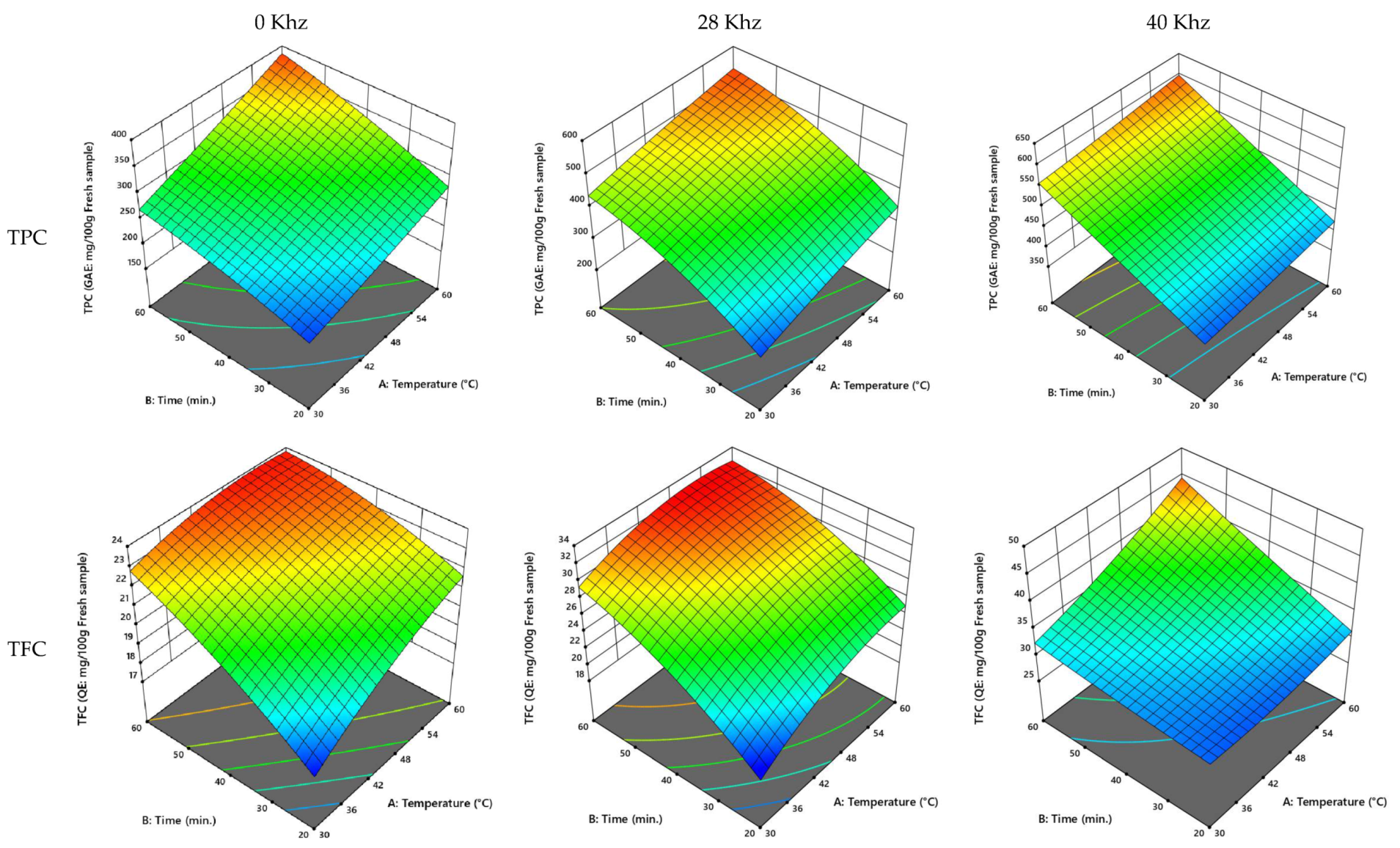
3.2.5. UAE Process Optimization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, H.E.; Alqahtani, N.K.; Darrag, H.M.; Ibrahim, H.M.; Emeka, P.M.; Badger-Emeka, L.I.; Matsunami, K.; Shehata, T.M.; Elsewedy, H.S. Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation. Plants 2021, 10, 735. [Google Scholar] [CrossRef] [PubMed]
- Zeyadi, M. Purification and characterization of peroxidase from date palm cv. Agwa fruits. Int. J. Food Prop. 2019, 22, 1910–1919. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, S.; Adiamo, O.; Ahmad, M.; Mudgil, P. Bioactive compounds from date fruit and seed as potential nutraceutical and functional food ingredients. Food Chem. 2020, 308, 125522. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Al-Shwyeh, H.A. Date Palm (Phoenix dactylifera L.) Fruit as Potential Antioxidant and Antimicrobial Agents. J. Pharm. Bioallied Sci. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Benmeziane-Derradji, F. Nutritional value, phytochemical composition, and biological activities of Middle Eastern and North African date fruit: An overview. Eur. Mediterr. J. Environ. Integr. 2019, 4, 1–11. [Google Scholar] [CrossRef]
- Hamad, I.; AbdElgawad, H.; Al Jaouni, S.; Zinta, G.; Asard, H.; Hassan, S.; Hegab, M.; Hagagy, N.; Selim, S. Metabolic Analysis of Various Date Palm Fruit (Phoenix dactylifera L.) Cultivars from Saudi Arabia to Assess Their Nutritional Quality. Molecules 2015, 20, 13620–13641. [Google Scholar] [CrossRef]
- Chaira, N.; Smaali, M.I.; Martinez-Tome, M.; Mrabet, A.; Murcia, M.A.; Ferchichi, A. Simple phenolic composition, flavonoid contents and antioxidant capacities in water-methanol extracts of Tunisian common date cultivars (Phoenix dactylifera L.). Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S7), 316–329. [Google Scholar] [CrossRef]
- AlFaris, N.A.; AlTamimi, J.Z.; AlGhamdi, F.A.; Albaridi, N.A.; Alzaheb, R.A.; Aljabryn, D.H.; Aljahani, A.H.; AlMousa, L.A. Total phenolic content in ripe date fruits (Phoenix dactylifera L.): A systematic review and meta-analysis. Saudi J. Biol. Sci. 2021, 28, 3566–3577. [Google Scholar] [CrossRef]
- Kuras, M.J.; Zielinska-Pisklak, M.; Duszynska, J.; Jablonska, J. Determination of the elemental composition and antioxidant properties of dates (Phoenix dactyliferia) originated from different regions. J. Food Sci. Technol. 2020, 57, 2828–2839. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekaran, M.; Bahkali, A.H. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology—Review. Saudi J. Biol. Sci. 2013, 20, 105–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghnimi, S.; Umer, S.; Karim, A.; Kamal-Eldin, A. Date fruit (Phoenix dactylifera L.): An underutilized food seeking industrial valorization. NFS J. 2017, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Revathi, S.; Govindarajan, R.K.; Rameshkumar, N.; Hakkim, F.L.; Mohammed, A.-B.; Krishnan, M.; Kayalvizhi, N. Anti-cancer, anti-microbial and antioxidant properties of Acacia nilotica and their chemical profiling. Biocatal. Agric. Biotechnol. 2017, 11, 322–329. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [Green Version]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Fatiha, B.; Hayette, L.; Mostepha Bachir, B. Optimized ultrasonic-assisted extraction of total phenolics and antioxidant activity of date (Phoenix dactylifera L.) using response surface methodology. Ann. Univ. Dun. Jos Galati 2018, 42, 9–22. [Google Scholar]
- Zahari, N.A.A.R.; Chong, G.H.; Abdullah, L.C.; Chua, B.L. Ultrasonic-Assisted Extraction (UAE) Process on Thymol Concentration from Plectranthus Amboinicus Leaves: Kinetic Modeling and Optimization. Processes 2020, 8, 322. [Google Scholar] [CrossRef] [Green Version]
- Al-Abdoulhadi, I.A.; Al-Ali, S.; Khurshid, K.; Al-Shryda, F.; Al-Jabr, A.M.; Abdallah, A.B. Assessing fruit characteristics to standardize quality norms in date cultivars of Saudi Arabia. Indian J. Sci. Technol. 2011, 4, 1262–1266. [Google Scholar] [CrossRef]
- Siddeeg, A.; Faisal Manzoor, M.; Haseeb Ahmad, M.; Ahmad, N.; Ahmed, Z.; Kashif Iqbal Khan, M.; Aslam Maan, A.; Mahr Un, N.; Zeng, X.-A.; Ammar, A.-F. Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Antioxidant Activity and Physicochemical Properties. Processes 2019, 7, 585. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Ouahida, D.; Mohammed Ridha, O.; Salah Eddine, L. Influence of extraction method on phytochemical composition and antioxidant activity from leaves extract of algerian Phoenix dactylifera L. Int. J. Curr. Pharm. Rev. Res. 2016, 7, 84–89. [Google Scholar]
- Hatamnia, A.A.; Abbaspour, N.; Darvishzadeh, R. Antioxidant activity and phenolic profile of different parts of Bene (Pistacia atlantica subsp. kurdica) fruits. Food Chem. 2014, 145, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.; Gullon, P.; Barroso, M.F.; Carvalho, A.P.; Domingues, V.F.; Gomes, A.M.; Becker, H.; Longhinotti, E.; Delerue-Matos, C. Brazilian fruit pulps as functional foods and additives: Evaluation of bioactive compounds. Food Chem. 2015, 172, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Thoo, Y.Y.; Ho, S.K.; Abas, F.; Lai, O.M.; Ho, C.W.; Tan, C.P. Optimal binary solvent extraction system for phenolic antioxidants from mengkudu (Morinda citrifolia) fruit. Molecules 2013, 18, 7004–7022. [Google Scholar] [CrossRef] [Green Version]
- Deffieux, T.; Demene, C.; Pernot, M.; Tanter, M. Functional ultrasound neuroimaging: A review of the preclinical and clinical state of the art. Curr. Opin. Neurobiol. 2018, 50, 128–135. [Google Scholar] [CrossRef]
- Castro-Puyana, M.; Marina, M.; Plaza, M. Water as Green Extraction Solvent: Principles and Reasons for its Use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Häckl, K.; Kunz, W. Some aspects of green solvents. C. R. Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Shahdadi, F.; Mirzaei, H.O.; Daraei Garmakhany, A. Study of phenolic compound and antioxidant activity of date fruit as a function of ripening stages and drying process. J. Food Sci. Technol. 2015, 52, 1814–1819. [Google Scholar] [CrossRef] [Green Version]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) peel antioxidants by response surface methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014. [Google Scholar] [CrossRef]
- Yan, Y.-L.; Yu, C.-H.; Chen, J.; Li, X.-X.; Wang, W.; Li, S.-Q. Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica. Carbohydr. Polym. 2011, 83, 217–224. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Wu, M.; Yang, S. Phenolic compounds from date pits: Ultrasonic-assisted extraction, antioxidant activity and component identification. J. Food Meas. Charact. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Almusallam, I.; Mohamed Ahmed, I.; Babiker, E.; Al Juhaimi, F.; Fadimu, G.; Osman, M.; Almaiman, S.; Ghafoor, K.; Alqah, H. Optimization of ultrasound-assisted extraction of bioactive properties from date palm (Phoenix dactylifera L.) spikelets using response surface methodology. LWT Food Sci. Technol. 2020, 140, 110816. [Google Scholar] [CrossRef]


| Independent “Factors” | Unit | The Variable Type | Level of Variation | |||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||
| Frequency a | Khz | Non-Continuous | 28 | -- | 40 | |
| Temperature b | °C | Continuous | 30 | 45 | 60 | |
| Time | min. | Continuous | 20 | 40 | 60 | |
| Cultivar | Type | Category | Agwa | Anbarah | Khalas | Reziz |
| Dependent “Response” | Abbreviation | Unit | ||||
| Total phenolic content | TPC | GAE: mg/100 g fresh sample | ||||
| Total flavonoid content | TFC | QE: mg/100 g fresh sample | ||||
| Ferric reducing antioxidant power asssy | FRAP | AE: mmol/100 g fresh sample | ||||
| Radical scavenging antioxidant capacity by ABTS | ABTS | Scavenging effect % | ||||
| Extraction Solvent | Responses | Date Palm Cultivars | |||
|---|---|---|---|---|---|
| Agwa | Anbarah | Khalas | Reziz | ||
| Ethanol:Distilled water (4:1) | Total phenolics (GAE: mg/100 g fresh weight) | 874.65 ± 12.7 | 923.86 ± 20.1 | 824.59 ± 14.4 | 771.41 ± 64.6 |
| Total flavonoids (QE: mg/100 g fresh weight) | 42.45 ± 3.5 | 61.21 ± 20.9 | 48.60 ± 19.6 | 52.08 ± 4.3 | |
| FRAP (AE: mmol/100 g fresh sample) | 1120 ± 54.3 | 1042 ± 89.3 | 944.13 ± 14.4 | 1023.43 ± 59.3 | |
| ABTS (Scavenging effect %) | 78.23 ± 4.9 | 74.12 ± 3.2 | 65.98 ± 5.2 | 72.62 ± 6.9 | |
| Distilled water | Total phenolics (GAE: mg/100 g fresh weight) | 232.12 ± 15.2 | 253.76 ± 14.6 | 190.10 ± 21.4 | 201.24 ± 15.5 |
| Total flavonoids (QE: mg/100 g fresh weight) | 16.43 ± 1.3 | 25.37 ± 3.6 | 18.93 ± 12.3 | 19.44 ± 14.1 | |
| FRAP (AE: mmol/100 g fresh sample) | 468.12 ± 23.5 | 433.24 ± 76.1 | 472.62 ± 9.2 | 532.45 ± 23.5 | |
| ABTS (Scavenging effect %) | 26.31 ± 2.2 | 33.24 ± 2.4 | 30.05 ± 1.4 | 25.15 ± 2.3 | |
| Cultivar | Power (kHz) | Temperature (°C) | Time (min) | TPC (GAE: mg/100 g Fresh Sample) | TFC (QE: mg/100 g Fresh Sample) | FRAP (AE: mmol/100 g Fresh Sample) | ABTS (Scavenging Effect %) |
|---|---|---|---|---|---|---|---|
| Ajwa | 0 | 50.36 | 42.87 | 332.57 | 24.25 | 576.64 | 34.62 |
| 28 | 54.78 | 51.12 | 553.25 | 30.44 | 896.50 | 66.93 | |
| 40 | 53.53 | 53.20 | 787.59 | 43.45 | 755.97 | 44.97 | |
| Anbarah | 0 | 51.61 | 49.51 | 346.33 | 29.47 | 577.17 | 39.58 |
| 28 | 51.657 | 48.873 | 499.439 | 35.985 | 764.903 | 49.376 | |
| 40 | 53.950 | 51.081 | 554.827 | 44.536 | 730.965 | 42.994 | |
| Khalase | 0 | 47.10 | 44.14 | 306.20 | 21.74 | 598.92 | 38.64 |
| 28 | 53.35 | 43.52 | 470.22 | 22.44 | 699.67 | 48.78 | |
| 40 | 48.91 | 45.25 | 753.46 | 27.10 | 630.28 | 32.37 | |
| Reziz | 0 | 47.13 | 44.76 | 307.29 | 22.88 | 597.05 | 40.33 |
| 28 | 51.82 | 45.56 | 472.44 | 31.06 | 788.53 | 47.79 | |
| 40 | 50.19 | 48.16 | 532.19 | 36.68 | 735.74 | 37.45 |
| Response | Ultrasonic Power | Agwa | Anbarah | Khalas | Reziz | |
|---|---|---|---|---|---|---|
| TPC | 0 kHz | Fold increase from water conventional extraction | 1.43 | 1.36 | 1.61 | 1.53 |
| Percentage achievement from ethanol conventional extraction | 38.02 | 37.49 | 37.13 | 39.83 | ||
| 28 kHz | Fold increase from water conventional extraction | 2.38 | 1.97 | 2.47 | 2.35 | |
| Percentage achievement from ethanol conventional extraction | 63.25 | 54.06 | 57.02 | 61.24 | ||
| 40 kHz | Fold increase from water conventional extraction | 3.39 | 2.19 | 3.96 | 2.64 | |
| Percentage achievement from ethanol conventional extraction | 90.05 | 60.06 | 91.37 | 68.99 | ||
| TFC | 0 kHz | Fold increase from water conventional extraction | 1.48 | 1.16 | 1.15 | 1.18 |
| Percentage achievement from ethanol conventional extraction | 57.12 | 48.14 | 44.73 | 43.92 | ||
| 28 kHz | Fold increase from water conventional extraction | 1.85 | 1.42 | 1.19 | 1.60 | |
| Percentage achievement from ethanol conventional extraction | 71.71 | 36.66 | 46.17 | 59.63 | ||
| 40 kHz | Fold increase from water conventional extraction | 2.64 | 1.76 | 1.43 | 1.89 | |
| Percentage achievement from ethanol conventional extraction | 102.37 | 72.76 | 55.76 | 70.42 | ||
| FRAP | 0 kHz | Fold increase from water conventional extraction | 1.23 | 1.33 | 1.27 | 1.12 |
| Percentage achievement from ethanol conventional extraction | 51.49 | 55.39 | 63.44 | 58.34 | ||
| 28 kHz | Fold increase from water conventional extraction | 1.92 | 1.77 | 1.48 | 1.48 | |
| Percentage achievement from ethanol conventional extraction | 80.04 | 73.41 | 74.11 | 77.05 | ||
| 40 kHz | Fold increase from water conventional extraction | 1.61 | 1.69 | 1.33 | 1.38 | |
| Percentage achievement from ethanol conventional extraction | 67.50 | 70.15 | 66.76 | 71.89 | ||
| ABTS | 0 kHz | Fold increase from water conventional extraction | 1.32 | 1.19 | 1.29 | 1.60 |
| Percentage achievement from ethanol conventional extraction | 44.25 | 53.41 | 58.56 | 55.53 | ||
| 28 kHz | Fold increase from water conventional extraction | 2.54 | 1.49 | 1.62 | 1.90 | |
| Percentage achievement from ethanol conventional extraction | 63.12 | 66.62 | 73.94 | 65.80 | ||
| 40 kHz | Fold increase from water conventional extraction | 1.71 | 1.29 | 1.08 | 1.49 | |
| Percentage achievement from ethanol conventional extraction | 57.49 | 58.01 | 49.06 | 51.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, H.; Al-Hajhoj, M.; Al-Saikhan, M.; Alqahtani, N.; Zayed, M.; Moawad, M.; Alsenaien, W.; Mohamed, M.E. Green Extraction of Date Palm Fruits via Ultrasonic-Assisted Approach: Optimizations and Antioxidant Enrichments. Processes 2022, 10, 1049. https://doi.org/10.3390/pr10061049
Mohamed H, Al-Hajhoj M, Al-Saikhan M, Alqahtani N, Zayed M, Moawad M, Alsenaien W, Mohamed ME. Green Extraction of Date Palm Fruits via Ultrasonic-Assisted Approach: Optimizations and Antioxidant Enrichments. Processes. 2022; 10(6):1049. https://doi.org/10.3390/pr10061049
Chicago/Turabian StyleMohamed, Hisham, Mohamed Al-Hajhoj, Mohamed Al-Saikhan, Nashi Alqahtani, Mohammad Zayed, Mahmoud Moawad, Waleed Alsenaien, and Maged E. Mohamed. 2022. "Green Extraction of Date Palm Fruits via Ultrasonic-Assisted Approach: Optimizations and Antioxidant Enrichments" Processes 10, no. 6: 1049. https://doi.org/10.3390/pr10061049
APA StyleMohamed, H., Al-Hajhoj, M., Al-Saikhan, M., Alqahtani, N., Zayed, M., Moawad, M., Alsenaien, W., & Mohamed, M. E. (2022). Green Extraction of Date Palm Fruits via Ultrasonic-Assisted Approach: Optimizations and Antioxidant Enrichments. Processes, 10(6), 1049. https://doi.org/10.3390/pr10061049








