A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment
Abstract
:1. Introduction
2. Overview of LDHs and LDH-Based Composites
2.1. General Structure and Properties of LDHs and Their Composites
2.1.1. Composition, Characteristics, and Preparation of LDHs
2.1.2. The Characteristics of Carbon Materials and Preparation of LDH–Carbon Composites
- (1)
- Biochar (BC)
- (2)
- Fullerene (C60)
- (3)
- Carbon nanotubes (CNTs)
- (4)
- Graphene (GN/RGO)
- (1)
- Direct mixing: The positively charged surface of LDHs and the negatively charged surface of carbon nanomaterials are assembled into LDH–carbon material composites through electrostatic force action. Liu et al. [35] used benzoic acid to intercalate an LDH, mixed the resulting material with a xylene solution containing C60, and stirred the mixture ultrasonically for 48 h at 70 °C to obtain a C60–LDH composite with C60-intercalated LDH. These C60–LDH materials can be obtained on a large scale by this simple, convenient, and low-cost preparation process. However, this method is not suitable to obtain a desired ideal structure because of the complicated interaction between carbon and the LDH.
- (2)
- Self-assembly: Relying on the force between molecules or ions, the combination of materials is changed from a disordered combination to an orderly combination. This method can control the material’s structure with required functions. Lestari et al. [36] co-precipitated ZnTi LDH in a homogeneous solution and then vigorously stirred Ag and C3N4 powder with the LDH in ultrapure water to complete the self-assembly reaction. The prepared material was combined by electrostatic force. Although the bonding process can be controlled, the overall material is unstable and requires further processing.
- (3)
- Growth in situ: A carbon material can be grown with an LDH as a substrate, or an LDH can also be grown using a carbon material as the substrate. In the preparation process, the carbon material adsorbs cations on its surface and these cations co-precipitate on the surface of the carbon material to form a new LDH. Similarly, the material can enter the inner layer of the LDH or use the LDH as a catalytic precursor to grow the carbon material. Li et al. [37] mixed the alkali solution of graphite oxide and the solution of Ni(NO3)2 and Al(NO3)3 to prepare graphene–LDH composites. This method mainly relies on the attraction of negatively charged carbon materials to positively charged cations of the LDH precursor solution. The size of the LDH prepared by this method is affected by the nanometer size of the carrier carbon material.
3. Research on the Application of LDH–C Materials
Application of LDH–C Composite as Environmental Remediation Materials
4. Mechanism Analysis of LDH–C as Various Remediation Materials
4.1. Mechanism Analysis of LDH–C as Adsorption Materials
4.1.1. Adsorption Mechanism of Non-Metal Elements
4.1.2. Adsorption Mechanism of Heavy Metals
4.1.3. Adsorption Mechanism of Organic Pollutants
4.2. Mechanism Analysis of LDH–C as Catalytic Materials
4.2.1. Mechanism as Electrocatalytic Materials
4.2.2. Mechanism as Photocatalytic Materials
4.2.3. Mechanism as Synergistic Materials for Adsorption and Photocatalysis
5. Conclusions and Prospects
- (1)
- The types of carbon materials that can be combined with LDHs lack diversity, especially in terms of biomass carbon materials. At present, there are many known types of biomass carbon materials but rare reports on biomass carbon materials that can produce composite LDHs on a large scale. In the future, research on the combination of biomass carbon and LDHs can be increased.
- (2)
- The mechanism analysis of the degradation of organic pollutants by LDH–C is not accurate enough. At present, the role of active radicals in the photocatalytic degradation reaction has been confirmed but the contribution of the carbon component in the composite material to the degradation of pollutants is still unclear and how the addition of carbon materials affects the transfer path or efficiency of photogenerated electrons is not detailed. In the future, the research on the mechanism of LDH–C degrading organic pollutants should be strengthened.
- (3)
- The application of research works on the LDH–C materials applied to practical wastewater treatment is not developed enough. It is necessary to transform the research from laboratory based to pilot plant and industrial-scale production and to study more parameters for the industrialization of such products.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Chen, M. Progress in the preparation and application of modified biochar for improved contaminant removal from water and wastewater. Bioresour. Technol. 2016, 214, 836–851. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ouyang, T.; Chen, J.; Wang, Z.; Liao, S.; Li, X.; Liu, Z.Q. Synthesis of nickel-iron layered double hydroxide via topochemical approach: Enhanced surface charge density for rapid hexavalent chromium removal. J. Colloid Interface Sci. 2022, 605, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Min, X.; Dong, Q.; Xu, S.; Wang, Y. Comparison of Zn-Al and Mg-Al layered double hydroxides for adsorption of perfluorooctanoic acid. Chemosphere 2022, 287, 132297. [Google Scholar] [CrossRef]
- Arif, M.; Liu, G.; Yousaf, B.; Ahmed, R.; Irshad, S.; Ashraf, A.; Zia-ur-Rehman, M.; Rashid, M.S. Synthesis, characteristics and mechanistic insight into the clays and clay minerals-biochar surface interactions for contaminants removal—A review. J. Clean. Prod. 2021, 310, 127548. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Sadiku, E.R.; Mokhena, T.C. Morphology, thermal stability, and flammability properties of polymer-layered double hydroxide (LDH) nanocomposites: A Review. Crystals 2020, 10, 612. [Google Scholar] [CrossRef]
- Xie, Z.H.; Zhou, H.Y.; He, C.S.; Pan, Z.C.; Yao, G.; Lai, B. Synthesis, application and catalytic performance of layered double hydroxide based catalysts in advanced oxidation processes for wastewater decontamination: A review. Chem. Eng. J. 2021, 414, 128713. [Google Scholar] [CrossRef]
- He, J.; Yang, Z.; Zhang, L.; Li, Y.; Pan, L. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method on γ-Al2O3 as catalytic material with high catalytic activity for methanol steam reforming. Int. J. Hydrog. Energy 2017, 42, 9930–9937. [Google Scholar] [CrossRef]
- Feng, X.; Yu, Z.; Long, R.; Li, X.; Shao, L.; Zeng, H.; Zeng, G.; Zuo, Y. Self-assembling 2D/2D (MXene/LDH) materials achieve ultra-high adsorption of heavy metals Ni2+ through terminal group modification. Sep. Purif. Technol. 2020, 253, 117525. [Google Scholar] [CrossRef]
- Olya, N.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. Construction of a novel corrosion protective composite film based on a core-shell LDH-Mo@SiO2 inhibitor nanocarrier with both self-healing/barrier functions. J. Taiwan Inst. Chem. Eng. 2020, 113, 406–418. [Google Scholar] [CrossRef]
- Soheili-Azad, P.; Yaftian, M.R.; Dorraji, M.S.S. Application of zinc/aluminum layered double hydroxide nanosorbent in a fixed-bed column for SPE-preconcentration followed by HPLC determination of diclofenac in biological and hospital wastewater samples. Microchem. J. 2019, 148, 270–276. [Google Scholar] [CrossRef]
- Lu, P.; Liu, Y.; Zhou, T.; Wang, Q.; Li, Y. Recent advances in layered double hydroxides (LDHs) as two-dimensional membrane materials for gas and liquid separations. J. Membr. Sci. 2018, 567, 89–103. [Google Scholar] [CrossRef]
- Allou, N.B.; Saikia, J.; Bordoloi, P.; Yadav, A.; Pal, M.; Goswamee, R.L. Layered double hydroxide and microwave assisted functionalized carbon based nanocomposites as controlled release vehicle for antibiotics. J. Drug Deliv. Sci. Technol. 2019, 49, 243–253. [Google Scholar] [CrossRef]
- Park, M.; Lee, C.I.; Lee, E.J.; Choy, J.H.; Kim, J.E.; Choi, J. Layered double hydroxides as potential solid base for beneficial remediation of endosulfan-contaminated soils. J. Phys. Chem. Solids 2004, 65, 513–516. [Google Scholar] [CrossRef]
- Erickson, K.L.; Bostrom, T.E.; Frost, R.L. A study of structural memory effects in synthetic hydrotalcites using environmental SEM. Mater. Lett. 2005, 59, 226–229. [Google Scholar] [CrossRef] [Green Version]
- Rosset, M.; Féris, L.A.; Perez-Lopez, O.W. Biogas dry reforming using Ni–Al-LDH catalysts reconstructed with Mg and Zn. Int. J. Hydrog. Energy 2021, 46, 20359–20376. [Google Scholar] [CrossRef]
- Yang, W.; Kim, Y.; Liu, P.K.; Sahimi, M.; Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem. Eng. Sci. 2002, 57, 2945–2953. [Google Scholar] [CrossRef]
- Lv, Z.; Yang, S.; Zhu, H.; Chen, L.; Alharbi, N.S.; Wakeel, M.; Wahid, A.; Chen, C. Highly efficient removal of As(V) by using NiAl layered double oxide composites. Appl. Surf. Sci. 2018, 448, 599–608. [Google Scholar] [CrossRef]
- Shen, J.C.; Zeng, H.Y.; Chen, C.R.; Xu, S. A facile fabrication of Ag2O-Ag/ZnAl-oxides with enhanced visible-light photocatalytic performance for tetracycline degradation. App. Clay Sci. 2020, 185, 105413. [Google Scholar] [CrossRef]
- Chen, M.; Liu, J.; Bi, Y.; Rehman, S.; Dang, Z.; Wu, P. Multifunctional magnetic MgMn-oxide composite for efficient purification of Cd(2+) and paracetamol pollution: Synergetic effect and stability. J. Hazard. Mater. 2020, 388, 122078. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Huang, Z.; Liu, J.; Li, Y.; Zhu, N.; Dang, Z.; Bi, Y. Environmental application of MgMn-layered double oxide for simultaneous efficient removal of tetracycline and Cd pollution: Performance and mechanism. J. Environ. Manag. 2019, 246, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- He, S.; An, Z.; Wei, M.; Evans, D.G.; Duan, X. Layered double hydroxide-based catalysts: Nanostructure design and catalytic performance. Chem. Commun. 2013, 49, 5912–5920. [Google Scholar] [CrossRef]
- Omwoma, S.; Chen, W.; Tsunashima, R.; Song, Y.F. Recent advances on polyoxometalates intercalated layered double hydroxides: From synthetic approaches to functional material applications. Coord. Chem. Rev. 2014, 258–259, 58–71. [Google Scholar] [CrossRef]
- Guo, X.; Ruan, Y.; Diao, Z.; Shih, K.; Su, M.; Song, G.; Chen, D.; Wang, S.; Kong, L. Environmental-friendly preparation of Ni–Co layered double hydroxide (LDH) hierarchical nanoarrays for efficient removing uranium (VI). J. Clean. Prod. 2021, 308, 127384. [Google Scholar] [CrossRef]
- Cosano, D.; Esquivel, D.; Romero, F.J.; Jiménez-Sanchidrián, C.; Ruiz, J.R. Microwave-assisted synthesis of hybrid organo-layered double hydroxides containing cholate and deoxycholate. Mater. Chem. Phys. 2019, 225, 28–33. [Google Scholar] [CrossRef]
- Barahuie, F.; Hussein, M.Z.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Drug delivery system for an anticancer agent, chlorogenate-Zn/Al-layered double hydroxide nanohybrid synthesised using direct co-precipitation and ion exchange methods. J. Solid State Chem. 2014, 217, 31–41. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, G.; Long, X.; Wang, Y.; Jiao, F. In situ topotactic fabrication of ZnS nanosheet by using ZnAl- layered double hydroxide template for enhanced tetracycline pollutant degradation activity. Mater. Sci. Semicond. Process. 2021, 134, 106007. [Google Scholar] [CrossRef]
- San Román, M.J.; Holgado, M.J.; Jaubertie, C.; Rives, V. Synthesis, characterisation and delamination behaviour of lactate-intercalated Mg,Al-hydrotalcite-like compounds. Solid State Sci. 2008, 10, 1333–1341. [Google Scholar] [CrossRef]
- Okamoto, K.; Iyi, N.; Sasaki, T. Factors affecting the crystal size of the MgAl-LDH (layered double hydroxide) prepared by using ammonia-releasing reagents. Appl. Clay Sci. 2007, 37, 23–31. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, W. Removal of borate by layered double hydroxides prepared through microwave-hydrothermal method. J. Water Process Eng. 2017, 17, 271–276. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xing, B.; Ding, Y.; Han, X.; Wang, S. A critical review of the production and advanced utilization of biochar via selective pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2020, 312, 123614. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L. Non-covalent immobilization of C60 in benzoic acid modified layered double hydroxides. Asian J. Chem. 2013, 25, 4703–4704. [Google Scholar] [CrossRef]
- Lestari, P.R.; Takei, T.; Kumada, N. Novel ZnTi/C3N4/Ag LDH heterojunction composite for efficient photocatalytic phenol degradation. J. Solid State Chem. 2021, 294, 121858. [Google Scholar] [CrossRef]
- Li, M.; Zhu, J.E.; Zhang, L.; Chen, X.; Zhang, H.; Zhang, F.; Xu, S.; Evans, D.G. Facile synthesis of NiAl-layered double hydroxide/graphene hybrid with enhanced electrochemical properties for detection of dopamine. Nanoscale 2011, 3, 4240–4246. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Fang, K.; Zhu, W.; Peng, Q.; Xie, Z. Enhanced nitrate removal by physical activation and Mg/Al layered double hydroxide modified biochar derived from wood waste: Adsorption characteristics and mechanisms. J. Environ. Chem. Eng. 2021, 9, 105184. [Google Scholar] [CrossRef]
- Hu, F.; Wang, M.; Peng, X.; Qiu, F.; Zhang, T.; Dai, H.; Liu, Z.; Cao, Z. High-efficient adsorption of phosphates from water by hierarchical CuAl/biomass carbon fiber layered double hydroxide. Colloids Surf. A 2018, 555, 314–323. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. LDH of NiZnFe and its composites with carbon nanotubes and data-palm biochar with efficient adsorption capacity for RB5 dye from aqueous solutions: Isotherm, kinetic, and thermodynamics studies. Curr. Appl. Phys. 2020, in press. [Google Scholar] [CrossRef]
- Wang, T.; Li, C.; Wang, C.; Wang, H. Biochar/MnAl-LDH composites for Cu (ΙΙ) removal from aqueous solution. Colloids Surf. A 2018, 538, 443–450. [Google Scholar] [CrossRef]
- Baruah, A.; Mondal, S.; Sahoo, L.; Gautam, U.K. Ni-Fe-layered double hydroxide/N-doped graphene oxide nanocomposite for the highly efficient removal of Pb(II) and Cd(II) ions from water. J. Solid State Chem. 2019, 280, 120963. [Google Scholar] [CrossRef]
- Cheng, X.; Deng, J.; Li, X.; Wei, X.; Shao, Y.; Zhao, Y. Layered double hydroxides loaded sludge biochar composite for adsorptive removal of benzotriazole and Pb(II) from aqueous solution. Chemosphere 2021, 287, 131966. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, H.; Sun, Y.; Xiao, T.; Tu, W.; Yuan, X.; Zeng, G.; Li, S.; Chew, J.W. Photogenerated charge transfer via interfacial internal electric field for significantly improved photocatalysis in direct Z-scheme oxygen-doped carbon nitrogen/CoAl-layered double hydroxide heterojunction. Appl. Catal. B 2018, 227, 530–540. [Google Scholar] [CrossRef]
- Ju, L.; Wu, P.; Yang, L. Synthesis of ZnAlTi-LDO supported C60@AgCl nanoparticles and their photocatalytic activity for photo-degradationof bisphenol A. Appl. Catal. B 2018, 224, 159–174. [Google Scholar] [CrossRef]
- Mureseanu, M.; Radu, T.; Andrei, R.D.; Darie, M.; Carja, G. Green synthesis of g-C 3 N 4/CuONP/LDH composites and derived g-C 3 N 4/MMO and their photocatalytic performance for phenol reduction from aqueous solutions. Appl. Clay Sci. 2017, 141, 1–12. [Google Scholar] [CrossRef]
- Jo, W.K.; Tonda, S. Novel CoAl-LDH/g-C3N4/RGO ternary heterojunction with notable 2D/2D/2D configuration for highly efficient visible-light-induced photocatalytic elimination of dye and antibiotic pollutants. J. Hazard. Mater. 2019, 368, 778–787. [Google Scholar] [CrossRef]
- Wang, P.; Ng, D.H.L.; Zhou, M.; Li, J. Freely standing MgAl-layered double hydroxides nanosheets and their derived metal oxides on g-C3N4 thin-layer designed for obtaining synergic effect of adsorption and photocatalysis. Appl. Clay Sci. 2019, 178, 105131. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, D.; Xu, W.; Fang, J.; Sun, J.; Liu, Z.; Chen, Y.; Liang, Y.; Fang, Z. Synergistic adsorption-photocatalytic degradation of different antibiotics in seawater by a porous g-C3N4/calcined-LDH and its application in synthetic mariculture wastewater. J. Hazard. Mater. 2021, 416, 126183. [Google Scholar] [CrossRef]
- Azalok, K.A.; Oladipo, A.A.; Gazi, M. UV-light-induced photocatalytic performance of reusable MnFe-LDO–biochar for tetracycline removal in water. J. Photochem. Photobiol. A 2021, 405, 112976. [Google Scholar] [CrossRef]
- Yang, X.; Gao, Y.; Zhao, Z.; Tian, Y.; Kong, X.; Lei, X.; Zhang, F. Three-dimensional spherical composite of layered double hydroxides/carbon nanotube for ethanol electrocatalysis. Appl. Clay Sci. 2021, 202, 105964. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Huang, J.; Dong, P.; Ji, J.; Li, J.; Cao, L.; Feng, L.; Jin, P.; Wang, C. Decorating CoNi layered double hydroxides nanosheet arrays with fullerene quantum dot anchored on Ni foam for efficient electrocatalytic water splitting and urea electrolysis. Chem. Eng. J. 2020, 390, 124525. [Google Scholar] [CrossRef]
- Dinari, M.; Allami, H.; Momeni, M.M. A high-performance electrode based on Ce-doped nickel-cobalt layered double hydroxide growth on carbon nanotubes for efficient oxygen evolution. J. Electroanal. Chem. 2020, 877, 114643. [Google Scholar] [CrossRef]
- Kumar, O.P.; Ashiq, M.N.; Shah, S.S.A.; Akhtar, S.; Al-Obaidi, M.A.; Mujtaba, I.M.; Rehman, A.U. Nanoscale ZrRGOCuFe layered double hydroxide composites for enhanced photocatalytic degradation of dye contaminant. Mater. Sci. Semicond. Process. 2021, 128, 105748. [Google Scholar] [CrossRef]
- Yekan Motlagh, P.; Khataee, A.; Sadeghi Rad, T.; Hassani, A.; Joo, S.W. Fabrication of ZnFe-layered double hydroxides with graphene oxide for efficient visible light photocatalytic performance. J. Taiwan Inst. Chem. E 2019, 101, 186–203. [Google Scholar] [CrossRef]
- Ou, B.; Wang, J.; Wu, Y.; Zhao, S.; Wang, Z. Efficient removal of Cr (VI) by magnetic and recyclable calcined CoFe-LDH/g-C3N4 via the synergy of adsorption and photocatalysis under visible light. Chem. Eng. J. 2020, 380, 122600. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Fan, Y.; Zhang, S. Layered double hydroxide modified biochar combined with sodium alginate: A powerful biomaterial for enhancing bioreactor performance to remove nitrate. Bioresour. Technol. 2021, 323, 124630. [Google Scholar] [CrossRef]
- Laipan, M.; Zhu, J.; Xu, Y.; Sun, L.; Zhu, R. Fabrication of layered double hydroxide/carbon nanomaterial for heavy metals removal. Appl. Clay Sci. 2020, 199, 105867. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Yu, H.; Yan, L.; Zhang, J.; Wang, B.; Du, B.; Xing, L. Magnetic graphene oxide/MgAl-layered double hydroxide nanocomposite: One-pot solvothermal synthesis, adsorption performance and mechanisms for Pb2+, Cd2+, and Cu2+. Chem. Eng. J. 2018, 341, 1–9. [Google Scholar] [CrossRef]
- Lyu, P.; Wang, G.; Wang, B.; Yin, Q.; Li, Y.; Deng, N. Adsorption and interaction mechanism of uranium (VI) from aqueous solutions on phosphate-impregnation biochar cross-linked Mg–Al layered double-hydroxide composite. Appl. Clay Sci. 2021, 209, 106146. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, B. Biotemplated fabrication of 3D hierarchically porous MgAl-LDH/CF composites with effective adsorption of organic dyes from wastewater. Ind. Eng. Chem. Res. 2020, 59, 16838–16850. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Meenakshi, S. Mechanistic performance of organic pollutants removal from water using Zn/Al layered double hydroxides imprinted carbon composite. Surf. Interfaces 2020, 20, 100581. [Google Scholar] [CrossRef]
- Nayak, S.; Mohapatra, L.; Parida, K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J. Mater. Chem. A 2015, 36, 18622–18635. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Bing, X.; Ng, D.H.L.; Cui, X.; Ji, F.; Kionga, D.D. ZnCr-LDH/N-doped graphitic carbon-incorporated g-C3N4 2D/2D nanosheet heterojunction with enhanced charge transfer for photocatalysis. Mater. Res. Bull. 2018, 102, 379–390. [Google Scholar] [CrossRef]


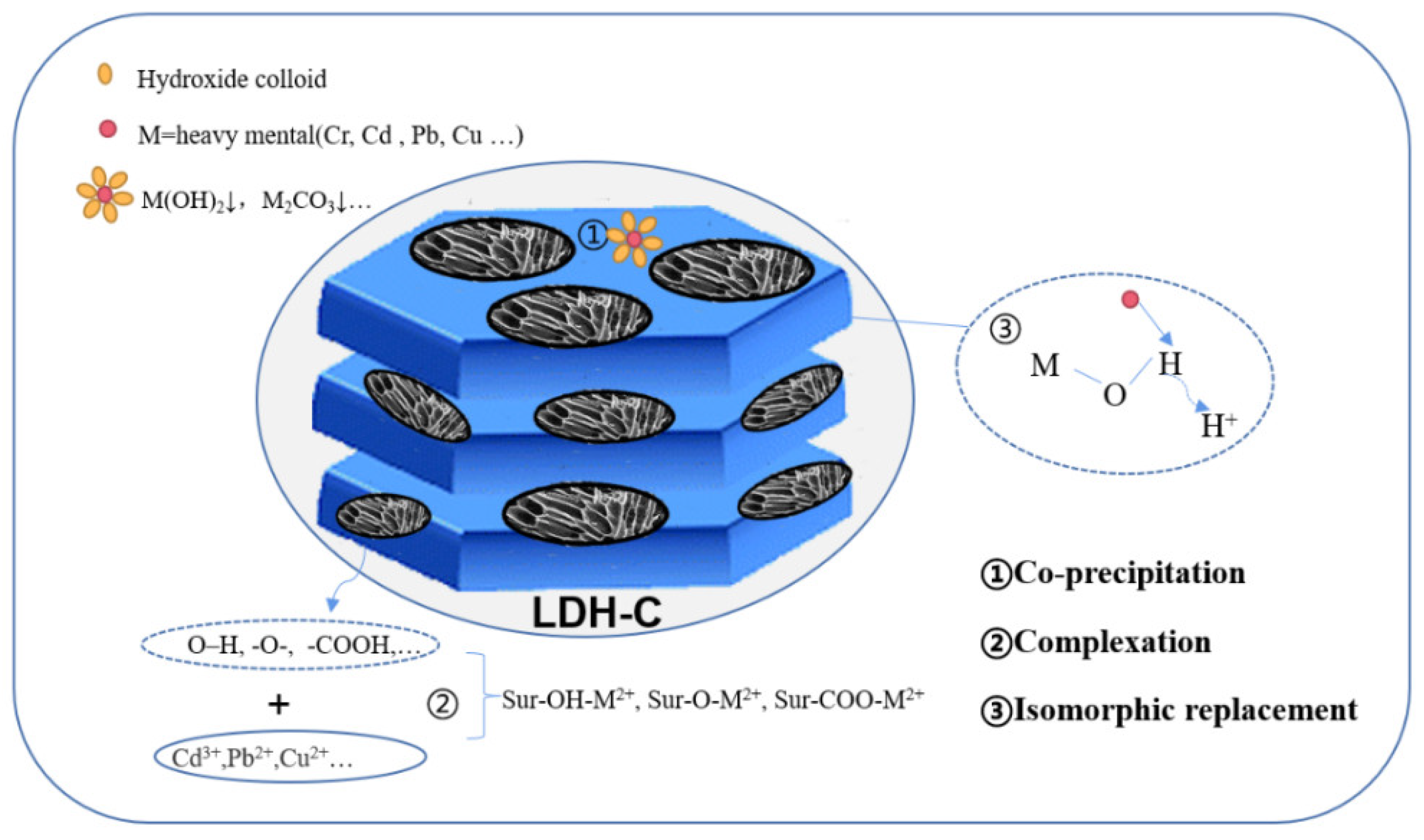
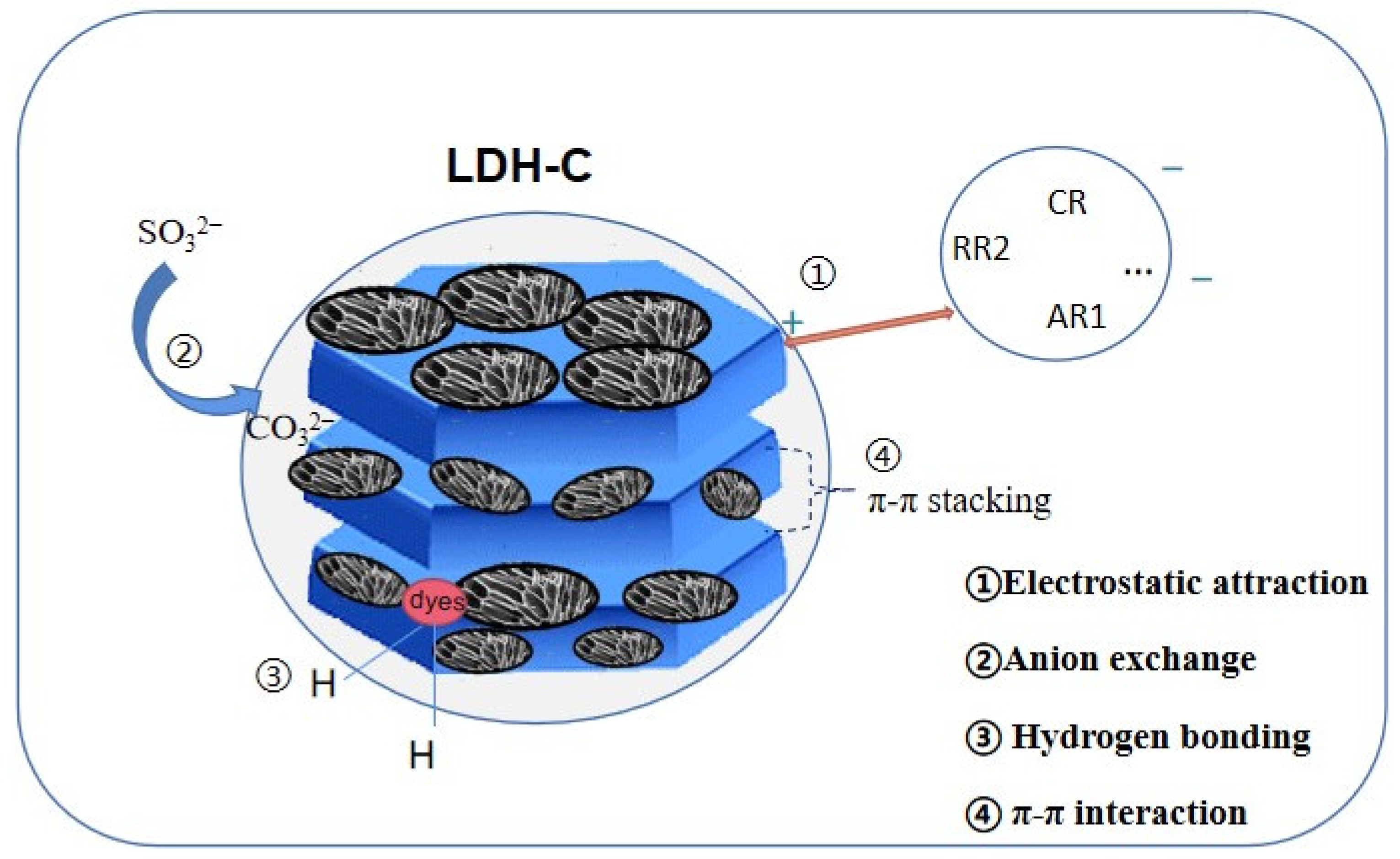


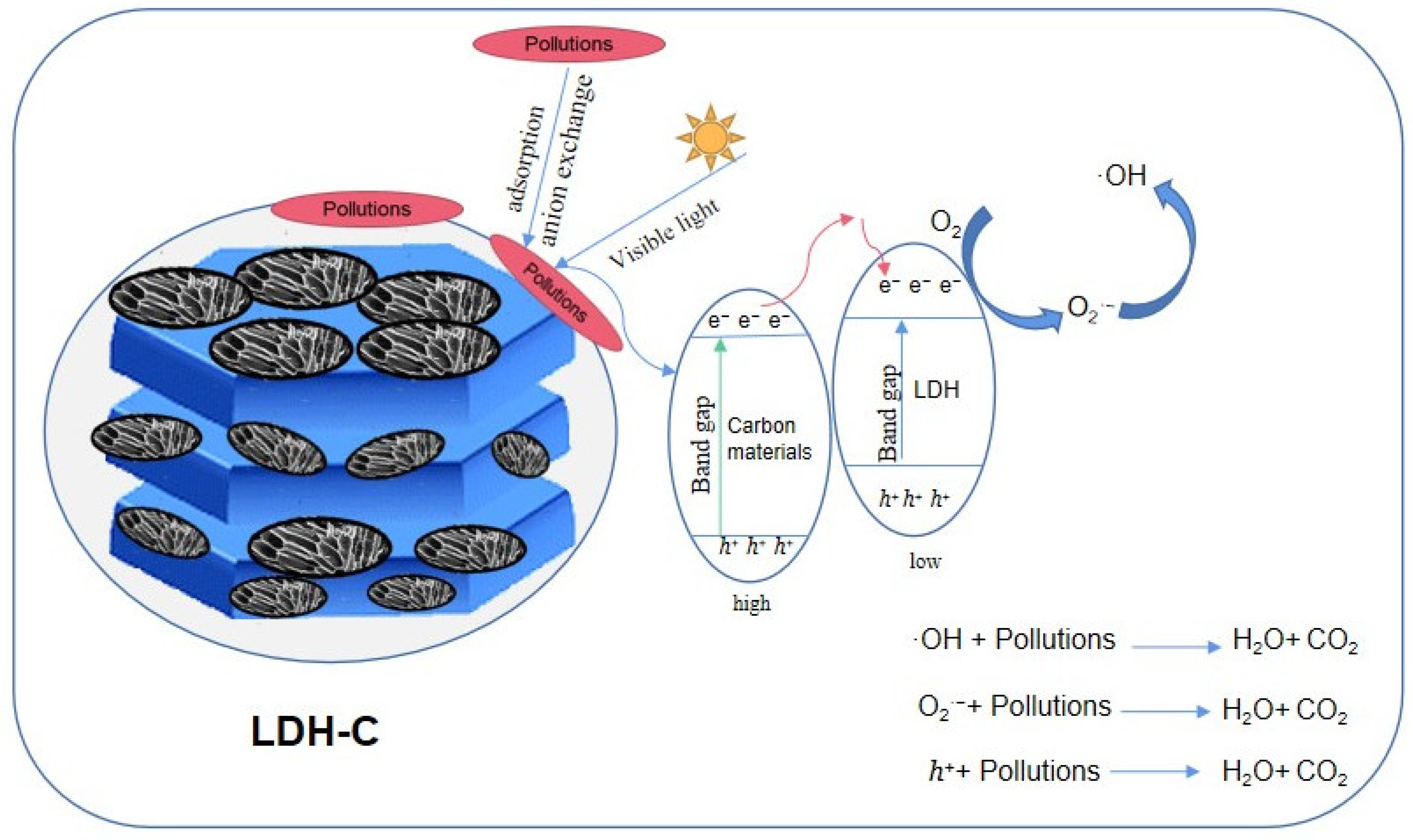
| Applications | Composites | Carbon Material | Synthetic Methods | Pollutants | Efficiency | References |
|---|---|---|---|---|---|---|
| Adsorption | MAC/MgAl LDO | Magnetite- activated carbon | Co-precipitation | Iodide | 86% | [38] |
| CuAl/CF LDH | Carbon fiber | One-pot hydrothermal technology | Phosphates | 100 mg·g−1 | [39] | |
| NiZnFe LDH/BC | Date palm biochar | Co-precipitation | RB5 dye | 90% | [40] | |
| MnAl LDH/BC | Oil tea camellia shells | Hydrothermal synthesis | Cu | 74.07 mg·g−1 | [41] | |
| NiFe CO3- LDH/NGO | NGO | Probe-sonication-mediated process | Cadmium and lead | 971 mg·g−1; 986 mg·g−1 | [42] | |
| ZnAl LDH/BC | Sludge biochar | Co-precipitation | BTA and Pb(II) | 239.6 mg·g−1; 226.1 mg·g−1 | [43] | |
| Photocatalysis | CoAl LDH/OCN | OCN | In situ hydrothermal method | Methyl orange | 0.09568 min−1 | [44] |
| C60@ZnAlTi LDH | C60 | Urea hydrolysis | Bisphenol A | 80% | [45] | |
| ZnAl LDH/g- C3N4/CuONP | g-C3N4 | Co-precipitation | Phenol | 94% | [46] | |
| CoAl LDH/CN/RGO | CN/RGO | One-step hydrothermal method | Congo red and tetracycline | 80%; 90% | [47] | |
| Adsorption and photocatalysis | MgAl CLDH/g-C3N4 | g-C3N4 | Co-precipitation | Congo red | 99.7% | [48] |
| MgZnAl LDH/g-C3N4 | g-C3N4 | Green template method | Tetracycline | 96.95% | [49] | |
| MnFe LDO/BC | Palm-seed-based biochar | Facile co-precipitation followed by calcination | Tetracycline | 98% | [50] | |
| Electrocatalysis | NiFe LDH/CNT | CNT | Urea hydrolysis | Ethanol | 30.5 mA·cm −2 | [51] |
| FQD/CoNi LDH/NF | FQD | Self-assembly process | Water and urea | 10 mA·cm −2 | [52] | |
| NiCoCe LDH/CNT | CNT | Solvothermal self-assembly process | Water | 10 mA·cm −2 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liu, C.; Rad, S.; He, H.; Qin, L. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes 2022, 10, 617. https://doi.org/10.3390/pr10040617
Huang Y, Liu C, Rad S, He H, Qin L. A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes. 2022; 10(4):617. https://doi.org/10.3390/pr10040617
Chicago/Turabian StyleHuang, Yongxiang, Chongmin Liu, Saeed Rad, Huijun He, and Litang Qin. 2022. "A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment" Processes 10, no. 4: 617. https://doi.org/10.3390/pr10040617
APA StyleHuang, Y., Liu, C., Rad, S., He, H., & Qin, L. (2022). A Comprehensive Review of Layered Double Hydroxide-Based Carbon Composites as an Environmental Multifunctional Material for Wastewater Treatment. Processes, 10(4), 617. https://doi.org/10.3390/pr10040617






