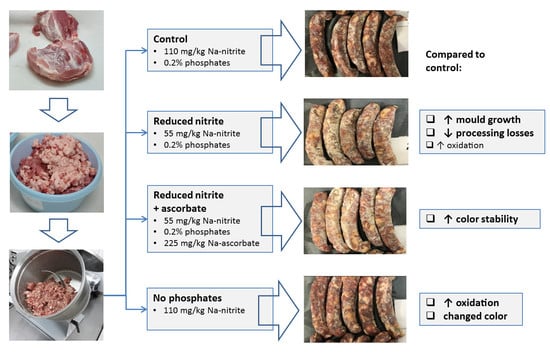
Reduced Use of Nitrites and Phosphates in Dry-Fermented Sausages
Abstract
:1. Introduction
2. Materials and Methods
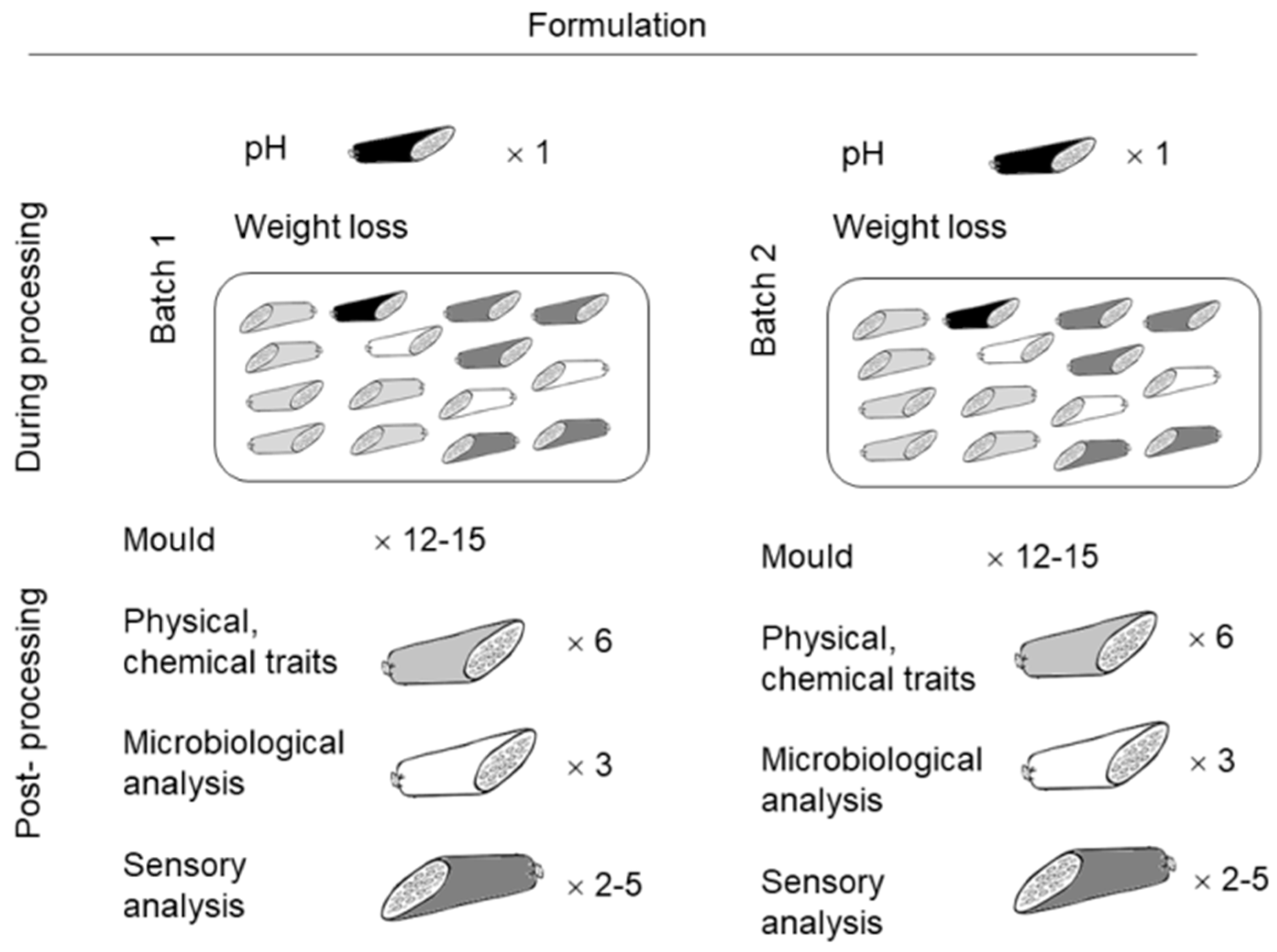
2.1. Dry-Fermented Sausages Formulations and Processing
2.2. Microbiological Parameters
2.3. Measurements of pH and Color
2.4. Instrumental Texture Measurement
2.5. Determination of Chemical Composition
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
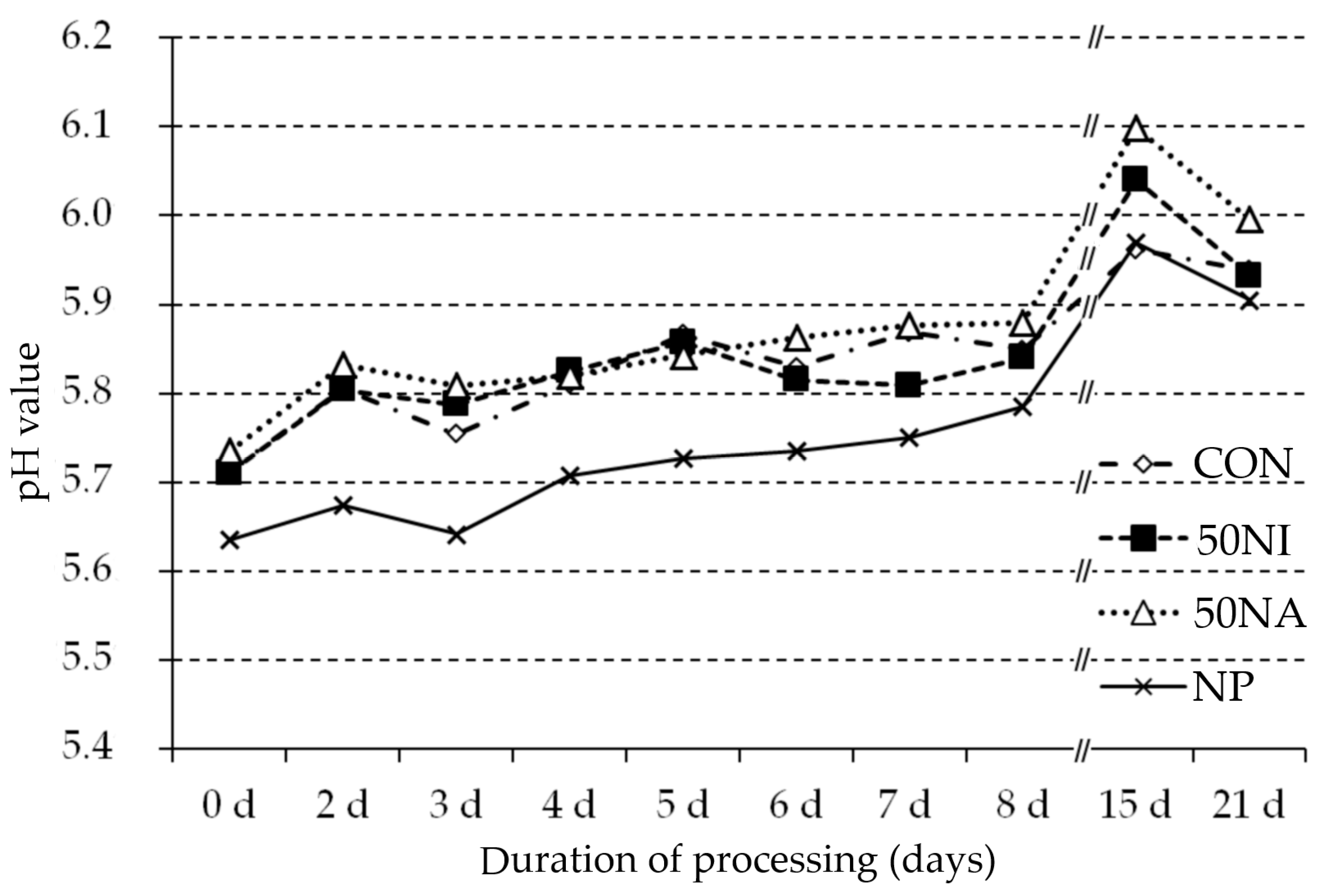
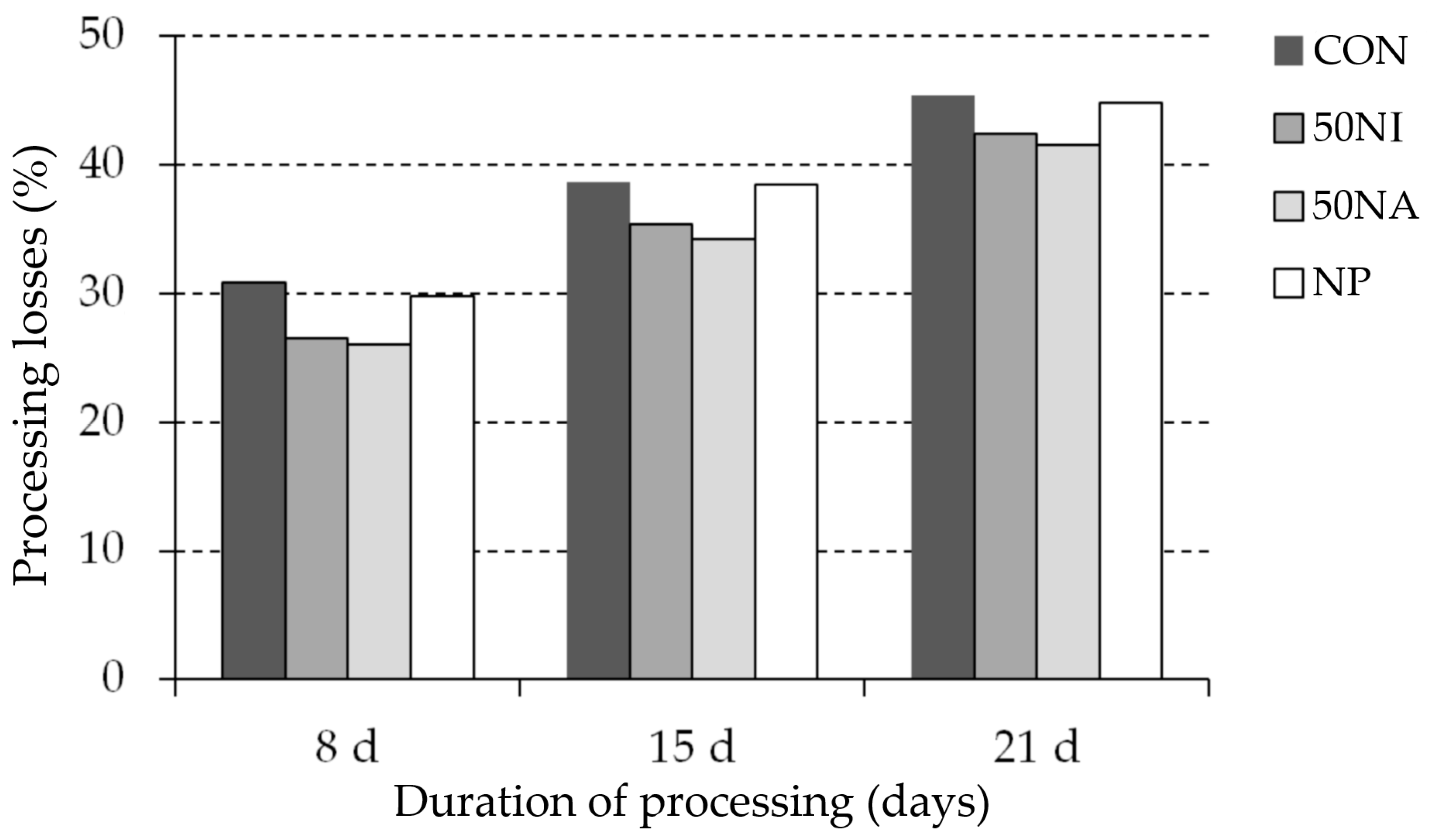
3.1. Changes during Processing (pH, Weight Loss), Sausage Surface, and Microbiological Profile
3.2. Instrumental Color Parameters
3.3. Sausage Physical Chemical Traits
3.4. Sausage Rheological and Sensory Traits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toldrá, F.; Flores, M. Sausages, Types of: Dry and Semidry. In Encyclopedia of Meat Sciences; Devine, C., Dikeman, M., Eds.; Academic Press: Oxford, UK, 2014; pp. 248–255. [Google Scholar]
- Flores, M.; Olivares, A. Flavour. In Handbook of Fermented Meat and Poultry; Toldrá, F., Ed.; John Wiley & Sons: Chichester, UK, 2015; pp. 217–225. [Google Scholar]
- Lücke, F.K. Utilization of microbes to process and preserve meat. Meat Sci. 1998, 56, 105–115. [Google Scholar] [CrossRef]
- Xiong, Y.L. Nonmeat ingredients and additives. In Handbook of Meat and Meat Processing, 2nd ed.; Hui, Y.L., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 573–588. [Google Scholar]
- Alhakoon, U.A.; Jayasena, D.D.; Ramachandra, S.; Jo, C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015, 45, 37–49. [Google Scholar] [CrossRef]
- Tompkin, R.B. Nitrite. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 169–236. [Google Scholar]
- Sindelar, J.J.; Milkowski, A.L. Sodium Nitrite in Processed Meat and Poultry Meats: A Review of Curing and Examining the Risk/Benefit of Use; American Meat Science Association: Champaign, IL, USA, 2011; Available online: https://info-nitrites.fr/wp-content/uploads/2016/06/nitrite_report.pdf (accessed on 29 October 2021).
- Pradhan, A.K.; Ivanek, R.; Grohn, Y.T.; Geornaras, I.; Sofos, J.N.; Wiedman, M. Quantitative risk assessment for Listeria monocytogenes in selected categories of meats: Impact of lactate and diacetate on listeriosis cases and deaths. J. Food. Prot. 2009, 72, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Parthasarthy, D.K.; Bryan, N.S. Sodium nitrite: The “cure” for nitric oxide insufficiency. Meat Sci. 2012, 92, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, J.J.; Milkowski, A.L. Human safety controversies surrounding nitrate and nitrite in the diet. Nitric Oxide 2012, 25, 259–266. [Google Scholar] [CrossRef]
- Sanchez-Echaniz, J.; Benito-Fernandez, J.; Mintegui-Raso, S. Methemoglobinemia and consumption of vegetables in infants. Pediatrics 2001, 107, 1024–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Escalante, A.; Dejenane, D.; Torrescano, G.; Beltran, J.A.; Roncales, P. The effects of ascorbic acid, taurine, carnosine and rosemary powder on colour and lipid stability of beef patties packaged in modified atmosphere. Meat Sci. 2001, 58, 421–429. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Izumi, K.; Cassens, R.G.; Greaser, M.L. Reaction of nitrite with ascorbic acid and its significant role in nitrite-cured foods. Meat Sci. 1989, 26, 141–153. [Google Scholar] [CrossRef]
- Varvara, M.; Bozzo, G.; Celano, G.; Disanto, C.; Pagliarone, C.N.; Celano, G.V. The use of ascorbic acid as a food additive: Technical-legal issues. Ital. J. Food. Saf. 2016, 5, 4313. [Google Scholar] [CrossRef] [Green Version]
- Haak, L.; Raes, K.; De Smet, S. Effect of plant phenolics, tocopherol and ascorbic acid on oxidative stability of pork patties. J. Sci. Food Agric. 2009, 89, 1360–1365. [Google Scholar] [CrossRef]
- Berardo, A.; De Maere, H.; Stavropoulou, D.A.; Rysman, T.; Leroy, F.; De Smet, S. Effect of sodium nitrite on protein and lipid oxidation in dry fermented sausages. Meat Sci. 2016, 121, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Roncáles, P. Additives. In Handbook of Fermented Meat and Poultry, 1st ed.; Toldrá, F., Ed.; Blackwell Publishing: Ames, IA, USA, 2007; pp. 77–86. [Google Scholar]
- Walz, F.H.; Gibis, M.; Schrey, M.; Herrmann, K.; Reichert, C.L.; Hinrichs, J.; Weiss, J. Inhibitory effect of phosphates on magnesium lactate efflorescence formation in dry-fermented sausages. Food Res. Int. 2017, 100, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Gassara, F.; Kouassi, A.P.; Kaur Brar, S.; Belkacemi, K. Green alternatives to nitrates and nitrites in meat-based products—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2133–2148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velasco, V.; Williams, P. Improving meat quality through natural antioxidants. Chil. J. Agric. Res. 2011, 71, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2007, 106, 1188–1194. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Opinion of the scientific panel on biological hazards on a request from the commission related to the effects of nitrites/nitrates on the microbiological safety of meat products. EFSA J. 2003, 14, 31. [Google Scholar]
- European Food Safety Authority (EFSA). Statement on nitrites in meat products. EFSA J. 2010, 8, 1538. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Fernández, M. Survival of Listeria innocua in dry fermented sausages and changes in the typical microbiota and volatile profile as affected by the concentration of nitrate and nitrite. Int. J. Food Microbiol. 2012, 153, 395–401. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Fernández, M. Effect of reducing nitrate and nitrite added to dry fermented sausages on the survival of Salmonella typhimurium. Food Res. Int. 2014, 62, 410–415. [Google Scholar] [CrossRef]
- Hospital, X.F.; Hierro, E.; Stringer, S.; Fernández, M. A study on the toxigenesis by Clostridium botulinum in nitrate and nitrite deduced dry fermented sausages. Int. J. Food Microbiol. 2016, 218, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Balev, D.; Vulkova, T.; Dragoev, S.; Zlatanov, M.; Bahtchevanska, S. A comparative study on the effect of some antioxidants on the lipid and pigment oxidation in dry-fermented sausages. Int. J. Food Sci. Technol. 2005, 40, 977–983. [Google Scholar] [CrossRef]
- Sebranek, J.G. An overview of functional non-meat ingredients in meat processing: The current toolbox. In Proceedings of the AMSA’s 68th Reciprocal Meat Conference, Lincoln, NE, USA, 14–17 June 2015; pp. 42–46. [Google Scholar]
- Zanardi, E.; Ghidini, S.; Battaglia, A.; Chizzolini, R. Lipolysis and lipid oxidation in fermented sausages depending on different processing conditions and different antioxidants. Meat Sci. 2004, 66, 415–423. [Google Scholar] [CrossRef]
- Perea-Sanz, L.; Montero, R.; Belloch, C.; Flores, M. Nitrate reduction in the fermentation process of salt reduced dry-sausages: Impact on microbial and physicochemical parameters and aroma profile. Int. J. Food Microbiol. 2018, 282, 84–91. [Google Scholar] [CrossRef] [Green Version]
- NLZOH. Smernice za Mikrobiološko Varnost Živil, ki so Namenjena Končnemu Potrošniku; University of Ljubljana: Ljubljana, Slovenia, 2019; Available online: https://www.vf.uni-lj.si/smernice-za-mikrobiolosko-varnost-zivil-ki-so-namenjena-koncnemu-potrosniku (accessed on 15 April 2021).
- ISO 6579; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Detection of Salmonella spp. International Organization for Standardization: Genève, Switzerland, 2002.
- ISO 7937; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Clostridium Perfringens. Colony-Count Technique; International Organization for Standardization: Genève, Switzerland, 2004.
- ISO 10273; Microbiology of the Food Chain—Horizontal Method for the Detection of Pathogenic Yersinia Enterocolitica. International Organization for Standardization: Genève, Switzerland, 2017.
- ISO 11290-1; Microbiology of the Food Chain. Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and of Listeria spp. International Organization for Standardization: Genève, Switzerland, 2017.
- ISO 6888-2; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Coagula-se-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 2: Technique Using Rabbit Plasma Fibrinogen Agar Medium. International Organization for Standardization: Genève, Switzerland, 1999.
- ISO 21528; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. International Organization for Standardization: Genève, Switzerland, 2017.
- Škrlep, M.; Čandek-Potokar, M.; Tomažin, U.; Batorek Lukač, N.; Flores, M. Properties and aromatic profile of dry-fermented sausages from Krškopolje pigs reared under organic and conventional regime. Animal 2018, 12, 1316–1323. [Google Scholar] [CrossRef] [Green Version]
- Batorek, N.; Škrlep, M.; Prunier, A.; Louveau, I.; Noblet, J.; Bonneau, M.; Čandek-Potokar, M. Effect of feed restriction on hormones, performance, carcass traits, and meat quality in immunocastrated pigs. J. Anim. Sci. 2012, 90, 4593–4603. [Google Scholar] [CrossRef] [Green Version]
- Pugliese, C.; Sirtori, F.; Škrlep, M.; Piasentier, E.; Calamai, L.; Franci, O.; Čandek-Potokar, M. The effect of ripening time on the chemical, textural, volatile and sensorial traits of Biceps femoris and Semimembranosus muscles of the Slovenian dry-cured ham Kraški pršut. Meat Sci. 2015, 100, 58–68. [Google Scholar] [CrossRef]
- Prevolnik, M.; Škrlep, M.; Janeš, L.; Velikonja Bolta, Š.; Škorjanc, D.; Čandek-Potokar, M. Accuracy of near infrared spectroscopy for prediction of chemical composition, salt content and free aminoacids in dry-cured ham. Meat Sci. 2011, 88, 299–304. [Google Scholar] [CrossRef]
- Lynch, S.M.; Frei, B. Mechanisms of copper- and iron-dependent oxidative modification of human low density lipoprotein. J. Lipid Res. 1993, 34, 1745–1753. [Google Scholar] [CrossRef]
- Piasenter, E.; Bianchi, P.; Cappellari, M.; Cavella, S.; Di Monaco, R.; Dinnella, C.; Favotto, S.; Galassi, S.; Laureati, M.; Marangon, A.; et al. Il prosciutto ed altri salumi crudi fermentati. In Atlante Sensoriale dei Prodotti Alimentare; Sinesio, F., Monteleone, E., Spinelli, S., Eds.; Techniche Nuove SPA: Milano, Italy, 2012; pp. 242–255. [Google Scholar]
- Knipe, L. Use of Phosphates in Sausage. Available online: https://meatsci.osu.edu/node/125 (accessed on 18 April 2021).
- Corral, S.; Belloch, C.; López-Diez, J.J.; Salvador, A.; Flores, M. Yeast inoculation as a strategy to improve the physico-chemical and sensory properties of reduced salt fermented sausages produced from entire male fat. Meat Sci. 2017, 123, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Stiebing, A.; Deder, I. Rohschinken un Rohwürste aus Eberfleisch. Fleischwirtschaft 2012, 92, 93–98. [Google Scholar]
- Szmanko, T.; Oziemblowski, M.; Dworecka, E.; Dobrowolska, D. Sensory quality and selected physicochemical properties of processed meat products produced in different plants. Acta Sci. Pol. Technol. Aliment. 2006, 5, 93–105. [Google Scholar]
- Tompkin, R.B.; Christiansen, L.N.; Shaparis, A.B. Effect of prior refrigeration on butulinal outgrowth in perishable canned cured meat when temperature abused. Appl. Environ. Microbiol. 1978, 35, 863–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govari, M.; Pexara, A. Nitrates and nitrites in meat products. J. Hell. Vet. Med. 2015, 66, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Ordoñez, A.; Hierro, E.M.; Bruna, J.M.; de la Hoz, L. Changes in the components of dry-fermented sausages during ripening. Crit. Rev. Food Sci. Nutr. 1999, 39, 329–367. [Google Scholar] [CrossRef]
- Acton, J.C.; Dick, R.L. Cured color development during fermented sausage processing. J. Food Sci. 1977, 42, 895–897. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Jaffe, R. Lipid peroxidation of muscle food as affected by NaCl. J. Agric. Food Chem. 1991, 39, 1017–1021. [Google Scholar] [CrossRef]
- Dunshea, F.R.; D’Souza, D.N.; Pethick, D.W.; Harper, G.S.; Warner, R.D. Effects of dietary factors and other metabolic modifiers on quality and nutritional value of meat. Meat Sci. 2005, 71, 8–38. [Google Scholar] [CrossRef]
- Flores, M.; Aristoy, M.C.; Toldrá, F. Curing agents affect aminopeptidase activity from porcine skeletal muscle. Z. Lebensm. Und-Forsch. A 1997, 205, 343–346. [Google Scholar] [CrossRef]
- Jaarsveld, F.P.V.; Naude, J.R.; Oelofsen, W. Effect of chemical and physical dry-curing parameters on cathepsin B, H and L from ostrich muscle. Meat Sci. 1998, 50, 223–233. [Google Scholar] [CrossRef]
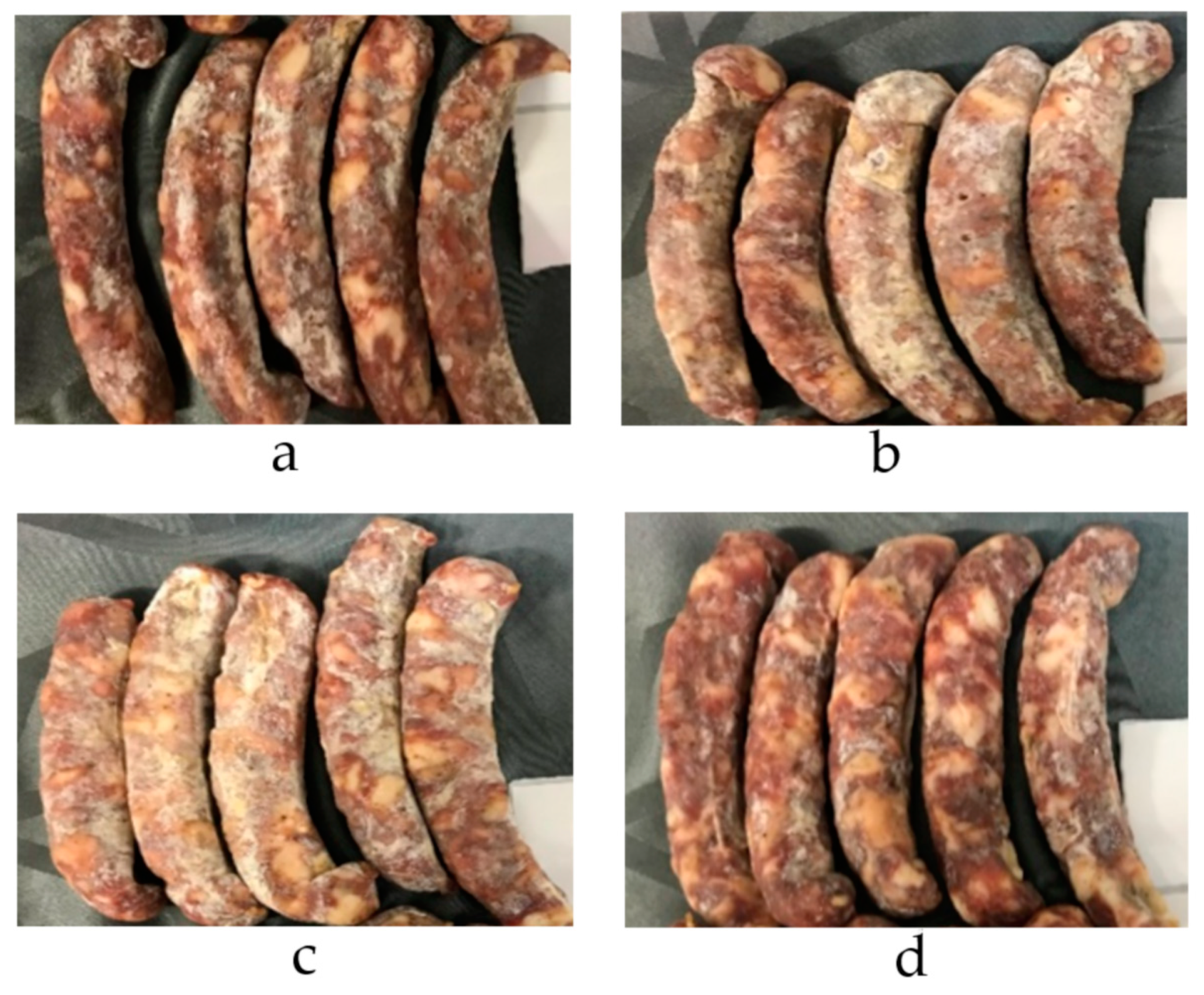
| CON | 50NI-CON a | 50NA-CON a | NP-CON a | RMSE | Effect of F | |
|---|---|---|---|---|---|---|
| Mold covering | 1.8 | 2.5 *** | 2.7 *** | −0.3 NS | 0.67 | *** |
| CON | 50NI-CON a | 50NA-CON a | NP-CON a | RMSE | Effect of F | |
|---|---|---|---|---|---|---|
| Fresh cut | ||||||
| L* | 43.2 | 1.5 NS | −2.1 NS | −3.6 * | 3.27 | ** |
| a* | 8.1 | −0.7 NS | 0.7 NS | −2.0 ** | 1.60 | ** |
| b* | 5.9 | 0.2 NS | −0.1 NS | −1.2 ** | 0.72 | *** |
| After storage and air exposure | ||||||
| L* | 42.8 | −0.3 NS | −1.0 NS | −1.9 NS | 2.38 | NS |
| a* | 8.5 | −0.4 NS | 1.1 * | −1.4 ** | 0.97 | *** |
| b* | 5.8 | 0.1 NS | 0.7 *** | −0.4 ‡ | 0.44 | *** |
| CON | 50NI-CON a | 50NA-CON a | NP-CON a | RMSE | Effect of F | |
|---|---|---|---|---|---|---|
| Lipids, g/kg | 310.2 | −5.1 NS | −3.6 NS | 0.8 NS | 25.56 | NS |
| Moisture, g/kg | 309.5 | 9.2 NS | 12.6 ‡ | −9.9 NS | 13.62 | * |
| Proteins, g/kg | 319.2 | −2.3 NS | −11.1 NS | 10.8 NS | 15.88 | * |
| PI, % | 9.8 | −0.2 NS | −3.5 NS | −0.1 NS | 0.45 | NS |
| Salt, g/kg | 59.6 | 0.2 NS | −0.6 NS | 2.8 * | 2.33 | ** |
| Final pH | 6.24 | −0.01 NS | −0.00 NS | −0.20 *** | 0.06 | *** |
| aw | 0.86 | 0.02 NS | 0.03 ** | 0.01 NS | 0.01 | ** |
| TBARS, µg MDA/kg | 3.3 | 1.7 * | −1.0 NS | 3.4 *** | 1.19 | *** |
| CON | 50NI-CON a | 50NA-CON a | NP-CON a | RMSE | Effect of F | |
|---|---|---|---|---|---|---|
| Hardness, N | 186.4 | 9.0 NS | 5.4 NS | −6.5 NS | 28.33 | NS |
| Cohesiveness | 0.39 | 0.03 NS | 0.04 ‡ | −0.04 ‡ | 0.043 | *** |
| Gumminess, N | 73.1 | 8.1 NS | 10.3 NS | −9.1 NS | 13.71 | ** |
| Springiness, mm | 3.6 | −0.0 NS | 0.2 NS | 0.1 NS | 0.29 | NS |
| Chewiness, N | 258.4 | 27.5 NS | 44.6 ‡ | −31.5 NS | 47.76 | ** |
| Adhesiveness, N * mm | −2.2 | −0.2 NS | −0.2 NS | −0.2 NS | 0.61 | NS |
| Y90 | 0.63 | 0.01 NS | 0.00 NS | 0.01 NS | 0.012 | NS |
| CON | 50NI-CON a | 50NA-CON a | NP-CON a | RMSE | Effect of F | |
|---|---|---|---|---|---|---|
| Color intensity | 6.36 | 0.41 NS | 0.53 NS | 0.73 NS | 1.31 | NS |
| Color brightness | 7.01 | −0.31 NS | 0.27 NS | −0.53 ‡ | 1.16 | * |
| Color uniformity | 7.69 | 0.14 NS | −0.01 NS | −0.42 NS | 0.95 | NS |
| Matured aroma | 7.24 | 0.46 NS | 0.01 NS | 0.04 NS | 1.04 | NS |
| Rancidity | 0.26 | 0.10 NS | 0.16 NS | 0.23 NS | 0.52 | NS |
| Off-tastes | 0.33 | 0.20 NS | 0.27 NS | 0.31 NS | 0.61 | NS |
| Chewiness | 5.93 | −0.28 NS | 0.29 NS | −0.19 NS | 1.35 | NS |
| Softness | 6.03 | −0.51 NS | 0.23 NS | −0.49 NS | 1.21 | NS |
| Juiciness | 6.05 | −0.59 NS | −0.07 NS | −0.68 NS | 1,63 | NS |
| Pastiness | 0.32 | 0.14 NS | 0.08 NS | 0.14 NS | 0.39 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škrlep, M.; Ozmec, M.; Čandek-Potokar, M. Reduced Use of Nitrites and Phosphates in Dry-Fermented Sausages. Processes 2022, 10, 821. https://doi.org/10.3390/pr10050821
Škrlep M, Ozmec M, Čandek-Potokar M. Reduced Use of Nitrites and Phosphates in Dry-Fermented Sausages. Processes. 2022; 10(5):821. https://doi.org/10.3390/pr10050821
Chicago/Turabian StyleŠkrlep, Martin, Manja Ozmec, and Marjeta Čandek-Potokar. 2022. "Reduced Use of Nitrites and Phosphates in Dry-Fermented Sausages" Processes 10, no. 5: 821. https://doi.org/10.3390/pr10050821
APA StyleŠkrlep, M., Ozmec, M., & Čandek-Potokar, M. (2022). Reduced Use of Nitrites and Phosphates in Dry-Fermented Sausages. Processes, 10(5), 821. https://doi.org/10.3390/pr10050821