Sorption of Cd2+ on Bone Chars with or without Hydrogen Peroxide Treatment under Various Pyrolysis Temperatures: Comparison of Mechanisms and Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Bone Char with/without H2O2 Pretreatment
2.3. Characterization
2.4. Batch Experiments and Analytical Methods
2.5. Desorption Study
3. Results and Discussion
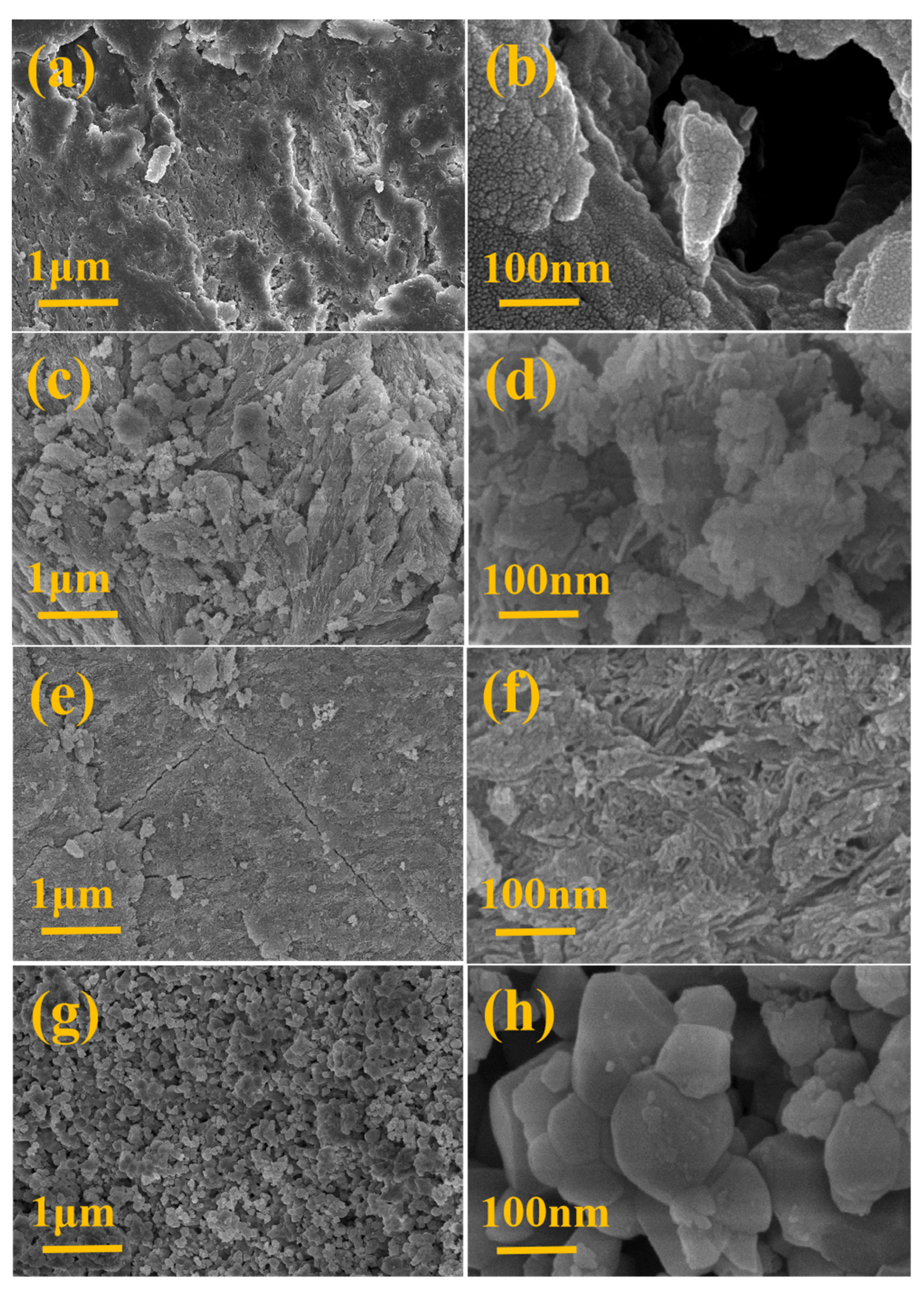
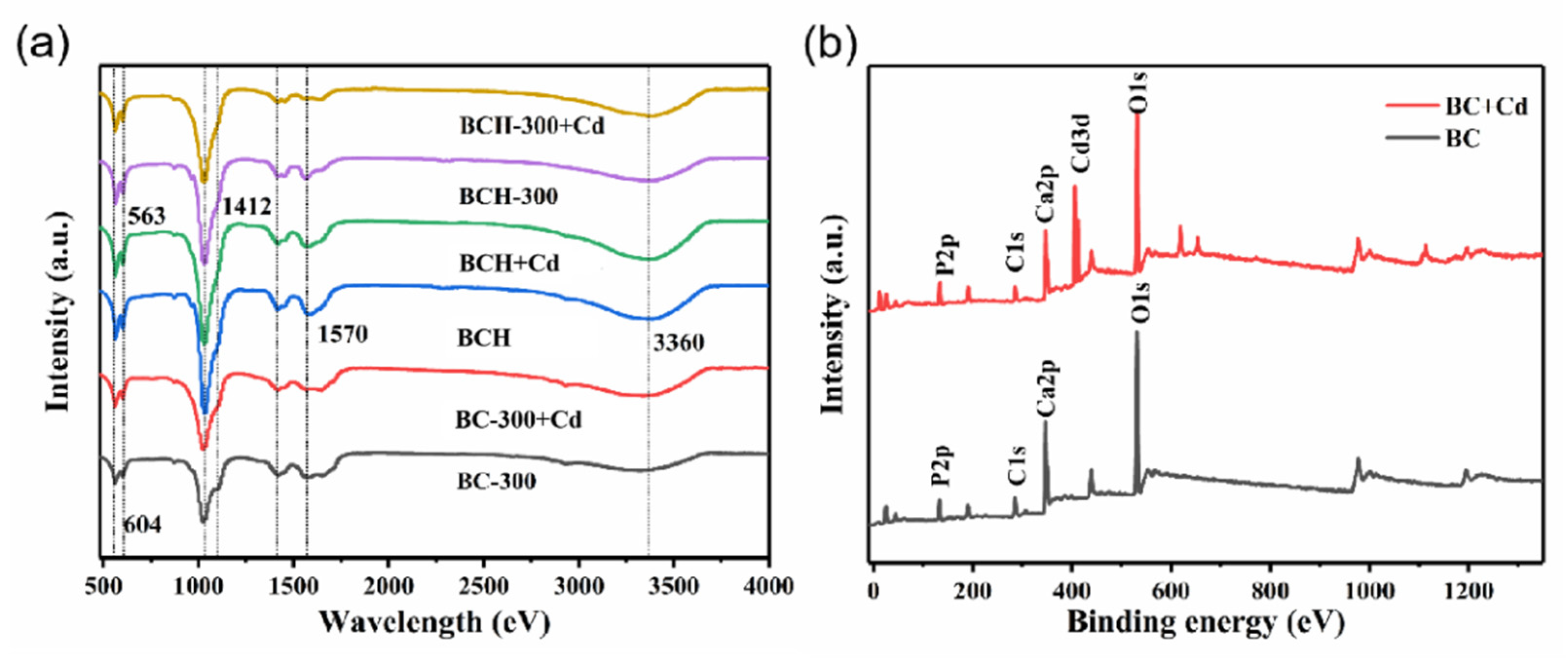
3.1. Characterization of BC and BCH Samples
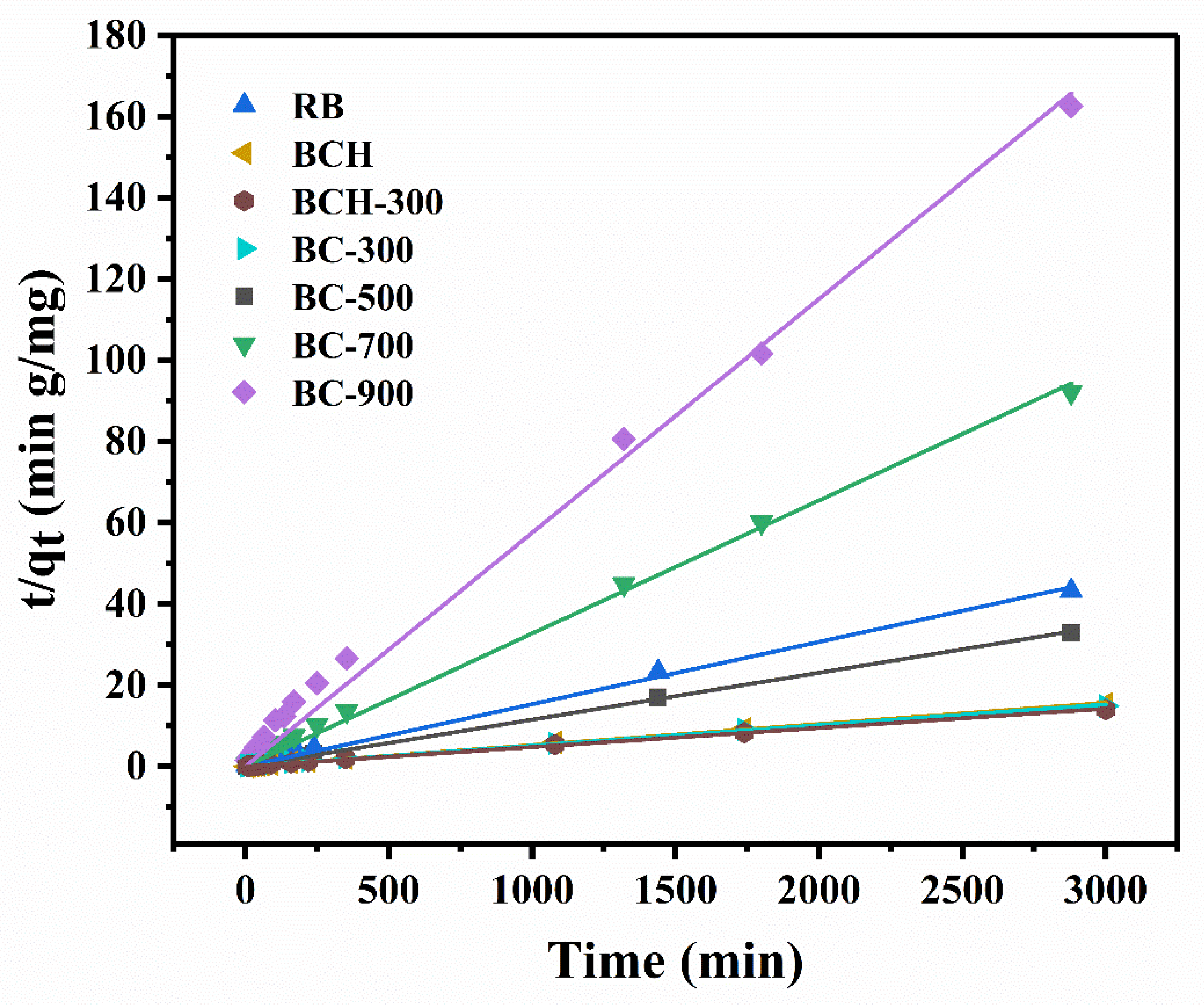
3.2. Adsorption Kinetics
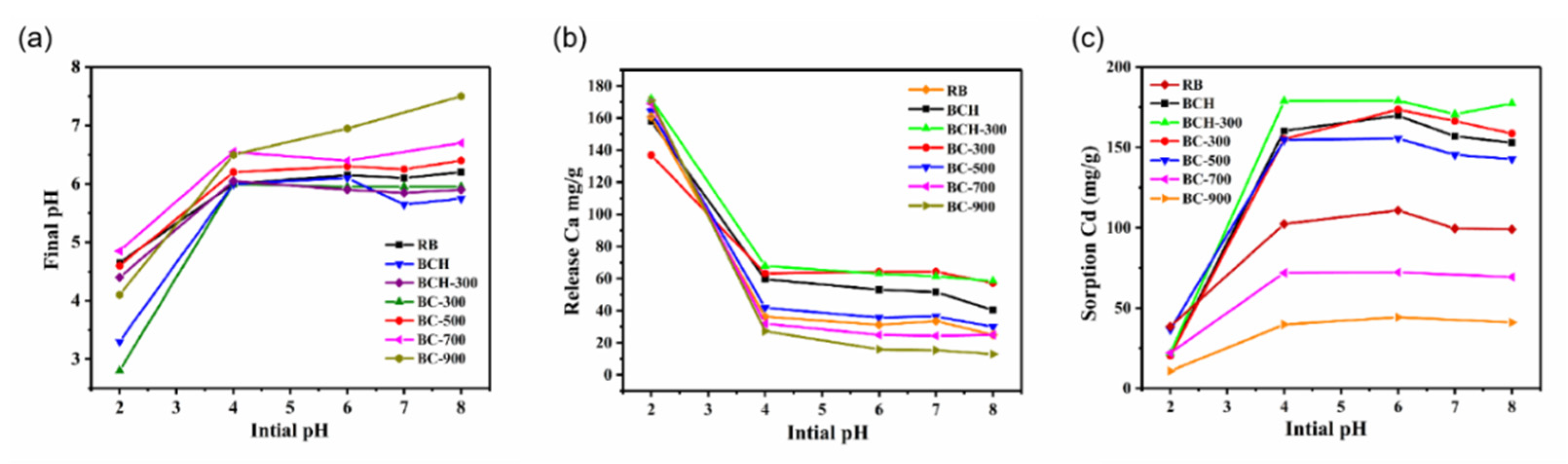
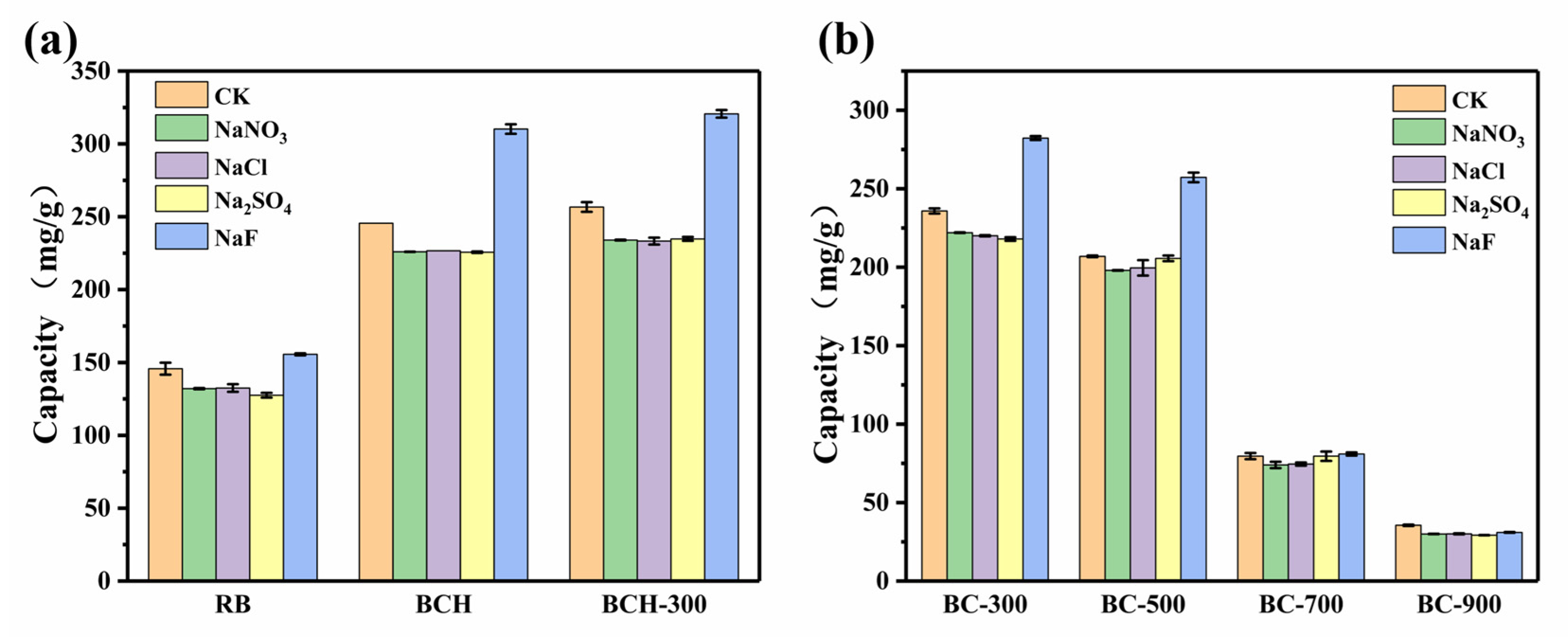
3.3. Effect of Solution pH and Ionic Strength
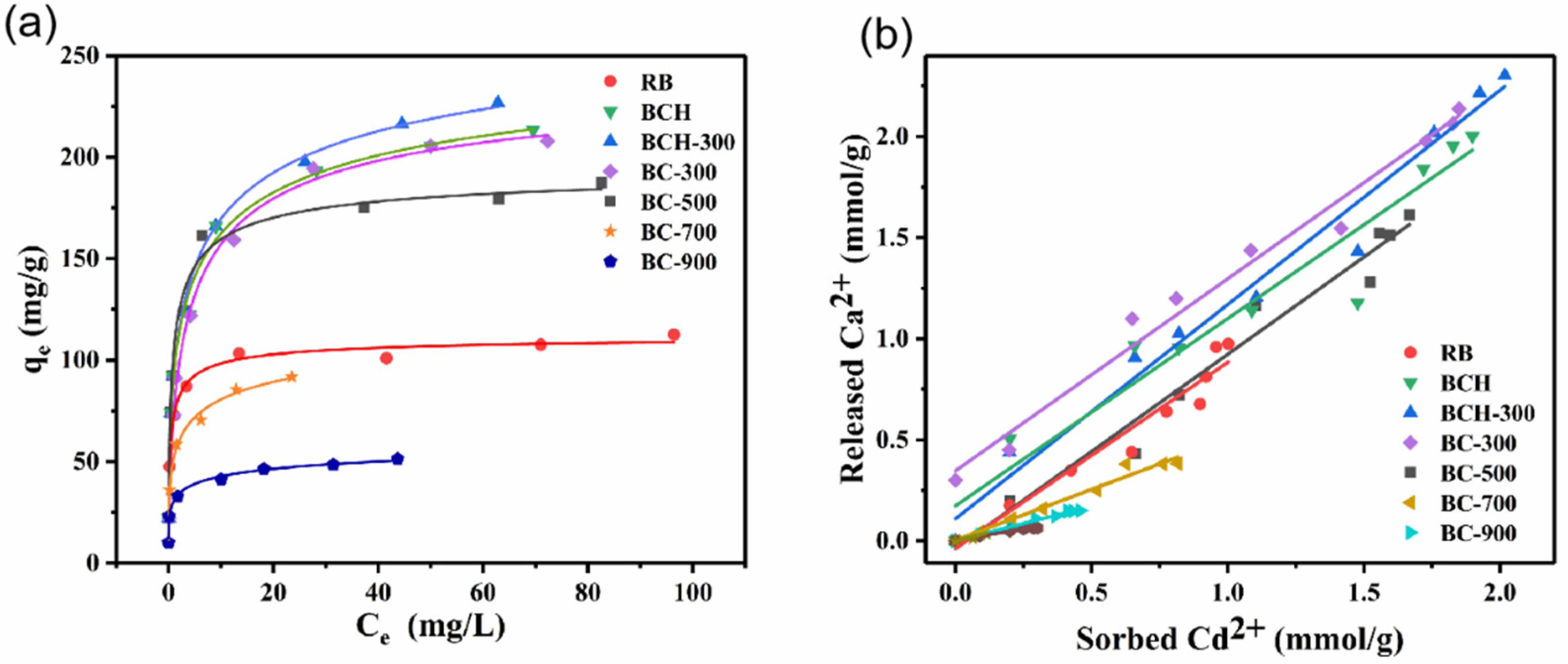
3.4. Adsorption Isotherms
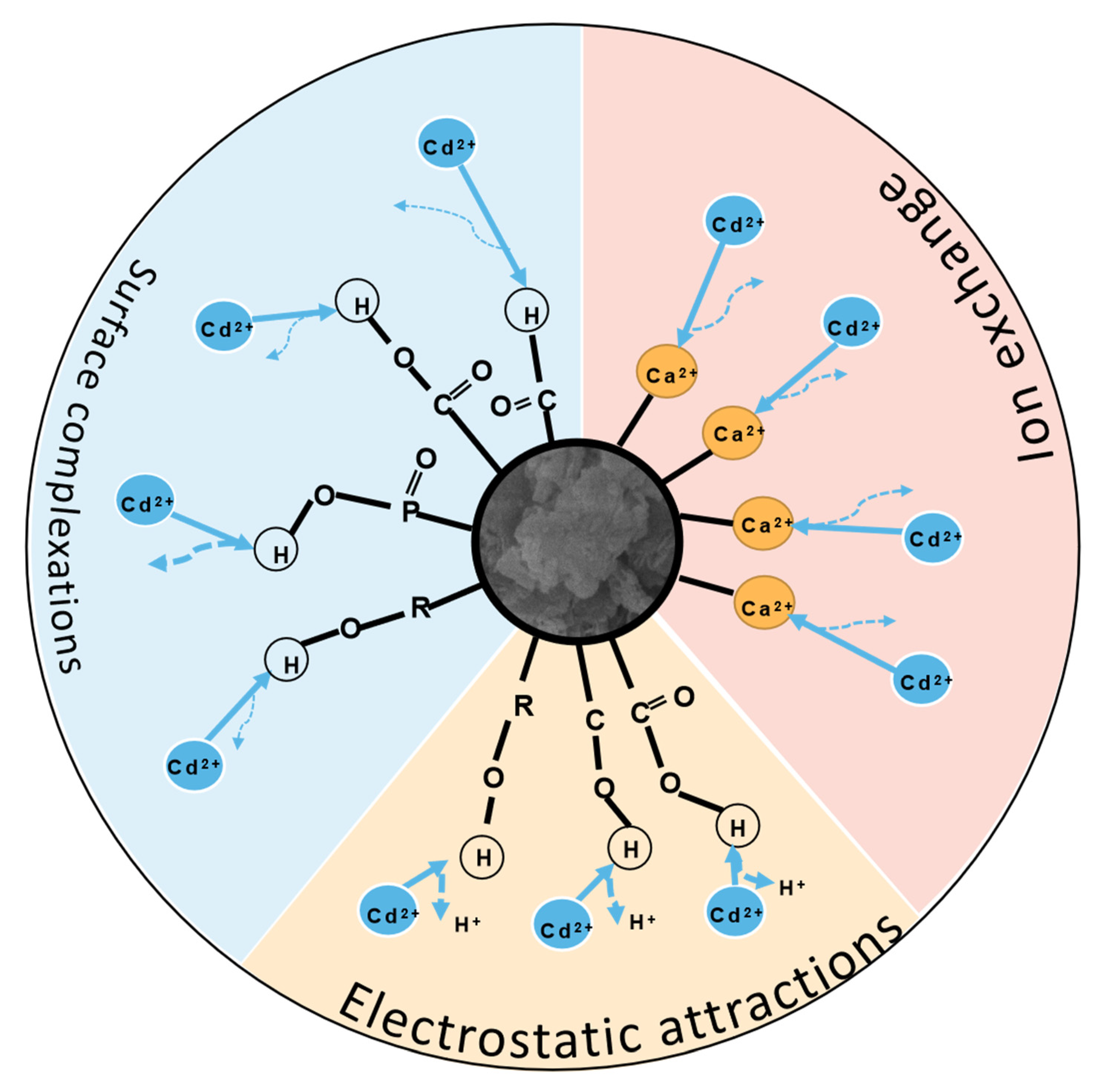
3.5. Sorption Mechanisms
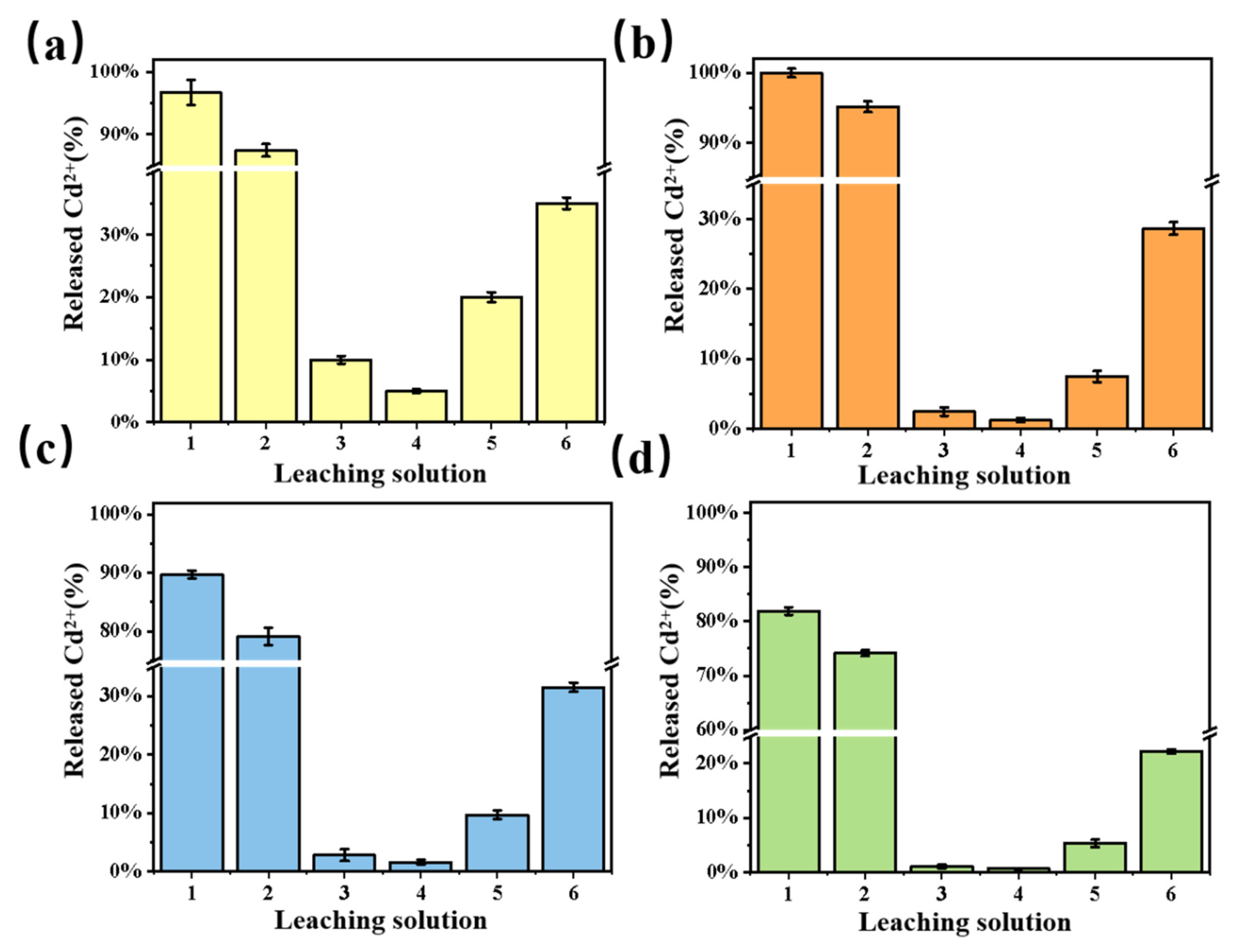
3.6. Desorption Study
3.7. Comparison of BCH with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Meunier, N.; Drogui, P.; Montané, C.; Hausler, R.; Mercier, G.; Blais, J.-F. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-H.; Ding, J.; Luan, Z.; Di, Z.; Zhu, Y.; Xu, C.; Wu, D.; Wei, B. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Assefi, M.; Maroufi, S.; Sahajwalla, V. Selective isolation of heavy metals from spent electronic waste solution by macroporous ion-exchange resins. J. Hazard. Mater. 2019, 371, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Vital, B.; Bartacek, J.; Ortega-Bravo, J.C.; Jeison, D. Treatment of acid mine drainage by forward osmosis: Heavy metal rejection and reverse flux of draw solution constituents. Chem. Eng. J. 2018, 332, 85–91. [Google Scholar] [CrossRef]
- Liang, Y.; Cao, X.; Zhao, L.; Arellano, E. Biochar- and phosphate-induced immobilization of heavy metals in contaminated soil and water: Implication on simultaneous remediation of contaminated soil and groundwater. Environ. Sci. Pollut. Res. 2014, 21, 4665–4674. [Google Scholar] [CrossRef]
- Zendehdel, M.; Ramezani, M.; Shoshtari-Yeganeh, B.; Cruciani, G.; Salmani, A. Simultaneous removal of Pb(II), Cd(II) and bacteria from aqueous solution using amino-functionalized Fe3O4/NaP zeolite nanocomposite. Environ. Technol. 2019, 40, 3689–3704. [Google Scholar] [CrossRef]
- Khraisheh, M.; Al-Degs, Y.; McMINN, W. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem. Eng. J. 2004, 99, 177–184. [Google Scholar] [CrossRef]
- Fernando, M.S.; de Silva, R.M.; de Silva, K.M.N. Synthesis, characterization, and application of nano hydroxyapatite and nanocomposite of hydroxyapatite with granular activated carbon for the removal of Pb2+ from aqueous solutions. Appl. Surf. Sci. 2015, 351, 95–103. [Google Scholar] [CrossRef]
- Schiewer, S.; Patil, S.B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903. [Google Scholar] [CrossRef]
- Xiong, B.; Zhang, Y.; Hou, Y.; Arp, H.P.H.; Reid, B.J.; Cai, C. Enhanced biodegradation of PAHs in historically contaminated soil by M. gilvum inoculated biochar. Chemosphere 2017, 182, 316–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chojnacka, K. Equilibrium and kinetic modelling of chromium(III) sorption by animal bones. Chemosphere 2005, 59, 315–320. [Google Scholar] [CrossRef]
- Deydier, E.; Guilet, R.; Sharrock, P. Beneficial use of meat and bone meal combustion residue: “an efficient low cost material to remove lead from aqueous effluent”. J. Hazard. Mater. 2003, 101, 55–64. [Google Scholar] [CrossRef]
- Banat, F.; Al-Asheh, S.; Mohai, F. Batch zinc removal from aqueous solution using dried animal bones. Sep. Purif. Technol. 2000, 21, 155–164. [Google Scholar] [CrossRef]
- Zahedifar, M.; Seyedi, N.; Shafiei, S.; Basij, M. Surface-modified magnetic biochar: Highly efficient adsorbents for removal of Pb(ΙΙ) and Cd(ΙΙ). Mater. Chem. Phys. 2021, 271, 124860. [Google Scholar] [CrossRef]
- Qian, Z.; Zengjiang, L.; Jinhui, Y.; Hongda, M.; Yi, L. Preparation of magnetic composite materials and Its Application in Heavy metal wastewater Treatment. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 1, p. 012001. [Google Scholar]
- Alkurdi, S.S.; Al-Juboori, R.A.; Bundschuh, J.; Bowtell, L.; McKnight, S. Effect of pyrolysis conditions on bone char characterization and its ability for arsenic and fluoride removal. Environ. Pollut. 2020, 262, 114221. [Google Scholar] [CrossRef] [PubMed]
- Alqadami, A.A.; Khan, M.A.; Otero, M.; Siddiqui, M.R.; Jeon, B.-H.; Batoo, K.M. A magnetic nanocomposite produced from camel bones for an efficient adsorption of toxic metals from water. J. Clean. Prod. 2018, 178, 293–304. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.; Silvestre-Albero, J.; Aguayo-Villarreal, I.; Mendoza-Castillo, D.; Bonilla-Petriciolet, A. A new synthesis route for bone chars using CO2 atmosphere and their application as fluoride adsorbents. Microporous Mesoporous Mater. 2015, 209, 38–44. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.; Leyva-Ramos, R.; Padilla-Ortega, E.; Perez, R.O.; Flores-Cano, J.; Berber-Mendoza, M. Adsorption capacity of bone char for removing fluoride from water solution. Role of hydroxyapatite content, adsorption mechanism and competing anions. J. Ind. Eng. Chem. 2014, 20, 4014–4021. [Google Scholar] [CrossRef]
- Dimović, S.; Smičiklas, I.; Plećaš, I.; Antonović, D.; Mitrić, M. Comparative study of differently treated animal bones for Co2+ removal. J. Hazard. Mater. 2009, 164, 279–287. [Google Scholar] [CrossRef]
- Wang, M.; Liu, Y.; Yao, Y.; Han, L.; Liu, X. Comparative evaluation of bone chars derived from bovine parts: Physicochemical properties and copper sorption behavior. Sci. Total Environ. 2020, 700, 134470. [Google Scholar] [CrossRef] [PubMed]
- Son, E.-B.; Poo, K.-M.; Chang, J.-S.; Chae, K.-J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Cruz-Briano, S.A.; Leyva-Ramos, R.; Moreno-Piraján, J.C.; Torres-Dosal, A.; Giraldo-Gutiérrez, L.; Labrada-Delgado, G.J.; Pérez, R.O.; Rodriguez-Estupiñan, J.P.; Lopez, S.Y.R.; et al. Use of bone char prepared from an invasive species, pleco fish (Pterygoplichthys spp.), to remove fluoride and Cadmium(II) in water. J. Environ. Manag. 2020, 256, 109956. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.C.; Thompson, T.J.U.; Evison, M.P.; Chamberlain, A.T.; Wess, T. Bone mineral change during experimental heating: An X-ray scattering investigation. Biomaterials 2003, 24, 5091–5097. [Google Scholar] [CrossRef]
- Tang, C.-M.; Fan, F.-Y.; Ke, Y.-C.; Lin, W.-C. Effects of electrode plate annealing treatment and the addition of hydrogen peroxide on improving the degradation of cobalt hydroxyapatite for bone repair. Mater. Chem. Phys. 2021, 259, 123962. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Yang, Y.; Huang, J.; Zimmerman, A.R.; Chen, H.; Hu, X.; Gao, B. Mechanisms and adsorption capacities of hydrogen peroxide modified ball milled biochar for the removal of methylene blue from aqueous solutions. Bioresour. Technol. 2021, 337, 125432. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Hu, R.; Chen, G. Micro-nano-engineered nitrogenous bone biochar developed with a ball-milling technique for high-efficiency removal of aquatic Cd(II), Cu(II) and Pb(II). J. Hazard. Mater. 2020, 387, 121980. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Borkowski, L.; Ginalska, G.; Ślósarczyk, A.; Kazarian, S.G. Structural transformation of synthetic hydroxyapatite under simulated in vivo conditions studied with ATR-FTIR spectroscopic imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 155–161. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, Y.; Zhu, Z.; Deng, H.; Ding, H.; Li, Y.; Zhang, L.; Lin, J. Preparation of a porous hydroxyapatite-carbon composite with the bio-template of sugarcane top stems and its use for the Pb(II) removal. J. Clean. Prod. 2018, 187, 650–661. [Google Scholar] [CrossRef]
- Smiciklas, I.; Onjia, A.; Raičević, S. Experimental design approach in the synthesis of hydroxyapatite by neutralization method. Sep. Purif. Technol. 2005, 44, 97–102. [Google Scholar] [CrossRef]
- Wang, B.; Gao, B.; Wan, Y. Entrapment of ball-milled biochar in Ca-alginate beads for the removal of aqueous Cd(II). J. Ind. Eng. Chem. 2018, 61, 161–168. [Google Scholar] [CrossRef]
- Smiciklas, I.; Dimović, S.; Plećaš, I.; Mitrić, M. Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res. 2006, 40, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. X-ray photoelectron and ion scattering spectroscopic surface analyses of amorphous and crystalline calcium phosphate nanoparticles with different chemical histories. Phys. Chem. Chem. Phys. 2020, 22, 5531–5547. [Google Scholar] [CrossRef] [PubMed]
- Piccirillo, C.; Moreira, I.S.; Novais, R.M.; Fernandes, A.J.S.; Pullar, R.C.; Castro, P.M.L. Biphasic apatite-carbon materials derived from pyrolysed fish bones for effective adsorption of persistent pollutants and heavy metals. J. Environ. Chem. Eng. 2017, 5, 4884–4894. [Google Scholar] [CrossRef]
- Rojas-Mayorga, C.K.; Castillo, D.I.M.; Bonilla-Petriciolet, A.; Silvestre-Albero, J. Tailoring the adsorption behavior of bone char for heavy metal removal from aqueous solution. Adsorpt. Sci. Technol. 2016, 34, 368–387. [Google Scholar] [CrossRef]
- Bekiaris, G.; Peltre, C.; Jensen, L.S.; Bruun, S. Using FTIR-photoacoustic spectroscopy for phosphorus speciation analysis of biochars. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 168, 29–36. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Wang, H.; Sarkar, B.; Shaheen, S.M.; Gielen, G.; Bolan, N.; Guo, J.; Che, L.; Sun, H.; et al. Animal carcass- and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: A trial for reclamation and improvement of degraded soils. J. Environ. Manag. 2020, 261, 110246. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.P.; Turner-Walker, G.; Rathod, J.; Liang, B.; Wang, C.-C.; Lee, Y.-C.; Sheu, H.-S. Sustainable phosphorus management in soil using bone apatite. J. Environ. Manag. 2021, 305, 114344. [Google Scholar] [CrossRef]
- Biswas, P.P.; Liang, B.; Turner-Walker, G.; Rathod, J.; Lee, Y.-C.; Wang, C.-C.; Chang, C.-K. Systematic changes of bone hydroxyapatite along a charring temperature gradient: An integrative study with dissolution behavior. Sci. Total Environ. 2021, 766, 142601. [Google Scholar] [CrossRef]
- Park, J.-H.; Yun, J.-J.; Kang, S.-W.; Kim, S.-H.; Cho, J.-S.; Wang, J.J.; Seo, D.-C. Removal of potentially toxic metal by biochar derived from rendered solid residue with high content of protein and bone tissue. Ecotoxicol. Environ. Saf. 2021, 208, 111690. [Google Scholar] [CrossRef]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Chen, S.B.; Ma, Y.B.; Chen, L.; Xian, K. Adsorption of aqueous Cd2+, Pb2+, Cu2+ ions by nano-hydroxyapatite: Single- and multi-metal competitive adsorption study. Geochem. J. 2010, 44, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Hu, C.; Huang, Q. Adsorption of Cu2+, Pb2+, and Cd2+ onto oiltea shell from water. Bioresour. Technol. 2019, 271, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Cho, D.-W.; Tsang, D.C.W.; Bolan, N.; Rinklebe, J.; Song, H. Fabrication of engineered biochar from paper mill sludge and its application into removal of arsenic and cadmium in acidic water. Bioresour. Technol. 2017, 246, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sitko, R.; Turek, E.; Zawisza, B.; Malicka, E.; Talik, E.; Heimann, J.; Gagor, A.; Feist, B.; Wrzalik, R. Adsorption of divalent metal ions from aqueous solutions using graphene oxide. Dalton Trans. 2013, 42, 5682–5689. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]

| Adsorbents Samples | Sorption Capacity (mg/g) | References |
|---|---|---|
| MBC-600 | Cd 165.77 | [28] |
| cauliflower leaves biochar | Cd 73.80 | [42] |
| banana peel biochar | Cd 121.31 | [42] |
| HAP | Cd 49.36 | [43] |
| oiltea shell | Cd 22.40 | [44] |
| biochar from paper mill sludge | Cd 41.6 | [45] |
| graphene oxide (GO) | Cd 530 | [46] |
| Hickory wood biochar | Cd 4.75 | [47] |
| BCH-300 | Cd 228.73 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Tang, H.; Jiang, L.; Chen, M.; Zhu, N.; Wu, P. Sorption of Cd2+ on Bone Chars with or without Hydrogen Peroxide Treatment under Various Pyrolysis Temperatures: Comparison of Mechanisms and Performance. Processes 2022, 10, 618. https://doi.org/10.3390/pr10040618
Guo Q, Tang H, Jiang L, Chen M, Zhu N, Wu P. Sorption of Cd2+ on Bone Chars with or without Hydrogen Peroxide Treatment under Various Pyrolysis Temperatures: Comparison of Mechanisms and Performance. Processes. 2022; 10(4):618. https://doi.org/10.3390/pr10040618
Chicago/Turabian StyleGuo, Qing, Hongmei Tang, Lu Jiang, Meiqing Chen, Nengwu Zhu, and Pingxiao Wu. 2022. "Sorption of Cd2+ on Bone Chars with or without Hydrogen Peroxide Treatment under Various Pyrolysis Temperatures: Comparison of Mechanisms and Performance" Processes 10, no. 4: 618. https://doi.org/10.3390/pr10040618
APA StyleGuo, Q., Tang, H., Jiang, L., Chen, M., Zhu, N., & Wu, P. (2022). Sorption of Cd2+ on Bone Chars with or without Hydrogen Peroxide Treatment under Various Pyrolysis Temperatures: Comparison of Mechanisms and Performance. Processes, 10(4), 618. https://doi.org/10.3390/pr10040618





