Effects of Some Weak Acids and Moringa oleifera Leaf Extract Powder on the Colour of Dried Apple
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Materials and Equipment
2.2. Experimental Design for the Drying of the Apples
2.3. Weight Loss
2.4. Colour Measurement
2.5. Profiling of the Moringa oleifera Extract Powder (MOLEP)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Model Adequacy
3.2. Effect of the Ascorbic Acid, Citric Acid, Potassium Sorbate, Moringa oleifera Leaf Extract Powder, Time, and Temperature on the Weight Loss of the Dried Apple Slices
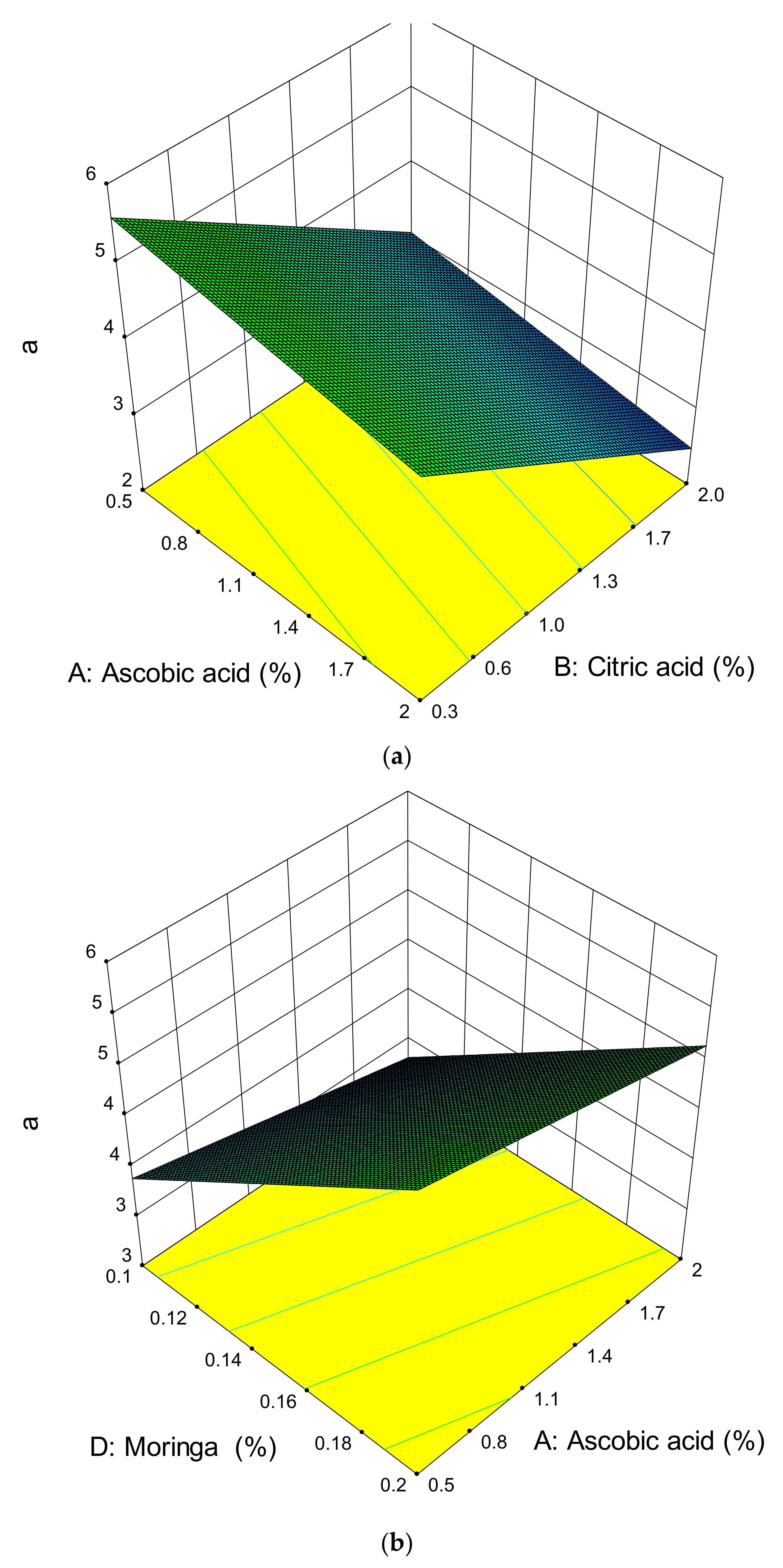
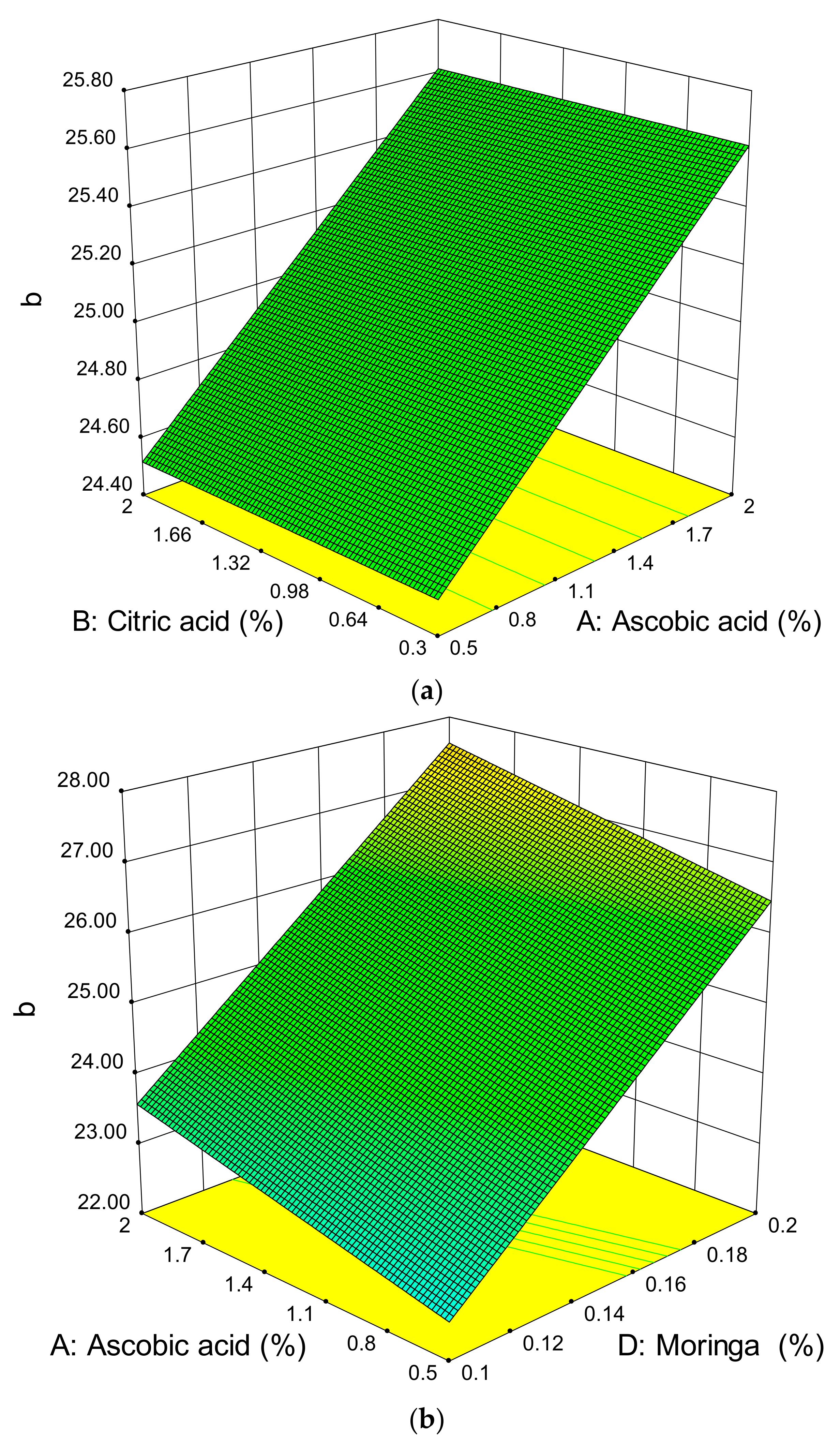
3.3. Effect of the Ascorbic Acid, Citric Acid, Potassium Sorbate, Moringa oleifera Leaf Extract Powder, Time, and Temperature on the Colour of Dried Apples
3.4. Optimum Combination of Some Weak Acids (Ascorbic Acid, Citric Acid and Potassium Sorbate), Moringa oleifera Leaf Extract Powder, Temperature, and Drying Time for the Apple Slices
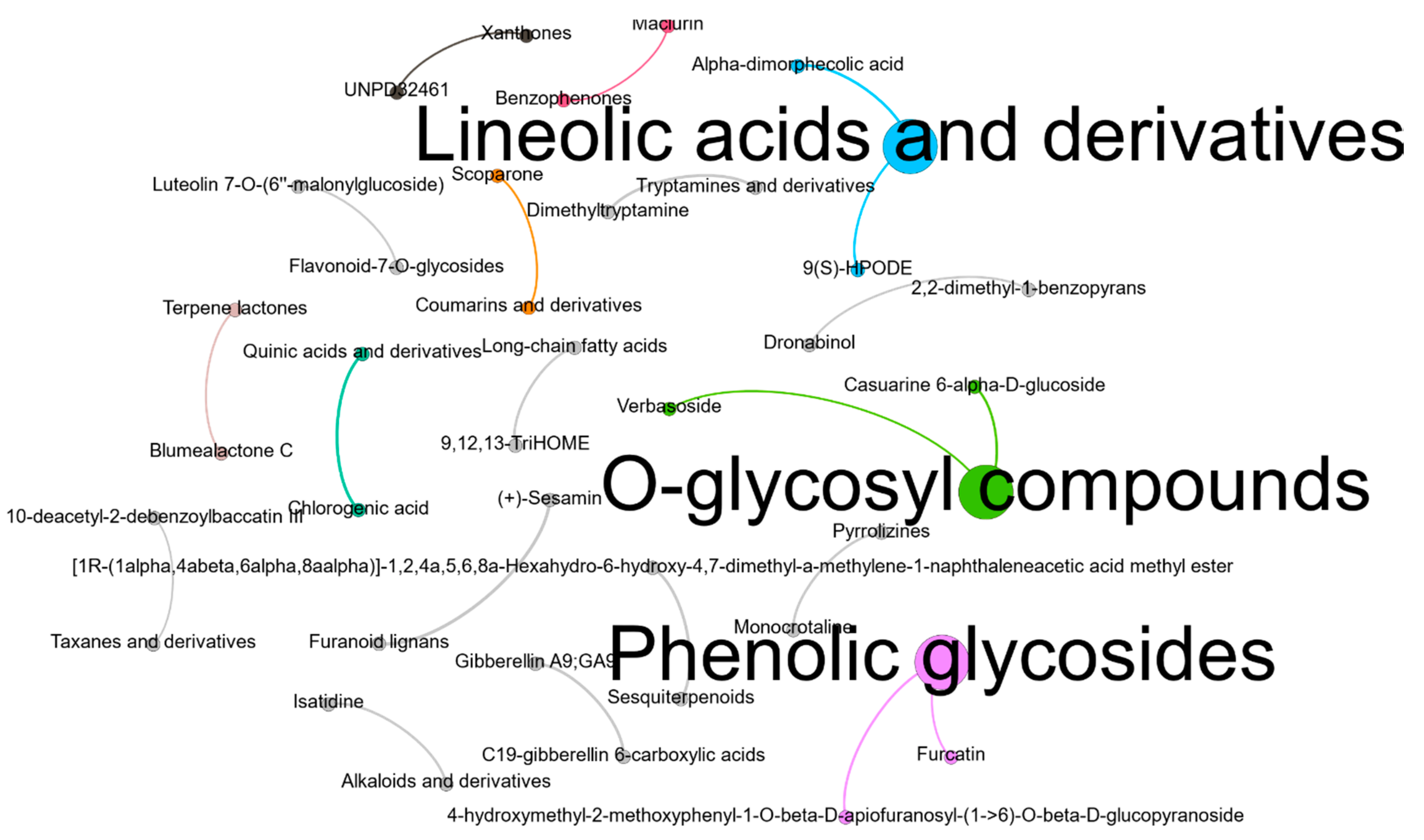
3.5. Phytochemical Constituents of the Moringa oleifera Leaf Extract Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Golisz, E.; Jaros, M.; Kalicka, M. Analysis of Convectional Drying Process of Peach. Tech. Sci. 2013, 16, 333–343. [Google Scholar]
- Denoya, G.I.; Polenta, G.A.; Apóstolo, N.M.; Budde, C.O.; Sancho, A.M.; Vaudagna, S.R. Optimization of high hydrostatic pressure processing for the preservation of minimally processed peach pieces. Innov. Food Sci. Emerg. Technol. 2015, 33, 84–93. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M.; Cortellino, G.; Spinelli, L.; Contini, D.; Herremans, E.; Bongaers, E.; Nemeth, A.; Leitner, M.; Verboven, P.; et al. Characterizing the tissue of apple air-dried and osmo-air-dried rings by X-CT and OCT and relationship with ring crispness and fruit maturity at harvest measured by TRS. Innov. Food Sci. Emerg. Technol. 2014, 24, 121–130. [Google Scholar] [CrossRef]
- Campbell, O.E.; Padilla-Zakour, O.I. Phenolic and carotenoid composition of canned peaches (Prunus persica) and apricots (Prunus armeniaca) as affected by variety and peeling. Food Res. Int. 2013, 54, 448–455. [Google Scholar] [CrossRef]
- Rizzolo, A.; Vanoli, M.; Cortellino, G.; Spinelli, L.; Torricelli, A. Quality characteristics of air-dried apple rings: Influence of storage time and fruit maturity measured by time-resolved reflectance spectroscopy. Procedia Food Sci. 2011, 1, 216–223. [Google Scholar] [CrossRef][Green Version]
- Lewicki, P.P.; Jakubczyk, E. Effect of hot air temperature on mechanical properties of dried apples. J. Food Eng. 2004, 64, 307–314. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Effect of natural antibrowning agents on color and related enzymes in fresh-cut fuji apples as an alternative to the use of ascorbic acid. J. Food Sci. 2008, 73, 267–272. [Google Scholar] [CrossRef]
- Brewer, S. Effects of Oxidation on Sensory Characteristics of Food Components during Processing and Storage. In Food Oxidants Antioxidants; CRC Press: Boca Raton, FL, USA, 2013; pp. 159–195. [Google Scholar]
- Huque, R.; Wills, R.B.H.; Pristijono, P.; Golding, J.B. Effect of nitric oxide (NO) and associated control treatments on the metabolism of fresh-cut apple slices in relation to development of surface browning. Postharvest Biol. Technol. 2013, 78, 16–23. [Google Scholar] [CrossRef]
- Lunadei, L.; Galleguillos, P.; Diezma, B.; Lleó, L.; Ruiz-Garcia, L. A multispectral vision system to evaluate enzymatic browning in fresh-cut apple slices. Postharvest Biol. Technol. 2011, 60, 225–234. [Google Scholar] [CrossRef]
- Verma, A.R.; Vijayakumar, M.; Mathela, C.S.; Rao, C.V. In vitro and in vivo antioxidant properties of different fractions of Moringa oleifera leaves. Food Chem. Toxicol. 2009, 47, 2196–2201. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Gritsanapan, W. Bioactive contents and free radical scavenging activity of Moringa oleifera leaf extract under different storage conditions. Ind. Crops Prod. 2013, 49, 419–421. [Google Scholar] [CrossRef]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Dahot, M.U. Antimicrobial activity of small protein of Moringa oleifera leaves. J. Islam. Acad. Sci. 1998, 11, 27–32. [Google Scholar]
- Mendonca, A.F. Mechanism of inhibitory action of potassium sorbate in Escherichia coli. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1992. [Google Scholar]
- Excalibur: The Complete Guide to Food Dehydration, 4th ed.; Prentince Hall Trade: Hoboken, NJ, USA, 2012; pp. 9–41, [Internet document]; Available online: https://www.amazon.com/Preserve-Naturally-Dehydration-Excalibur-1-Feb-1984/dp/B012HTWZTK (accessed on 18 August 2020).
- Deng, Y.; Zhao, Y. Effect of pulsed vacuum and ultrasound osmopretreatments on glass transition temperature, texture, microstructure and calcium penetration of dried apples (Fuji). LWT-Food Sci. Technol. 2008, 41, 1575–1585. [Google Scholar] [CrossRef]
- Cárcel, J.A.; García-Pérez, J.V.; Sanjuán, N.; Mulet, A. Influence of pre-treatment and storage temperature on the evolution of the colour of dried persimmon. LWT-Food Sci. Technol. 2010, 43, 1191–1196. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Xu, B.; Chen, K.; Yang, Q.; Zhang, Q. Burdock fructooligosaccharide enhances biocontrol of Rhodotorula mucilaginosa to postharvest decay of peaches. Carbohydr. Polym. 2013, 98, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, N.C.; Briones, V.; Buera, P.; Aguilera, J.M. Microstructure affects the rate of chemical, physical and color changes during storage of dried apple discs. J. Food Eng. 2008, 85, 222–231. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, M.; Adhikari, B.; Yang, Z. Effect of microwave air spouted drying arranged in two and three-stages on the drying uniformity and quality of dehydrated carrot cubes. J. Food Eng. 2015, 177, 80–89. [Google Scholar] [CrossRef]
- Kutyła-Olesiuk, A.; Nowacka, M.; Wesoły, M.; Ciosek, P. Evaluation of organoleptic and texture properties of dried apples by hybrid electronic tongue. Sens. Actuators B Chem. 2013, 187, 234–240. [Google Scholar] [CrossRef]
- Orikasa, T.; Koide, S.; Okamoto, S.; Imaizumi, T.; Muramatsu, Y.; Takeda, J.I.; Shiina, T.; Tagawa, A. Impacts of hot air and vacuum drying on the quality attributes of kiwifruit slices. J. Food Eng. 2014, 125, 51–58. [Google Scholar] [CrossRef]
- Limbo, S.; Piergiovanni, L. Shelf life of minimally processed potatoes: Part 1. Effects of high oxygen partial pressures in combination with ascorbic and citric acids on enzymatic browning. Postharvest Biol. Technol. 2006, 39, 254–264. [Google Scholar] [CrossRef]
- Goyeneche, R.; Agüero, M.V.; Roura, S.; Di Scala, K. Application of citric acid and mild heat shock to minimally processed sliced radish: Color evaluation. Postharvest Biol. Technol. 2014, 93, 106–113. [Google Scholar] [CrossRef]
- Ioannou, I.; Ghoul, M. Prevention of Enzymatic Browning in Fruit and Vegetables. Eur. Sci. J. 2013, 9, 1857–7881. [Google Scholar]
- Barbagallo, R.N.; Chisari, M.; Patanè, C. Use in vivo of natural anti-browning agents against polyphenol oxidase activity in minimally processed eggplant. Chem. Eng. Trans. 2012, 27, 49–54. [Google Scholar] [CrossRef]
- Abd-Elhady, M. Effect of citric acid, calcium lactate and low temperature prefreezing treatment on the quality of frozen strawberry. Ann. Agric. Sci. 2014, 59, 69–75. [Google Scholar] [CrossRef]
- Rocculi, P.; Galindo, F.G.; Mendoza, F.; Wadsö, L.; Romani, S.; Rosa, M.D.; Sjöholm, I. Effects of the application of anti-browning substances on the metabolic activity and sugar composition of fresh-cut potatoes. Postharvest Biol. Technol. 2007, 43, 151–157. [Google Scholar] [CrossRef]
- Javdani, Z.; Ghasemnezhad, M.; Zare, S. A comparison of heat treatment and ascorbic acid on controlling enzymatic browning of fresh-cuts apple fruit. Int. J. Agric. Crop Sci. 2013, 5, 186–193. [Google Scholar]
- Koushesh Saba, M.; Sogvar, O.B. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. LWT Food Sci. Technol. 2016, 66, 165–171. [Google Scholar] [CrossRef]
- Li, Y.; Wills, R.B.H.; Golding, J.B. Sodium chloride, a cost effective partial replacement of calcium ascorbate and ascorbic acid to inhibit surface browning on fresh-cut apple slices. LWT-Food Sci. Technol. 2015, 64, 503–507. [Google Scholar] [CrossRef]
- Chow, Y.N.; Louarme, L.; Bonazzi, C.; Nicolas, J.; Billaud, C. Apple polyphenoloxidase inactivation during heating in the presence of ascorbic acid and chlorogenic acid. Food Chem. 2011, 129, 761–767. [Google Scholar] [CrossRef]
- Salaha, M.I.; Kallithraka, S.; Marmaras, I.; Koussissi, E.; Tzourou, I. A natural alternative to sulphur dioxide for red wine production: Influence on colour, antioxidant activity and anthocyanin content. J. Food Compos. Anal. 2008, 21, 660–666. [Google Scholar] [CrossRef]
- Siddiq, A.; Anwar, F.; Manzoor, M.; Fatima, A. Antioxidant Activity of Different Solvent Extracts of Moringa oleifera Leaves under Accelerated Storage of Sunflower Oil. Asian J. Plant Sci. 2005, 4, 630–635. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Verma, A.K.; Swarup, D. Moringa oleifera leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int. J. Food Sci. Technol. 2012, 47, 585–591. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Biela, M.; Rimarčík, J.; Senajová, E.; Kleinová, A.; Klein, E. Antioxidant action of deprotonated flavonoids: Thermodynamics of sequential proton-loss electron-transfer. Phytochemistry 2020, 180, 112528. [Google Scholar] [CrossRef]
- Messaadia, L.; Bekkar, Y.; Benamira, M.; Lahmar, H. Predicting the antioxidant activity of some flavonoids of Arbutus plant: A theoretical approach. Chem. Phys. Impact 2020, 1, 100007. [Google Scholar] [CrossRef]
- Dong, W.; Chen, D.; Chen, Z.; Sun, H.; Xu, Z. Antioxidant capacity differences between the major flavonoids in cherry (Prunus pseudocerasus) in vitro and in vivo models. LWT 2021, 141, 110938. [Google Scholar] [CrossRef]
- De Marino, S.; Festa, C.; Zollo, F.; Incollingo, F.; Raimo, G.; Evangelista, G.; Iorizzi, M. Antioxidant activity of phenolic and phenylethanoid glycosides from Teucrium polium L. Food Chem. 2012, 133, 21–28. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Bibi, A.; Ishaq, M.S.; Afridi, M.S.; Kanwal, F.; Zakir, M.; Fatima, F. Phytochemical analysis, antioxidant activity, fatty acids composition, and functional group analysis of Heliotropium bacciferum. Sci. World J. 2014, 2014, 829076. [Google Scholar] [CrossRef] [PubMed]
- Hichri, F.; Ben Salah, N.; Omri, A.; Hossan, A.S.M.; Ben Jannet, H. New antioxidant C-glycosyl flavone and flavonol derivatives from the Tunisian Achille acretica L. S. Afr. J. Bot. 2018, 116, 1–5. [Google Scholar] [CrossRef]


| Factor Level (xi) | |||
|---|---|---|---|
| Factor | Notation | Low (−1) | High (+1) |
| Ascorbic acid (%) | X1 | 0.5 | 2.0 |
| Citric acid (%) | X2 | 0.3 | 2.0 |
| Potassium sorbate (%) | X3 | 0.1 | 0.3 |
| Moringa oleifera (%) | X4 | 0.1 | 0.2 |
| Time (hours) | X5 | 7 | 15 |
| Temperature (°C) | X6 | 57 | 70 |
| Independent Variables (%) | ||||||
|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | X4 | X5 | X6 |
| 1A | 2.00 | 0.30 | 0.30 | 0.10 | 15 | 57 |
| 2A | 1.25 | 1.15 | 0.20 | 0.15 | 11 | 64 |
| 3A | 0.50 | 2.00 | 0.30 | 0.10 | 7 | 70 |
| 4A | 0.50 | 0.30 | 0.30 | 0.20 | 7 | 57 |
| 4B | 0.50 | 0.30 | 0.30 | 0.20 | 7 | 57 |
| 2B | 1.25 | 1.15 | 0.20 | 0.15 | 11 | 64 |
| 5A | 2.00 | 2.00 | 0.30 | 0.20 | 15 | 70 |
| 6A | 2.00 | 0.30 | 0.10 | 0.10 | 7 | 70 |
| 1B | 2.00 | 0.30 | 0.30 | 0.10 | 15 | 57 |
| 7A | 0.50 | 2.00 | 0.10 | 0.10 | 15 | 57 |
| 5B | 2.00 | 2.00 | 0.30 | 0.20 | 15 | 70 |
| 8A | 2.00 | 2.00 | 0.10 | 0.20 | 7 | 57 |
| 9A | 0.50 | 0.30 | 0.10 | 0.20 | 15 | 70 |
| 2C | 1.25 | 1.15 | 0.20 | 0.15 | 11 | 64 |
| 9B | 0.50 | 0.30 | 0.10 | 0.20 | 15 | 70 |
| 6B | 2.00 | 0.30 | 0.10 | 0.10 | 7 | 70 |
| 8B | 2.00 | 2.00 | 0.10 | 0.20 | 7 | 57 |
| 7B | 0.50 | 2.00 | 0.10 | 0.10 | 15 | 57 |
| 3B | 0.50 | 2.00 | 0.30 | 0.10 | 7 | 70 |
| Colour | |||
|---|---|---|---|
| Model Parameter | Lightness (L*) | Redness (+a*) | Yellowness (+b*) |
| p-value | 0.0836 | 0.0112 # | 0.0106 # |
| F-value | 2.52 | 4.72 | 4.78 |
| R2 | 0.4918 | 0.6448 | 0.6478 |
| Predicted R2 | −0.2213 | 0.1779 | 0.1882 |
| Adeq precision | 4.566 | 6.136 | 6.555 |
| Independent Variables (%) | |||||||
|---|---|---|---|---|---|---|---|
| Run | X1 | X2 | X3 | X4 | X5 | X6 | Weight Loss (%) 1 |
| 1 | 2.00 | 0.30 | 0.30 | 0.10 | 15 | 57 | 85.4 ± 0.4 |
| 2 | 1.25 | 1.15 | 0.20 | 0.15 | 11 | 64 | 87.8 ± 5.3 |
| 3 | 0.50 | 2.00 | 0.30 | 0.10 | 7 | 70 | 86.6 ± 4.3 |
| 4 | 0.50 | 0.30 | 0.30 | 0.20 | 7 | 57 | 90.2 ± 3.8 |
| 5 | 2.00 | 2.00 | 0.30 | 0.20 | 15 | 70 | 88.3 ± 2.0 |
| 6 | 2.00 | 0.30 | 0.10 | 0.10 | 7 | 70 | 88.2 ± 6.1 |
| 7 | 0.50 | 2.00 | 0.10 | 0.10 | 15 | 57 | 87.8 ± 3.8 |
| 8 | 2.00 | 2.00 | 0.10 | 0.20 | 7 | 57 | 85.4 ± 3.4 |
| 9 | 0.50 | 0.30 | 0.10 | 0.20 | 15 | 70 | 90.3 ± 6.5 |
| X1 | X2 | X3 | X4 | X5 | X6 | Colour Parameters 1 | |||
|---|---|---|---|---|---|---|---|---|---|
| Run | (%) | (%) | (%) | (%) | (h) | (°C) | L* | a* | b* |
| 1 | 2.00 | 0.30 | 0.30 | 0.10 | 15 | 57 | 78.9 ± 2.6 | 4.0 ± 2.5 | 22.8 ± 4.3 |
| 2 | 1.25 | 1.15 | 0.20 | 0.15 | 11 | 64 | 77.8 ± 1.4 | 3.7 ± 1.1 | 25.3 ± 1.9 |
| 3 | 0.50 | 2.00 | 0.30 | 0.10 | 7 | 70 | 81.90 ± 1.2 | 2.1 ± 0.3 | 21.3 ± 1.9 |
| 4 | 0.50 | 0.30 | 0.30 | 0.20 | 7 | 57 | 75.0 ± 4.4 | 6.7 ± 2.6 | 23.9 ± 4.7 |
| 5 | 2.00 | 2.00 | 0.30 | 0.20 | 15 | 70 | 82.0 ± 1.2 | 3.4 ± 0.3 | 28.0 ± 0.8 |
| 6 | 2.00 | 0.30 | 0.10 | 0.10 | 7 | 70 | 78.2 ± 0.7 | 4.3 ± 0.9 | 24.5 ± 2.0 |
| 7 | 0.50 | 2.00 | 0.10 | 0.10 | 15 | 57 | 81.4 ± 0.5 | 2.4 ± 0.5 | 23.5 ± 0.5 |
| 8 | 2.00 | 2.00 | 0.10 | 0.20 | 7 | 57 | 77.7 ± 3.7 | 3.7 ± 1.8 | 27.0 ± 0.8 |
| 9 | 0.50 | 0.30 | 0.10 | 0.20 | 15 | 70 | 76.0 ± 5.7 | 6.5 ± 1.2 | 29.2 ± 0.8 |
| p-Value | |||
|---|---|---|---|
| Model Parameter | Lightness (L*) | Redness (+a*) | Yellowness (+b) |
| Ascorbic acid | 0.7034 | 0.3646 | 0.3151 |
| Citric Acid | 0.0539 # | 0.0022 # | - |
| Potassium sorbate | - | - | 0.0714 |
| MOLEP | 0.0149 # | 0.0136 # | 0.0022 # |
| Time | 0.8403 | 0.8169 | 0.1307 |
| Temperature | 0.8849 | 0.8698 | 0.1817 |
| Average Rt (min) | Average Mz | Structure Rank 1 | Formula | Total Score | Ontology | Classification |
|---|---|---|---|---|---|---|
| 7.097 | 570.093 | UNPD32461 | C30H21NO11 | 5.902 | Xanthones | Alkaloid |
| 7.186 | 205.063 | Scoparone | C11H10O4 | 5.961 | Coumarins and derivatives | Flavonoid |
| 7.490 | 261.026 | Maclurin | C13H10O6 | 5.438 | Benzophenones | Flavonoid |
| 7.602 | 353.102 | Chlorogenic acid | C16H18O9 | 7.943 | Quinic acids and derivatives | Flavonoid |
| 7.707 | 323.145 | Blumealactone C | C17H24O6 | 5.829 | Terpene lactones | Flavonoid |
| 7.824 | 315.129 | Gibberellin A9; GA9 | C19H24O4 | 7.719 | C19-gibberellin 6-carboxylic acids | Phytohormone |
| 8.115 | 353.108 | (+)-Sesamin | C20H18O6 | 8.836 | Furanoid lignans | Flavonoid |
| 8.404 | 447.160 | 4-hydroxymethyl-2-methoxyphenyl-1-O-beta-D-apiofuranosyl-(1->6)-O-beta-D-glucopyranoside | C19H28O12 | 6.893 | Phenolic glycosides | Flavonoid |
| 8.718 | 427.185 | Furcatin | C20H28O10 | 6.067 | Phenolic glycosides | Flavonoid |
| 9.006 | 461.175 | Verbasoside | C20H30O12 | 5.839 | O-glycosyl compounds | Flavonoid |
| 9.126 | 439.192 | 10-deacetyl-2-debenzoylbaccatin III | C22H32O9 | 5.483 | Taxanes and derivatives | Alkaloid |
| 9.295 | 324.129 | Monocrotaline | C16H23NO6 | 7.420 | Pyrrolizines | Alkaloid |
| 9.987 | 261.157 | [1R-(1alpha,4abeta,6alpha,8aalpha)]-1,2,4a,5,6,8a-Hexahydro-6-hydroxy-4,7-dimethyl-a-methylene-1-naphthaleneacetic acid methyl ester | C16H22O3 | 7.599 | Sesquiterpenoids | Flavonoid |
| 10.227 | 533.098 | Luteolin 7-O-(6′′-malonylglucoside) | C24H22O14 | 5.766 | Flavonoid-7-O-glycosides | Flavonoid |
| 10.478 | 187.126 | Dimethyltryptamine | C12H16N2 | 7.863 | Tryptamines and derivatives | Alkaloid |
| 10.628 | 366.138 | Isatidine | C18H25NO7 | 6.541 | Alkaloids and derivatives | Alkaloid |
| 10.954 | 366.137 | Casuarine 6-alpha-D-glucoside | C14H25NO10 | 7.134 | O-glycosyl compounds | Alkaloid |
| 12.919 | 329.249 | 9,12,13-TriHOME | C18H34O5 | 6.984 | Long-chain fatty acids | Fatty Acid |
| 13.938 | 313.261 | Dronabinol | C21H30O2 | 6.485 | 2,2-dimethyl-1-benzopyrans | Alkaloid |
| 14.123 | 311.242 | 9(S)-HPODE | C18H32O4 | 5.954 | Lineolic acids and derivatives | Fatty acid |
| 14.351 | 295.244 | Alpha-dimorphecolic acid | C18H32O3 | 6.983 | Lineolic acids and derivatives | Fatty acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arendse, W.; Jideani, V. Effects of Some Weak Acids and Moringa oleifera Leaf Extract Powder on the Colour of Dried Apple. Processes 2022, 10, 206. https://doi.org/10.3390/pr10020206
Arendse W, Jideani V. Effects of Some Weak Acids and Moringa oleifera Leaf Extract Powder on the Colour of Dried Apple. Processes. 2022; 10(2):206. https://doi.org/10.3390/pr10020206
Chicago/Turabian StyleArendse, Washiela, and Victoria Jideani. 2022. "Effects of Some Weak Acids and Moringa oleifera Leaf Extract Powder on the Colour of Dried Apple" Processes 10, no. 2: 206. https://doi.org/10.3390/pr10020206
APA StyleArendse, W., & Jideani, V. (2022). Effects of Some Weak Acids and Moringa oleifera Leaf Extract Powder on the Colour of Dried Apple. Processes, 10(2), 206. https://doi.org/10.3390/pr10020206






