Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of the Volatile Oil
2.2. Gas Chromatographic Analysis
2.3. Antimicrobial Activity Determination
2.3.1. Test Microorganisms
2.3.2. Disk Diffusion Assays
2.4. Molecular Docking
2.4.1. Preparation of the Ligand
2.4.2. Preparation of the Receptors
3. Results and Discussion
3.1. Phytochemical Analysis of R. officinalis L. Essential Oil
3.2. Antimicrobial Activity
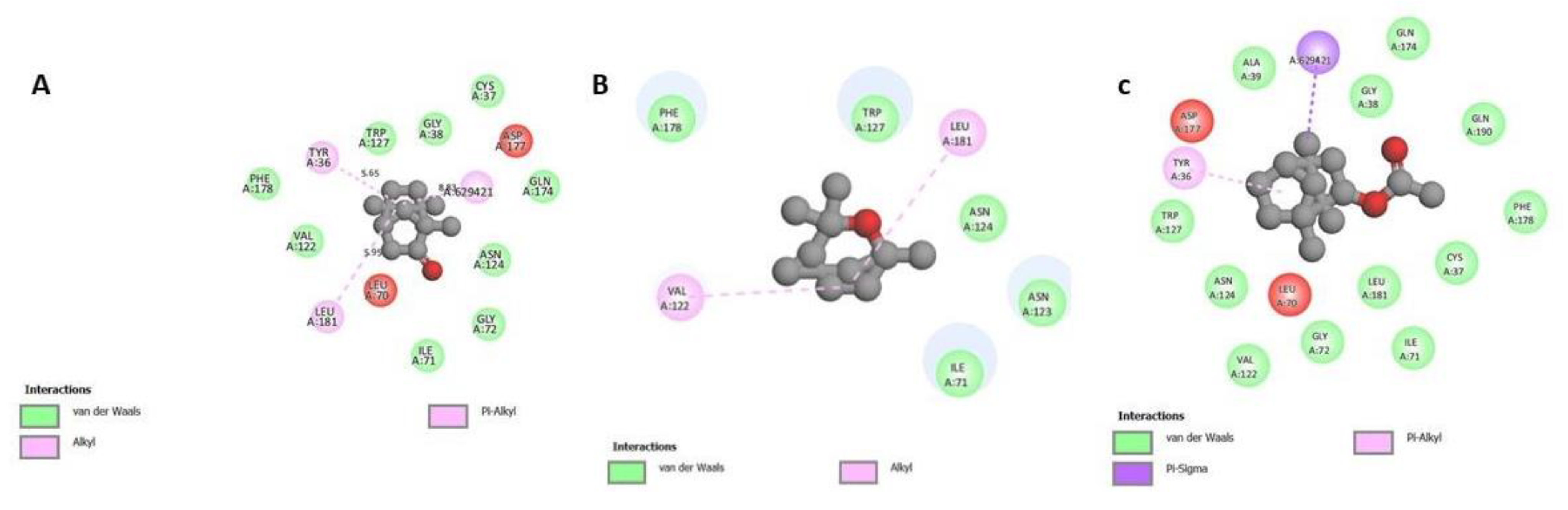
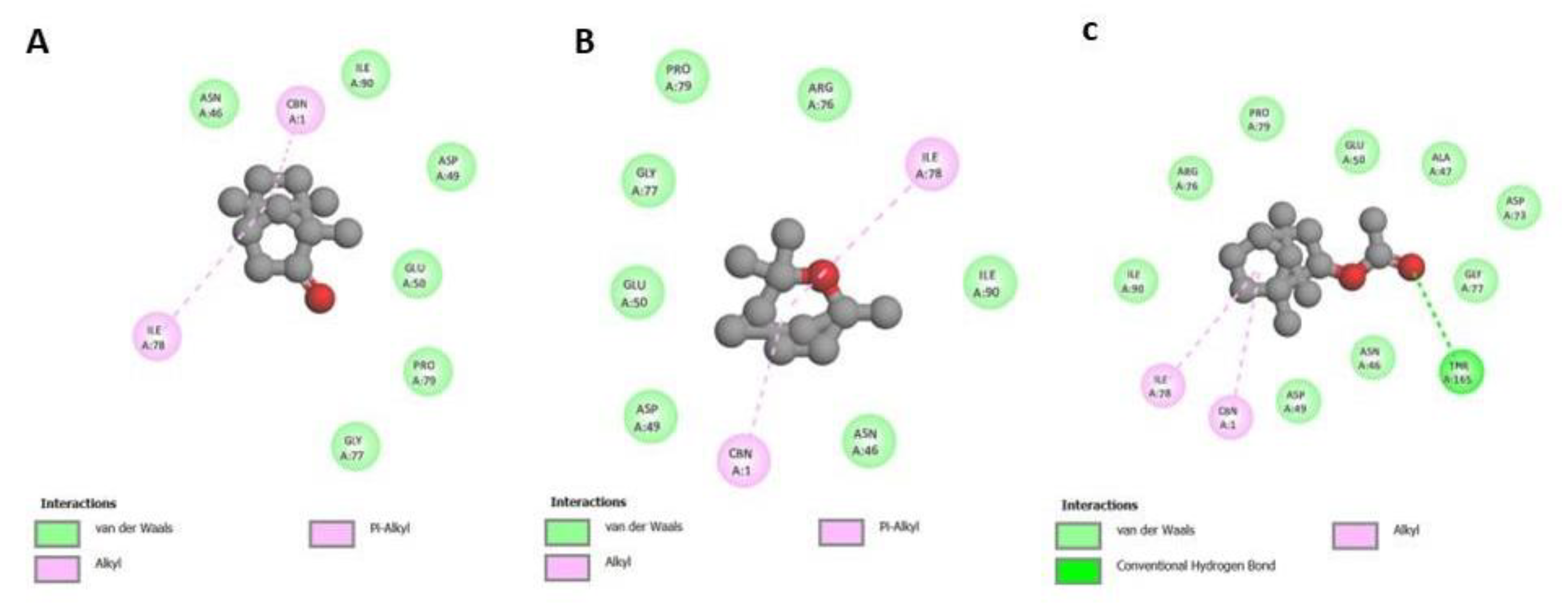
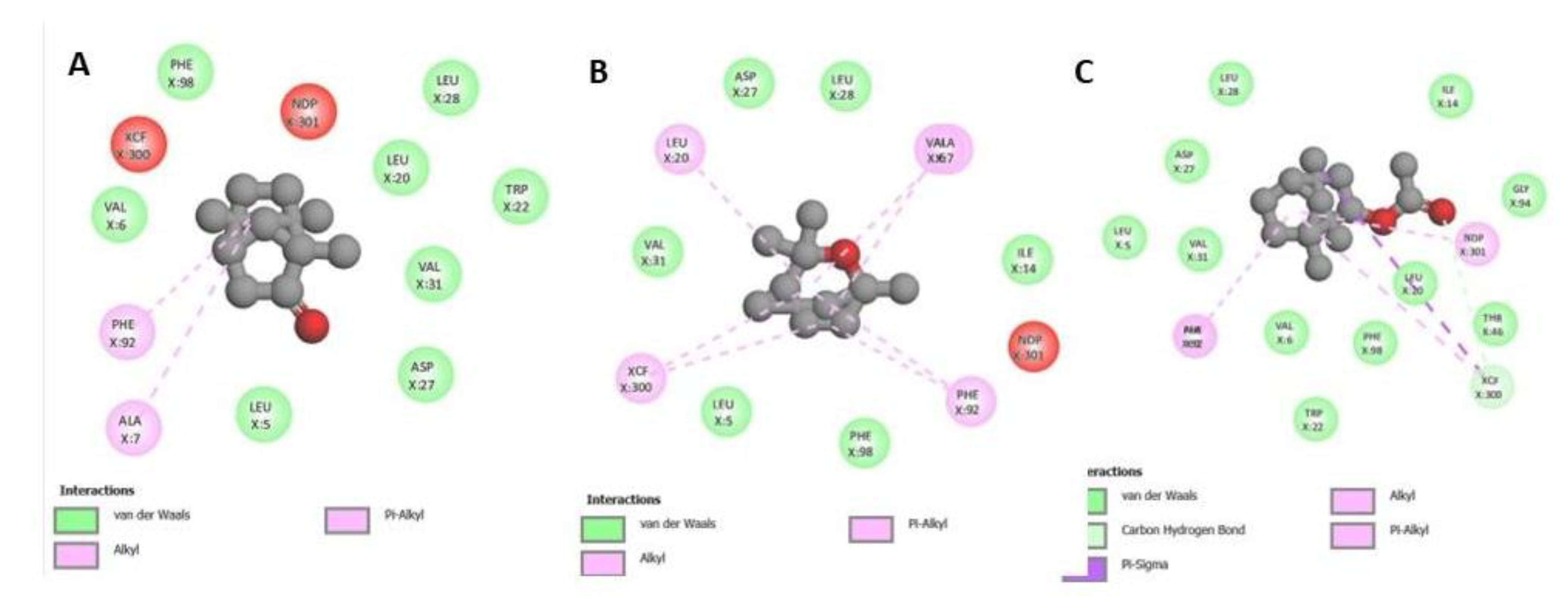
3.3. Molecular Docking
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.C.; Lorenzi, H. Botânica Sistemática: Guia Ilustrado Para Identificação Das Famílias de Angiospermas Da Flora Brasileira, Baseado Em APG II; Instituto Plantarum: Nova Odessa, 2005; ISBN 978-85-86714-21-4. [Google Scholar]
- Quézel, P.; Santa, S. Nouvelle Flore de L’Algérie et des Régions Désertiques Méridionales; Editions du Centre National de la Recherche Scientifique: Paris, France, 1962. [Google Scholar]
- González-Trujano, M.E.; Peña, E.I.; Martínez, A.L.; Moreno, J.; Guevara-Fefer, P.; Déciga-Campos, M.; López-Muñoz, F.J. Evaluation of the Antinociceptive Effect of Rosmarinus Officinalis L. Using Three Different Experimental Models in Rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Milyuhina, A.K.; Zabodalova, L.A.; Kyzdarbek, U.; Romazyaeva, I.R.; Klyuchko, N.Y. In Vitro Antibacterial and Antioxidant Activity of Rosmarinus Officinalis. E3S Web. Conf. 2021, 285, 05012. [Google Scholar] [CrossRef]
- Hamidpour, R.; Elias, G.; Hamidpour, S. Rosmarinus Officinalis (Rosemary): A Novel Therapeutic Agent for Antioxidant, Antimicrobial, Anticancer, Antidiabetic, Antidepressant, Neuroprotective, AntiInflammatory, and Anti-Obesity Treatment. Biomed. J. Sci. Tech. Res. 2017, 1, 1098–1103. [Google Scholar]
- Pérez-Fons, L.; Garzón, M.T.; Micol, V. Relationship between the Antioxidant Capacity and Effect of Rosemary (Rosmarinus officinalis L.) Polyphenols on Membrane Phospholipid Order. J. Agric. Food. Chem. 2010, 58, 161–171. [Google Scholar] [CrossRef]
- Yu, M.-H.; Choi, J.-H.; Chae, I.-G.; Im, H.-G.; Yang, S.-A.; More, K.; Lee, I.-S.; Lee, J. Suppression of LPS-Induced Inflammatory Activities by Rosmarinus officinalis L. Food Chem. 2013, 136, 1047–1054. [Google Scholar] [CrossRef]
- Bakirel, T.; Bakirel, U.; Keleş, O.U.; Ulgen, S.G.; Yardibi, H. In Vivo Assessment of Antidiabetic and Antioxidant Activities of Rosemary (Rosmarinus officinalis) in Alloxan-Diabetic Rabbits. J. Ethnopharmacol. 2008, 116, 64–73. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Singletary, K.; MacDonald, C.; Wallig, M. Inhibition by Rosemary and Carnosol of 7,12-Dimethylbenz[a]Anthracene (DMBA)-Induced Rat Mammary Tumorigenesis and in Vivo DMBA-DNA Adduct Formation. Cancer Lett. 1996, 104, 43–48. [Google Scholar] [CrossRef]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of Skin Tumorigenesis by Rosemary and Its Constituents Carnosol and Ursolic Acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation Effect of Rosemary (Rosmarinus officinalis) on Human Ovarian Cancer Cells in Vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; García-Cañas, V.; Rocamora-Reverte, L.; Gómez-Martínez, Á.; Ferragut, J.A.; Cifuentes, A. Effect of Rosemary Polyphenols on Human Colon Cancer Cells: Transcriptomic Profiling and Functional Enrichment Analysis. Genes Nutr. 2013, 8, 43–60. [Google Scholar] [CrossRef]
- Habtemariam, S. The Therapeutic Potential of Rosemary (Rosmarinus officinalis) Diterpenes for Alzheimer’s Disease. Evid. Based Complement. Alternat. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Surburg, H.; Panten, J. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley: Hoboken, NJ, USA, 2006; p. 8214. [Google Scholar] [CrossRef]
- Jawad, A.M.; Allawi, A.K.; Ewadh, H.M. Essential Oils of Rosemary as Antimicrobial Agent against Three Types of Bacteria. Med. J. Babylon 2018, 15, 53. [Google Scholar] [CrossRef]
- Napoli, E.M.; Siracusa, L.; Saija, A.; Speciale, A.; Trombetta, D.; Tuttolomondo, T.; La Bella, S.; Licata, M.; Virga, G.; Leone, R.; et al. Wild Sicilian Rosemary: Phytochemical and Morphological Screening and Antioxidant Activity Evaluation of Extracts and Essential Oils. Chem. Biodivers. 2015, 12, 1075–1094. [Google Scholar] [CrossRef]
- Khalil, M.I.M.; Ibrahim, M.M.; El-Gaaly, G.A.; Sultan, A.S. Trigonella foenum (Fenugreek) Induced Apoptosis in Hepatocellular Carcinoma Cell Line, HepG2, Mediated by Upregulation of P53 and Proliferating Cell Nuclear Antigen. BioMed Res. Int. 2015, 2015, e914645. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream 2005, 16, 65–120. [Google Scholar]
- Wiley/NBS Registry of Mass Spectral Data. Available online: https://analyticalscience.wiley.com/do/10.1002/sepspec.9780471628866 (accessed on 25 October 2022).
- Lazreg-Aref, H.; Bel Hadj Salah, K.; Fekih, A.; Chemli, R.; Mars, M.; Aouni, M.; Chaumon, J.; Said, K. Variability in Antimicrobial Activity of Latex from Two Varieties of Ficus Carica. Afr. J. Microbiol. Res. 2011, 5, 665–672. [Google Scholar] [CrossRef]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant Activity of Rosemary (Rosmarinus officinalis L.) Essential Oil and Its Hepatoprotective Potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Bernardes, W.A.; Lucarini, R.; Tozatti, M.G.; Flauzino, L.G.B.; Souza, M.G.M.; Turatti, I.C.C.; Andrade e Silva, M.L.; Martins, C.H.G.; da Silva Filho, A.A.; Cunha, W.R. Antibacterial Activity of the Essential Oil from Rosmarinus Officinalis and Its Major Components against Oral Pathogens. Z. Naturforsch. C J. Biosci. 2010, 65, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, M.M.; Chalchat, J.-C. Chemical Composition and Antifungal Activity of Rosemary (Rosmarinus Officinalis L.) Oil from Turkey. Int. J. Food Sci. Nutr. 2008, 59, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, N.; Fu, Y.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Liu, X.-L. Chemical Composition and Antimicrobial Activity of the Essential Oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Gachkar, L.; Yadegari, D.; Rezaei, M.B.; Taghizadeh, M.; Astaneh, S.A.; Rasooli, I. Chemical and Biological Characteristics of Cuminum Cyminum and Rosmarinus Officinalis Essential Oils. Food Chem. 2007, 102, 898–904. [Google Scholar] [CrossRef]
- Bendeddouche, M.S.; Benhassaini, H.; Hazem, Z.; Romane, A. Essential Oil Analysis and Antibacterial Activity of Rosmarinus Tournefortii from Algeria. Nat. Prod. Commun. 2011, 6, 1511–1514. [Google Scholar] [CrossRef]
- Pintore, G.; Usai, M.; Bradesi, P.; Juliano, C.; Boatto, G.; Tomi, F.; Chessa, M.; Cerri, R.; Casanova, J. Chemical Composition and Antimicrobial Activity of Rosmarinus officinalis L. Oils from Sardinia and Corsica. Flavour Fragr. J. 2002, 17, 15–19. [Google Scholar] [CrossRef]
- Soković, M.; van Griensven, L.J.L.D. Antimicrobial Activity of Essential Oils and Their Components against the Three Major Pathogens of the Cultivated Button Mushroom, Agaricus Bisporus. Eur. J. Plant Pathol. 2006, 116, 211–224. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical Composition and Antifungal Activity of the Essential Oils of Lavandula Pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1292. [Google Scholar] [CrossRef]
- Peng, Y.; Yuan, J.; Liu, F.; Ye, J. Determination of Active Components in Rosemary by Capillary Electrophoresis with Electrochemical Detection. J. Pharm. Biomed. Anal. 2005, 39, 431–437. [Google Scholar] [CrossRef]
- Ugur, A.; Sarac, N.; Duru, M.E. Antimicrobial Activity and Chemical Composition of Senecio Sandrasicus on Antibiotic Resistant Staphylococci. Nat. Prod. Commun. 2009, 4, 579–584. [Google Scholar] [CrossRef]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of Antilisterial Action of Cilantro Oil on Vacuum Packed Ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]


| Receptors | ID | Resolution (Å) | Class |
|---|---|---|---|
| TyRS | 1jij | 3.20 Å | Ligase |
| DNA gyrase | 1KZN | 2.30 Å | Isomerase |
| DHFR | 3fyv | 2.20 Å | Oxidoreductase |
| Compound Name | Chemical Formula | Molecular Weight (g/mol) | RT (min) | % Area |
|---|---|---|---|---|
| Alpha-Pinene | C10H16 | 136.23 | 6.094 | 3.85 |
| Camphene | C10H16 | 136.23 | 6.339 | 4.1 |
| Beta-Pinene | C10H16 | 136.23 | 6.784 | 3.41 |
| Eucalyptol | C10H18O | 154.25 | 7.654 | 17.38 |
| Gamma-Terpinene | C10H16 | 136.23 | 8.079 | 0.28 |
| Camphor | C10H16O | 152.23 | 9.469 | 10.42 |
| Borneol | C10H18O | 154.25 | 9.809 | 9.78 |
| Terpinen-4-ol | C10H18O | 154.25 | 9.951 | 1.17 |
| Alpha-Terpineol | C10H18O | 154.25 | 10.15 | 3.77 |
| Bornyl acetate | C12H20O2 | 196.29 | 11.511 | 26.6 |
| Beta-Caryophyllene | C15H24 | 204.35 | 13.365 | 7.8 |
| Alpha-Caryophyllene | C15H24 | 204.35 | 13.79 | 1.06 |
| Cadina-1(10),4-diene | C15H24 | 204.35 | 14.613 | 0.32 |
| Caryophyllene oxide | C15H24O | 220.35 | 15.416 | 1.19 |
| Bicyclo [4.4.0]dec-1-ene, 2-isopropyl-5-methyl-9-methylene- | C15H24 | 204.35 | 16.05 | 0.33 |
| Linoleic acid | C18H32O2 | 280.4 | 20.995 | 0.5 |
| Gamma-Sitosterol | C29H50O | 414.7 | 27.33 | 0.89 |
| Lupeol | C30H50O | 426.7 | 28.559 | 7.18 |
| Affinities (Kcal/mol) | |||
|---|---|---|---|
| TyrRS | DNA Gyrase | DHFR | |
| Camphor | −4.8 | −4.5 | −6.0 |
| Eucalyptol | −4.8 | −4.6 | −5.8 |
| Bornyl acetate | −4.9 | −4.9 | −6.0 |
| Clorobiocin | −8.2 | −9.1 | - |
| SCHEMBL2181345 | - | - | −6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, A.; Al Kamaly, O.; Alanazi, A.S.; Noman, O. Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches. Processes 2022, 10, 2422. https://doi.org/10.3390/pr10112422
Saleh A, Al Kamaly O, Alanazi AS, Noman O. Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches. Processes. 2022; 10(11):2422. https://doi.org/10.3390/pr10112422
Chicago/Turabian StyleSaleh, Asmaa, Omkulthom Al Kamaly, Ashwag S. Alanazi, and Omar Noman. 2022. "Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches" Processes 10, no. 11: 2422. https://doi.org/10.3390/pr10112422
APA StyleSaleh, A., Al Kamaly, O., Alanazi, A. S., & Noman, O. (2022). Phytochemical Analysis and Antimicrobial Activity of Rosmarinus officinalis L. Growing in Saudi Arabia: Experimental and Computational Approaches. Processes, 10(11), 2422. https://doi.org/10.3390/pr10112422






