Large-Scale Production of Size-Adjusted β-Cell Spheroids in a Fully Controlled Stirred-Tank Reactor
Abstract
1. Introduction
| Cell Type | Cell Name | Application | Bioreactor Type; Scalability | Production Scale [mL] | Stirrer Type | Reynolds Number Re [−] and Flow Range ** | Seeding Density [Cells mL−1] | Size Range [µm] | Yield | Agglomeration Technique | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Islets/ β-cell lines | INS-1 | Bioprocess model for diabetes therapy | STR (Infors HT); scalable | 1000 | 30-SPB; 45-SPB; Rushton | Re = 11,000–18,000; turbulent | 5 × 105 | 40– 50 | 22 × 103 spheroids mL−1; 1.1 × 103 IEQs mL−1; 22 × 106 spheroids batch−1; 1.1 × 106 IEQs batch−1; | Controlled: isotropic conditions; surface erosion (dSph/λ < 3); narrow size distribution; size restricted | This study |
| MIN6 | Research studies and clinical application | Spinner flask (ProCulture, Corning); semi-scalable | Not specified | Straight blade paddle impeller * | Re = 2300 (d = 40 mm, 60 rpm); non-turbulent | 0.2–0.4 × 105 | 100–400 | ~0.7 × 106 cells mL−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction | [26] | |
| Primary neonatal porcine pancreatic islet cells | Scalable process to expand pancreatic endocrine tissue for cell therapy | Spinner flask (Corning); semi-scalable | 100 | Magnetic stir bars | - | 1.3 × 105 | Not specified | 1 × 106 cells mL−1; 1 × 108 cells batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction | [25] | |
| ESC | CyT49 | Diabetes therapy | 6-well plates; not scalable | 5.5 | No stirrer | Non-turbulent | 10 × 105 | 100–200 | ~1000 aggregates mL−1; ~5500 aggregates batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction | [32] |
| Royan H5 and H6 | Bioprocess development for production of aggregates | Spinner flask (Cellspin; Integra Biosciences); semi-scalable | 100 | Magnetic pendulums * | - | 2–10 × 105 | 140–200 | 2 × 106 cells mL−1; 2 × 108 cells batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction; addition of shear protectant | [24] | |
| iPSC | hCBiPSC2 | Development of suspension culture for iPSCs | STR (cellferm®, DASGIP AG); scalable | 100 | 45° 0°, 45°60°, 60°60° impeller | Re ≈ 2500 (d = 40 mm, 60 rpm); Non-turbulent | 4–5 × 105 | 50–150 | 2 × 106 cells mL−1; 2 × 108 cells batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction | [33] |
| hHSC_1285i_iPS2 | Development of high-density bioprocessing | STR (DASbox, Eppendorf AG); scalable | 150–500 | Eight-blade impeller (60° pitched) | Re = 1200–2600 (d = 30 mm); non-turbulent | 5 × 105 | 50–400 | 2.9 × 106 cells mL−1; 4.6–14.5 × 108 cells batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction | [34] | |
| hiPSC1 and hiPSC4 | Bioprocess development for production of iPSCs aggregates | Spinner flask (Cellspin; Integra Biosciences); semi-scalable | 100 | Magnetic pendulums * | - | 2–10 × 105 | 140–200 | 1.8 × 106 cells mL−1; 1.8 × 108 cells batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction; addition of shear protectant | [24] | |
| Tumor cells | MCF7, BT474, HCC1954, HCC1806, A549, H460, H157, H1650 and HT29 | 3D cancer models | Baffled spinner flasks (Corning® Life Sciences); semi-scalable | 125–500 | Straight blade paddle impeller | Re = 2300–3800 (d = 40 mm, 60–100 rpm); non-turbulent | 2 × 105 | 81–298 (depending on cell type) | 1000–1500 spheroids mL−1; 0.16–0.63 × 106 spheroids batch−1 | Uncontrolled: non-isotropic; broad size distribution; no size restriction | [27] |
2. Materials and Methods
2.1. Cells and Culture Medium
2.2. Static Spheroid Formation
2.3. Shaking Flask Cultivation
2.4. Production of Spheroids in a STR
2.5. Calcein/Ethidium Staining to Analyze Particle Count, Size, Circularity, and Areas of Green and Red Particles
2.6. Particle Size Distribution
2.7. Assessment of Viability
2.8. Determination of Growth Rate and Expansion Factor
2.9. Assessment of β-Cell Functionality
2.10. Statistical Analysis
3. Results and Discussion
3.1. Differences in Static and Dynamic Formation of INS-1 Spheroids
3.2. Influence of Seeding Density on INS-1 Spheroid Formation in Shaking Flasks
3.3. Influence of Power Input on INS-1 Spheroid Formation in Shaking Flasks
3.4. Determination of INS-1 Spheroid Strength in Shaking Flasks
3.5. INS-1 Spheroid Formation in a Stirred-Tank Bioreactor Using Different Stirrer Types
3.6. Correlation between Predicted and Measured INS-1 Spheroid Size in STR
3.7. Growth and Viability of the INS-1 Spheroids Produced in the STR
3.8. Online Monitoring of Spheroid Growth and Destruction
3.9. Glucose-Stimulated Insulin Secretion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas Ninth Edition 2019. 2020. Available online: www.diabetesatlas.org (accessed on 12 April 2021).
- Mir-Coll, J.; Moede, T.; Paschen, M.; Neelakandhan, A.; Valladolid-Acebes, I.; Leibiger, B.; Biernath, A.; Ämmälä, C.; Leibiger, I.B.; Yesildag, B.; et al. Human Islet Microtissues as an In Vitro and an In Vivo Model System for Diabetes. Int. J. Mol. Sci. 2021, 22, 1813. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. The Islets of Langerhans; Springer Netherlands: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-3270-6. [Google Scholar]
- Benthuysen, J.R.; Carrano, A.C.; Sander, M. Advances in β cell replacement and regeneration strategies for treating diabetes. J. Clin. Investig. 2016, 126, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Scharfmann, R.; Didiesheim, M.; Richards, P.; Chandra, V.; Oshima, M.; Albagli, O. Mass production of functional human pancreatic β-cells: Why and how? Diabetes Obes. Metab. 2016, 18 (Suppl. 1), 128–136. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, J.T.; Hamid, M.; Guo-Parke, H.; McClenaghan, N.H.; Gomis, R.; Flatt, P.R. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J. Biol. Chem. 2011, 286, 21982–21992. [Google Scholar] [CrossRef] [PubMed]
- Ravassard, P.; Hazhouz, Y.; Pechberty, S.; Bricout-Neveu, E.; Armanet, M.; Czernichow, P.; Scharfmann, R. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J. Clin. Investig. 2011, 121, 3589–3597. [Google Scholar] [CrossRef]
- Scharfmann, R.; Pechberty, S.; Hazhouz, Y.; von Bülow, M.; Bricout-Neveu, E.; Grenier-Godard, M.; Guez, F.; Rachdi, L.; Lohmann, M.; Czernichow, P.; et al. Development of a conditionally immortalized human pancreatic β cell line. J. Clin. Investig. 2014, 124, 2087–2098. [Google Scholar] [CrossRef]
- Ntamo, Y.; Samodien, E.; Burger, J.; Muller, N.; Muller, C.J.F.; Chellan, N. In vitro Characterization of Insulin-Producing β-Cell Spheroids. Front. Cell Dev. Biol. 2020, 8, 623889. [Google Scholar] [CrossRef]
- Petry, F.; Weidner, T.; Czermak, P.; Salzig, D. Three-Dimensional Bioreactor Technologies for the Cocultivation of Human Mesenchymal Stem/Stromal Cells and Beta Cells. Stem Cells Int. 2018, 2018, 2547098. [Google Scholar] [CrossRef]
- Bernard, A.B.; Lin, C.-C.; Anseth, K.S. A microwell cell culture platform for the aggregation of pancreatic β-cells. Tissue Eng. Part C Methods 2012, 18, 583–592. [Google Scholar] [CrossRef]
- Bhang, S.H.; Jung, M.J.; Shin, J.-Y.; La, W.-G.; Hwang, Y.H.; Kim, M.J.; Kim, B.-S.; Lee, D.Y. Mutual effect of subcutaneously transplanted human adipose-derived stem cells and pancreatic islets within fibrin gel. Biomaterials 2013, 34, 7247–7256. [Google Scholar] [CrossRef] [PubMed]
- Achilli, T.-M.; Meyer, J.; Morgan, J.R. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin. Biol. Ther. 2012, 12, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Lammert, E.; Thorn, P. The Role of the Islet Niche on Beta Cell Structure and Function. J. Mol. Biol. 2020, 432, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 94. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Cockram, C.S.; Flyvbjerg, A.; Goldstein, B.J. Textbook of Diabetes; Wiley-Blackwell: Oxford, UK, 2010; ISBN 9781444324808. [Google Scholar]
- Friberg, A.S.; Brandhorst, H.; Buchwald, P.; Goto, M.; Ricordi, C.; Brandhorst, D.; Korsgren, O. Quantification of the islet product: Presentation of a standardized current good manufacturing practices compliant system with minimal variability. Transplantation 2011, 91, 677–683. [Google Scholar] [CrossRef]
- Gmyr, V.; Bonner, C.; Lukowiak, B.; Pawlowski, V.; Dellaleau, N.; Belaich, S.; Aluka, I.; Moermann, E.; Thevenet, J.; Ezzouaoui, R.; et al. Automated digital image analysis of islet cell mass using Nikon’s inverted eclipse Ti microscope and software to improve engraftment may help to advance the therapeutic efficacy and accessibility of islet transplantation across centers. Cell Transplant. 2015, 24, 1–9. [Google Scholar] [CrossRef]
- Buchwald, P.; Bernal, A.; Echeverri, F.; Tamayo-Garcia, A.; Linetsky, E.; Ricordi, C. Fully Automated Islet Cell Counter (ICC) for the Assessment of Islet Mass, Purity, and Size Distribution by Digital Image Analysis. Cell Transplant. 2016, 25, 1747–1761. [Google Scholar] [CrossRef]
- Huang, H.-H.; Harrington, S.; Stehno-Bittel, L. The Flaws and Future of Islet Volume Measurements. Cell Transplant. 2018, 27, 1017–1026. [Google Scholar] [CrossRef]
- Tanaka, H.; Tanaka, S.; Sekine, K.; Kita, S.; Okamura, A.; Takebe, T.; Zheng, Y.-W.; Ueno, Y.; Tanaka, J.; Taniguchi, H. The generation of pancreatic β-cell spheroids in a simulated microgravity culture system. Biomaterials 2013, 34, 5785–5791. [Google Scholar] [CrossRef]
- Zuellig, R.A.; Cavallari, G.; Gerber, P.; Tschopp, O.; Spinas, G.A.; Moritz, W.; Lehmann, R. Improved physiological properties of gravity-enforced reassembled rat and human pancreatic pseudo-islets. J. Tissue Eng. Regen. Med. 2017, 11, 109–120. [Google Scholar] [CrossRef]
- Abbasalizadeh, S.; Larijani, M.R.; Samadian, A.; Baharvand, H. Bioprocess development for mass production of size-controlled human pluripotent stem cell aggregates in stirred suspension bioreactor. Tissue Eng. Part C Methods 2012, 18, 831–851. [Google Scholar] [CrossRef]
- Chawla, M.; Bodnar, C.A.; Sen, A.; Kallos, M.S.; Behie, L.A. Production of islet-like structures from neonatal porcine pancreatic tissue in suspension bioreactors. Biotechnol. Prog. 2006, 22, 561–567. [Google Scholar] [CrossRef]
- Lock, L.T.; Laychock, S.G.; Tzanakakis, E.S. Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J. Biotechnol. 2011, 151, 278–286. [Google Scholar] [CrossRef]
- Santo, V.E.; Estrada, M.F.; Rebelo, S.P.; Abreu, S.; Silva, I.; Pinto, C.; Veloso, S.C.; Serra, A.T.; Boghaert, E.; Alves, P.M.; et al. Adaptable stirred-tank culture strategies for large scale production of multicellular spheroid-based tumor cell models. J. Biotechnol. 2016, 221, 118–129. [Google Scholar] [CrossRef]
- Murray, H.E.; Paget, M.B.; Downing, R. Preservation of glucose responsiveness in human islets maintained in a rotational cell culture system. Mol. Cell. Endocrinol. 2005, 238, 39–49. [Google Scholar] [CrossRef]
- Chmiel, H. (Ed.) Bioprozesstechnik; Spektrum Akademischer Verl.: Heidelberg, Germany, 2011; ISBN 9783827424761. [Google Scholar]
- Petry, F.; Salzig, D. Impact of Bioreactor Geometry on Mesenchymal Stem Cell Production in Stirred-Tank Bioreactors. Chem. Ing. Tech. 2021, 93, 1537–1554. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Schulz, T.C.; Young, H.Y.; Agulnick, A.D.; Babin, M.J.; Baetge, E.E.; Bang, A.G.; Bhoumik, A.; Cepa, I.; Cesario, R.M.; Haakmeester, C.; et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS ONE 2012, 7, e37004. [Google Scholar] [CrossRef]
- Olmer, R.; Lange, A.; Selzer, S.; Kasper, C.; Haverich, A.; Martin, U.; Zweigerdt, R. Suspension culture of human pluripotent stem cells in controlled, stirred bioreactors. Tissue Eng. Part C Methods 2012, 18, 772–784. [Google Scholar] [CrossRef]
- Manstein, F.; Ullmann, K.; Kropp, C.; Halloin, C.; Triebert, W.; Franke, A.; Farr, C.-M.; Sahabian, A.; Haase, A.; Breitkreuz, Y.; et al. High density bioprocessing of human pluripotent stem cells by metabolic control and in silico modeling. Stem Cells Transl. Med. 2021, 10, 1063–1080. [Google Scholar] [CrossRef]
- Peter, C.P. Auslegung Geschüttelter Bioreaktoren für Hochviskose und Hydromechanisch Empfindliche Fermentationssysteme. Ph.D. Thesis, Rheinisch-Westfälischen Technischen Hochschule Aachen, Aachen, Germany, 2007. [Google Scholar]
- Büchs, J.; Maier, U.; Milbradt, C.; Zoels, B. Power consumption in shaking flasks on rotary shaking machines: I. Power consumption measurement in unbaffled flasks at low liquid viscosity. Biotechnol. Bioeng. 2000, 68, 589–593. [Google Scholar] [CrossRef]
- Büchs, J.; Maier, U.; Milbradt, C.; Zoels, B. Power consumption in shaking flasks on rotary shaking machines: II. Nondimensional description of specific power consumption and flow regimes in unbaffled flasks at elevated liquid viscosity. Biotechnol. Bioeng. 2000, 68, 594–601. [Google Scholar] [CrossRef]
- Joo, D.J.; Kim, J.Y.; Lee, J.I.; Jeong, J.H.; Cho, Y.; Ju, M.K.; Huh, K.H.; Kim, M.S.; Kim, Y.S. Manufacturing of insulin-secreting spheroids with the RIN-5F cell line using a shaking culture method. Transplant. Proc. 2010, 42, 4225–4227. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.; Aguiar, T.; Carvalho, S.B.; Silva, M.M.; Gomes, R.A.; Carrondo, M.J.T.; Gomes-Alves, P.; Peixoto, C.; Serra, M.; Alves, P.M. Bioprocess integration for human mesenchymal stem cells: From up to downstream processing scale-up to cell proteome characterization. J. Biotechnol. 2017, 248, 87–98. [Google Scholar] [CrossRef]
- Serra, M.; Brito, C.; Sousa, M.F.Q.; Jensen, J.; Tostões, R.; Clemente, J.; Strehl, R.; Hyllner, J.; Carrondo, M.J.T.; Alves, P.M. Improving expansion of pluripotent human embryonic stem cells in perfused bioreactors through oxygen control. J. Biotechnol. 2010, 148, 208–215. [Google Scholar] [CrossRef]
- Jarvis, P.; Jefferson, B.; Gregory, J.; Parsons, S.A. A review of floc strength and breakage. Water Res. 2005, 39, 3121–3137. [Google Scholar] [CrossRef]
- KOBAYASHI, M.; Adachi, Y.; OOI, S. Effect of Particle Size on Breakup of Flocs in a Turbulent Flow. Proc. Hydraul. Eng. 2001, 45, 1249–1253. [Google Scholar] [CrossRef][Green Version]
- Mühle, K.; Domasch, K. Stability of particle aggregates in flocculation with polymers. Chem. Eng. Processing Process Intensif. 1991, 29, 1–8. [Google Scholar] [CrossRef]
- Oyegbile, B.; Ay, P.; Narra, S. Flocculation kinetics and hydrodynamic interactions in natural and engineered flow systems: A review. Environ. Eng. Res. 2016, 21, 1–14. [Google Scholar] [CrossRef]
- Schubert, H.; Mühle, K. The role of turbulence in unit operations of particle technology. Adv. Powder Technol. 1991, 2, 295–306. [Google Scholar] [CrossRef]
- Wengeler, R.; Nirschl, H. Turbulent hydrodynamic stress induced dispersion and fragmentation of nanoscale agglomerates. J. Colloid Interface Sci. 2007, 306, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Cruz, P.E.; Santana, P.C.; Aunins, J.G.; Carrondo, M.J. Formation and disruption of animal cell aggregates in stirred vessels: Mechanisms and kinetic studies. Chem. Eng. Sci. 1995, 50, 2747–2764. [Google Scholar] [CrossRef]
- Yeung, A.; Gibbs, A.; Pelton, R. Effect of Shear on the Strength of Polymer-Induced Flocs. J. Colloid Interface Sci. 1997, 196, 113–115. [Google Scholar] [CrossRef]
- Sart, S.; Tsai, A.-C.; Li, Y.; Ma, T. Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Eng. Part B Rev. 2014, 20, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Parker, D. Floc Breakup in Turbulent Flocculation Processes. J. Sanit. Eng. 1972, 98, 79–99. [Google Scholar] [CrossRef]
- David, B. Use of hydrodynamic shear stress to analyze cell adhesion. In Principles of Cellular Engineering: Understanding the Biomolecular Interface; King, M.R., Ed.; Elsevier Academic Press: Amsterdam, Boston, 2006; pp. 51–80. ISBN 9780123693921. [Google Scholar]
- Böhm, L.; Hohl, L.; Bliatsiou, C.; Kraume, M. Multiphase Stirred Tank Bioreactors—New Geometrical Concepts and Scale-up Approaches. Chem. Ing. Tech. 2019, 91, 1724–1746. [Google Scholar] [CrossRef]
- Henzler, H.J. Particle stress in bioreactors. Adv. Biochem. Eng. Biotechnol. 2000, 67, 35–82. [Google Scholar] [CrossRef]
- Jüsten, P.; Paul, G.C.; Nienow, A.W.; Thomas, C.R. Dependence of mycelial morphology on impeller type and agitation intensity. Biotechnol. Bioeng. 1996, 52, 672–684. [Google Scholar] [CrossRef]
- Langer, G.; Deppe, A. Zum Verständnis der hydrodynamischen Beanspruchung von Partikeln in turbulenten Rührerströmungen. Chem. Ing. Tech. 2000, 72, 31–41. [Google Scholar] [CrossRef]
- Wollny, S. Experimentelle und Numerische Untersuchungen zur Partikelbeanspruchung in Gerührten (Bio-)Reaktoren. Doctoral Thesis, Technische Universität Berlin, Berlin, Germany, 2010. [Google Scholar]
- Collignon, M.-L.; Delafosse, A.; Crine, M.; Toye, D. Axial impeller selection for anchorage dependent animal cell culture in stirred bioreactors: Methodology based on the impeller comparison at just-suspended speed of rotation. Chem. Eng. Sci. 2010, 65, 5929–5941. [Google Scholar] [CrossRef]
- Jirout, T.; Rieger, F. Impeller design for mixing of suspensions. Chem. Eng. Res. Des. 2011, 89, 1144–1151. [Google Scholar] [CrossRef]
- Torizal, F.G.; Kim, S.M.; Horiguchi, I.; Inamura, K.; Suzuki, I.; Morimura, T.; Nishikawa, M.; Sakai, Y. Production of homogenous size-controlled human induced pluripotent stem cell aggregates using ring-shaped culture vessel. J. Tissue Eng. Regen. Med. 2022, 16, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Kahn-Krell, A.; Pretorius, D.; Ou, J.; Fast, V.G.; Litovsky, S.; Berry, J.; Liu, X.M.; Zhang, J. Bioreactor Suspension Culture: Differentiation and Production of Cardiomyocyte Spheroids From Human Induced Pluripotent Stem Cells. Front. Bioeng. Biotechnol. 2021, 9, 674260. [Google Scholar] [CrossRef]
- Isidro, I.A.; Vicente, P.; Pais, D.A.M.; Almeida, J.I.; Domingues, M.; Abecasis, B.; Zapata-Linares, N.; Rodriguez-Madoz, J.R.; Prosper, F.; Aspegren, A.; et al. Online monitoring of hiPSC expansion and hepatic differentiation in 3D culture by dielectric spectroscopy. Biotechnol. Bioeng. 2021, 118, 3610–3617. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-H.; Ramachandran, K.; Stehno-Bittel, L. A replacement for islet equivalents with improved reliability and validity. Acta Diabetol. 2013, 50, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.M.J.; Ricordi, C.; Hering, B.J.; Auchincloss, H.; Lindblad, R.; Robertson, R.P.; Secchi, A.; Brendel, M.D.; Berney, T.; Brennan, D.C.; et al. International trial of the Edmonton protocol for islet transplantation. N. Engl. J. Med. 2006, 355, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.J.; Lee, K.; Nienow, A.W.; Thomas, R.J.; Smith, M.; Thomas, C.R. Expansion of human mesenchymal stem cells on microcarriers. Biotechnol. Lett. 2011, 33, 2325–2335. [Google Scholar] [CrossRef]
- Pattappa, G.; Heywood, H.K.; de Bruijn, J.D.; Lee, D.A. The metabolism of human mesenchymal stem cells during proliferation and differentiation. J. Cell. Physiol. 2011, 226, 2562–2570. [Google Scholar] [CrossRef]
- Heyter, A.; Wollny, S. Einfluss verschiedener Stromstörerausführungen auf die Bewehrung eines mehrstufigen Rührbehälters. Chem. Ing. Tech. 2017, 89, 416–423. [Google Scholar] [CrossRef]
- Kraume, M. Mischen und Rühren: Grundlagen und Moderne Verfahren; Wiley-VCH: Weinheim, Germany, 2003; ISBN 3527307095. [Google Scholar]
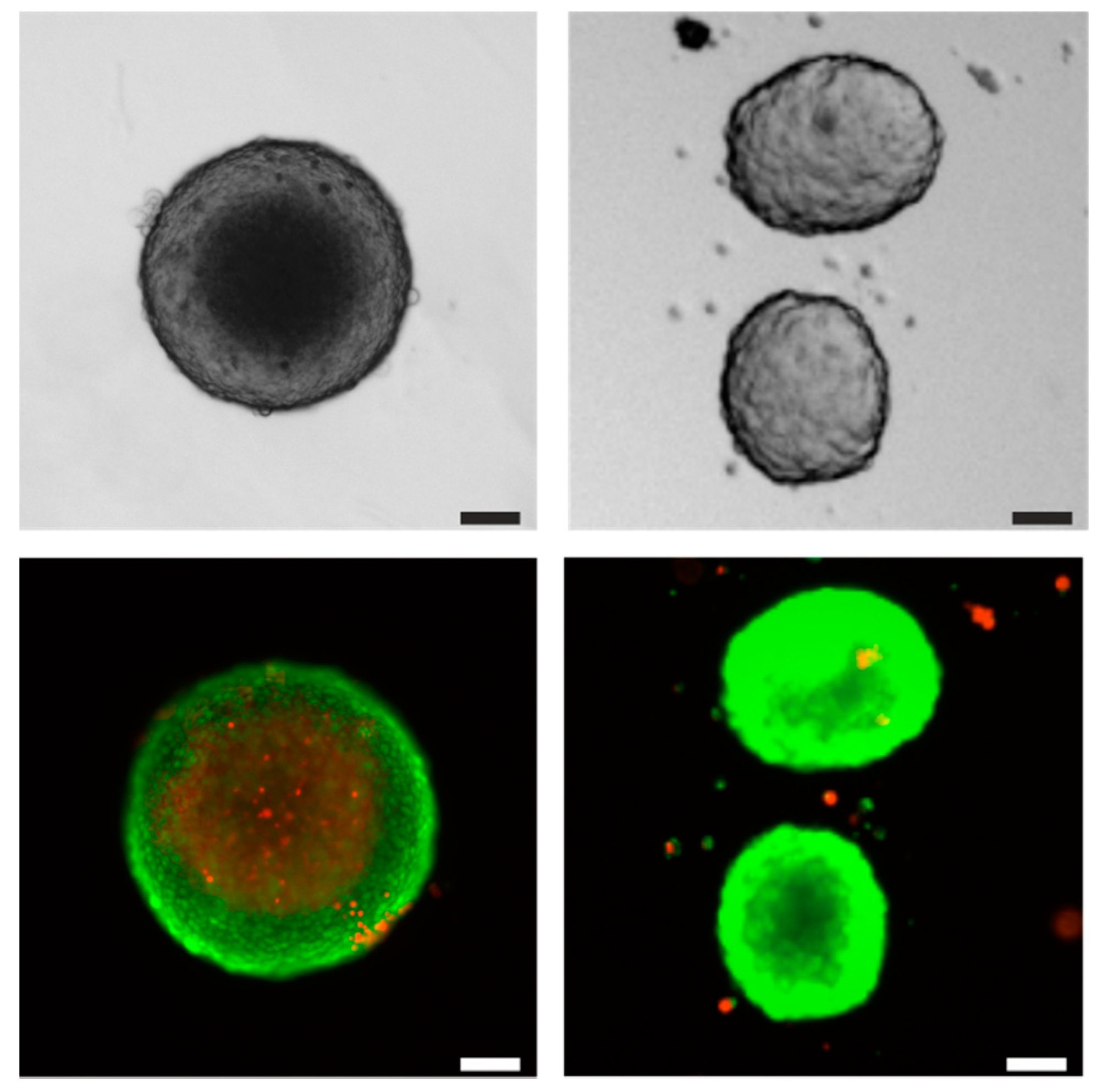


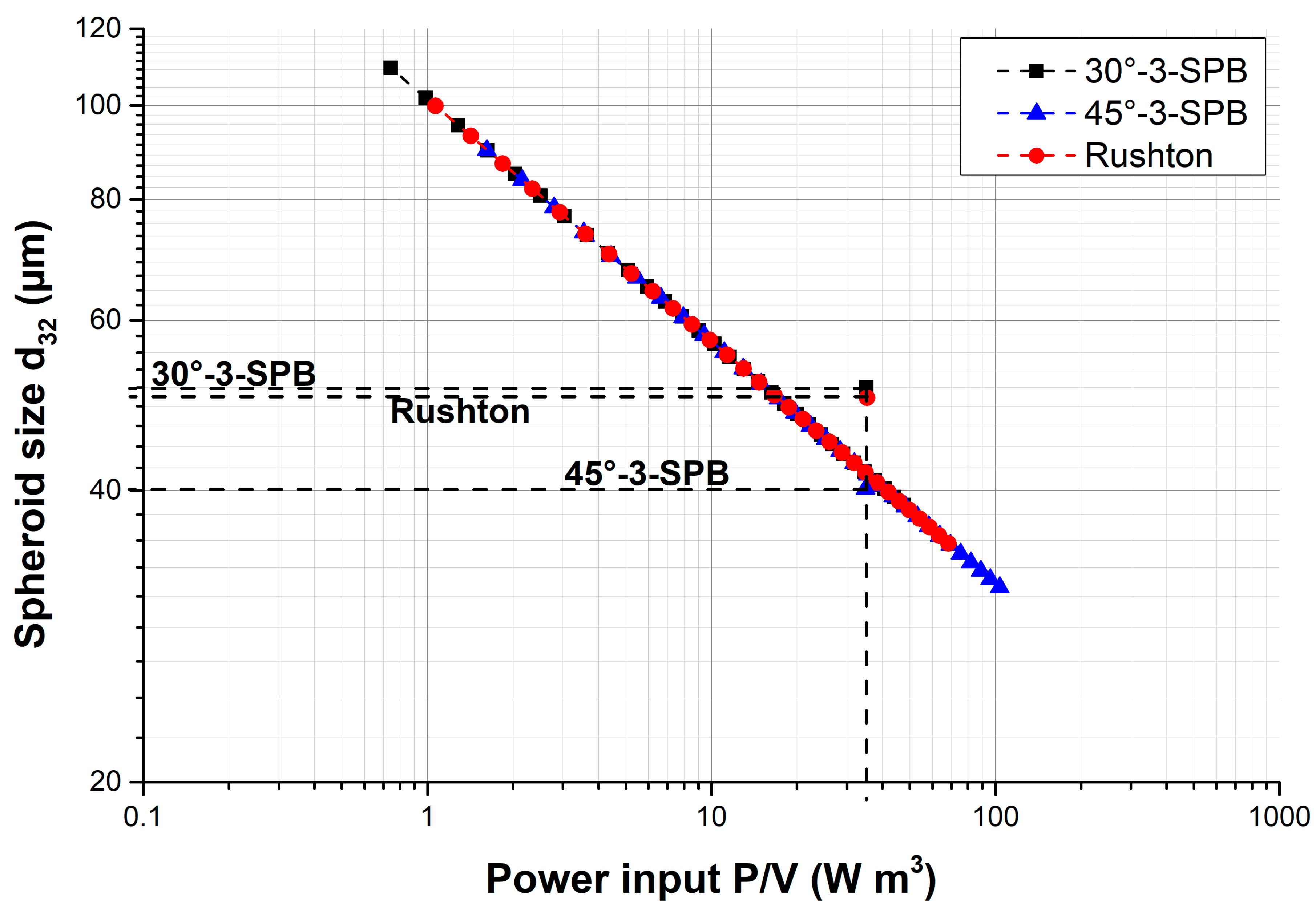
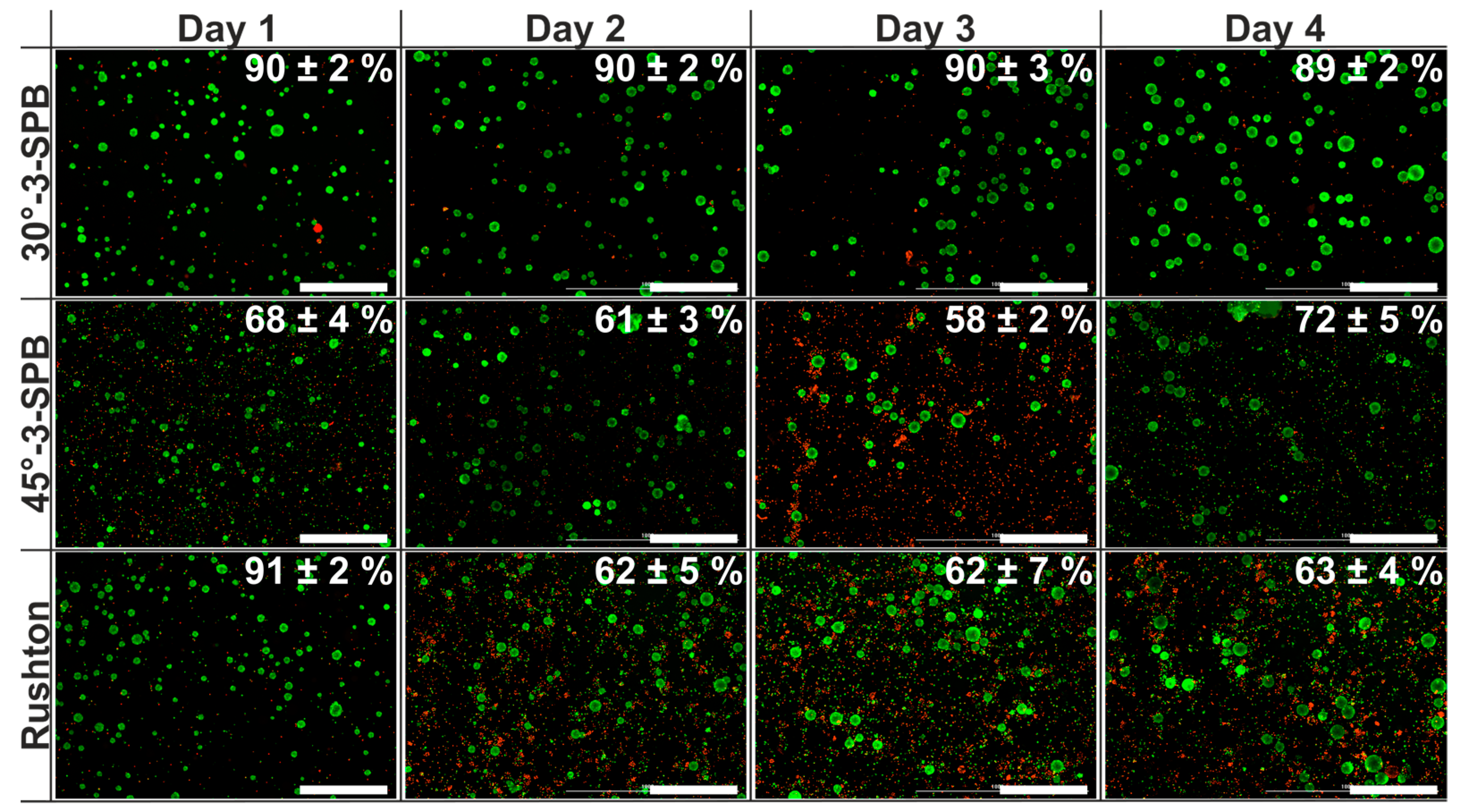

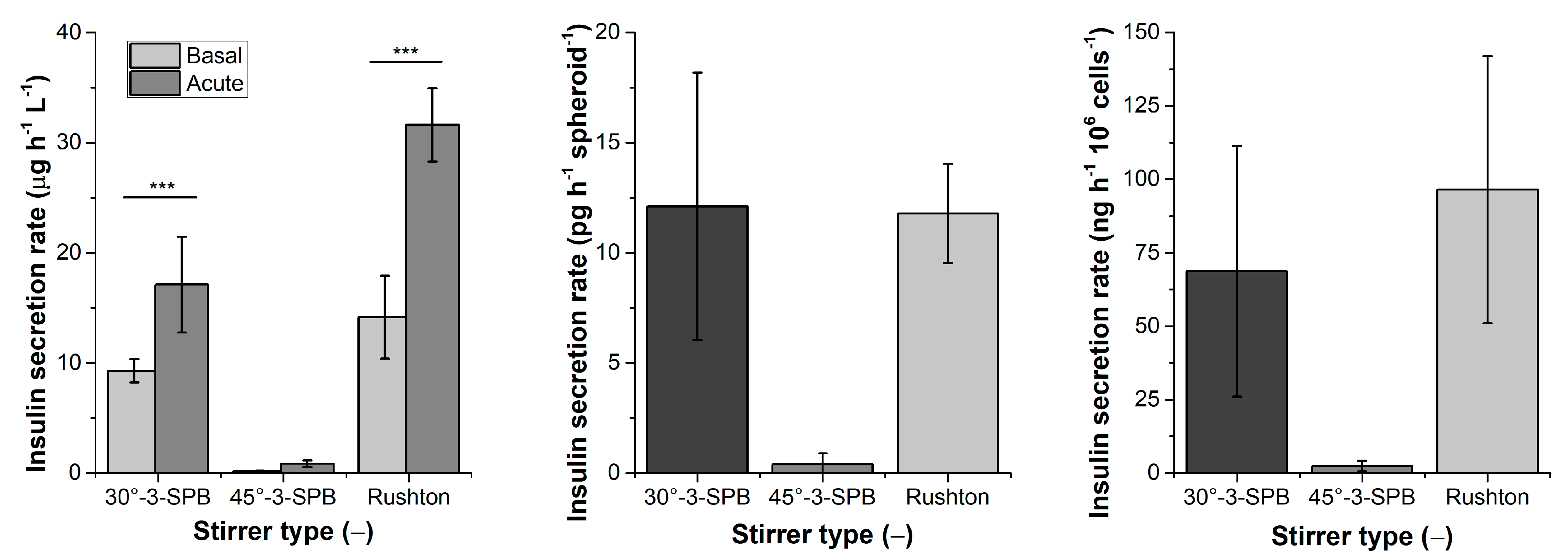
| Stirrer Type | Np [−] | d32 [µm] | Spheroid Formation Efficiency [%] | Span Width of Distribution [µm] | EDCF [kW m−3 s−1] | Stirrer Tip Speed [m s−1] | εmax/ [−] |
|---|---|---|---|---|---|---|---|
| Shaking flask | - | 94 ± 12 | 7.7 ± 1.4 | 134 ± 11 | - | - | - |
| 30°-3-SPB | 1.1 | 51.5 ± 1.2 | 60 ± 3 | 66 ± 7 | 0.9 | 0.62 | 5 |
| 45°-3-SPB | 2.4 | 40 ± 3 | 8.7 ± 1.4 | 51 ± 6 | 0.6 | 0.49 | 6 |
| Rushton | 4.0 | 50.6 ± 1.6 | 33 ± 2 | 61 ± 3 | 1.6 | 0.46 | 13 |
| Stirrer Type | µVol [d−1] | tD,Vol [d] | Acute Insulin Secretion [µg h−1 L−1] | SI [−] | YSpheroids [Spheroids L−1] | YIEQs [IEQs L−1] | Ycells [Cells L−1] |
|---|---|---|---|---|---|---|---|
| 30°-3-SPB | 0.38 ± 0.018 | 1.81 ± 0.09 | 32 ± 3 | 3.2 ± 1.2 | 18 × 106 ± 2 × 106 | 1.4 × 106 ± 0.6 × 106 | 2 × 108 ± 0.1 × 108 |
| 45°-3-SPB | 0.4 ± 0.2 | 1.9 ± 1.0 | - | - | 14 × 106 ± 4 × 106 | 0.3 × 106 ± 0.2 × 106 | 0.8 × 108 ± 0.4 × 108 |
| Rushton | 0.27 ± 0.07 | 2.7 ± 0.7 | 17 ± 4 | 2.7 ± 1.0 | 22 × 106 ± 0.6 × 106 | 1.1 × 106 ± 0.4 × 106 | 2.2 × 108 ± 0.2 × 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petry, F.; Salzig, D. Large-Scale Production of Size-Adjusted β-Cell Spheroids in a Fully Controlled Stirred-Tank Reactor. Processes 2022, 10, 861. https://doi.org/10.3390/pr10050861
Petry F, Salzig D. Large-Scale Production of Size-Adjusted β-Cell Spheroids in a Fully Controlled Stirred-Tank Reactor. Processes. 2022; 10(5):861. https://doi.org/10.3390/pr10050861
Chicago/Turabian StylePetry, Florian, and Denise Salzig. 2022. "Large-Scale Production of Size-Adjusted β-Cell Spheroids in a Fully Controlled Stirred-Tank Reactor" Processes 10, no. 5: 861. https://doi.org/10.3390/pr10050861
APA StylePetry, F., & Salzig, D. (2022). Large-Scale Production of Size-Adjusted β-Cell Spheroids in a Fully Controlled Stirred-Tank Reactor. Processes, 10(5), 861. https://doi.org/10.3390/pr10050861






