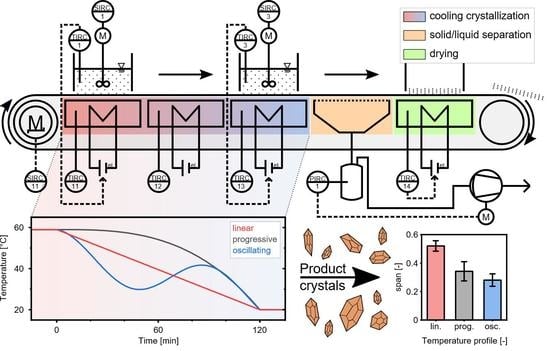
Cooling Crystallization with Complex Temperature Profiles on a Quasi-Continuous and Modular Plant
Abstract
:1. Introduction
2. Materials and Methods
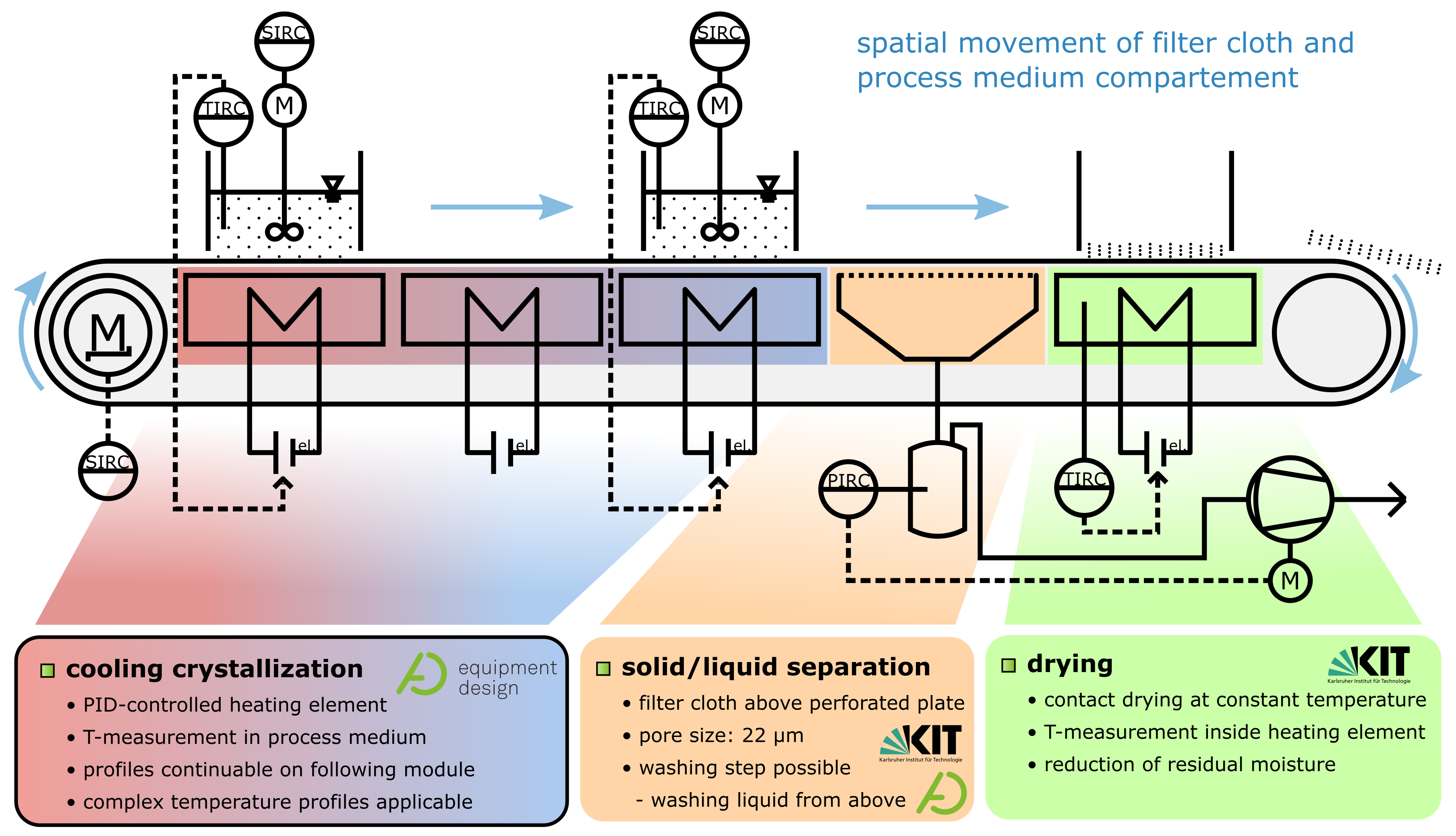
2.1. Quasi Continuous Filter Belt Crystallizer Design
2.2. Experimental Setup
2.3. Temperature Control
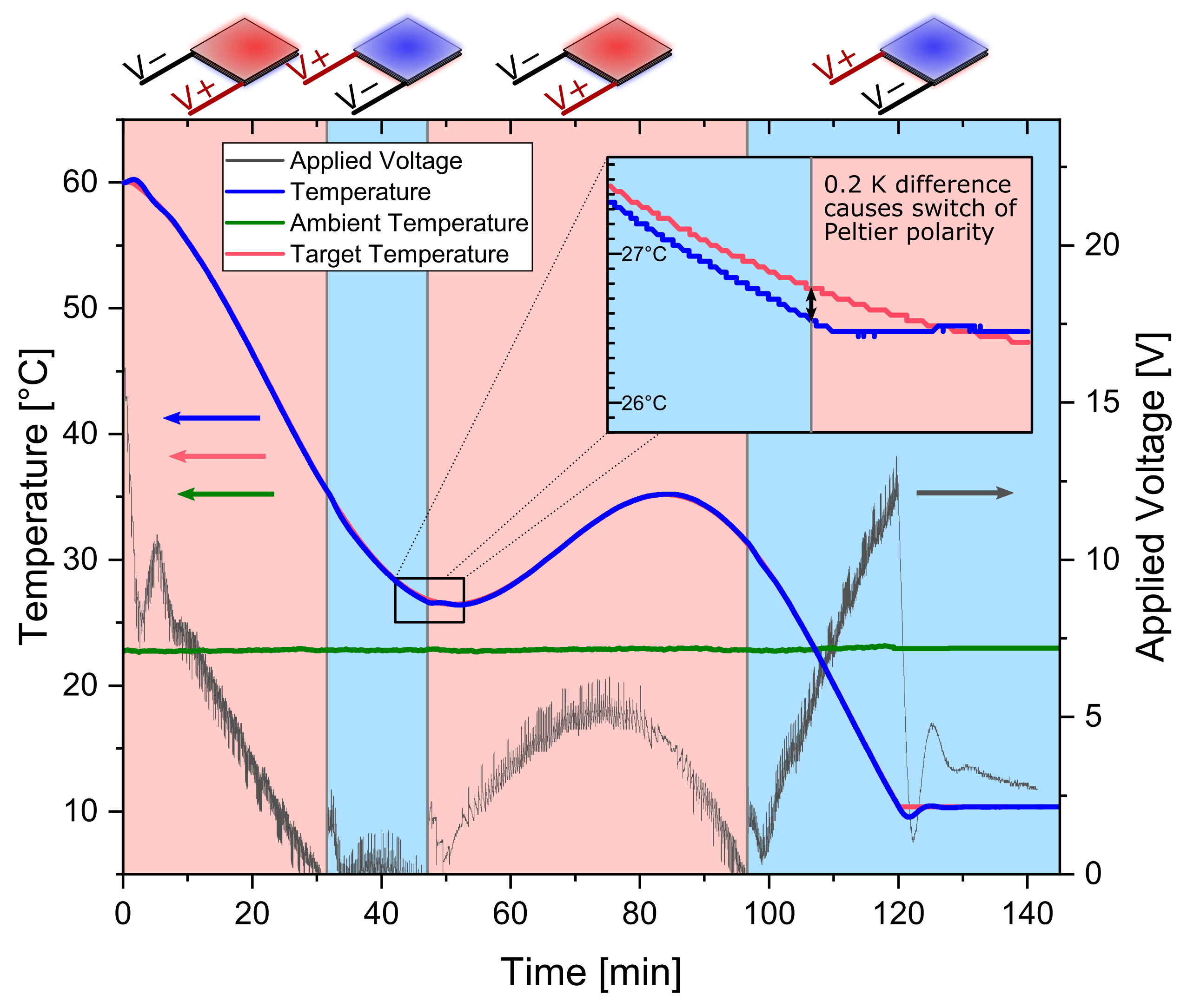
2.3.1. Peltier Elements
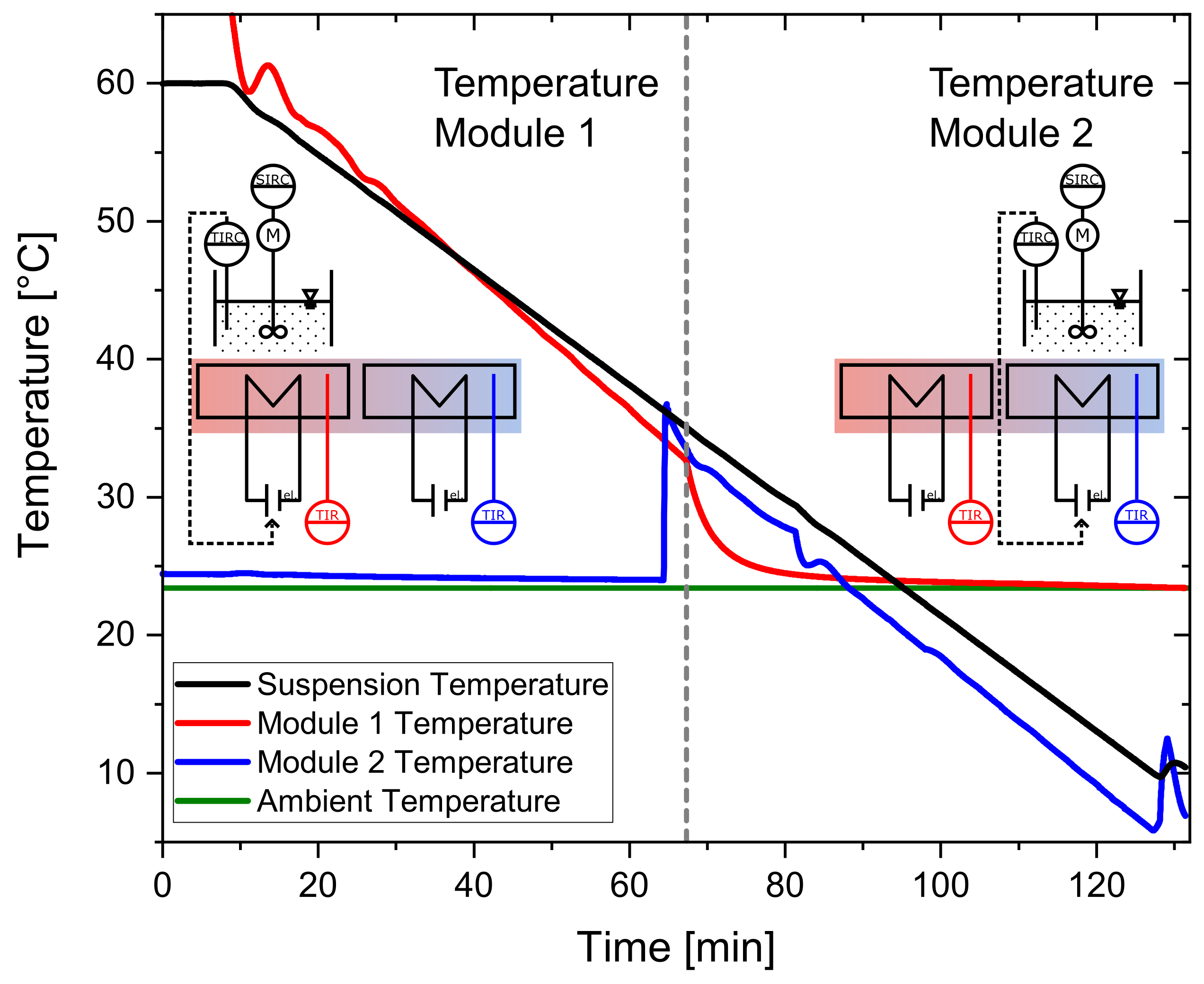
2.3.2. Temperature Profiles
2.3.3. Experimental Procedure
2.3.4. Analytic
3. Results and Discussion
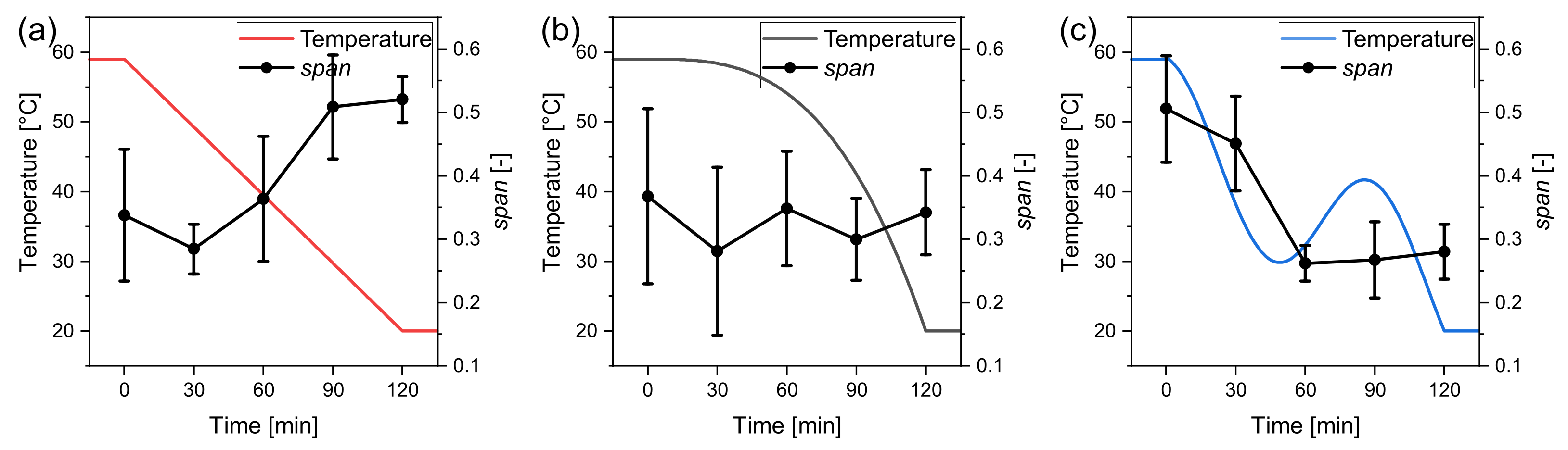
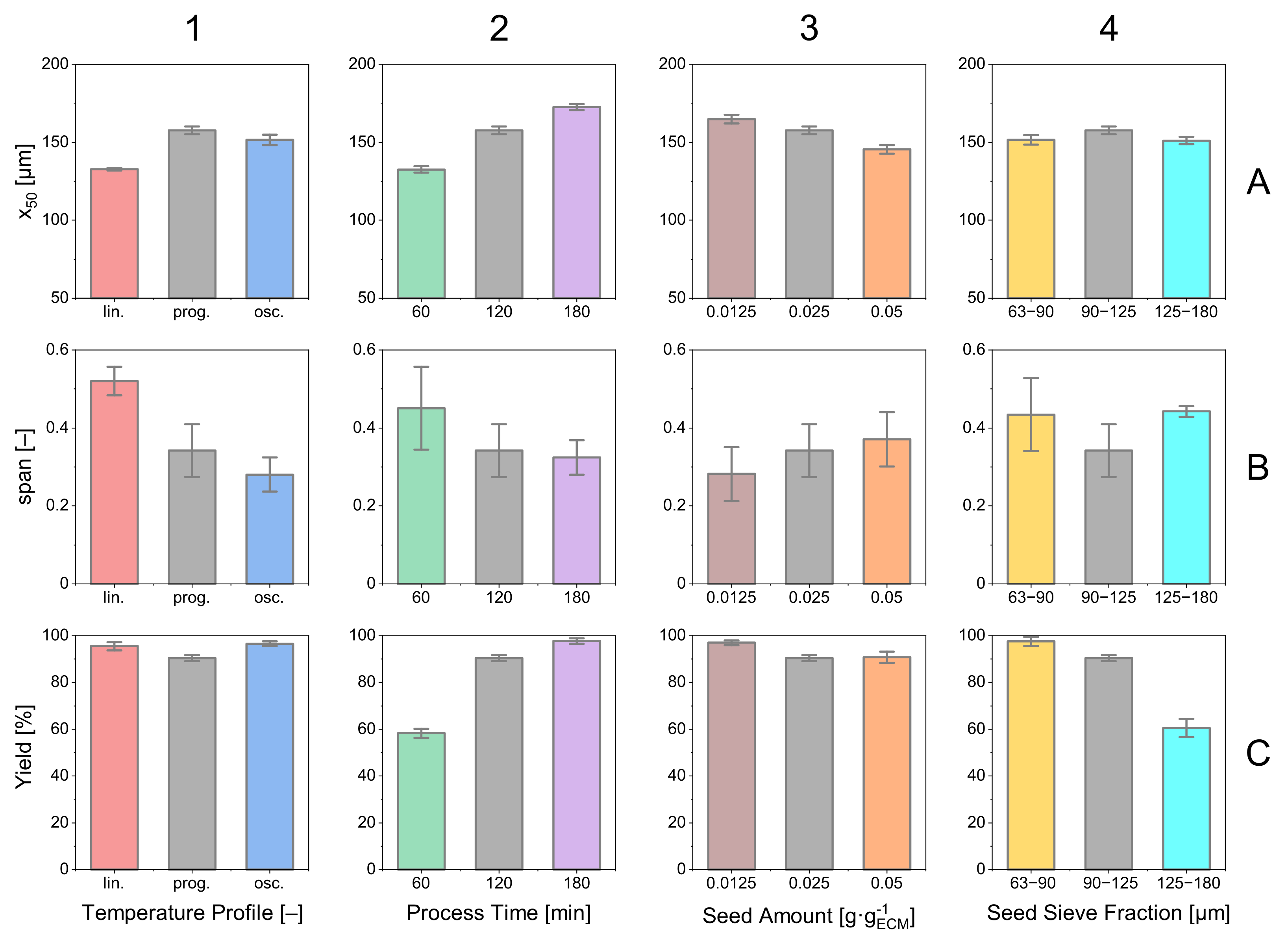
3.1. Temperature Profiles
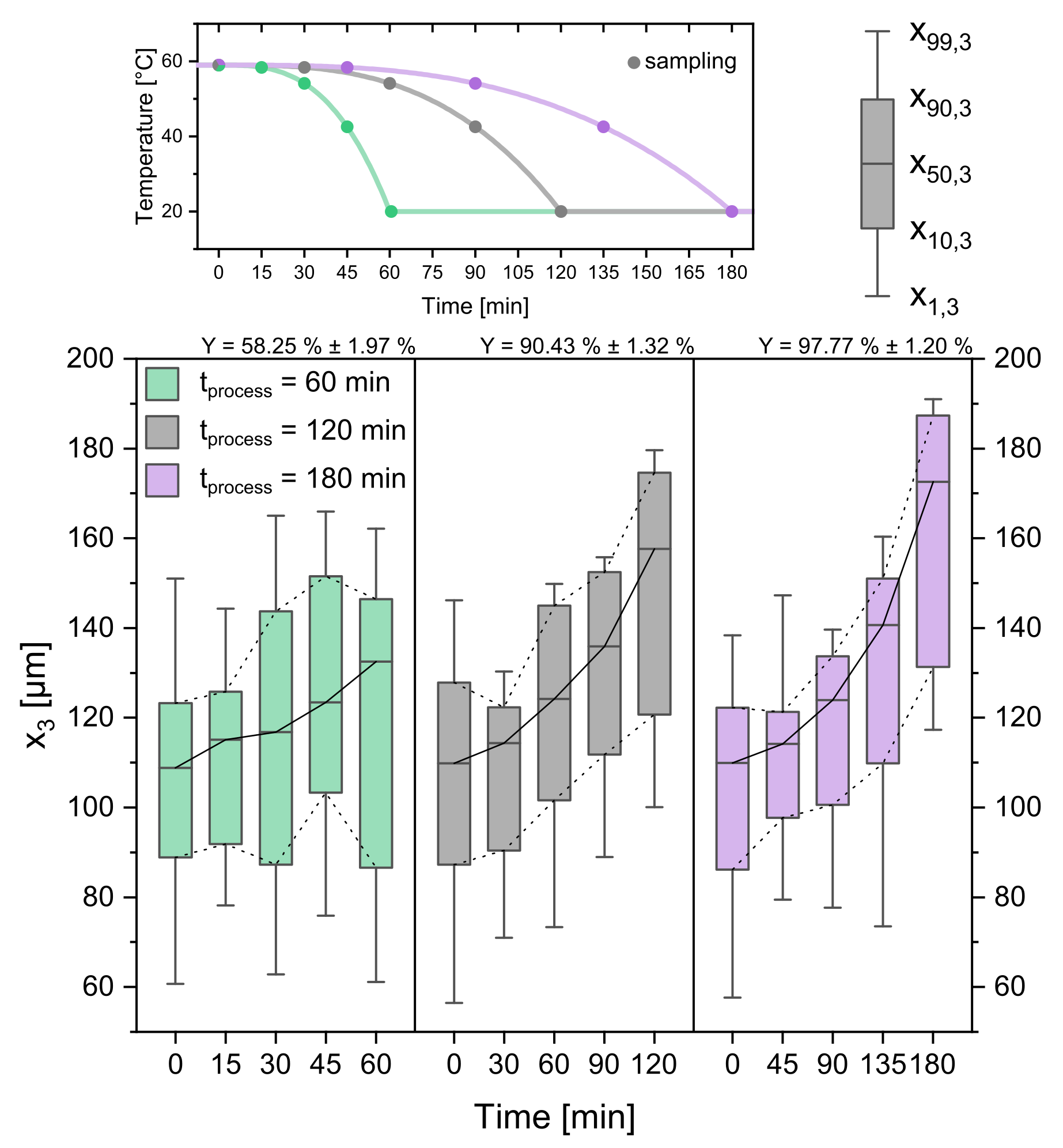
3.2. Process Time
3.3. Seed Crystal Content
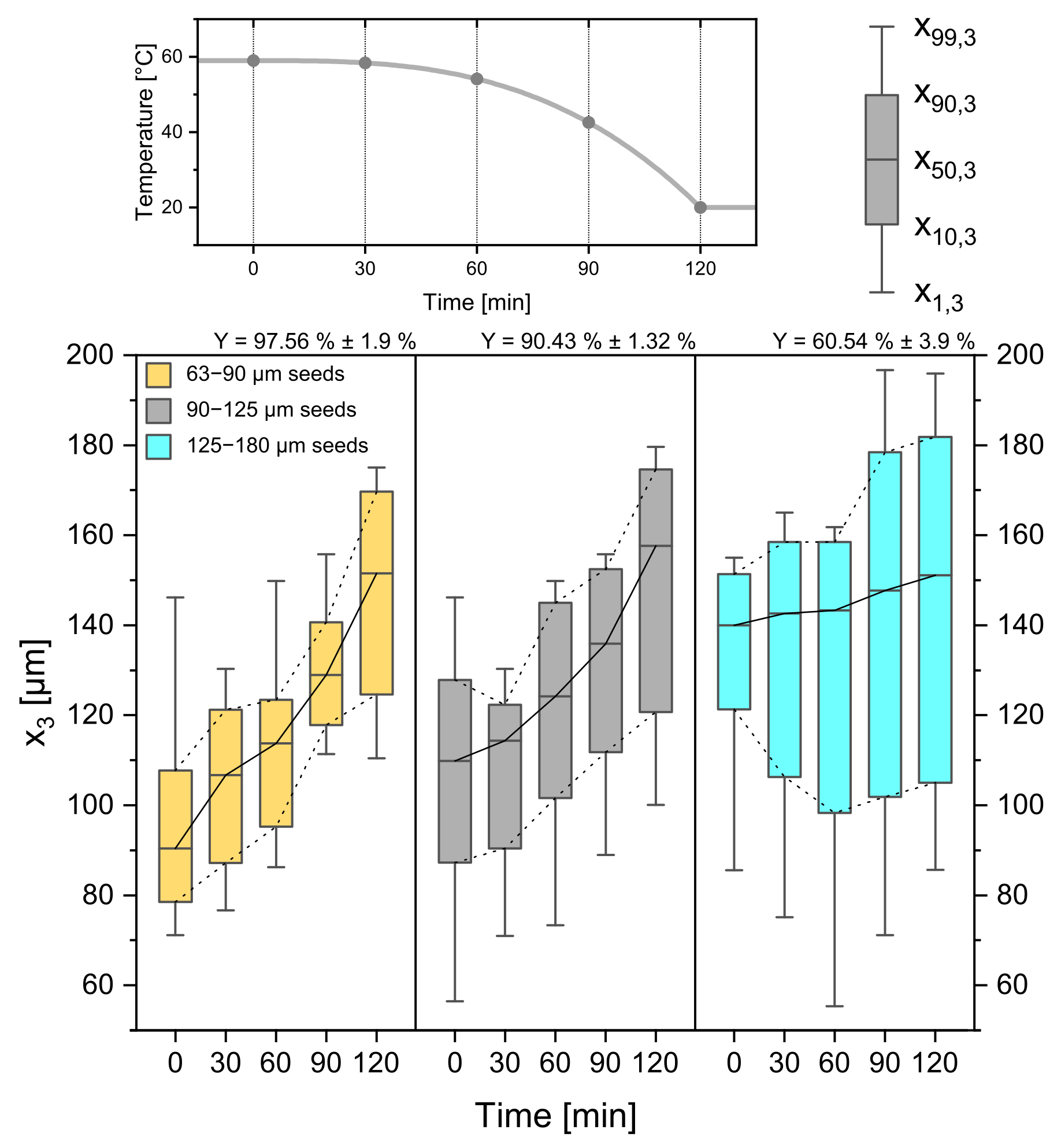
3.4. Seed Crystal Size
3.5. Parameter Study: Summary
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABS | Acrylonitrile butadiene styrene |
| CAD | Computer aided design |
| CSD | Crystal size distribution |
| ECM | Excess crystal mass |
| EtOH | Ethanol |
| IQR | Interquantile range |
| LND | Lognormal distribution |
| PLA | Polylactic acid |
| QCFBC | Quasi continuous filter belt crystallizer |
| Latin Symbols | |
| max. excess crystal mass [g] | |
| crystal growth rate [µm min] | |
| interquantile range [µm] | |
| mass of product crystals [g] | |
| mass of evaporated water [g] | |
| mass of filter cake [g] | |
| mass of mother liquor [g] | |
| seed crystal mass [g] | |
| remaining mother liquor after filtration [g] | |
| t | time [min] |
| total process time [min] | |
| T | temperature [°C] |
| end temperature of profile [°C] | |
| temperature profile function [°C] | |
| start temperature of profile [°C] | |
| saturation concentration [g g] | |
| water content [g g] | |
| crystal diameter [µm] | |
| theoretical final loading [g g] | |
| initial loading [g g] | |
| relative yield [%] | |
| Greek Symbols | |
| seed crystal mass fraction [g g] |
References
- Domokos, A.; Nagy, B.; Szilágyi, B.; Marosi, G.; Nagy, Z.K. Integrated Continuous Pharmaceutical Technologies—A Review. Org. Process Res. Dev. 2021, 25, 721–739. [Google Scholar] [CrossRef]
- Schaber, S.D.; Gerogiorgis, D.I.; Ramachandran, R.; Evans, J.M.B.; Barton, P.I.; Trout, B.L. Economic Analysis of Integrated Continuous and Batch Pharmaceutical Manufacturing: A Case Study. Ind. Eng. Chem. Res. 2011, 50, 10083–10092. [Google Scholar] [CrossRef] [Green Version]
- Melches, C.; Plate, H.; Schürhoff, J.; Buchfink, R. The Steps from Batchwise to Continuous Crystallization for a Fine Chemical: A Case Study. Crystals 2020, 10, 542. [Google Scholar] [CrossRef]
- Lier, S.; Paul, S.; Ferdinand, D.; Grünewald, M. Modulare Verfahrenstechnik: Apparateentwicklung für wandlungsfähige Produktionssysteme. Chem. Ing. Tech. 2016, 88, 1444–1454. [Google Scholar] [CrossRef]
- Lang, J.; Stenger, F.; Schütte, R. Chemieanlagen der Zukunft-Unikate und/oder Module. Chem. Ing. Tech. 2012, 6, 883–884. [Google Scholar] [CrossRef]
- Bieringer, T.; Buchholz, S.; Kockmann, N. Future Production Concepts in the Chemical Industry: Modular-Small-Scale-Continuous. Chem. Eng. Technol. 2013, 36, 900–910. [Google Scholar] [CrossRef]
- Lier, S.; Wörsdörfer, D.; Grünewald, M. Wandlungsfähige Produktionskonzepte: Flexibel, Mobil, Dezentral, Modular, Beschleunigt. Chem. Ing. Tech. 2015, 87, 1147–1158. [Google Scholar] [CrossRef]
- Baldea, M.; Edgar, T.F.; Stanley, B.L.; Kiss, A.A. Modular manufacturing processes: Status, challenges, and opportunities. AIChE J. 2017, 63, 4262–4272. [Google Scholar] [CrossRef] [Green Version]
- Stankiewicz, A.; Moulijn, J.A. Process Intensification. Ind. Eng. Chem. Res. 2002, 41, 1920–1924. [Google Scholar] [CrossRef]
- Nagy, Z.K.; El Hagrasy, A.; Litster, J. (Eds.) Continuous Pharmaceutical Processing, 1st ed.; AAPS Advances in the Pharmaceutical Sciences Series; Springer: Cham, Switzerland, 2020; Volume 42. [Google Scholar] [CrossRef]
- Wong, S.Y.; Tatusko, A.P.; Trout, B.L.; Myerson, A.S. Development of Continuous Crystallization Processes Using a Single-Stage Mixed-Suspension, Mixed-Product Removal Crystallizer with Recycle. Cryst. Growth Des. 2012, 12, 5701–5707. [Google Scholar] [CrossRef]
- Ferguson, S.; Ortner, F.; Quon, J.; Peeva, L.; Livingston, A.; Trout, B.L.; Myerson, A.S. Use of Continuous MSMPR Crystallization with Integrated Nanofiltration Membrane Recycle for Enhanced Yield and Purity in API Crystallization. Cryst. Growth Des. 2014, 14, 617–627. [Google Scholar] [CrossRef]
- Ferguson, S.; Morris, G.; Hao, H.; Barrett, M.; Glennon, B. Characterization of the anti-solvent batch, plug flow and MSMPR crystallization of benzoic acid. Chem. Eng. Sci. 2013, 104, 44–54. [Google Scholar] [CrossRef]
- Alvarez, A.J.; Myerson, A.S. Continuous Plug Flow Crystallization of Pharmaceutical Compounds. Cryst. Growth Des. 2010, 10, 2219–2228. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Neugebauer, P.; Scheibelhofer, O.; Khinast, J.G. Crystal Engineering in Continuous Plug-Flow Crystallizers. Cryst. Growth Des. 2017, 17, 6432–6444. [Google Scholar] [CrossRef] [PubMed]
- Schmalenberg, M.; Kreis, S.; Weick, L.K.; Haas, C.; Sallamon, F.; Kockmann, N. Continuous Cooling Crystallization in a Coiled Flow Inverter Crystallizer Technology—Design, Characterization, and Hurdles. Processes 2021, 9, 1537. [Google Scholar] [CrossRef]
- Hohmann, L.; Greinert, T.; Mierka, O.; Turek, S.; Schembecker, G.; Bayraktar, E.; Wohlgemuth, K.; Kockmann, N. Analysis of Crystal Size Dispersion Effects in a Continuous Coiled Tubular Crystallizer: Experiments and Modeling. Cryst. Growth Des. 2018, 18, 1459–1473. [Google Scholar] [CrossRef]
- Hohmann, L.; Löbnitz, L.; Menke, C.; Santhirakumaran, B.; Stier, P.; Stenger, F.; Dufour, F.; Wiese, G.; zur Horst-Meyer, S.; Kusserow, B.; et al. Continuous Downstream Processing of Amino Acids in a Modular Miniplant. Chem. Eng. Technol. 2018, 41, 1152–1164. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, S.; Macaringue, E.G.J.; Zhang, T.; Gong, J.; Wang, J. Recent Progress in Continuous Crystallization of Pharmaceutical Products: Precise Preparation and Control. Org. Process Res. Dev. 2020, 24, 1785–1801. [Google Scholar] [CrossRef]
- Myerson, A. Handbook of Industrial Crystallization; Butterworth-Heinemann: Oxford, UK, 2002. [Google Scholar]
- Wang, T.; Lu, H.; Wang, J.; Xiao, Y.; Zhou, Y.; Bao, Y.; Hao, H. Recent progress of continuous crystallization. J. Ind. Eng. Chem. 2017, 54, 14–29. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, S.; Du, S.; Wang, J.; Gong, J. Progress of Pharmaceutical Continuous Crystallization. Engineering 2017, 3, 354–364. [Google Scholar] [CrossRef]
- Jiang, M.; Braatz, R.D. Designs of continuous-flow pharmaceutical crystallizers: Developments and practice. Cryst. Eng. Comm 2019, 21, 3534–3551. [Google Scholar] [CrossRef] [Green Version]
- Schmalenberg, M.; Mensing, L.; Lindemann, S.; Krell, T.; Kockmann, N. Miniaturized draft tube baffle crystallizer for continuous cooling crystallization. Chem. Eng. Res. Des. 2022, 178, 232–250. [Google Scholar] [CrossRef]
- Dobler, T.; Buchheiser, S.; Gleiß, M.; Nirschl, H. Development and Commissioning of a Small-Scale, Modular and Integrated Plant for the Quasi-Continuous Production of Crystalline Particles. Processes 2021, 9, 663. [Google Scholar] [CrossRef]
- Löbnitz, L. Auslegung des Separationsprozesses und Entwicklung neuer Verfahrenskonzepte zur Integrierten Produktion und Separation kristalliner Aminosäuren; Karlsruher Institut für Technologie: Karlsruhe, Germany, 2020. [Google Scholar]
- Deutsches Institut für Normung. Agitators and Baffles for Agitator Vessels; Types, Terms and Main Dimensions; Deutsches Institut für Normung: Berlin, Germany, 1992. [Google Scholar]
- Wilson, A.J.C. Atlas der Zuckerkristalle-Atlas of Sugar Crystals by G. Vavrinecz. Acta Crystallogr. 1966, 20, 152. [Google Scholar] [CrossRef] [Green Version]
- Choong, K.L.; Smith, R. Novel strategies for optimization of batch, semi-batch and heating/cooling evaporative crystallization. Chem. Eng. Sci. 2004, 59, 329–343. [Google Scholar] [CrossRef]
- Choong, K.L.; Smith, R. Optimization of batch cooling crystallization. Chem. Eng. Sci. 2004, 59, 313–327. [Google Scholar] [CrossRef]
- Moscosa-Santillán, M.; Bals, O.; Fauduet, H.; Porte, C.; Delacroix, A. Study of batch crystallization and determination of an alternative temperature-time profile by on-line turbidity analysis—Application to glycine crystallization. Chem. Eng. Sci. 2000, 55, 3759–3770. [Google Scholar] [CrossRef]
- Abu Bakar, M.R.; Nagy, Z.K.; Rielly, C.D. Investigation of the Effect of Temperature Cycling on Surface Features of Sulfathiazole Crystals during Seeded Batch Cooling Crystallization. Cryst. Growth Des. 2010, 10, 3892–3900. [Google Scholar] [CrossRef]
- Bakar, M.R.A.; Nagy, Z.K.; Rielly, C.D. Seeded Batch Cooling Crystallization with Temperature Cycling for the Control of Size Uniformity and Polymorphic Purity of Sulfathiazole Crystals. Org. Process Res. Dev. 2009, 13, 1343–1356. [Google Scholar] [CrossRef]
- Kim, S.; Wei, C.; Kiang, S. Crystallization Process Development of an Active Pharmaceutical Ingredient and Particle Engineering via the Use of Ultrasonics and Temperature Cycling. Org. Process Res. Dev. 2003, 7, 997–1001. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Wu, W. Application of temperature cycling for crystal quality control during crystallization. Cryst. Eng. Comm. 2016, 18, 2222–2238. [Google Scholar] [CrossRef]
- Schmalenberg, M.; Sallamon, F.; Haas, C.; Kockmann, N. Temperature-Controlled Minichannel Flow-Cell for Non-Invasive Particle Measurements in Solid-Liquid Flow. In Proceedings of the ASME 2020 18th International Conference on Nanochannels, Microchannels, and Minichannels, Virtual, 13–15 July 2020. [Google Scholar] [CrossRef]
- Bouchard, A.; Hofland, G.W.; Witkamp, G.J. Properties of Sugar, Polyol, and Polysaccharide Water–Ethanol Solutions. J. Chem. Eng. Data 2007, 52, 1838–1842. [Google Scholar] [CrossRef]
- Schneider, F.; Schliephake, D.; Klimmek, A. Über die Viskosität von Reinen Saccharoselösungen. Zucker 1963, 16, 465–473. [Google Scholar]
- Chianese, A. Fines Removal. In Industrial Crystallization Process Monitoring and Control; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; Chapter 15; pp. 175–184. [Google Scholar] [CrossRef]
- Frede, T.A.; Maier, M.C.; Kockmann, N.; Gruber-Woelfler, H. Advances in Continuous Flow Calorimetry. Org. Process Res. Dev. 2022, 26, 267–277. [Google Scholar] [CrossRef]



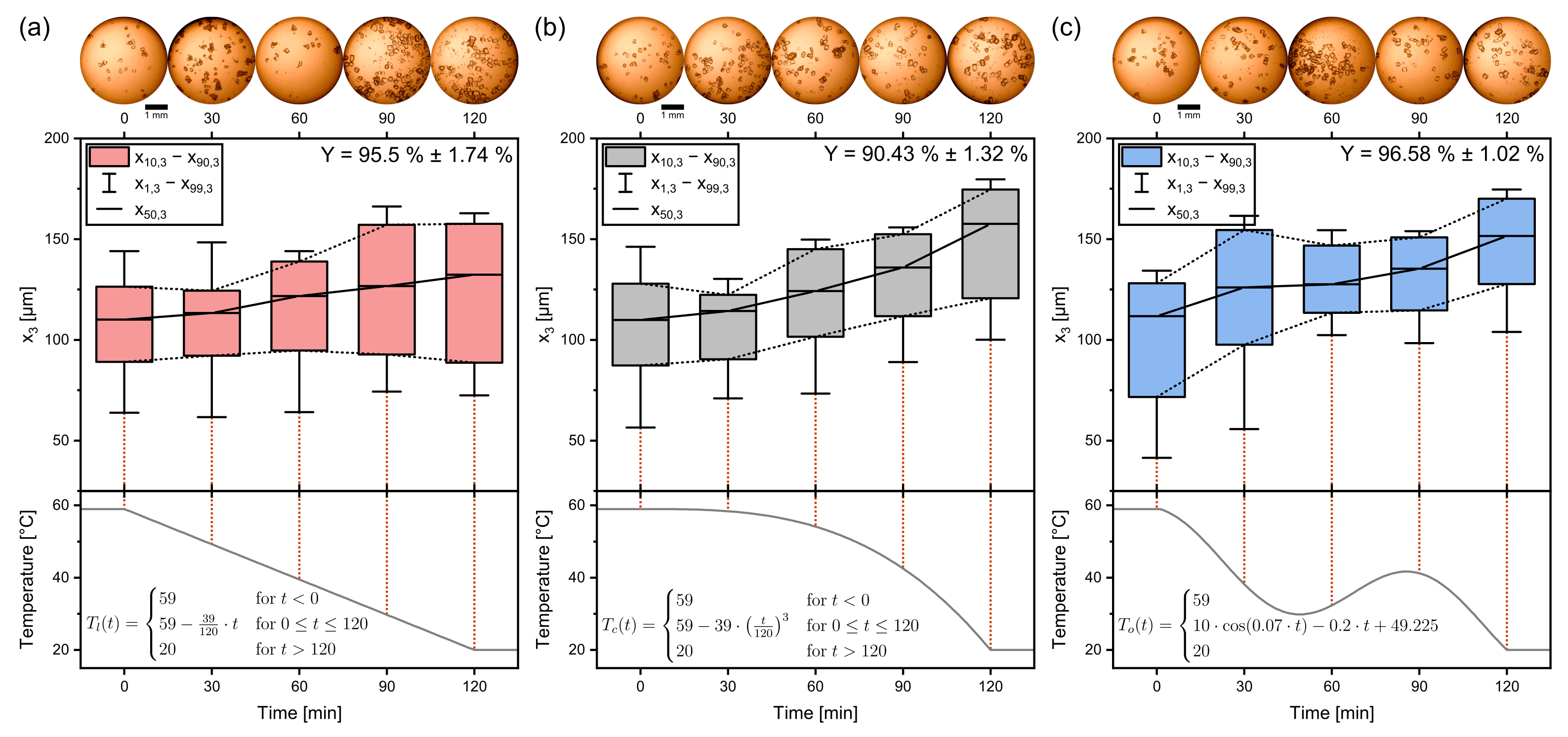

| “Low” | “Standard” | “High” | |
|---|---|---|---|
| temperature profile [-] | linear | progressive | oscillating |
| process time [min] | 60 | 120 | 180 |
| seed crystal amount [gg] | 0.0125 | 0.025 | 0.05 |
| seed sieve size [µm] | 63–90 | 90–125 | 125–180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höving, S.; Oldach, B.; Kockmann, N. Cooling Crystallization with Complex Temperature Profiles on a Quasi-Continuous and Modular Plant. Processes 2022, 10, 1047. https://doi.org/10.3390/pr10061047
Höving S, Oldach B, Kockmann N. Cooling Crystallization with Complex Temperature Profiles on a Quasi-Continuous and Modular Plant. Processes. 2022; 10(6):1047. https://doi.org/10.3390/pr10061047
Chicago/Turabian StyleHöving, Stefan, Bastian Oldach, and Norbert Kockmann. 2022. "Cooling Crystallization with Complex Temperature Profiles on a Quasi-Continuous and Modular Plant" Processes 10, no. 6: 1047. https://doi.org/10.3390/pr10061047
APA StyleHöving, S., Oldach, B., & Kockmann, N. (2022). Cooling Crystallization with Complex Temperature Profiles on a Quasi-Continuous and Modular Plant. Processes, 10(6), 1047. https://doi.org/10.3390/pr10061047