Carcass Composition and Physicochemical Characteristics of Meat from Pork Chains Based on Native and Hybrid Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Production Chains, and Slaughter Procedure
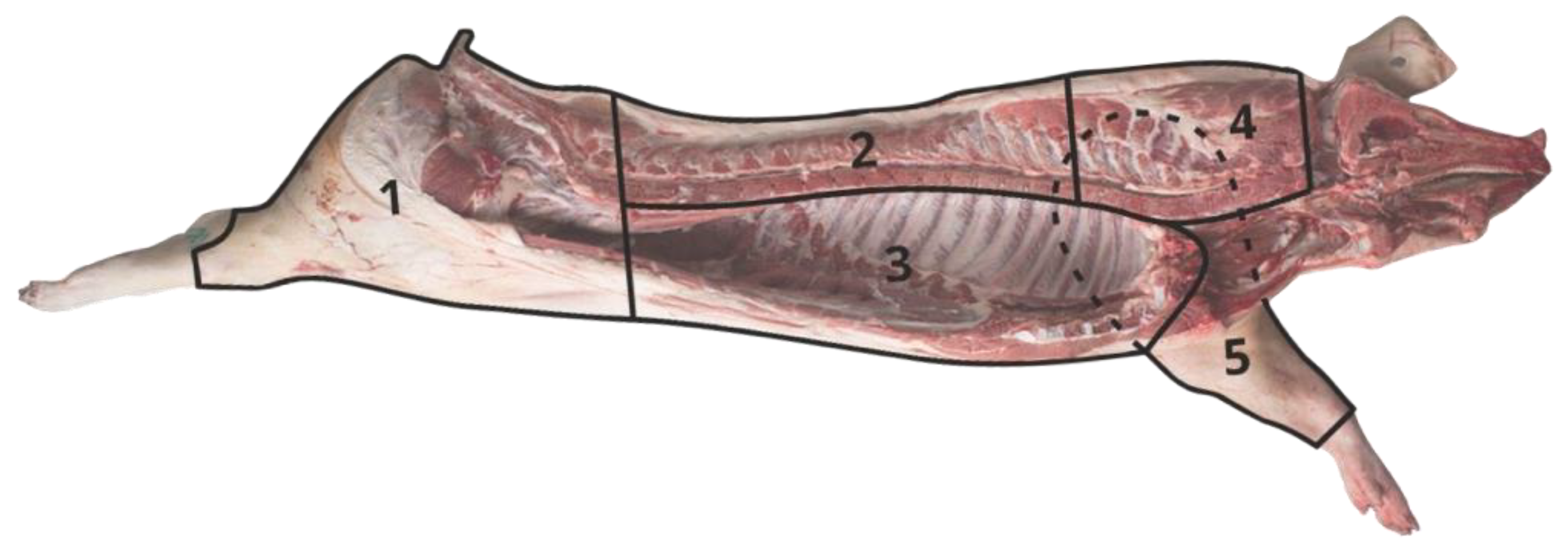
2.2. Carcass Traits
2.2.1. Meat Quality Traits
2.2.2. Chemical Composition and Fatty Acids
2.3. Statistical Analysis
3. Results and Discussion
3.1. Carcass Traits
3.2. Meat Quality
3.3. Chemical Composition and Fatty Acid Profile
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kasprzyk, A.; Bogucka, J. Meat quality of Pulawska breed pigs and image of longissimus lumborum muscle microstructure compared to commercial DanBred and Naima hybrids. Arch. Anim. Breed. 2020, 63, 293–301. [Google Scholar] [CrossRef]
- Pietrosemoli, S.; Tang, C. Animal Welfare and Production Challenges Associated with Pasture Pig Systems: A Review. Agriculture 2020, 10, 223. [Google Scholar] [CrossRef]
- Bonneau, M.; Lebret, B. Production systems and influence on eating quality of pork. Meat Sci. 2010, 84, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Candek-Potokar, M.; Zlender, B.; Lefaucheur, L.; Bonneau, M. Effects of age and/or weight at slaughter on longissimus dorsi muscle: Biochemical traits and sensory quality in pigs. Meat Sci. 1998, 48, 287–300. [Google Scholar] [CrossRef]
- Đurkin, I.; Dadić, M.; Brkić, D.; Lukić, B.; Kušec, G.; Mikolin, M.; Jerković, I. Influence of gender and slaughter weight on meat quality traits of heavy pigs. Acta Agric. Slov. 2012, 100, 3. [Google Scholar]
- Gan, M.; Shen, L.; Chen, L.; Jiang, D.; Jiang, Y.; Li, Q.; Chen, Y.; Ge, G.; Liu, Y.; Xu, X.; et al. Meat Quality, Amino Acid, and Fatty Acid Composition of Liangshan Pigs at Different Weights. Animals 2020, 10, 822. [Google Scholar] [CrossRef] [PubMed]
- Nevrkla, P.; Kapelański, W.; Václavková, E.; Hadaš, Z.; Cebulska, A.; Horký, P. Meat Quality and Fatty Acid Profile of Pork and Backfat from an Indigenous Breed and A Commercial Hybrid of Pigs. Ann. Anim. Sci. 2017, 17, 1215–1227. [Google Scholar] [CrossRef] [Green Version]
- Galian, M.; Poto, Á.; Santaella, M.; Peinado, B. Effects of the rearing system on the quality traits of the carcass, meat and fat of the Chato Murciano pig. Anim. Sci. J. 2008, 79, 487–497. [Google Scholar] [CrossRef]
- Ambrosio, R.L.; Smaldone, G.; Di Paolo, M.; Vollano, L.; Ceruso, M.; Anastasio, A.; Marrone, R. Effects of Different Levels of Inclusion of Apulo-Calabrese Pig Meat on Microbiological, Physicochemical and Rheological Parameters of Salami during Ripening. Animals 2021, 11, 3060. [Google Scholar] [CrossRef]
- Fortin, A.; Robertson, W.M.; Tong, A.K.W. The eating quality of Canadian pork and its relationship with intramuscular fat. Meat Sci. 2005, 69, 297–305. [Google Scholar] [CrossRef]
- Sadurní, J.R. Joint genetic improvement of lean meat content and intramuscular fat in pork. Prod. Anim. 2004, 199, 50–62. [Google Scholar]
- Kasprzyk, A. Characteristics of genetic parameters and genetic gain in breeding herd of PL pigs over 25-year breeding work period. Arch. Tierz. 2007, 50, 107–115. [Google Scholar]
- Nieto, R.; García-Casco, J.; Lara, L.; Palma-Granados, P.; Izquierdo, M.; Hernandez, F.; Dieguez, E.; Luis Duarte, J.; Batorek-Lukač, N. Ibérico (Iberian) Pig. In European Local Pig Breeds—Diversity and Performance. A Study of Project Treasure; Čandek Potokar, M., Nieto, R.M., Eds.; IntechOpen: London, UK, 2019; pp. 115–141. [Google Scholar]
- Wood, J.D.; Richardson, R.I.; Nute, G.R.; Fisher, A.V.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.B.; MacFie, H.J.H.; Smith, W.C.; Chadwick, J.P.; Ellis, M.; Laird, R. Fatty acid composition of backfat in large white pigs selected for low backfat thickness. Meat Sci. 1978, 2, 289–300. [Google Scholar] [CrossRef]
- Margeta, V.; Gvozdanović, K.; Kušec, G.; Djurkin Kušec, I.; Batorek-Lukač, N. Black Slavonian (Crna slavonska) Pig. In European Local Pig Breeds—Diversity and Performance. A Study of Project Treasure; Čandek Potokar, M., Nieto, R.M., Eds.; IntechOpen: London, UK, 2019; pp. 87–101. [Google Scholar]
- Karolyi, D.; Salajpal, K.; Sinjeri, Ž.; Kovačić, D.; Jurić, I.; Đikić, M. Meat quality, blood stress indicators and trimmed cut yield comparison of Black Slavonian pig with modern pigs in the production of Slavonian kulen. Acta Agric. Slov. 2004, 1, 67–72. [Google Scholar]
- Gvozdanović, K.; Margeta, V.; Kušec, I.D.; Margeta, P.; Radišić, Ž.; Kušec, G. Comparison of carcass and meat quality traits of Black Slavonian pigs regarding the duration of fattening period. Arch. Zootec. 2018, 1, 205–208. [Google Scholar]
- FAO. Regulation on Classification and Identification of Beef, Pig and Sheep Carcasses and on Labelling of Meat from Beef Younger Than 12 Months; Pub. L. No. NN 71/2018, LEX-FAOC135783; FAO: Rome, Italy, 2018. [Google Scholar]
- Commission of the European Communities. Development of Uniform Methods of Pig Carcase Classification in EC; Information on Agriculture Series No. 70; CEC: Brussels, Luxembourg, 1979. [Google Scholar]
- Christensen, L.B. Drip loss sampling in porcine m. longissimus dorsi. Meat Sci. 2003, 63, 469–477. [Google Scholar] [CrossRef]
- ISO 1442:1997; Meat and Meat Products—Determination of Moisture Content (Reference Method). ISO: Geneva, Switzerland, 1997.
- ISO 1443:1973; Meat and Meat Products—Determination of Total Fat Content. ISO: Geneva, Switzerland, 1993.
- ISO 937:1978; Meat and Meat Products—Determination of Nitrogen Content (Reference Method). ISO: Geneva, Switzerland, 1978.
- ISO 3100-1:1991; Meat and Meat Products—Sampling and Preparation of Test Samples—Part 1: Sampling. ISO: Geneva, Switzerland, 1991.
- Aldai, N.; Kramer, J.K.G.; Cruz-Hernandez, C.; Santercole, V.; Delmonte, P.; Mossoba, M.; Dugan, M.E.R. Appropriate extraction and methylation techniques for lipids analysis. In Fat and Fatty Acids in Poultry Nutrition and Health; Cherian, G., Poureslami, R., Eds.; Context Products Ltd.: Packington, UK, 2012; pp. 249–278. [Google Scholar]
- Petrović, M.; Kezić, N.; Bolanča, V. Optimization of the GC method for routine analysis of the fatty acid profile in several food samples. Food Chem. 2010, 122, 285–291. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Dell Inc. Dell Statistica (Data Analysis Software System), Version 12; Dell: Round Rock, TX, USA, 2015; Volume 12. [Google Scholar]
- Kassambara, A.; Kassambara, M.A. Package ‘ggpubr’. R Package Version 0.3.5; R Foundation for Statistical Computing: Vienna, Austria, 2012. [Google Scholar]
- Gómez-Rubio, V. ggplot2-elegant graphics for data analysis. J. Stat. Softw. 2017, 77, 1–3. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing Version 4.0.2; R Development Core Team: Vienna, Austria, 2020. [Google Scholar]
- Dai, F.; Feng, D.; Cao, Q.; Ye, H.; Zhang, C.; Xia, W.; Zuo, J. Developmental differences in carcass, meat quality and muscle fibre characteristics between the Landrace and a Chinese native pig. S. Afr. J. Anim. Sci. 2010, 39, 267–273. [Google Scholar] [CrossRef]
- Wojtysiak, D.; Połtowicz, K. Carcass quality, physico-chemical parameters, muscle fibre traits and myosin heavy chain composition of m. longissimus lumborum from Puławska and Polish Large White pigs. Meat Sci. 2014, 97, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Renaudeau, D.; Mourot, J. A comparison of carcass and meat quality characteristics of Creole and Large White pigs slaughtered at 90 kg BW. Meat Sci. 2007, 76, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Poklukar, K.; Čandek-Potokar, M.; Batorek Lukač, N.; Tomažin, U.; Škrlep, M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals 2020, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.M.; Bressan, M.C.; Amaral, A.J.; Bettencourt, C.; Santos-Silva, J.; Moreira, O.; Gama, L.T. Body weight and ultrasound measurements over the finishing period in Iberian and F1 Large White × Landrace pigs raised intensively or in free-range conditions. Livest. Sci. 2019, 229, 170–178. [Google Scholar] [CrossRef]
- Latorre, M.A.; Lázaro, R.; Valencia, D.G.; Medel, P.; Mateos, G.G. The effects of gender and slaughter weight on the growth performance, carcass traits, and meat quality characteristics of heavy pigs. J. Anim. Sci. 2004, 82, 526–533. [Google Scholar] [CrossRef]
- Lebret, B.; Dourmad, J.Y.; Mourot, J.; Pollet, P.Y.; Gondret, F. Production performance, carcass composition, and adipose tissue traits of heavy pigs: Influence of breed and production system. Sci. J. Anim. Sci. 2014, 92, 3543–3556. [Google Scholar] [CrossRef] [Green Version]
- García-Gudiño, J.; Izquierdo, M.; Ayuso, D.; del Rosario, A.I.; Duarte, J.L.; Pérez, M.A.; Hernandez-García, F.I. Effect of pre-slaughter weight and sex on commercial meat cut yields of Iberian pigs. Acta Agric. Slov. 2013, 4, 101–104. [Google Scholar]
- Mayoral, A.I.; Dorado, M.; Guillén, M.T.; Robina, A.; Vivo, J.M.; Vázquez, C.; Ruiz, J. Development of meat and carcass quality characteristics in Iberian pigs reared outdoors. Meat Sci. 1999, 52, 315–324. [Google Scholar] [CrossRef]
- Bertol, T.M.; Oliveira, E.A.; Coldebella, A.; Kawski, V.L.; Scandolera, A.J.; Warpechowski, M.B. Meat quality and cut yield of pigs slaughtered over 100kg live weight. Arq. Bras. Med. Vet. Zootec. 2015, 67, 1166–1174. [Google Scholar] [CrossRef] [Green Version]
- Bressan, M.C.; Almeida, J.; Santos Silva, J.; Bettencourt, C.; Francisco, A.; Gama, L.T. Carcass characteristics and fat depots in Iberian and F1 Large White × Landrace pigs intensively finished or raised outdoors in oak-tree forests. J. Anim. Sci. 2016, 94, 2592–2602. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. The effect of stress on livestock and meat quality prior to and during slaughter. Int. J. Study Anim. Probl. 1980, 1, 313–337. [Google Scholar]
- Lebret, B.; Ecolan, P.; Bonhomme, N.; Méteau, K.; Prunier, A. Influence of production system in local and conventional pig breeds on stress indicators at slaughter, muscle and meat traits and pork eating quality. Animal 2015, 9, 1404–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiorano, G.; Gambacorta, M.; Tavaniello, S.; D’andrea, M.; Stefanon, B.; Pilla, F.; Andrea, M.D. Growth, carcass and meat quality of Casertana, Italian Large White and Duroc x (Landrace x Italian Large White) pigs reared outdoors. Ital. J. Anim. Sci. 2013, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Martins, J.M.; Fialho, R.; Albuquerque, A.; Neves, J.; Freitas, A.; Nunes, J.T.; Charneca, R. Growth, blood, carcass and meat quality traits from local pig breeds and their crosses. Animal 2020, 14, 636–647. [Google Scholar] [CrossRef]
- Sirtori, F.; Crovetti, A.; Zilio, D.M.; Pugliese, C.; Acciaioli, A.; Campodoni, G.; Bozzi, R.; Franci, O. Effect of sire breed and rearing system on growth, carcass composition and meat traits of Cinta Senese crossbred pigs. Ital. J. Anim. Sci. 2011, 10, e47. [Google Scholar] [CrossRef]
- Serra, X.; Gil, F.; Pérez-Enciso, M.; Oliver, M.A.; Vázquez, J.M.; Gispert, M.; Díaz, I.; Moreno, F.; Latorre, R.; Noguera, J.L. A comparison of carcass, meat quality and histochemical characteristics of Iberian (Guadyerbas line) and Landrace pigs. Livest. Prod. Sci. 1998, 56, 215–223. [Google Scholar] [CrossRef]
- Estévez, M.; Morcuende, D.; Cava, R. Oxidative and colour changes in meat from three lines of free-range reared Iberian pigs slaughtered at 90 kg live weight and from industrial pig during refrigerated storage. Meat Sci. 2003, 65, 1139–1146. [Google Scholar] [CrossRef]
- Alfeo, V.; Velotto, S.; de Camillis, S.; Stasi, T.; Todaro, A. Variation in meat quality characteristics between Landrace and Sicilian pig. Ital. J. Food Sci. 2019, 31, 800–807. [Google Scholar]
- Bejerholm, A.C.; Barton-Gade, P. Effect of intramuscular fat level on eating quality of pig meat. In Proceedings of the 30th European Meeting of Meat Research Workers, Ghent, Belgium, 24–29 August 1986; pp. 389–391. [Google Scholar]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of intramuscular fat content on the quality of pig meat—2. Consumer acceptability of m. longissimus lumborum. Meat Sci. 1999, 53, 67–72. [Google Scholar] [CrossRef]
- Kühn, H.; Heydeck, D.; Hugou, I.; Gniwotta, C. In Vivo Action of 15-Lipoxygenase in Early Stages of Human Atherogenesis. J. Clin. Investig. 1997, 99, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Furman, M.; Malovrh, Š.; Levart, A.; Kovač, M. Fatty acid composition of meat and adipose tissue from Krškopolje pigs and commercial fatteners in Slovenia. Arch. Anim. Breed. 2010, 53, 73–84. [Google Scholar] [CrossRef]
- Franco, D.; Carballo, J.; Bermñudez, R.; Lorenzo, J.M. Effect of Genotype and Slaughter Age on Carcass Traits and Meat Quality of the Celta Pig Breed in Extensive System. Ann. Anim. Sci. 2016, 16, 259–273. [Google Scholar] [CrossRef] [Green Version]
- Nevrkla, P.; Václavková, E.; Horký, P.; Kamanová, V.; Hadaš, Z.; Rečková, Z.; Máchal, L. Growth and Meat Quality of Prestice Black-Pied and (Landrace × Large White) × Duroc Pigs. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 66, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez-Escriche, N.; Magallón, E.; Gonzalez, E.; Tejeda, J.F.; Noguera, J.L. Genetic parameters and crossbreeding effects of fat deposition and fatty acid profiles in Iberian pig lines. J. Anim. Sci. 2016, 94, 28–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zhou, L.; Zhang, J.; Liu, X.; Zhang, Y.; Cai, L.; Zhang, W.; Cui, L.; Yang, J.; Ji, J.; et al. A large-scale comparison of meat quality and intramuscular fatty acid composition among three Chinese indigenous pig breeds. Meat Sci. 2020, 168, 108182. [Google Scholar] [CrossRef]
- Connor, W.E. Importance of n−3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, 171S–175S. [Google Scholar] [CrossRef]
- Yu, K.; Shu, G.; Yuan, F.; Zhu, X.; Gao, P.; Wang, S.; Wang, L.; Xi, Q.; Zhang, S.; Zhang, Y.; et al. Fatty Acid and Transcriptome Profiling of Longissimus Dorsi Muscles between Pig Breeds Differing in Meat Quality. Int. J. Biol. Sci. 2013, 9, 108–118. [Google Scholar] [CrossRef]
- Barea, R.; Isabel, B.; Nieto, R.; López-Bote, C.; Aguilera, J.F. Evolution of the fatty acid profile of subcutaneous back-fat adipose tissue in growing Iberian and Landrace × Large White pigs. Animal 2013, 7, 688–698. [Google Scholar] [CrossRef] [Green Version]
- Hallenstvedt, E.; Øverland, M.; Rehnberg, A.; Kjos, N.P.; Thomassen, M. Sensory quality of short- and long-term frozen stored pork products. Influence of diets varying in polyunsaturated fatty acid (PUFA) content and iodine value. Meat Sci. 2012, 90, 244–251. [Google Scholar] [CrossRef]
- Rubio, B.; Martínez, B.; García-Cachán, M.D.; Rovira, J.; Jaime, I. Effect of the packaging method and the storage time on lipid oxidation and colour stability on dry fermented sausage salchichón manufactured with raw material with a high level of mono and polyunsaturated fatty acids. Meat Sci. 2008, 80, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Simonetti, A.; Intaglietta, I.; Gambacorta, E. Fatty acids composition, cholesterol and vitamin E contents of Longissimus dorsi and Semitendinosus muscles of Suino Nero Lucano pigs slaughtered at two different weights. Anim. Prod. Sci. 2015, 55, 1037–1043. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Zhu, L.; Li, X.W.; Si, T. Evaluation of the Chinese indigenous pig breed Dahe and crossbred Dawu for growth and carcass characteristics, organ weight, meat quality and intramuscular fatty acid and amino acid composition. Animal 2011, 5, 1485–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Composition | Finisher-BS | Finisher-PIC |
|---|---|---|
| Dry matter, % | - | 88.340 |
| Crude protein, % | 13.230 | 13.008 |
| ME, MJ/kg | 12.91 | 12.81 |
| Crude fibre, % | 5.840 | 5.999 |
| Crude fat, % | 2.840 | 3.224 |
| Ash, % | 4.360 | 4.406 |
| Methionine, % | - | 0.265 |
| Methionine + cysteine, % | 0.410 | 0.476 |
| Lysine, % | 0.710 | 0.696 |
| Threonine, % | - | 0.452 |
| Thryptofane, % | - | 0.1498 |
| Ca, % | 0.740 | 0.7458 |
| Phosphate, % | - | 0.365 |
| Moisture, % | - | 11.312 |
| Vitamin K, mg/kg | - | 4877.530 |
| Vitamin A, IJ/kg | 5200.000 | 6400.401 |
| Vitamin D3, IJ/kg | 960.000 | 960.000 |
| Vitamin E, mg/kg | - | 40.000 |
| Phytase | 400.000 | 400.000 |
| Neutral detergent fibre, % | - | 14.677 |
| Acid detergent fibre, % | - | 9.500 |
| Trait | NAT Pork Chain | INT Pork Chain | F Value | Significance (p) |
|---|---|---|---|---|
| Hot half-carcass weight, kg | 69.81 ± 3.81 | 69.51 ± 1.62 | 0.116 | 0.7382 |
| Dressing percentage, % | 86.29 ± 1.56 | 85.48 ± 1.13 | 1.380 | 0.2612 |
| TP fat thickness, mm | 60.63 ± 6.72 | 28.13 ± 4.22 | 1.615 | 0.0001 |
| TP muscle thickness, mm | 66.25 ± 8.61 | 81.00 ± 7.80 | 17.067 | 0.0031 |
| Carcass length, cm | 97.63 ± 2.83 | 101.88 ± 3.83 | 4.470 | 0.0244 |
| Ham length, cm | 34.38 ± 2.72 | 37.75 ± 1.75 | 10.332 | 0.0107 |
| Ham circumference, cm | 78.00 ± 2.88 | 84.38 ± 2.33 | 29.600 | 0.0004 |
| Ham weight, kg | 15.99 ± 1.24 | 17.28 ± 1.03 | 5.121 | 0.0401 |
| Ham in the carcass, % | 22.94 ± 1.95 | 24.92 ± 1.29 | 5.773 | 0.0308 |
| Loin weight, kg | 12.70 ± 1.26 | 11.38 ± 0.48 | 7.743 | 0.0148 |
| Loin in the carcass, % | 18.18 ± 1.29 | 16.42 ± 0.58 | 12.456 | 0.0034 |
| Shoulder weight, kg | 10.68 ± 1.07 | 11.60 ± 1.06 | 3.036 | 0.1034 |
| Shoulder in the carcass, % | 15.34 ± 1.88 | 16.74 ± 1.51 | 2.665 | 0.1250 |
| Belly/rib weight, kg | 14.69 ± 1.46 | 13.20 ± 1.10 | 5.344 | 0.0366 |
| Belly/rib in the carcass, % | 21.02 ± 1.38 | 19.04 ± 1.42 | 8.083 | 0.0131 |
| Neck weight, kg | 5.49 ± 0.91 | 5.51 ± 0.51 | 0.004 | 0.9520 |
| Neck in the carcass, % | 7.88 ± 1.42 | 7.94 ± 0.67 | 0.013 | 0.9109 |
| Tenderloin weight, kg | 0.40 ± 0.04 | 0.75 ± 0.11 | 68.092 | 0.0001 |
| Tenderloin in the carcass, % | 0.57 ± 0.07 | 1.08 ± 0.16 | 69.702 | 0.0001 |
| Cut | Tissue | NAT Pork Chain | INT Pork Chain | F Value | Significance (p) | ||
|---|---|---|---|---|---|---|---|
| Weight, kg | Share, % | Weight, kg | Share, % | ||||
| Ham | Muscle | 6.77 ± 0.48 | 42.44 ± 3.11 | 10.61 ± 0.76 | 61.42 ± 1.97 | 145.744 | 0.0001 |
| Fat | 7.85 ± 0.95 | 48.97 ± 3.32 | 4.67 ± 0.49 | 27.05 ± 2.82 | 70.114 | 0.0001 | |
| Bones | 1.37 ± 0.16 | 8.58 ± 0.78 | 1.99 ± 0.24 | 11.53 ± 1.09 | 36.543 | 0.0001 | |
| Loin | Muscle | 3.05 ± 0.45 | 24.06 ± 2.78 | 5.50 ± 0.55 | 48.35 ± 3.96 | 96.252 | 0.0001 |
| Fat | 8.42 ± 1.04 | 66.18 ± 3.86 | 4.21 ± 0.48 | 37.01 ± 4.34 | 107.214 | 0.0001 | |
| Bones | 1.24 ± 0.16 | 9.77 ± 1.32 | 1.67 ± 0.19 | 14.64 ± 1.34 | 23.554 | 0.0004 | |
| Shoulder | Muscle | 4.99 ± 1.03 | 46.43 ± 5.86 | 6.45 ± 0.67 | 55.59 ± 3.06 | 11.257 | 0.0048 |
| Fat | 4.78 ± 0.56 | 45.08 ± 5.94 | 3.92 ± 0.52 | 33.76 ± 2.58 | 10.121 | 0.0068 | |
| Bones | 0.90 ± 0.10 | 8.49 ± 0.83 | 1.23 ± 0.10 | 10.65 ± 1.02 | 41.786 | 0.0001 | |
| Belly/rib | Muscle | 3.97 ± 0.59 | 26.98 ± 2.76 | 6.15 ± 0.8 | 46.59 ± 4.42 | 37.432 | 0.0001 |
| Fat | 9.80 ± 1.09 | 66.66 ± 2.65 | 5.66 ± 0.62 | 42.96 ± 4.25 | 86.938 | 0.0001 | |
| Bones | 0.93 ± 0.10 | 6.36 ± 0.84 | 1.39 ± 0.37 | 10.45 ± 2.27 | 11.484 | 0.0045 | |
| Neck | Muscle | 2.21 ± 0.39 | 40.44 ± 3.78 | 3.09 ± 0.38 | 56.03 ± 3.26 | 20.853 | 0.0005 |
| Fat | 2.58 ± 0.52 | 46.82 ± 2.73 | 1.51 ± 0.23 | 27.34 ± 3.43 | 28.210 | 0.0002 | |
| Bones | 0.69 ± 0.15 | 12.75 ± 2.19 | 0.91 ± 0.11 | 16.63 ± 1.92 | 10.823 | 0.0055 | |
| Total in cuts | Muscle | 21.38 ± 1.53 | 35.68 ± 2.11 | 32.56 ± 2.03 | 54.51 ± 2.00 | 334.414 | 0.0001 |
| Fat | 33.42 ± 2.41 | 55.74 ± 2.48 | 19.96 ± 1.48 | 33.45 ± 2.45 | 327.792 | 0.0001 | |
| Bones | 5.13 ± 0.47 | 8.58 ± 0.83 | 7.19 ± 0.57 | 12.04 ± 0.74 | 77.405 | 0.0001 | |
| Trait | NAT Pork Chain | INT Pork Chain | F Value | Significance (p) |
|---|---|---|---|---|
| pH45 | 6.44 ± 0.11 | 6.08 ± 0.19 | 22.010 | 0.0005 |
| pH24 | 5.92 ± 0.39 | 5.45 ± 0.05 | 11.670 | 0.0043 |
| EZ drip, % | 2.40 ± 2.44 | 7.46 ± 3.18 | 12.719 | 0.0032 |
| L* | 45.94 ± 4.11 | 56.29 ± 3.78 | 27.479 | 0.0003 |
| a* | 10.46 ± 1.41 | 11.23 ± 2.68 | 0.519 | 0.4834 |
| b* | 2.18 ± 1.22 | 3.85 ± 2.08 | 3.827 | 0.0708 |
| c* | 10.72 ± 1.55 | 11.93 ± 3.13 | 0.956 | 0.3450 |
| h° | 11.23 ± 5.76 | 17.84 ± 6.63 | 4.528 | 0.0517 |
| Trait | NAT Pork Chain | INT Pork Chain | F Value | Significance (p) |
|---|---|---|---|---|
| Collagen, % | 1.11 ± 0.03 | 1.30 ± 0.06 | 51.983 | 0.0002 |
| Moisture, % | 72.11 ± 2.27 | 73.27 ± 2.45 | 0.730 | 0.4129 |
| Protein, % | 21.75 ± 0.94 | 24.57 ± 0.98 | 25.885 | 0.0006 |
| IMF, % | 5.16 ± 3.34 | 1.60 ± 0.78 | 6.467 | 0.0294 |
| Fatty Acid, % of Total Fatty Acids | Pork Chain | F Value | Significance (p) | |
|---|---|---|---|---|
| NAT | INT | |||
| C10:0 | 0.089 ± 0.04 | 0.000 ± 0.00 | Inf. | 0.0036 |
| C12:0 | 0.077 ± 0.03 | 0.000 ± 0.00 | Inf. | 0.0002 |
| C14:0 | 1.459 ± 0.04 | 1.430 ± 0.02 | 0.003 | 0.5894 |
| C14:1 | 0.016 ± 0.01 | 0.000 ± 0.00 | 8.100 | 0.3770 |
| C15:0 | 0.014 ± 0.10 | 0.260 ± 0.10 | 0.986 | 0.0001 |
| C16:0 | 26.534 ± 0.90 | 28.690 ± 0.10 | 0.693 | 0.0009 |
| C16:1 n-7 | 4.353 ± 0.69 | 2.710 ± 0.59 | 1.251 | 0.0013 |
| C17:0 | 0.131 ± 0.10 | 0.370 ± 0.08 | 3.500 | 0.0005 |
| C17:1 | 0.155 ± 0.03 | 0.210 ± 0.03 | 3.300 | 0.0538 |
| C18:0 | 11.891 ± 1.78 | 16.120 ± 1.90 | 18.902 | 0.0013 |
| C18:1 n-9 | 48.410 ± 3.68 | 40.410 ± 2.90 | 16.664 | 0.0185 |
| C18:2 n-6 | 5.100 ± 1.34 | 7.730 ± 1.25 | 21.516 | 0.0560 |
| C18:3 n-3 | 0.108 ± 0.05 | 0.000 ± 0.00 | Inf. | 0.0041 |
| C19:0 | 0.010 ± 0.01 | 0.000 ± 0.00 | Inf. | 0.4011 |
| C20:0 | 0.240 ± 0.04 | 0.330 ± 0.03 | 5.000 | 0.0208 |
| C20:4 n-6 | 1.191 ± 0.50 | 0.340 ± 0.44 | 1.645 | 0.1259 |
| C22:0 | 0.064 ± 0.18 | 0.490 ± 0.20 | 1.569 | 0.0001 |
| C22:1 n-9 | 0.025 ± 0.10 | 0.260 ± 0.08 | 0.986 | 0.0001 |
| C22:2 n-6 | 0.015 ± 0.01 | 0.000 ± 0.01 | Inf. | 0.3809 |
| C24:0 | 0.059 ± 0.03 | 0.000 ± 0.02 | Inf. | 0.1189 |
| C24:1 n-9 | 0.057 ± 0.03 | 0.000 ± 0.02 | Inf. | 0.0420 |
| ∑ MUFA, % | 53.017 ± 1.826 | 43.867 ± 0.514 | 67.932 | 0.0001 |
| ∑ PUFA, % | 6.4140 ± 1.297 | 8.280 ± 0.187 | 5.754 | 0.0533 |
| ∑ SFA, % | 40.569 ± 0.690 | 46.856 ± 0.370 | 187.588 | 0.0001 |
| ∑ n-6, % | 6.306 ± 1.285 | 8.280 ± 0.187 | 6.5708 | 0.0423 |
| ∑ n-3, % | 0.108 ± 0.107 | 0000 ± 0.00 | 117.946 | 0.0001 |
| PUFA/SFA | 0.158 ± 0.031 | 0.177 ± 0.007 | 1.0568 | 0.3430 |
| IA | 1.260 ± 0.312 | 1.189 ± 0.024 | 0.1424 | 0.7189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kušec, G.; Komlenić, M.; Gvozdanović, K.; Sili, V.; Krvavica, M.; Radišić, Ž.; Kušec, I.D. Carcass Composition and Physicochemical Characteristics of Meat from Pork Chains Based on Native and Hybrid Pigs. Processes 2022, 10, 370. https://doi.org/10.3390/pr10020370
Kušec G, Komlenić M, Gvozdanović K, Sili V, Krvavica M, Radišić Ž, Kušec ID. Carcass Composition and Physicochemical Characteristics of Meat from Pork Chains Based on Native and Hybrid Pigs. Processes. 2022; 10(2):370. https://doi.org/10.3390/pr10020370
Chicago/Turabian StyleKušec, Goran, Miodrag Komlenić, Kristina Gvozdanović, Velimir Sili, Marina Krvavica, Žarko Radišić, and Ivona Djurkin Kušec. 2022. "Carcass Composition and Physicochemical Characteristics of Meat from Pork Chains Based on Native and Hybrid Pigs" Processes 10, no. 2: 370. https://doi.org/10.3390/pr10020370
APA StyleKušec, G., Komlenić, M., Gvozdanović, K., Sili, V., Krvavica, M., Radišić, Ž., & Kušec, I. D. (2022). Carcass Composition and Physicochemical Characteristics of Meat from Pork Chains Based on Native and Hybrid Pigs. Processes, 10(2), 370. https://doi.org/10.3390/pr10020370








